 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This work aims to study the effect of degree of protein aggregation on the surface and rheological properties of whey protein isolate dispersions with different concentrations (80, 100 and 120 g/kg), pHs (5.5 or 6.5) or NaCl contents (1 or 2 g/kg). Dispersions were thermally treated at 70, 75 and 80°C for different times. By mixing aggregated with native protein dispersions, different degrees of aggregation (0%, 20%, 40%, 60% and 80%) were obtained. Surface tension of the dispersions was determined by the pendant drop method, whilst their rheological properties were obtained from flow curve tests. A slight effect of the degree of aggregation over diffusion was found, which indicates that native proteins dominated the decrease in surface tension. The rheological behavior of dispersions with NaCl addition changed from Newtonian to shear thinning for aggregation degrees above 20%, whilst all dispersions at pH 5.5 and 6.5 presented a shear thinning behavior.
RESUMEN
Este trabajo apunta a estudiar el efecto del grado de agregación de proteínas en las propiedades de superficie y reológicas de dispersiones de ASP con diferente concentración de proteína (80, 100 y 120 g/kg), pH (5,5 ó 6,5) o contenido de NaCl (1 ó 2 g/kg). Las dispersiones fueron tratadas térmicamente a 70, 75 y 80°C por diferentes tiempos. Mediante la mezcla de dispersiones de proteína agregada y nativa, se obtuvieron dispersiones con grados de agregación diferente. La tensión superficial de las dispersiones se determinó fue determinada por el método de la gota colgante, mientras que las propiedades reológicas se obtuvieron desde ensayos de curva de flujo. Se encontró un efecto menor del grado de agregación sobre la difusión, lo cual indica que las proteínas nativas dominaron la disminución de la tensión superficial. La reología de las dispersiones con adición de NaCl cambió desde un comportamiento Newtoniano a uno pseudoplástico para grados de agregación sobre el 20%; mientras que todas las dispersiones a pH 5,5 ó 6,5 presentaron un comportamiento pseudoplástico.
1. Introduction
Whey protein isolate (WPI) is one of the highest quality proteins because of their amino acid profile and rapid digestibility (Devries & Phillips, Citation2015). The use of WPI is based on specific nutritional applications, such as sports performance, nutrition supplements and medical foods, where formulations can benefit from very low lactose levels and extremely high protein levels (Agarwal, Beausire, Patel, & Patel, Citation2015; Boland, Citation2011). However, the variety of functional groups present in WPI allows them to stabilize water-, fat- and air-based structures (i.e. gels, emulsions and foams, respectively) (Boland, Citation2011; Kulozik, Citation2007). WPI is mainly composed of β-lactoglobulin (50%), α-lactalbumin (15–20%) and bovine serum albumin (5–7%), which are well known for their surface activity and colloid-stabilizing characteristics (Martinez, Carrera-Sánchez, Rodríguez-Patino, & Pilosof, Citation2009; Schmitt, Bovay, Rouvet, Shojaei-Rami, & Kolodziejczyk, Citation2007), whereas proteins, lactose, minerals and fat are the remainder compounds (Boland, Citation2011).
WPI has been widely used for stabilizing foams because of their amphiphilic nature. Rapid diffusion of protein molecules to the air–water interface followed by molecular rearrangement allows proteins to decrease the surface tension and stabilize the gas phase (Germain & Aguilera, Citation2014). The adsorption and unfolding process result from the amphiphilic (polar/nonpolar regions) nature of proteins (Germain & Aguilera, Citation2014; Mahmoudi, Axelos, & Riaublanc, Citation2011; Mezzenga, Schurtenberger, Burbidge, & Michel, Citation2005). Globular proteins are relatively flexible and can rearrange their tertiary structures to facilitate adsorption at interfaces (Germain & Aguilera, Citation2014; Mezzenga et al., Citation2005). In addition, it has been demonstrated that surface tension of proteins dispersions correlated to their foaming properties (Mahmoudi, Axelos, & Riaublanc, Citation2011; Rullier, Novales, & Axelos, Citation2008; Tamm, Sauer, Scampicchio, & Drusch, Citation2012). Another key property in foam stabilization is the apparent viscosity of the continuous phase. Increasing viscosity of continuous phase delays instability phenomena, like draining, disproportionation and coalescence (Mezzenga et al., Citation2005). However, whey protein dispersions with low viscosity reduce their application in aerated food products (Mleko & Foegeding, Citation1999). According to Boland (Citation2011), dispersions of about 15% of WPI are needed to generate stable foams.
It is known that thermal treatment induces changes in the functional performance of whey proteins. Whey proteins, β-lactoglobulin and α-lactalbumin are molecules that are particularly heat sensitive in a targeted manner to make use of their potential to create or optimize food structures (Kulozik, Citation2007). In general, partial heat denaturation improves protein surface activity because of enhanced molecular flexibility (Bals & Kulozik, Citation2003; Germain & Aguilera, Citation2014). Controlled aggregation of whey proteins could be an intentional way of modifying functionality (Purwanti et al., Citation2011; Rullier et al., Citation2008; Schmitt et al., Citation2007). Generally, protein aggregates are produced by heating a whey protein solution below its critical gelation concentration, which is about 12% w/w (Purwanti et al., Citation2011). In contrast, intensive heat-induced aggregation and polymerization of whey proteins impaired foaming properties (Bals & Kulozik, Citation2003). On the other hand, thermal treatment is able to lead to thickening behavior or increase in the apparent viscosity of whey protein dispersions (Bals & Kulozik, Citation2003; Mleko & Foegeding, Citation1999; Purwanti et al., Citation2011) because of protein unfolding and the increasing interactions between the reactive groups of protein molecule and the aqueous phase, and further formation of protein aggregates and/or polymers. As demonstrated by Mleko and Foegeding (Citation1999), heat-aggregated WPI at 4% w/v resulted in a similar viscosity as carbohydrate-based hydrocolloids dispersions at 1% w/v.
