 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Ultrasonic-assisted extraction of flavonoids from Camellia fascicularis leaves was optimized using response surface methodology. The optimal extracting conditions of flavonoids were determined to be the ratio of liquid to raw material of 60 mL/g, ethanol concentration of 40%, extraction temperature of 72.3°C, and extraction time of 1.6 h, which contributed to the highest flavonoids yield of 4.765%. The crude flavonoids were purified through an AB-8 macroporous adsorption resin column, eluted with ethanol concentration of 40%, and purified flavonoids were obtained. In vitro antioxidant assay demonstrated that purified flavonoids showed significant antioxidant capacities in a concentration-dependent manner for scavenging hydroxyl radical, superoxide anion radical, 2,2-diphenyl-1-picrylhydrazyl radical, and azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt radical. Overall, ultrasonic-assisted extraction was found to be an effective method of extracting flavonoids from C. fascicularis leaves, which might be explored as potential natural antioxidant.
RESUMEN
Mediante el uso de la metodología de superficies de respuesta (msr) se optimizó la extracción de flavonoides obtenidos de hojas de Camellia fascicularis asistida por ultrasonido. Al respecto, se estableció que las condiciones óptimas para la extracción de flavonoides son: ratio de líquido a materia prima de 60 mL/g, concentración de etanol de 40%, temperatura de extracción de 72,3°C y tiempo de extracción de 1,6 horas; a partir de estas condiciones se determinó que el máximo rendimiento de los flavonoides es 4,765%. Empleando una columna de resina de adsorción macroporosa AB-8 se purificaron los flavonoides crudos, siendo posteriormente eluidos con etanol en una concentración de 40%. El ensayo mediante antioxidante in vitro demostró que, dependiendo de su concentración, los flavonoides purificados tienen capacidades antioxidantes significativas para eliminar radicales de hidroxilo, anión superóxido, DPPH y ABTS. En general, se constató que la extracción asistida por ultrasonido constituye un método eficaz para la extracción de flavonoides de hojas de C. fascicularis, los cuales pueden ser sometidos a investigación como un potente antioxidante natural.
Introduction
Camellia fascicularis Hung T. Chang (Theaceae), one of Camellia species, is a ornamental and valuable plants species with yellow flower (Min & Bartholomew, Citation2007; Zhang & Ren, Citation1998). It was distributed in Gejiu City, Maguan County, and Hekou County of Yunnan province with about 663 single plants (Min & Bartholomew, Citation2007; Zhang, Zhang, Zhang, Wang, & Ping Citation2015). Because of limited distribution and suffered destruction, it have been listed precious tree species in Yunnan province. According to the IUCN evaluation criteria, C. fascicularis, with another 650 kinds of angiosperm, belongs to ‘critically endangered’ grades (Wang & Xie, Citation2004; Xie & Wang, Citation2007). The plants of series of Camellia L. have been explored as an important biological material used in food industry because of the high content of bioactive components, including flavonoids (Azuma, Santos, & Lago, Citation2011; Maran, Manikandan, Priya, & Gurumoorthi, Citation2013), polysaccharides (Feng et al., Citation2014; Zhang, Citation2015), polyphenols (Georgiev, Zhelev, & Georgieva, Citation2014; Zielinski et al., Citation2015), and saponins (He et al., Citation2014; Liu, Li, Xu, & Han, Citation2016) in their flowers and leaves. Meanwhile, bioactive components extracted from Camellia chrysancha have been proven to exhibit potent biological activity (Oku, Ogawa, Iwaoka, Yu, & Kagota, Citation2011; Peng, Yu, Feng, Wang, & Shi, Citation2012; Song, Wang, Zheng, & Huang, Citation2011; Wang et al., Citation2016; Wei et al., Citation2015).
In recent years, flavonoids, derived from plants, have attracted broad attention for a wide range of biological functions such as antioxidant (Boudkhili et al., Citation2015; Sait et al., Citation2015), antitumor (Guo, Citation2013; Radhika, Ghoshal, & Chatterjee, Citation2012), immunological (Ciftci et al., Citation2015; Liu, Zhao, Ma, Ding, & Su, Citation2012), antimicrobial (Dzoyem, Hamamoto, Ngameni, Ngadjui, & Sekimizu, Citation2013; Liu et al., Citation2010), and antihyperlipidemic (Srinivasan & Pari, Citation2013; Zhang, Wu, Ma, Cheng, & Liu, Citation2015) activities. However, there was almost no attention paid to the flavonoids from C. fascicularis. Up to now, there was no investigation carried out on the optimization of ultrasonic extraction and antioxidant capacity of flavonoids from leaves of C. fascicularis.
Ultrasonic-assisted extraction is also one of the important techniques for extracting functional components from different plant materials. Due to the mechanical effects of ultrasound, ultrasonic-assisted extraction, which can shorten extraction time, save solvent, increase extraction yield, and avoid high temperature to the destruction of the active ingredients, has obvious advantages in the extraction of natural products. Response surface methodology (RSM) is widely employed for constructing and exploring estimated functional relationship between a response variable and design variables and used to model and optimize biochemical and biotechnological process related to food systems (Hammi, Jdey, Abdelly, Majdoub, & Ksouri, Citation2015; Liyana-Pathirana & Shahidi, Citation2005; Lu et al., Citation2013).
