ABSTRACT
The oxygen can have an adverse effect on the quality of some foods leading to a decrease in their shelf-life. Several approaches have been applied to remove the oxygen concentration in packed food; among them, oxygen scavengers have been widely used to preserve oxygen-sensitive foods. In the present study, an experimental set-up was designed to evaluate the effectiveness of oxygen scavengers, in removing oxygen from butter containers. Two types of oxygen absorbers adhesive labels and sachets and two caps with and without adjustable closure were tested. The plastic caps used in the study were characterized by differential scanning calorimetry and Fourier transform infrared spectroscopy. The oxygen concentration in the headspace of the containers was monitored using a gas analyser. The best results were achieved with the oxygen absorber sachets and using caps with adjustable closure. Under these conditions, the oxygen concentration inside the container remained below 3% during 150 h.
RESUMEN
El oxígeno puede tener un efecto adverso en la calidad de algunos alimentos que puede conducir a una disminución de su vida útil. Se han aplicado varios métodos para eliminar o reducir la concentración de oxígeno en los alimentos envasados; entre ellos los absorbedores de oxígeno han sido ampliamente utilizados para conservar alimentos sensibles al oxígeno. En este estudio se diseñó un experimento para evaluar la eficacia de los absorbedores de oxígeno, basados en la oxidación de polvo de hierro, en la eliminación del oxígeno de los recipientes de mantequilla. Se ensayaron dos tipos de absorbedores, etiquetas adhesivas y bolsitas y dos tipos de tapas con y sin cierre ajustable. Las tapas de plástico utilizadas en el estudio se caracterizaron por calorimetría de barrido diferencial (DSC) y espectroscopía infrarroja por transformada de Fourier (FTIR). Se controló la concentración de oxígeno en el espacio de cabeza de los recipientes utilizando un analizador de gas. Los experimentos se realizaron simulando las condiciones de almacenamiento de la mantequilla. Los mejores resultados se obtuvieron con los absorbedores de oxígeno tipo bolsitas y con tapas con cierre ajustable. En estas condiciones, la concentración de oxígeno dentro del recipiente permaneció por debajo del 3% durante 150 h.
1. Introduction
Oxygen is one of the major causes of food deterioration. It is responsible for undesirable reactions and processes that can occur in foodstuffs such as, rancidity of fats and fatty foods, discoloration of certain foods (i.e. meat products, herbs, tea, dried vegetables and so on) and loss of oxygen sensitive micronutrients (i.e. vitamin C, vitamin E, β-carotene, etc.). Moreover, its presence favours the growth of moulds, yeast and aerobic bacteria (Anthierens et al., Citation2011; Busolo & Lagaron, Citation2012; Byun, Darby, Cooksey, Dawson, & Whiteside, Citation2011; de Kruijfy et al., Citation2002; Kerry, O’Grady, & Hogan, Citation2006; López-Rubio et al., Citation2004; Mills, Citation2005; Sängerlaub et al., Citation2013; Suppakul, Miltz, Sonneveld, & Bigger, Citation2003). Also unwanted odours can appear (e.g. as a result of the lipid oxidation) which make the product unacceptable by the consumers (Barriuso, Astiasarán, & Ansorena, Citation2013; Coupland & McClements, Citation1996; Karpińska, Borowski, & Danowska-Oziewicz, Citation2001).
To control the oxygen concentration is crucial to protect the packed foods. Different technologies have been employed to remove the oxygen including vacuum packaging, active modified atmosphere packaging and oxygen scavengers (Busolo & Lagaron, Citation2012; Byun et al., Citation2011; Kerry et al., Citation2006; Mu et al., Citation2013; Sängerlaub et al., Citation2013). The latter ones have been successfully applied to eliminate oxygen from different packed food items such as nuts, meat products, bakery products, cheese, coffee, fish, pasta, fruit juices, vegetables and so on (Charles, Sanchez, & Gontard, Citation2006; López-Cervantes, Sánchez-Machado, Pastorelli, Rijk, & Paseiro-Losada, Citation2003; Restuccia et al., Citation2010; Vermeiren, Devlieghere, van Beest, de Kruijf, & Debevere, Citation1999).
In a study conducted by Pastorelli, Valzacchi, Rodriguez, and Simoneau (Citation2006), the oxygen scavengers were employed in the storage of hazelnuts with satisfactory results. The oxygen content was reduced until 0.001% in samples stored with oxygen scavengers, while in the samples without the active system, the O2% was 18%. Antunez, Botero Omary, Rosentrater, Pascall, and Winstone (Citation2012) also found promising results in the use of oxygen absorbers for the conservation of preservative-free flour tortillas. These active systems have as well proven to be effective to extend the shelf-life of wheat crackers (Berenzon & Saguy, Citation1998). The oxygen absorbers have also demonstrated to be efficient in avoiding discolouration in ground beef (Gill & McGinnis, Citation1995). Mohan, Ravishankar, and Srinivasagopal (Citation2008) reported another application of oxygen scavenger technology for the improvement of the shelf-life of catfish (Pangasius sutchi) steaks. With this active system, the oxygen level inside the packages was greatly reduced and consequently the shelf-life of the products was considerably increased.