The interfacial behavior and foaming properties of whey proteins (β-lactoglobulin, α-lactalbumin and bovine serum albumin) have been the subject of extensive research on native state at varying protein concentration, pH and ionic strength. However, as most of the dairy products are subjected to heat treatment, this thermal process may induce denaturation and aggregation; hence, it is very likely that a fraction of the whey proteins are no longer in their native state, but in a self-assembled aggregated or polymerized state (Davis & Foegeding, Citation2004; Mahmoudi et al., Citation2011; Rullier et al., Citation2008; Schmitt et al., Citation2007). As protein aggregates are complex structures whose size, morphology, surface charge and surface hydrophobicity are dependent on formation conditions, their surface behavior differs from non-aggregated proteins (Guyomarc’h et al., Citation2015; Nicolai & Durand, Citation2013; Ryan, Zhong, & Foegeding, Citation2013; Schmitt et al., Citation2007). The resulting interfacial and foaming properties will thus be a combination of that of native whey proteins and aggregates/polymers (Davis & Foegeding, Citation2004). The most probable explanation for this effect is a combination of the fast diffusion of monomeric/native protein at the air/water interface, in order to decrease surface tension coupled with the subsequent formation of a viscoelastic network at the interface by soluble aggregates (Schmitt et al., Citation2007).
From a technological and practical point of view, controlling the number, size, morphology, surface charge and surface hydrophobicity of heat-formed protein aggregates to improve foaming properties of WPI dispersions is not an easy task. Hence, we used an approach of mixing aggregated/polymerized WPI proteins with native WPI dispersions (Davis & Foegeding, Citation2004) to evaluate the interfacial and rheological behavior of WPI dispersions, as key factors in determining quality attributes of food foams. Therefore, the objective of this work was to study the effect of degree of protein aggregation on the surface and rheological properties of WPI dispersions at different pHs and levels of NaCl addition.
2. Materials and methods
2.1. Raw materials
WPI (Provon 190, Glanbia Nutritionals Inc., USA) was used as surface active agent. WPI is mainly composed of proteins β-lactoglobulin and α-lactoalbumin. According to the supplier, the WPI composition was 926.2 g protein/kg dry solids, 33.4 g water/kg product and 29.9 g ash/kg product. The pH of the native WPI dispersion was about 6.3. Hydrochloric acid (HCl), sodium hydroxide (NaOH) and sodium chloride (NaCl) of analytical grade were used (Merck, Darmstadt, Germany).
2.2. Methods
2.2.1. Formation of WPI dispersions
Native dispersions of WPI (80, 100 and 120 g/kg protein basis) with addition of NaCl (1 or 2 g/kg) or at two different pHs (5.5 or 6.5) in ultra-pure water (resistivity of 15 MΩ cm) were prepared by slow stirring (200 rpm) at 25°C for 2 h, avoiding foam formation. NaCl levels (1 or 2 g/kg) were chosen based on a previous study on cold gelation of WPI foams (Orrego, Troncoso, & Zúñiga, Citation2015), and the pH of these dispersions was not modified (pH ~6.5). The dispersion pH (5.5 or 6.5) was carefully adjusted with 1 N HCl or 1 N NaOH solutions (Sigma-Aldrich Corp., St. Louis, MO, USA). The WPI dispersions were left at 4°C for at least 12 h to allow complete hydration of the protein.
2.2.2. Thermal treatment of WPI dispersions
In order to induce aggregation of the WPI dispersions, samples (3 mL) were heated in test tubes (inner diameter: 9 mm; thickness 1 mm; length: 120 mm) by immersing in a water bath (Memmert, model Basic WNB, Germany) at constant temperatures of 70, 75 or 80°C, reaching the bath temperature after 3.5 s. The choice of the thermal treatment time () was based on the methodology described by Kerstens, Murray and Dickinson (Citation2006). Briefly, the tubes were taken out of the water bath for observation at regular intervals (every 1 min, then every 30 s and finally every 10 s), and the gel point was defined empirically as the time at which a tube could be turned upside-down without observable downwards flow of its contents, indicating the formation of a self-supporting gel (Kerstens et al., Citation2006). Thermal treatment times before dispersions became a gel (liquid samples without lumps) were chosen for each experimental condition under the consideration that at this condition, the dispersions were fully aggregated (Davis & Foegeding, Citation2004; Orrego et al., Citation2015). According to Ryan et al. (Citation2013), near-complete aggregation of native proteins can occur in the order of minutes when heating WPI, depending on protein dispersion and heating process conditions. After the thermal treatment (), the samples were cooled to ambient temperature (25°C). Different mass ratios of aggregated WPI dispersions (0%, 20%, 40%, 60% and 80%) were generated by mixing native with thermally treated samples (Davis & Foegeding, Citation2004). All procedures were done in triplicate.
Table 1. Combination of the process conditions studied for complete aggregation of the WPI dispersions.
Tabla 1. Combinación de las condiciones de proceso estudiadas para la agregación completa de las dispersiones de ASP.