In this study, C. fascicularis leaves were taken to extract flavonoids. Based on the single factor experiment, the extraction process was optimized by central composite design (CCD). In addition, the fraction was purified from crude flavonoids. The antioxidant scavenging effect of purified flavonoids was evaluated by in vitro antioxidant assay, including hydroxyl radical scavenging capacity, superoxide anion radical scavenging capacity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity, and azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical scavenging capacity. This study is to provide theoretical basis for further exploitation and utilization of C. fascicularis.
Materials and methods
Materials
The C. fascicularis leaves were collected from Hekou branch of administration bureau of Daweishan National Nature Reserve in Hekou of Yunnan province, China. The leaves were washed, dried until the moisture content was about 3% at 60°C, pulverized into powder, sieved through 60 meshes, and then stored in a dry and shade place until use.
Chemicals
DPPH, tris(hydroxymethyl)aminomethane(tris), 2,2′-ABTS, ascorbic acid (abbreviation for Vc), pyrogallol, and 1,10-phenanthroline were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Rutin was purchased from National Institutes for Food and Drug Control (Beijing, China). Tea polyphenols (TP) were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd (Beijing, China). AB-8 macroporous adsorption resin was purchased from The Chemical Plant of NanKai University (Tianjin, China). All other chemical reagents were of analytical grade.
Ultrasonic-assisted extraction of flavonoids
The extraction process was based on the previous method with minor modification (Liu, Ma, Liu, Yang, & Zhang, Citation2014). The dried leaves powder was defatted with ether. The pretreated samples were soaked in ethanol solution and then extracted in an ultrasound cleaner (SB25-12DTDS, Ningbo Xin Yi Ultrasound instrument Co., Ltd., Zhejiang, China) at a frequency of 45 kHz and power of 500 W. The leaves extract was collected by filtration and concentrated using a rotary evaporator (R210, BUCHI Labortechnik AG, Flawil, Switzerland) at 55°C under vacuum. The concentrated extract was dried using freeze dryer (FD5-3, SIM International Group Co., Ltd., Newark, NJ, USA). The single factor experiment was performed with the factors as follows: ratio of liquid to raw material, ethanol concentration, extraction temperature, and extraction time. Each single factor experiment was repeated thrice.
Measurement of flavonoids
The standard curve was constructed using solutions of rutin at 0.008, 0.016, 0.032, 0.048, and 0.064 mg/mL, respectively. Using the concentration of rutin in standard solution (C) as abscissa and the absorbency (A) as ordinate, the linear chart was constructed. The regression equation was A = 11.7591C + 0.0043 (R2 = 0.9991). The contents of flavonoids were measured according to colorimetric method with slight modification (Zhu, Wang, Liu, Xia, & Tang, Citation2010). A volume of 1 mL flavonoids extract was accurately removed in a volumetric flask (25 mL) by adding 10 mL of ethanol (50%) solution, 1 mL of NaNO2 (5%) solution, shaken up and standing for 6 min. Second, 1 mL of the Al(NO3)3 (10%) solution was added to the volumetric flask, shaken and was left to standing for 6 min. Finally, 10 mL of the NaOH (4%) solution was added to the volumetric flask and followed by adding ethanol (50%) solution to the scale, shaken and left to stand for 15 min before determination. A wavelength of 510 nm was used to determine the content of flavonoids in samples by a Shimadzu UV-2600 spectrophotometer (Japan) against the same mixture solution, without using the sample as a blank. Each experiment was repeated thrice.
Experimental design
On the basis of single-factor test, four independent variables, including ratio of liquid to raw material, ethanol concentration, extraction temperature, and extraction time, significantly affected the yield of flavonoids extraction. A CCD was applied to statistically study the effects of four independent variables, namely, the liquid to raw material (X1), ethanol concentration (X2), extraction temperature (X3), and extraction time (X4) on the yield of flavonoids (Y). The independent variables were designated as ‘−α (−2),’ ‘−1,’ ‘0,’ ‘+1,’ and ‘+α (+2)’ for low, intermediate, and high levels, and the axial or star points, respectively. The value of α was fixed at 2 to make the design a rotatable one. The independent variables and their levels in CCD are listed in . The complete design consisted of 30 experimental runs and the independent variables and experimental results in CCD are given in . The behaviour of the system was explained by the following quadratic equation:
Table 1. Independent variables and their levels in CCD.
Tabla 1. Variables independientes y sus niveles en DCC (diseño compuesto central).
Table 2. CCD for independent variables and experimental results of the response surface methodology.
Tabla 2. DCC para las variables independientes y resultados experimentales de la metodología de superficies de respuesta.
where b0, b1, bii, and bij are the constant tem, regression coefficients of the liner effects, quadratic effects, and interaction effects between the variables, respectively. xi and xj are the levels of the independent variables.
The Design-Expert software (trial version 8.0.6.1, Stat-Ease Inc., Minneapolis, MN, USA) was used to perform the complete CCD design. The correlation coefficient, R2, adjusted correlation coefficient, , and lack-of-fit test were taken to evaluated the model adequacy. Regression analysis and three-dimensional (3D) response surface plots were plotted to determine optimum conditions for extraction yield of flavonoids. Under optimal extraction conditions, the experiments for five verification runs were conducted to verify the availability of the statistical experimental strategies.
Preparation of purified fractions of flavonoids
The purification assay was based on the previous method with modification (Wen, Liu, Zhang, & Guo, Citation2013). The pretreated leaves powder was extracted under the optimal conditions, and the crude flavonoids extract was dissolved and purified through an AB-8 macroporous adsorption resin column, which eluted with ethanol concentration of 40%. The purified flavonoids were collected, concentrated under vacuum, lyophilized, and then stored for further study.
Antioxidant capacities of purified flavonoids
Assay of hydroxyl radical scavenging capacity
The antioxidant capacity of scavenging hydroxyl radical was determined according to the method with slight modification (Shen et al., Citation2014). The samples were first dissolved in deionized water for a series of different concentration solutions (0.2, 0.4, 0.6, 0.8, and 1 mg/mL). Then, the sample solution (1 mL) was mixed with 1,10-phenanthroline ethanol solution (5 mM, 1 mL), potassium phosphate buffer (100 mM, pH 7.4, 4.5 mL), ferrous sulfate (1 mM, 1 mL), and H2O2 (0.1%, 1 mL) at 37°C for 60 min. The absorbance of the mixture was measured at 510 nm against a blank. The capacity of scavenging hydroxyl radical was calculated by following equation:
where A0 is the absorbance of the mixture solution which the sample is replaced by deionized water, A1 is the absorbance of the mixture solution which the sample is replaced by H2O2, and A2 is the absorbance of the mixture solution with sample.
Assay of superoxide anion radical scavenging capacity
The scavenging ability of superoxide anion radical was measured by the method described by Shen et al. and Tumbas et al. with a minor modification (Shen et al., Citation2014; Tumbas, Canadanovic-Brunet, Gille, Dilas, & Cetkovic, Citation2010). The samples were dissolved in deionized water and diluted a series of sample solutions with different concentrations (0.05, 0.1, 0.15, 0.2, and 0.25 mg/mL), respectively. A aliquot of each sample (1 mL) was mixed with 4.5 mL of 50 mM Tris–HCL buffer (pH 8.2), shaken up, and was incubated at 25°C for 20 min. Then, the reaction mixture was mixed with 0.3 mL of 3 mM pyrogallol, shaken up and was incubated at 25°C for 5 min. The absorbance was measured at 325 nm. The capacity of scavenging superoxide anion radical was calculated by following equation:
where A1 is the average absorbance value of the sample and A0 is the absorbance value of the control, which the sample is replaced by deionized water.
Assay of DPPH radical scavenging capacity
The scavenging effect of DPPH radicals was carried out by the previous method with minor modification (Balasumdram, Ai, Sambanthamurthi, Sundram, & Samman, Citation2005). A volume of 0.5 mL flavonoid samples at different concentrations (0.05, 0.1, 0.15, 0.2, and 0.25 mg/mL) were incubated with 4.0 mL ethanol solution of DPPH (0.1 mM) at room temperature for 30 min in dark, respectively. The absorbance was recorded at 517 nm using standard Vc as a positive. The DPPH radical scavenging capacity was calculated using the following equation:
where A1 is the absorbance of a mixture of sample and DPPH solution and A0 is the absorbance of the control reaction in which the sample is replaced by ethanol.
Assay of ABTS radical scavenging capacity
The scavenging effect of ABTS radicals was carried out by the previous method with minor modification (Chen & Yen, Citation2007; Re et al., Citation1999). The ABTS stock solution (ABTS radical cation, ABTS+) was first produced by reacting ABTS solution (7 mM) with 2.45 mM potassium persulfate, with the proportion of 1:2, and allowing the mixture to stand in the dark at room temperature for 12–18 h. Then, the stock solution was diluted with ethanol to an absorbance of 0.700 ± 0.020 at 734 nm and equilibrated at 30°C; the ABTS working solution was obtained. For the study of the ABTS scavenging capacity, the samples were dissolved in deionized water and diluted a series of sample solutions with different concentrations (0.05, 0.1, 0.15, 0.2, and 0.25 mg/mL), respectively. A aliquot of each sample (0.1 mL) was mixed with 4 mL ABTS working solution, shaken up, and was placed at room temperature for 6 min in dark, respectively. The absorbance was measured at 734 nm. The capacity of scavenging ABTS radical was calculated by following equation:
where A0 is the absorbance of the mixture solution which the sample is replaced by ethanol, A1 is the absorbance of the mixture solution with sample and ABTS working solution, and A2 is the absorbance of the mixture solution which ABTS working solution is replaced ethanol.
Statistical analysis
All data were expressed as mean ± standard deviation from triplicate. Statistical analysis was evaluated by analysis of variance. Difference was considered to be statistically significant if P < 0.05.
Results and discussion
Statistical analysis and the model fitting
The extraction yield of the flavonoids from C. fascicularis leaves was optimized with the RSM based on the independent variables, including ratio of liquid to raw material, ethanol concentration, extraction temperature, and extraction time. The results of the optimization experiments are given in . By applying multiple regression analysis on the experimental data, the response variable and test variables were exhibited by the following second-order polynomial equation:
As shown in , the correlation coefficient (R2) was calculated to be 0.9585 suggesting that the yield variation of 95.85% was attributed to be the variable factors; the value of adjusted correlation coefficient (adjusted R2 = 0.9198) also demonstrated that the regression model was suitable for the actual situation. The data showed a good fit with Equation (5) and the highly significant of the model, which was statistically acceptable at P < 0.01 level (P < 0.0001). Moreover, the lack of fit with P value of 0.2479 indicated that the model equation was qualified to predict the extraction yield of flavonoids within the range of experimental variables. However, the analysis showed that the studied factors influence the flavonoids yield in the following order: ethanol concentration (F-value = 150.79) > ratio ofliquid to raw material (F-value = 1126.19) > extraction time (F value = 10.81) > extraction temperature (F-value = 0.033).