2.3. Measurements
2.3.1. Dynamic surface tension measurements of WPI dispersions
For the surface tension experiments, the WPI dispersions were diluted to a factor of 10 using ultra-pure water, previously adjusted to the sample pH. This dilution was required to bring out interfacial properties among samples and minimize the viscosity effect on protein diffusion to the air–liquid interface (Schmitt et al., Citation2007; Zúñiga, Kulozik, & Aguilera, Citation2011). Changes in the WPI surface tension were measured by using an automated contact angle goniometer (Ramé-Hart Inc., model 250-F4, NJ, USA). Dynamic surface tension (DST) measurement (τ) was based on the pendant drop method, where a drop (9 μL) of WPI dispersion at 25°C was delivered and allowed to stand at the tip of the needle for 300 s to achieve protein adsorption at the air–water interface. According to Rullier et al. (Citation2008) and Tamm et al. (Citation2012), as foam formation occurs in a few seconds, it is more pertinent to study the adsorption kinetics at short times when attempting to explain the ability of proteins to adsorb at air/water interfaces.
Drop images (captured at different time intervals) were taken with a CCD camera. DST measurement of the WPI dispersions was calculated analyzing the drop profile by image analysis with the DROPimage Advanced software (Ramé-Hart Inc., NJ, USA) and then by fitting the Laplace equation to the drop shape. To validate the results, it was corroborated experimentally that the interfacial tension of the pure water/air system was 72.8 ± 0.2 mN/m (Zúñiga, Skurtys, Osorio, Aguilera, & Pedreschi, Citation2012).
Results from the DST measurements were interpreted in terms of surface pressure, defined as the decrease in surface tension of a pure solvent caused by the addition of the protein
where Π is the surface pressure of the WPI dispersion (mN/m), τw is the surface tension of pure water (72.8 mN/m at 25°C) and τp is the surface tension of the WPI dispersion (mN/m) at the same temperature.
Values of the diffusion rate constant (kdiff) were obtained from Π against time1/2 plots. Assuming that diffusion toward the interface controls the adsorption process, a plot of Π against time1/2 will be linear for short times, and the slope of this plot will be the diffusion rate constant (Martinez et al., Citation2009; Tamm et al., Citation2012).
2.3.2. Rheological measurements of WPI dispersions
Rheological tests were made in a controlled shear rate rheometer (Anton Paar, ReolabQC, Austria). Samples were carefully poured into the rheometer cup and allowed to stand 1 min before shearing. Test temperature was set at 25°C using a Peltier controller (Peltier RheolabQC plus, C-PTD180/AIR/QC). The test geometry was a coaxial cylinder geometry conforming to DIN 53018 (bob radius: 13.331 mm; length: 40.005 mm; cup radius: 14.46 mm). Rheological characterization of the WPI dispersions was performed using a flow curve test, where the shear rate was increased linearly from 1 to 100 s−1 during 400 s.
The experimental flow curves of WPI dispersions over the range of shear rates were described by the power law model as follows:
where σ is the shear stress (Pa), K is the consistency index (Pa sn), is the shear rate (s−1) and n is the flow behavior index (dimensionless). For a Newtonian dispersion n = 1, and for a dispersion which exhibits shear thinning behavior n < 1.
2.3.3. Microstructure of the WPI aggregates
The WPI aggregates microstructure was observed by transmission electron microscopy (TEM) using the negative staining method described by Zúñiga, Tolkach, Kulozik and Aguilera (Citation2010). A drop of WPI dispersion diluted to a factor of 10 was deposited onto a carbon-support film on a copper grid. The excess of sample was removed after 30 s using filter paper. Contrast between aggregates and image background was achieved by negative staining by adding a droplet of 20 g/kg uranil acetate solution for 60 s. Any excess of staining agent was removed again by a filter paper. After drying the grid at room temperature for 5 min, micrographs were made using a TEM (Philips Tecnai 12 BioTwin, Eindhoven, The Netherlands) operating at 80 kV. Images in digital format (TIFF) without compression (1.44 MB) were recorded.
2.4. Statistical analysis of data
Analysis of variance tests were used to analyze the data at a confidence level of 95% using Statgraphics Centurion XVI software (Manugistics Inc., Statistical Graphics Corporation, Rockville, MD, USA). Differences between samples were evaluated by multiple range test according to least significant differences multiple comparison method.
3. Results and discussion
3.1. DST of WPI dispersions
The DST decreased with time due to protein diffusion and adsorption at the air/water interface (data not shown). According to Tripp, Magda and Andrade (Citation1995), DST curves could be divided into four periods: (1) an induction period in which surface tension remains nearly equal to the pure solvent value, (2) a rapid rate of decrease in surface tension with time (−10.0 to −0.2 mN/m min), (3) a mesoequilibrium period of surface tension (−0.2 to −0.001 mN/m min) and (4) an equilibrium/steady period of surface tension (−0.001–0.0 mN/m min). In this study, the DST curves did not show an induction period, probably because more than 50% of the monolayer surface concentration was obtained during drop formation at the tip of the needle. The latter is due to a relatively high protein concentration and a rapid adsorption of WPI dispersions at the air/water interface. For systems with very low protein concentration (<0.01 mg/mL), different induction periods have been reported (Tripp et al., Citation1995). However, the other three regimes have been observed for diluted protein dispersions (1 g/kg) (Schmitt et al., Citation2007). A special case was reported by Tamm et al. (Citation2012) who measured the surface properties of concentrated milk-derived protein dispersions. The authors applied pendant drop tensiometry to determine protein diffusion by using modified DST measurements, in order to characterize the protein adsorption process on an initially empty surface. Tamm and coworkers observed the first three periods, where the adsorption process was accelerated with increasing protein concentration (10, 20 and 30 g/kg of WPI). In opposition to the results obtained for these authors, in this study, only the second period was observed from the DST curves in view of the relatively short measuring times and the relatively high WPI dispersion concentration (8–12 g/kg) used. For native protein, we determined that the surface tension decreased from 60.5 to 49.2 mN/m for samples with NaCl addition, and from 61.3 to 47.8 mN/m for samples with change in their pH, results in agreement with literature data for WPI dispersions. Schmitt et al. (Citation2007) and Schmitt, Bovay and Rouvet (Citation2014) reported values of surface tension between 72 and 52 mN/m for WPI dispersions at 1 g/kg, and values between 68 and 50 mN/m for WPI concentrations ranging from 0.5 to 3.5 g/kg at pH 5.0. Schmitt et al. (Citation2007) argued that it is reasonable to expect similar DST kinetics at small time scales (~50 s), because an initial decrease in surface tension is mainly due to diffusion of native proteins. In a latter work, Schmitt et al. (Citation2014) observed that the slopes of the initial decreasing phase were almost the same even if the WPI concentration varied by a factor of almost 10.