Table 3. ANOVA for response surface quadratic model analysis of variance.
Tabla 3. ANOVA para el modelo cuadrático de superficies de respuesta del análisis de la varianza
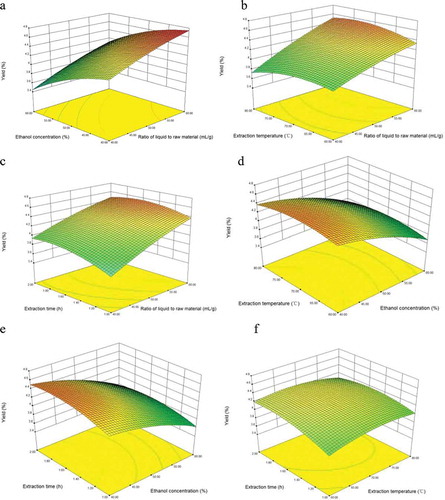
The extraction yield can also be predicted from the 3D response surface plot (), which graphically revealed the sensitivity of the response value towards the change in the variable. ) showed the effects of ethanol concentration and ratio of liquid to raw material on the extraction yield of flavonoids when the extraction temperature and extraction time were set at 70°C and 1.5 h. It was obvious that the yield increased significantly as the ratio of liquid to raw material increased, it agreed with , as the linear effect (P < 0.05) was significant; but the yield decreased significantly as the ethanol concentration increased, the ethanol concentration had a more significant effect on flavonoids yield than the ratio of liquid to raw material. showed the effects of extraction temperature and ratio of liquid to raw material on the extraction yield of flavonoids when the ethanol concentration and extraction time were set at 50% and 1.5 h. The yield increased significantly as the ratio of liquid to raw material increased. When ratio of liquid to raw material kept in a low level, the flavonoids yield increased at first and then decreased with the increase of extraction temperature. These results might be attributed to the thermal degradation (Lu et al., Citation2013). From the profile of , the ratio of liquid to raw material had a more significant effect on yield than the extraction temperature. showed the effects of extraction time and ratio of liquid to raw material on the extraction yield of flavonoids when the ethanol concentration and extraction temperature were set at 50% and 70°C. The yield increased significantly as the ratio of liquid to raw material increased, and the yield increased slowly as extraction time increased. From the profile of , the ratio of liquid to raw material had a more significant effect on yield than the extraction time. showed the effects of extraction temperature and ethanol concentration on the extraction yield of flavonoids when the ratio of liquid to raw material and extraction time were set at 50 mL/g and 1.5 h. It was obvious that the yield decreased significantly as ethanol concentration increased. When ethanol concentration kept in a low level, the yield increased slowly at first and then decreased with the increase of ethanol concentration. showed the effects of extraction time and ethanol concentration on the extraction yield of flavonoids when the ratio of liquid to raw material and extraction temperature were set at 50 mL/g and 70°C. The yield increased as extraction time increased, but the yield decreased as ethanol concentration increased. showed the effects of extraction time and extraction temperature on the extraction yield of flavonoids when the ratio of liquid to raw material and ethanol concentration were set at 50 mL/g and 50%. The yield increased slowly as extraction temperature increased. During the initial extraction period (lower temperature), the yield increased with the extraction time increased, at higher extraction temperature periods, the yield declined with an increase in the extraction time.
Figure 1. Response surface (3D) plots showing the effects of variables on the extraction yield of flavonoids. (a) Ethanol concentration and ratio of liquid to raw material. (b) Extraction temperature and ratio of liquid to raw material. (c) Extraction time and ratio of liquid to raw material. (d) Extraction temperature and ethanol concentration. (e) Extraction time and ethanol concentration. (f) Extraction time and extraction temperature.
Figura 1. Gráficos en 3D de superficies de respuesta que muestran los efectos de las variables en el rendimiento de la extracción de flavonoides. (a) Concentración de etanol y ratio de líquido a materia prima. (b) Temperatura de extracción y ratio de líquido a materia prima. (c) Tiempo de extracción y ratio de líquido a materia prima. (d) Temperatura de extracción y concentración de etanol. (e) Tiempo de extracción y concentración de etanol. (f) Tiempo de extracción y temperatura de extracción.
Verification of results
Under the optimized conditions for ratio of liquid to raw material of 60 mL/g, ethanol concentration of 40%, extraction temperature of 72.3°C, and extraction time of 1.6 h, five parallel verification experiments were carried out. There was no obvious difference between the experimental value (4.743%) and predicted value (4.759%). The result indicates the validation of the RSM model.
Purification of flavonoids
Crude flavonoids were extracted from the C. fascicularis leaves by ultrasonic-assisted extraction. The crude flavonoids were purified through an AB-8 macroporous adsorption resin column with the conditions as follows: the adsorption conditions for sample loading velocity, concentration and pH value were 2 BV/h, 1.8 mg/mL and 5, respectively, and the desorption conditions were achieved by using 2 BV of 40% ethanol as elution solvent at a flow rate of 1 BV/h. Then, the purified flavonoids were collected, concentrated under vacuum, and lyophilized.
Antioxidant capacities
Assay of hydroxyl radical scavenging capacity
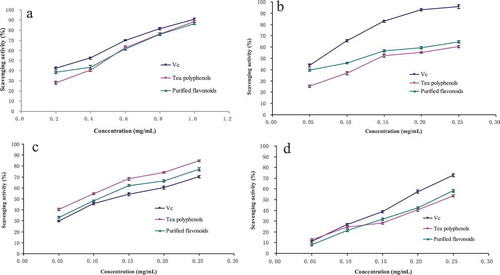
The hydroxyl radical is considered as one of the important reactive oxygen species, which can be formed in biological cells via the Fenton reaction and cause the general processes of aging and tissue damage (Shen et al., Citation2014; Zhong, Wang, He, & He, Citation2010). As shown in , all samples possessed obviously scavenging ability in dose-dependent pattern at all concentration. At a concentration of 0.4 mg/mL, the scavenging effects of Vc, TP, and purified flavonoids were 52.45%, 40.65%, and 43.54% for hydroxyl radicals, respectively. But with further increase of the concentration, the hydroxyl radical scavenging ability of TP increased obviously, which was higher than those of purified flavonoids, and still lower than those of Vc. The IC50 values of Vc, TP, and purified flavonoids were 0.322, 0.481, and 0.425 mg/mL, respectively. The result indicated that Vc had the higher scavenging capacity than TP and purified flavonoids, and the scavenging capacity of TP was close to that of purified flavonoids from C. fascicularis leaves. The possible antioxidant mechanism is that flavonoids can combine with the radical ions, which are needed for radical chain reaction (Shen et al., Citation2014). These results were in agreement with Li, Liu, Zheng, and Tang (Citation2009). Their study showed that hydroxyl radical scavenging capacity of Vc was higher than flavonoids extracted by ethanol from P. communis trin leaf (Li et al., Citation2009).
Figure 2. Scavenging capacities of ascorbic acid (abbreviation for Vc), tea polyphenols, and purified flavonoids on radicals. (a) Hydroxyl radical. (b) Superoxide anion radical. (c) DPPH radical. (d) ABTS radical. Each value is the mean ± SD of triplicate measurements.
Figura 2. Capacidad de eliminación de radicales mediante ácido ascórbico (Vc), polifenoles de té y flavonoides purificados. (a) Radical de hidroxilo. (b) Radical de anión superóxido. (c) Radical de DPPH. (d) Radical de ABTS. Todos los valores son la media ± DE de mediciones por triplicado.
Assay of superoxide anion radical scavenging capacity
Superoxide anion radical is the first generation of oxygen-free radical and also the predecessor of all the oxygen-free radicals, which could be converted to other oxygen-free radical. Therefore, scavenging of superoxide anion radical is very important for effectively reducing the generation of oxygen-free radicals (Luo, Guo, Hu, Shi, & Qian, Citation2011). As shown in , all samples were capable of scavenging superoxide anion radical in dose-dependent manner. At a concentration of 0.25 mg/mL, the scavenging effects of Vc, TP, and purified flavonoids were 95.85%, 60.36%, and 64.57% for superoxide anion radicals, respectively. The IC50 values of Vc, TP, and purified flavonoids were 0.051, 0.173, and 0.125 mg/mL, respectively. The result proved that purified flavonoids from C. fascicularis leaves had the higher scavenging capacity than TP, but lower than Vc. These results were in agreement with Luo et al. (Citation2011). Their study showed that superoxide anion radical scavenging capacity of Vc was higher than TP and purified flavonoids, and scavenging capacity of purified flavonoids was higher than TP (Luo et al., Citation2011).
Assay of DPPH radical scavenging capacity
DPPH radical is used to evaluate free radical scavenging effect of natural compounds, and the mechanism is that the DPPH-free radical bonds the hydrogen donated by antioxidant, forming into DPPH–H, a stable non-radical form (Kedare & Singh, Citation2011; Matthaus, Citation2002). As shown in , the scavenging effects of DPPH radical increased with increase in the sample concentration. The scavenging ability on DPPH radical of TP was higher than that of Vc and purified flavonoids with the increase of concentration. At a concentration of 0.25 mg/mL, the scavenging effects of Vc, TP, and purified flavonoids were 70.23%, 84.78%, and 76.98% for DPPH radicals, respectively. The IC50 values of Vc, TP, and purified flavonoids were 0.139, 0.083, and 0.116 mg/mL, respectively. Compared with flavonoids from P. communis trin leaf, which were extracted by hot water and ethanol, the scavenging effect of flavonoids was higher than that of Vc, when the concentration was greater than 0.12 mg/mL (Li et al., Citation2009). The result showed that purified flavonoids from C. fascicularis leaves had a remarkable effect on scavenging DPPH radical.