Surface pressure (Π) was calculated from DST data (). Theoretically, the evolution of Π is result of adsorption and unfolding at the air–water interface of protein molecules from the bulk. The driving force for the adsorption process is the concentration difference between current and the equilibrium surface pressure (Wang & Narsimhan, Citation2005). The surface pressure of native protein dispersions was dependent on the extrinsic conditions of the milieu (pH or NaCl addition). For example, at the end of the experimental period for the DST test, native samples at pH 6.5 showed a Π value of 24.9 mN/m ()), whereas those with NaCl addition of 1 g/kg reached a value of 23.6 mN/m (b)). Besides, it was observed that samples at different pHs and NaCl concentrations showed a sharp increase in Π at short times (up to 15 s) and, after that, the rate of change in Π was smaller. The native protein induced the highest increase in Π and presented a statistical difference (p < 0.05) with respect to aggregated dispersions independently of the aggregation degree and milieu conditions, results in agreement with previous works (Nicorescu et al., Citation2008; Rullier et al., Citation2008; Schmitt et al., Citation2007). It is known that during thermal treatment of globular proteins, aggregation/polymerization of protein molecules occurs (Zúñiga et al., Citation2010), and the rate and extent of this phenomenon is function of processing (e.g. protein concentration and temperature–time relationship) and environmental conditions (e.g. pH and ionic strength). Aggregates, being larger in size than native protein molecules, diffused slower to the air–water interface (Rullier et al., Citation2008; Schmitt et al., Citation2007; Zhu & Damodaran, Citation1994), explaining the results observed in . However, it has been conjectured that the presence of aggregates/polymers improves the foaming properties of WPI dispersions by forming a viscoelastic network in the thin films (mainly governed by disulfide bond formation and hydrophobic interactions) at the interface (Rullier et al., Citation2008; Schmitt et al., Citation2007).
Figure 1. Effect of aggregation degree on surface pressure of WPI dispersions at 120 g/kg, treated at 80°C and (a) pH 6.5 and (b) NaCl addition 1 g/kg. Lines are merely a visual guide.
Figura 1. Efecto del grado de agregación sobre la presión superficial de dispersiones de ASP a 120 g/kg, tratadas a 80°C y A) pH 6,5 y B) adición de NaCl a 1 g/kg. Las líneas son sólo una guía visual.
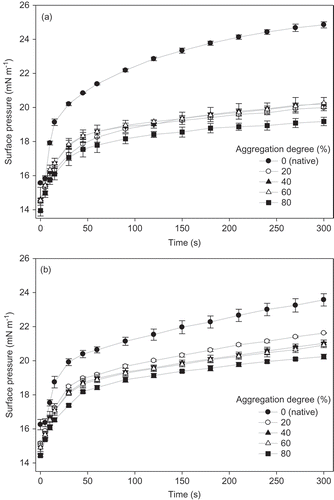
As shown in a), at the end of the test, the reduction of Π for aggregated samples was 20%, 19%, 18% and 23% for samples with aggregation degrees of 20%, 40%, 60% and 80%, respectively. In the case of b), it can be observed that differences in Π values between native protein and aggregated protein dispersions are lower than those with pH modification, giving percentages of reduction at the end of the test of 8%, 10%, 11% and 13% for samples with aggregation degrees of 20%, 40%, 60% and 80%, respectively. The salt addition at a fixed pH is equivalent to lowering the pH toward isoelectric point at a fixed ionic strength. In both cases, the electrostatic interactions are reduced by either increasing the screening or by decreasing the charge density (Nicolai, Britten, & Schmitt, Citation2011). The effect of changing the electrostatic interactions between proteins is stronger for a pH change than for an increase in the ionic strength. This behavior could be attributed to a lower repulsion between proteins during thermal treatment and, subsequently, it is possible to obtain larger aggregates for pH change than NaCl addition. In fact, a lower increase in Π was observed for WPI dispersions at pH 5.5 or 6.5 than those with 1 or 2 g/kg of NaCl. Moreover, it can be observed from that at the end of the DST assay, equilibrium values for Π were not reached.