Assay of ABTS radical scavenging capacity
ABTS radical, as the DPPH radical, is used to evaluate free radical scavenging effect of natural compounds, and the mechanism is that the preformed radical monocation of ABTS+ is generated by oxidation of ABTS with potassium persulfate and is reduced in the presence of such hydrogen-donating antioxidants; the influences of both the concentration of antioxidant and duration of reaction on the inhibition of the radical cation absorption are taken into account when determining the antioxidant capacity (Re et al., Citation1999). As shown in , the scavenging effects of ABTS radical increased with increase in the sample concentration. With further increase of the concentration, when concentration was greater than 0.1 mg/mL, the ABTS radical scavenging ability of Vc and purified flavonoids increased obviously, which both were higher than that of TP. At a concentration of 0.25 mg/mL, the scavenging effects of Vc, TP, and purified flavonoids were 72.66%, 53.66%, and 58.23% for ABTS radicals, respectively. The IC50 values of Vc, TP, and purified flavonoids were 0.178, 0.242, and 0.223 mg/mL, respectively. Compared with Eriobotrya japonica (Thunb.) Lindl. leaves flavonoids, which were extracted with the ultrasonic method, flavonoids exhibited stronger scavenging effect for ABTS radical at 300 mg/L (Fu, Tang, Rang, Peng, & Jiang, Citation2015). The result indicated that Vc had the higher scavenging capacity for ABTS radical than TP and purified flavonoids, and the scavenging capacity of TP was close to that of purified flavonoids from C. fascicularis leaves.
Conclusions
In this study, CCD and RSM were used to optimize the ultrasonic-assisted extraction of flavonoids from C. fascicularis leaves. The optimal extraction conditions were obtained from RSM as following: ratio of liquid to raw material of 60 mL/g, ethanol concentration of 40%, extraction temperature of 72.3°C, and extraction time of 1.6 h. Under this optimized condition, the highest yield of flavonoids was 4.765%. Furthermore, the crude flavonoids were purified by eluting with ethanol concentration of 40% through an AB-8 macroporous adsorption resin column. The purified flavonoids exhibited obviously antioxidant capacities, including hydroxyl radical scavenging capacity, superoxide anion radical scavenging capacity, DPPH radical scavenging capacity, and ABTS radical scavenging capacity. This is the first report on the antioxidant capacities of flavonoids extracted from C. fascicularis using ultrasonic-assisted extraction. In the future, the flavonoids from C. fascicularis leaves might be investigated as potential natural antioxidant, which will be used in the functional food ingredients and medicinal production.
Acknowledgements
This work is supported by the foundation of Ministry of Science and Technology of the Republic of China of The National Spark Program Project: [Grant Number 2014GA830016], and by the foundation of The Forestry Department of Yunnan Province of National Park Pilot Construction Project: [Grant Number 2136299]. The authors gratefully acknowledge the support of Hanlong Dan, Han Li, and Rong Miao during the development of this scientific work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Azuma, C.M., Santos, F.C.S.D., & Lago, J.H.G. (2011). Flavonoids and fatty acids of Camellia japonica leaves extract. Revista Brasileira De Farmacognosia, 21, 1159–1162. doi:10.1590/S0102-695X2011005000128
- Balasumdram, N., Ai, T.Y., Sambanthamurthi, R., Sundram, K., & Samman, S. (2005). Antioxidant properties of palm fruit extract. Asia Pacific Journal of Clinical Nutrition. 14, 319–324. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16326638
- Boudkhili, M., Greche, H., Misbahi, H., Giovanelli, S., Noccioli, C., Pistelli, L., & Aarab, L. (2015). Isolation and antioxidant activity of flavonoids from Coriaria myrtifolia methanolic extract. Chemistry of Natural Compounds, 51, 141–142. doi:10.1007/s10600-015-1222-y
- Chen, H.Y., & Yen, G.C. (2007). Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chemistry, 101, 686–694. doi:10.1016/j.foodchem.2006.02.047
- Ciftci, O., Ozcan, C., Kamisli, O., Cetin, A., Basak, N., & Aytac, B. (2015). Hesperidin, a citrus flavonoid, has the ameliorative effects against experimental autoimmune encephalomyelitis (EAE) in a C57BL/J6 Mouse Model. Neurochemical Research, 40, 1111–1120. doi:10.1007/s11064-015-1571-8
- Dzoyem, J.P., Hamamoto, H., Ngameni, B., Ngadjui, B.T., & Sekimizu, K. (2013). Antimicrobial action mechanism of flavonoids from dorstenia species. Drug Discoveries & Therapeutics, 7, 66–72. doi:10.5582/ddt.2013.v7.2.66
- Feng, S., Cheng, H., Fu, L., Ding, C., Zhang, L., Yang, R., & Zhou, Y. (2014). Ultrasonic-assisted extraction and antioxidant activities of polysaccharides from camellia oleifera leaves. International Journal of Biological Macromolecules, 68, 7–12. doi:10.1016/j.ijbiomac.2014.04.026
- Fu, X.D., Tang, C.F., Rang, L., Peng, K.L., & Jiang, H.T. (2015). Antioxidant effect of flavonoids in extracts from the Eriobotrya japonica (Thunb.) Lindl. Leaves. Science and Technology of Food Industy, 36, 135–139. (in Chinese). doi:10.13386/j.issn1002-0306.2015.01.020
- Georgiev, K., Zhelev, I., & Georgieva, S. (2014). Total phenolic compounds and tannins content of bancha green tea (Camellia sinensis) depending on extraction conditions. Scripta Scientifica Pharmaceutica, 1, 48–51. doi:10.14748/ssp.v1i1.605
- Guo, L.X. (2013). Antitumor effects and mechanisms of total saponin and total flavonoid extracts from Patrinia villosa (Thunb.) juss. African Journal of Pharmacy & Pharmacology, 7, 165–171. doi:10.5897/AJPP12.761
- Hammi, K.M., Jdey, A., Abdelly, C., Majdoub, H., & Ksouri, R. (2015). Optimization of ultrasound-assisted extraction of antioxidant compounds from tunisian zizyphus lotus fruits using response surface methodology. Food Chemistry, 184, 80–89. doi:10.1016/j.foodchem.2015.03.047
- He, J., Wu, Z.Y., Zhang, S., Zhou, Y., Zhao, F., Peng, Z.Q., & Hu, Z.W. (2014). Optimization of microwave-assisted extraction of tea saponin and its application on cleaning of historic silks. Journal of Surfactants & Detergents, 17, 919–928. doi:10.1007/s11743-013-1523-8
- Kedare, S.B., & Singh, R.P. (2011). Genesis and development of DPPH method of antioxidant assay. Journal of Food Science and Technology, 48, 412–422. doi:10.1007/s13197-011-0251-1
- Li, R.G., Liu, L.W., Zheng, H.Y., & Tang, L.L. (2009). Antioxidant properties of P. communis trin flavonoids extracts. Acta Agriculturae Boreali-Occidentalis Sinica, 18, 310–314. (in Chinese). Retrieved from http://www.xbnyxb.net/ch/reader/create_pdf.aspx?file_no=20090466
- Liu, B., Ma, Y., Liu, Y., Yang, Z., & Zhang, L. (2014). Ultrasonic-assisted extraction and antioxidant activity of flavonoids from Adinandra nitida leaves. Tropical Journal of Pharmaceutical Research, 12, 1045–1051. doi:10.4314/tjpr.v12i6.27
- Liu, H., Mou, Y., Zhao, J.L., Wang, J.H., Zhou, L.G., Wang, M.A., … Yang, F.Y. (2010). Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules, 15, 7933–7945. doi:10.3390/molecules15117933
- Liu, T., Zhao, J., Ma, L., Ding, Y., & Su, D. (2012). Hepatoprotective effects of total triterpenoids and total flavonoids from Vitis viniferal against immunological liver injury in mice. Evidence-Based Complementary and Alternative Medicine, 2012, 1–8. doi:10.1155/2012/969386
- Liu, Y., Li, Z., Xu, H., & Han, Y. (2016). Extraction of saponin from Camellia oleifera abel cake by a combination method of alkali solution and acid isolation. Journal of Chemistry, 10, 1–8. doi:10.1155/2016/6903524
- Liyana-Pathirana, C., & Shahidi, F. (2005). Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chemistry, 93, 47–56. doi:10.1016/j.foodchem.2004.08.050
- Lu, J., Zhou, C., Rong, Q., Xu, Y.Y., Zhou, B., & Li, Z.H. (2013). Optimization of microwave-assisted extraction of flavonoids from Cryptotaenia japonica hassk using response surface methodology. Advance Journal of Food Science and Technology. 5, 310–317. Retrieved from http://maxwellsci.com/print/ajfst/v5-310-317.pdf
- Luo, Y.Q., Guo, H., Hu, L.F., Shi, L.W., & Qian, J.Q. (2011). Antioxidant activity of flavonoids from bamboo leaves. Food Science and Technology, 36, 201–203. (in Chinese). Retrieved from http://www.ixueshu.com/document/720104517f97441d318947a18e7f9386.html
- Maran, J.P., Manikandan, S., Priya, B., & Gurumoorthi, P. (2013). Box-behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L.) leaves. Journal of Food Science and Technology, 52, 92–104. doi:10.1007/s13197-013-0985-z
- Matthaus, B. (2002). Antioxidant activity of extracts obtained from residues of different oilseeds. Journal of Agricultural & Food Chemistry, 50, 3444–3452. doi:10.1021/jf011440s
- Min, T.L., & Bartholomew, B. (2007). Theaceae, Cemellia. In P.H. Raven & L.B. Zhang (Eds.), Flora of China (Vol. 12, pp. 367–370). Beijing: Science Press & St. Louis: Missouri Botanical Garden Press.