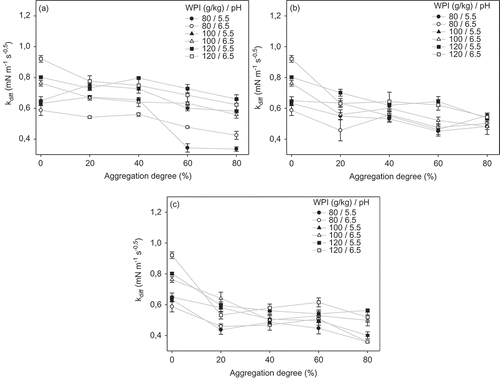
The diffusion rate constant (kdiff) represents the diffusional migration of proteins to the air–water interface, behavior that decreases surface tension. shows the kdiff values (obtained from Π against time1/2 plots) for native and thermally treated dispersions at different protein concentrations and pH. In all cases (), it was observed that the highest kdiff values were obtained for native protein dispersions. For aggregated protein dispersions, a clear effect of protein concentration or pH on kdiff values was not observed. For all conditions studied, a slight decrease in kdiff was found with increasing the aggregation degree, being not significant (p > 0.05) in the most cases.
Figure 2. Effect of aggregation degree and pH of WPI dispersions on the diffusion rate constant for different thermal treatments: (a) 70, (b) 75 and (c) 80°C.
Figura 2. Efecto del grado de agregación y pH de dispersiones de ASP en la constante de velocidad de difusión para diferentes tratamientos térmicos: A) 70°C, B) 75°C, y C) 80°C.
Values of kdiff for thermally treated protein dispersions at different protein and NaCl concentrations can be seen in . As seen from results in , in all cases (a–c)), the highest kdiff values were obtained for native protein dispersions. However, samples with protein concentration of 120 g/kg presented kdiff values statistically higher than the other concentrations of protein. There was a trend in decrease in kdiff with increasing the aggregation degree (), which was more evident in this case.
Figure 3. Effect of aggregation degree and NaCl addition of WPI dispersions on the diffusion rate constant for different thermal treatments: (a) 70, (b) 75 and (c) 80°C.
Figura 3. Efecto del grado de agregación y adición de NaCl de dispersiones de ASP en la constante de velocidad de difusión para diferentes tratamientos térmicos: A) 70°C, B) 75°C, and C) 80°C.
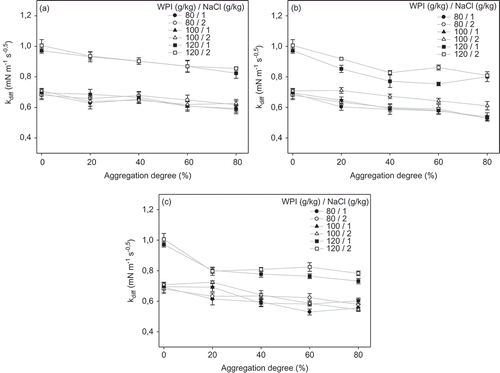
Because kdiif values are calculated for short times (within the first 10 s of the measurements), it seems probable that the only difference between kdiif values is in the native protein concentration. Hence, kdiif values will be dominated for the amount of native protein and not by the physicochemical properties of WPI aggregates/polymers (e.g. size, morphology, surface charge and surface hydrophobicity), which explains the trends observed in and .
On the other hand, kdiff values ranged from 0.34 to 0.92 mN/m s0.5 for WPI dispersions with pH modification, and from 0.53 to 1.01 mN/m s0.5 for dispersions with NaCl addition. Tamm et al. (Citation2012) found kdiff values ranging from 19.5 to 31.5 mN/m s0.5 for WPI dispersions with protein concentration between 10 and 30 g/kg. Differences found between the Tamm’s work and this study are due to the modification made by these authors to the pendant drop method, who initially generated a drop with no protein present, and afterward they incorporated a WPI dispersion by using a concentric needle. With this method, a protein-free interface is established at the beginning of the experiment, after which protein diffuses at the air–water interface generating a sharp increase in Π which provokes high values of the initial slope of the Π against time1/2 curve. Although the methodology reported by these authors is ideal for the determination of protein diffusion toward the interface, the diffusion kinetics obtained is strongly affected by the driving force, which is increased at the beginning, when no surfactant is present at the interface. The Tamm’s methodology does not necessarily address the phenomenon occurring in real systems (e.g. foam formation), where during the interface-forming process, surfactant is present in the system when the interface is formed.
3.2. Rheological behavior of WPI dispersions
The results obtained from the rheological studies indicated that higher values of shear stress are obtained for dispersions with higher WPI concentration and with an increased degree of protein aggregation (). No significant effect was observed (p > 0.05) by pH and NaCl addition in rheograms obtained for the different dispersions. At aggregation degrees above 40%, the increase in shear stress values was more significant (p < 0.05). Bals and Kulozik (Citation2003) observed that the higher the denaturation degree, the higher was the viscosity of WPI dispersions because of the unfolding of the proteins and the increased interactions between the reactive groups of the protein molecules and the aqueous phase. The hydrodynamic size of the protein molecules increased with aggregation degree (Zúñiga et al., Citation2010), and consequently an increase in apparent viscosity due to formation of aggregates/polymers via sulfhydryl–disulfide interchange reactions and/or non-covalent bonding (Zhu & Damodaran, Citation1994; Zúñiga et al., Citation2010).
Figure 4. Rheograms for WPI dispersions (120 g/kg) heat treated at 75°C. (a) Dispersion at pH 6.5 and (b) dispersion with NaCl addition of 1 g/kg. Lines are merely a visual guide.
Figura 4. Reogramas para dispersiones de ASP (120 g/kg) tratadas térmicamente a 75°C. A) Dispersión a pH 6,5, y B) dispersión con adición de NaCl a 1 g/kg. Las líneas son sólo una guía visual.
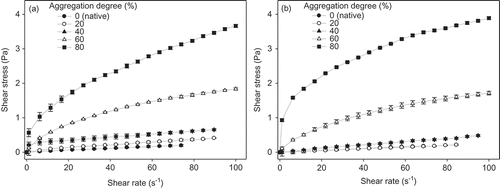
From rheograms of WPI dispersions, it is possible to observe that the dispersions with aggregation degrees between 0% and 40% showed a linear relationship between shear rate and shear stress; however, this performance changed for aggregation degrees of 60% and 80%. This behavior was observed for all experimental conditions. In order to verify and quantify this fact, rheograms were fitted to the Power Law rheological model (Equation (2)), finding a good agreement between the experimental data and the model (R2 between 0.8841 and 0.9997). By applying this rheological model, the flow behavior index (n) allows determining if a fluid has a Newtonian (n ~ 1.0), shear thinning (n < 1.0) or shear thickening behavior (n > 1.0). WPI dispersions with NaCl addition showed a Newtonian behavior, with n values ranging from 0.93 to 1.19, for aggregation degrees of 0% and 20%, whilst for aggregation degrees between 40% and 80%, a shear thinning behavior was found with n values ranging from 0.27 to 0.83. For all dispersions at pHs 5.5 or 6.5, the n values ranged from 0.29 to 0.89, indicating a shear thinning behavior independent of the aggregation degree.
The consistency coefficient (K) was plotted against aggregation degree of the WPI dispersions for different pHs and NaCl concentrations ( and ). It can be observed that the increase in the thermal treatment temperature augmented the K values (). This behavior was enhanced with an increase in the aggregation degree of the dispersions. In general, for aggregation degrees above 40%, the increase in K values was more evident. Besides, the effect of pH on the K values was more noticeable at higher thermal treatment temperatures and for aggregation degrees above 40%. As expected, higher WPI concentrations in dispersions generated higher K values.
Figure 5. Effect of aggregation degree and pH of WPI dispersions on the consistency coefficient for different thermal treatments: (a) 70, (b) 75 and (c) 80°C.
Figura 5. Efecto del grado de agregación y pH de dispersiones de ASP en el coeficiente de consistencia para diferentes tratamientos térmicos: A) 70°C, B) 75°C, y C) 80°C.
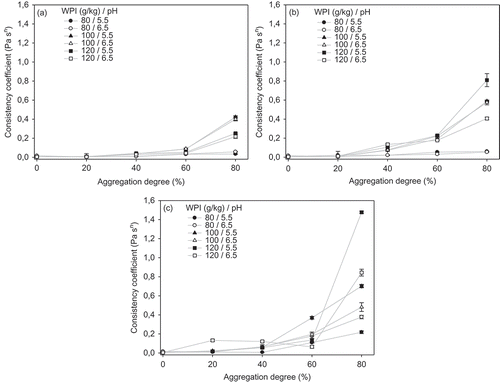
Figure 6. Effect of aggregation degree of WPI and addition of NaCl on dispersions consistency coefficient for different thermal treatments: (a) 70, (b) 75 and (c) 80°C.
Figura 6. Efecto del grado de agregación de ASP y adición de NaCl en el coeficiente de consistencia de las dispersiones para diferentes tratamientos térmicos: A) 70°C, B) 75°C, y C) 80°C.
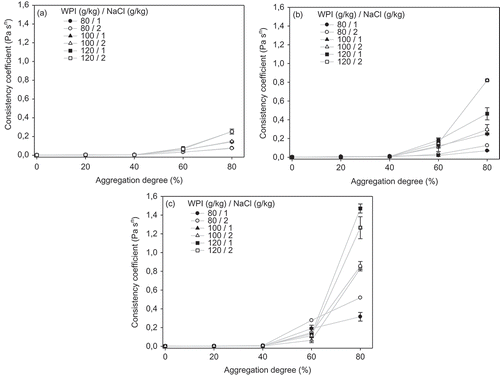
The effect of NaCl addition and aggregation degree at different thermal treatment temperatures on K values is shown in . K values were not affected by the thermal treatment temperatures, NaCl addition and WPI concentration for aggregation degrees up to 40%. Above this latter value, the effect of thermal treatment on K values becomes evident. The impact of concentration is clear at aggregation degree of 80% for thermal treatments at 70 and 75°C, and for aggregation degrees of 60% and 80% for thermal treatments at 80°C. The effect of NaCl addition can be visualized only for aggregation degrees above 40% and thermal treatment temperatures of 75 and 80°C.
3.3. Microstructure of WPI aggregates
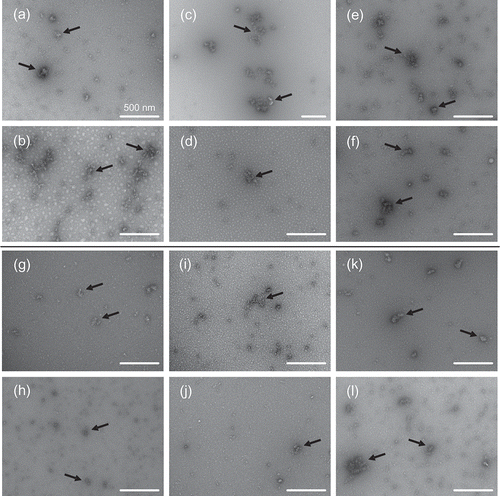
The effect of process conditions on the morphology of WPI aggregates was evaluated by TEM as shown in . Aggregates took the form of white masses due to the negative staining procedure employed. TEM images also depict regions of excessive stain due to the negative staining preparation method. Compact and spherical morphology was revealed by TEM for all experimental conditions. Aggregates of larger size were found at higher WPI concentration, probably because of the higher probability of contact between monomeric proteins to form aggregates. Besides, higher temperatures of thermal treatment led to formation of larger aggregates. The effect of NaCl addition or change in the dispersions pH was too weak to allow satisfactory observation of differences of the aggregates formed; it is likely that the extent of the thermal treatment masked the influence of the electrostatic change due to NaCl addition or pH change. As discussed earlier, the change in DST (determined by the pendant drop method) for the WPI dispersions was not affected by the morphology or the physicochemical properties of the aggregates formed, because monomeric/native proteins first migrated to the air–water interface. On the other hand, rheological properties were strongly affected by the aggregated proteins concentration, because of the increased protein–solvent interaction and amount of aggregates of the dispersions.