- Oku, H., Ogawa, Y., Iwaoka, E., Yu, Y., & Kagota, S. (2011). Preventive effects of the extract of kinka-cha, a folk tea, on a rat model of metabolic syndrome. Journal of Natural Medicines, 65, 610–616. doi:10.1007/s11418-011-0523-0
- Peng, X., Yu, D.Y., Feng, B.M., Wang, Y.Q., & Shi, L.Y. (2012). A new acylated flavonoid glycoside from the flowers of Camellia nitidissima and its effect on the induction of apoptosis in human lymphoma U937 cells. Journal of Asian Natural Products Research, 14, 799–804. doi:10.1080/10286020.2012.691475
- Radhika, M., Ghoshal, N., & Chatterjee, A. (2012). Comparison of effectiveness in antitumor activity between flavonoids and polyphenols of the methanolic extract of roots of potentilla fulgens in breast cancer cells. Journal of Complementary & Integrative Medicine, 9. Article 24. doi:10.1515/1553-3840.1644
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26, 1231–1237. doi:10.1016/S0891-5849(98)00315-3
- Sait, S., Hamrizeghichi, S., Boulekbachemakhlouf, L., Madani, K., Rigou, P., Brighenti, V., … Pellati, F. (2015). HPLC-UV/DAD and ESI-MS(n) analysis of flavonoids and antioxidant activity of an algerian medicinal plant: Paronychia argentea Lam. Journal of Pharmaceutical and Biomedical Analysis, 111, 231–240. doi:10.1016/j.jpba.2015.03.027
- Shen, S.A., Cheng, H.R., Li, X., Li, T., Yuan, M., Zhou, Y.H., & Ding, C.B. (2014). Effects of extraction method on antioxidant activities of polysaccharides from camellia seed cake. European Food Research and Technology, 238, 1015–1021. doi:10.1007/s00217-014-2183-2
- Song, L.X., Wang, X.S., Zheng, X.Q., & Huang, D.J. (2011). Polyphenolic antioxidant profiles of yellow camellia. Food Chemistry, 129, 351–357. doi:10.1016/j.foodchem.2011.04.083
- Srinivasan, S., & Pari, L. (2013). Antihyperlipidemic effect of diosmin: A citrus flavonoid on lipid metabolism in experimental diabetic rats. Journal of Functional Foods, 5, 484–492. doi:10.1016/j.jff.2012.12.004
- Tumbas, V., Canadanovic-Brunet, J., Gille, L., Dilas, S., & Cetkovic, G. (2010). Superoxide anion radical scavenging activity of bilberry (Vaccinium myrtillus L.). Journal of Berry Research, 1, 13–23. doi:10.3233/BR-2010-002
- Wang, S., & Xie, Y. (2004). China species red list, Vol.1: Red list. Beijing: Higher Education Press. (in Chinese).
- Wang, W., Liu, H., Wang, Z., Qi, J., Yuan, S., Zhang, W., … Jia, A.Q. (2016). Phytochemicals from Camellia nitidissima Chi inhibited the formation of advanced glycation end-products by scavenging methylglyoxal. Food Chemistry, 205, 204–211. doi:10.1016/j.foodchem.2016.03.019
- Wei, J.B., Li, X., Song, H., Liang, Y.H., Pan, Y.Z., Ruan, J.X., … Su, Z.H. (2015). Characterization and determination of antioxidant components in the leaves of Camellia chrysantha (Hu) Tuyama based on composition–activity relationship approach. Journal of Food and Drug Analysis, 23, 40–48. doi:10.1016/j.jfda.2014.02.003
- Wen, Y., Liu, Y.T., Zhang, J.M., & Guo, L. (2013). Purification of total flavonoids from Buddleja officinalis by ab-8 macroporous adsorption resin. Advanced Materials Research, 641-642, 988–992. doi:10.4028/www.scientific.net/AMR.641-642.988
- Xie, Y., & Wang, S. (2007). Conservation status of Chinese species: (1) Overview. Integrative Zoology, 2, 26–35. doi:10.1111/j.1749-4877.2007.00039.x
- Zhang, G.L., Zhang, G.S., Zhang, K., Wang, D., & Ping, S.H. (2015). Investigation and analysis of wild Camellia fascicularis in Yunnan province. Forestry Science and Technology of Guangdong Province, 31, 45–48. (in Chinese). doi:10.3969/j.issn.1006-4427.2015.01.009
- Zhang, H.T., & Ren, S.X. (1998). Camellia fascicularis Chang. In Z.Y. Wu & H.B. Cui (Eds.), Flora Republicae Popularis Sinicae (Vol. 49, pp. 105). Beijing: Science Press. (in Chinese).
- Zhang, Y., Wu, L.Y., Ma, Z.S., Cheng, J., & Liu, J.B. (2015). Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of flavonoids from corn silk on stz-induced diabetic mice. Molecules, 21, E7. doi:10.3390/molecules21010007
- Zhang, Z. (2015). Extraction and free radical scavenging activity of polysaccharide from ‘anji baicha’ (Camellia sinensis (L.) O. kuntze). International Journal of Biological Macromolecules, 84, 161–165. doi:10.1016/j.ijbiomac.2015.12.016
- Zhong, K., Wang, Q., He, Y., & He, X. (2010). Evaluation of radical scavenging polysaccharides with ultrasonic extraction on in S1580 tumor mice models. International Journal of Biological Macromolecules, 47, 356–360. doi:10.1016/j.ijbiomac.2010.05.022
- Zhu, H.B., Wang, Y.Z., Liu, Y.X., Xia, Y.L., & Tang, T. (2010). Analysis of flavonoids in portulaca oleracea L. by UV-Vis spectrophotometry with comparative study on different extraction technologies. Food Analytical Methods, 3, 90–97. doi:10.1007/s12161-009-9091-2
- Zielinski, A.A.F., Granato, D., Alberti, A., Nogueira, A., Demiate, I.M., & Haminiuk, C.W.I. (2015). Modelling the extraction of phenolic compounds and in vitro antioxidant activity of mixtures of green, white and black teas (Camellia sinensis, l. kuntze). Journal of Food Science and Technology -Mysore-, 52, 6966–6977. doi:10.1007/s13197-015-1797-0