Figure 7. TEM micrographs of WPI aggregates for different protein dispersions: (a) 80 g/kg, 70°C, 1 g/kg NaCl; (b) 80 g/kg, 80°C, 1 g/kg NaCl; (c) 100 g/kg, 75°C, 1 g/kg NaCl; (d) 100 g/kg, 75°C, 2 g/kg NaCl; (e) 120 g/kg, 70°C, 2 g/kg NaCl; (f) 120 g/kg, 80°C, 2 g/kg NaCl; (g) 80 g/kg, 70°C, pH 5.5; (h) 80 g/kg, 80°C, pH 5.5; (i) 100 g/kg, 75°C, pH 5.5; (j) 100 g/kg, 75°C, pH 6.5; (k) 120 g/kg, 70°C, pH 6.5 and (l) 120 g/kg, 80°C, pH 6.5.
Figura 7. Micrografías TEM de agregados de ASP para diferentes dispersiones de proteína: (A) 80 g/kg, 70°C, 1 g/kg NaCl; (B) 80 g/kg, 80°C, 1 g/kg NaCl; (C) 100 g/kg, 75°C, 1 g/kg NaCl; (D) 100 g/kg, 75°C, 2 g/kg NaCl; (E) 120 g/kg, 70°C, 2 g/kg NaCl; (F) 120 g/kg, 80°C, 2 g/kg NaCl; (G) 80 g/kg, 70°C, pH 5,5; (H) 80 g/kg, 80°C, pH 5,5; (I) 100 g/kg, 75°C, pH 5,5; (J) 100 g/kg, 75°C, pH 6,5; (K) 120 g/kg, 70°C, pH 6,5; y (L) 120 g/kg, 80°C, pH 6,5.
4. Conclusions
The surface and rheological properties of WPI dispersions can be modified by varying the aggregation degree at different pHs and levels of NaCl addition. The diffusional migration (represented by the diffusion rate constant, kdiff) of proteins to the air–water interface was slightly affected by aggregation degree, confirming that native proteins play a dominant role in the decrease of surface tension at short times. In addition, it was possible to observe that WPI dispersions with NaCl addition showed a Newtonian rheological behavior for aggregation degrees below 20%, and above this value, the flow behavior was shear thinning; however, for all the WPI dispersions with pH change, the flow behavior was always shear thinning. Mixing of native and aggregated WPI dispersions can be an alternative to improve foaming properties because the fraction of native protein dominates the adsorption process at the air–water interface and the aggregated fraction gives the necessary apparent viscosity for stabilizing foams.
Acknowledgments
R.N. Zúñiga thanks the financial support of CONICYT by means of the FONDECYT Project 1140031 Controlled protein denaturation for the engineering design of aerated food products with enhanced textural and nutritional properties.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agarwal, S., Beausire, R.L.W., Patel, S., & Patel, H. (2015). Innovative uses of milk protein concentrates in product development. Journal of Food Science, 80(S1), A23–A29. doi:10.1111/1750-3841.12807
- Bals, A., & Kulozik, U. (2003). Effect of pre-heating on the foaming properties of whey protein isolate using a membrane foaming apparatus. International Dairy Journal, 13(11), 903–908. doi:10.1016/S0958-6946(03)00111-0
- Boland, M. (2011). Whey proteins. In G.O. Phillips & P.A. Williams (Eds.), Handbook of food proteins (pp. 30–55). New York, NY: Woodhead Publishing Limited.
- Davis, J.P., & Foegeding, E.A. (2004). Foaming and interfacial properties of polymerized whey protein isolate. Journal of Food Science, 69(5), C404–C410. doi:10.1111/j.1365-2621.2004.tb10706.x
- Devries, M.C., & Phillips, S.M. (2015). Supplemental protein in support of muscle mass and health: Advantage whey. Journal of Food Science, 80(S1), A8–A15. doi:10.1111/1750-3841.12802
- Germain, J.C., & Aguilera, J.M. (2014). Multi-scale properties of protein-stabilized foams. Food Structure, 1(1), 55–70. doi:10.1016/j.foostr.2014.01.001
- Guyomarc’h, F., Famelart, M.-H., Henry, G., Gulzar, M., Leonil, J., Hamon, P., … Croguennec, T. (2015). Current ways to modify the structure of whey proteins for specific functionalities - a review. Dairy Science & Technology, 95(6), 795–814. doi:10.1007/s13594-014-0190-5
- Kerstens, S., Murray, B.S., & Dickinson, E. (2006). Microstructure of β-lactoglobulin-stabilized emulsions containing non-ionic surfactant and excess free protein: Influence of heating. Journal of Colloid and Interface Science, 296(1), 332–341. doi:10.1016/j.jcis.2005.08.046
- Kulozik, U. (2007). Structuring dairy products by means of processing and matrix design. In J.M. Aguilera & P.J. Lillford (Eds.), Food materials science. Principles and practice (pp. 439–473). New York, NY: Springer.
- Mahmoudi, N., Axelos, M.A.V., & Riaublanc, A. (2011). Interfacial properties of fractal and spherical whey protein aggregates. Soft Matter, 7, 7643–7654. doi:10.1039/C1SM05262D
- Martinez, M.J., Carrera-Sánchez, C., Rodríguez-Patino, J.M., & Pilosof, A.M.R. (2009). Interactions in the aqueous phase and adsorption at the air–water interface of caseinoglycomacropeptide (GMP) and β-lactoglobulin mixed systems. Colloids and Surfaces B: Biointerfaces, 68(1), 39–47. doi:10.1016/j.colsurfb.2008.09.006
- Mezzenga, R., Schurtenberger, P., Burbidge, A., & Michel, M. (2005). Understanding foods as soft materials. Nature Materials, 4, 729–740. doi:10.1038/nmat1496
- Mleko, S., & Foegeding, E.A. (1999). Formation of whey protein polymers: Effects of a two-step heating process on rheological properties. Journal of Texture Studies, 30(2), 137–149. doi:10.1111/j.1745-4603.1999.tb00207.x
- Nicolai, T., Britten, M., & Schmitt, C. (2011). β-lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocolloids, 25(8), 1945–1962. doi:10.1016/j.foodhyd.2011.02.006
- Nicolai, T., & Durand, D. (2013). Controlled food protein aggregation for new functionality. Current Opinion in Colloid & Interface Science, 18(4), 249–256. doi:10.1016/j.cocis.2013.03.001
- Nicorescu, I., Loisel, C., Vial, C., Riaublanc, A., Djelveh, G., Cuvelier, G., & Legrand, J. (2008). Combined effect of dynamic heat treatment and ionic strength on denaturation and aggregation of whey proteins - Part I. Food Research International, 41(7), 707–713. doi:10.1016/j.foodres.2008.05.003
- Orrego, M., Troncoso, E., & Zúñiga, R.N. (2015). Aerated whey protein gels as new food matrices: Effect of thermal treatment over microstructure and textural properties. Journal of Food Engineering, 163, 37–44. doi:10.1016/j.jfoodeng.2015.04.027
- Purwanti, N., Smiddy, M., Van der Goot, A.J., De Vries, R., Alting, A., & Boom, R. (2011). Modulation of rheological properties by heat-induced aggregation of whey protein solution. Food Hydrocolloids, 25(6), 1482–1489. doi:10.1016/j.foodhyd.2011.02.027
- Rullier, B., Novales, B., & Axelos, M.A.V. (2008). Effect of protein aggregates on foaming properties of α-lactoglobulin. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 330(2–3), 96–102. doi:10.1016/j.colsurfa.2008.07.040
- Ryan, K.N., Zhong, Q., & Foegeding, E.A. (2013). Use of whey protein soluble aggregates for thermal stability - A hypothesis paper. Journal of Food Science, 78(8), R1105–R1115. doi:10.1111/1750-3841.12207
- Schmitt, C., Bovay, C., & Rouvet, M. (2014). Bulk self-aggregation drives foam stabilization properties of whey protein microgels. Food Hydrocolloids, 42(1), 139–148. doi:10.1016/j.foodhyd.2014.03.010
- Schmitt, C., Bovay, C., Rouvet, M., Shojaei-Rami, S., & Kolodziejczyk, E. (2007). Whey protein soluble aggregates from heating with NaCl: Physicochemical, interfacial, and foaming properties. Langmuir, 23(8), 4155–4166. doi:10.1021/la0632575
- Tamm, F., Sauer, G., Scampicchio, M., & Drusch, S. (2012). Pendant drop tensiometry for the evaluation of the foaming properties of milk-derived proteins. Food Hydrocolloids, 27(2), 371–377. doi:10.1016/j.foodhyd.2011.10.013
- Tripp, B.C., Magda, J.J., & Andrade, J.D. (1995). Adsorption of globular proteins at the air/water interface as measured via dynamic surface tension: Concentration dependence, mass-transfer considerations, and adsorption kinetics. Journal of Colloid and Interface Science, 173(1), 16–27. doi:10.1006/jcis.1995.1291
- Wang, Z., & Narsimhan, G. (2005). Interfacial dilatational elasticity and viscosity of β-lactoglobulin at air−water interface using pulsating bubble tensiometry. Langmuir, 21(10), 4482–4489. doi:10.1021/la047374g
- Zhu, H., & Damodaran, S. (1994). Heat-induced conformational changes in whey protein isolate and its relation to foaming properties. Journal of Agricultural and Food Chemistry, 42(4), 846–855. doi:10.1021/jf00040a002
- Zúñiga, R.N., Kulozik, U., & Aguilera, J.M. (2011). Ultrasonic generation of aerated gelatin gels stabilized by whey protein β-lactoglobulin. Food Hydrocolloids, 25(5), 958–967. doi:10.1016/j.foodhyd.2010.09.010
- Zúñiga, R.N., Skurtys, O., Osorio, F., Aguilera, J.M., & Pedreschi, F. (2012). Physical properties of emulsion-based hydroxypropyl methylcellulose films: Effect of their microstructure. Carbohydrate Polymers, 90(2), 1147–1158. doi:10.1016/j.carbpol.2012.06.066
- Zúñiga, R.N., Tolkach, A., Kulozik, U., & Aguilera, J.M. (2010). Kinetics of formation and physicochemical characterization of β-lactoglobulin aggregates. Journal of Food Science, 75(5), E261–E268. doi:10.1111/j.1750-3841.2010.01805.x