The oxygen scavengers available are based on different mechanisms of action mainly: chemical systems (powdered iron oxide, catechol, ferrous carbonate, iron-sulphur, sulphite salt-copper sulphate, photosensitive dye oxidation, ascorbic acid oxidation, catalytic conversion of oxygen by platinum catalyst) and enzymatic systems (glucose oxidase-glucose, alcohol oxidase-ethanol vapour) (Ozdemir & Floros, Citation2004).
These active systems are presented in different forms: sachets, adhesive labels cards or sheets. The sachets are not appropriate for liquid and semi-liquid foods.
The last trends in the active food packaging field are devoted to incorporate the oxygen scavengers into the packaging materials (Antunez et al., Citation2012; Byun et al., Citation2011; Cecchi, Passamonti, & Cecchi, Citation2010; Dainelli, Gontard, Spyropoulos, Zondervan-van den Beuken, & Tobback, Citation2008; Vermeiren et al., Citation1999). Several examples have been described in the literature. Galdi, Nicolais, Di Maio, and Incarnato (Citation2008) reported the development of an active material by means of the incorporation of oxygen scavengers into a polyethylene terephthalate (PET) matrix during the extrusion process. The developed films have demonstrated effective in preventing discoloration in fresh apple slices when compared with the samples packed with the control PET films without the oxygen scavenger.
A new oxygen scavenging system consisted of a polyolefin nanocomposite containing an iron-based kaolinite is described in a work carried out by Busolo and Lagaron (Citation2012). Sängerlaub et al. (Citation2013) developed iron-based oxygen scavenging multilayer films by extrusion for food packaging applications.
These last systems are preferred by consumers and represent a different option to the conventional sachets and labels. But, however, the sachets based on iron powder oxidation present a higher oxygen scavenging capacity and from the commercial point of view are the most widely used (Busolo & Lagaron, Citation2012; de Kruijfy et al., Citation2002; Galdi et al., Citation2008; Kerry et al., Citation2006; Vermeiren et al., Citation1999).
Butter is a food highly susceptible to lipid oxidation, this phenomenon leads to the development of undesirable odours, resulting in an alteration of the organoleptic properties and the nutritional value, and therefore decreasing its shelf-life (Ayar, Özcan, Akgül, & Akin, Citation2001; Barriuso et al., Citation2013; Mallia, Escher, & Schlichtherle-Cerny, Citation2008; Mallia et al., Citation2008; Panseri, Soncin, Chiesa, & Biondi, Citation2011).
In the present work, an experimental set-up was designed to assess the effectiveness of oxygen scavengers in reducing or eliminating the oxygen levels from butter containers. Two different forms, sachets and adhesive labels, and two different types of caps with and without adjustable closure were evaluated. The assays were performed simulating real conditions of storage. Additionally, the plastic caps used in the study were characterized by differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR). The oxygen transmission through the cap in the closed package was also evaluated.
2. Materials and methods
2.1. Materials
Two commercial oxygen scavengers, adhesive labels (ATCO OS 200) and sachets (ATCO LH 210), were obtained from Laboratoires Standa (Caen, France). The specifications of both systems are presented in . According the supplier’s instructions, the oxygen absorber ATCO OS-200 must be used in atmospheres with relative humidity (RH) higher than 85% RH whereas the oxygen scavenger ATCO LH 210 can be used in either humid or dry atmospheres.
Table 1. Characteristics of the oxygen scavengers used in the study.
Tabla 1. Características de los absorbedores de oxígeno usados en el estudio.
All packaging samples were provided by Feiraco (Feiraco Sociedad Cooperativa Galega, Ames, A Coruña, Spain) and are shown in . The circular and hermetic packaging is made of tinplate can and the capacity is 250 mL. On top of package is an easy open lid made of tin. There are two caps that can be employed once the easy open lid is removed: cap with adjustable closure and cap without adjustable closure.
Figure 1. (a, b) Butter packed in the packaging and (c) adjustable closure cap and (d) no adjustable cap.
Figura 1. (a, b) Mantequilla envasada y (c) tapa de cierre ajustable y (d) tapa de cierre no ajustable.
These containers are intended to hold butter particularly sensitive to oxidation because of its fatty acid composition. This butter presents a higher content in polyunsaturated fatty acids particularly in omega-3 fatty acids and in conjugated linoleic acid compared with a conventional butter. These differences are attributed to the milk used in its elaboration.
2.2. Experimental procedure
An experimental set-up was designed in order to evaluate the effectiveness of oxygen scavengers in removing residual oxygen in butter containers.
Two series of tests were carried out in order to evaluate the different oxygen scavengers: sachets and adhesive labels. In the first sequence of assays, adhesive labels were checked. The labels were pasted to the internal face of the plastic cap ()). The cans were filled with 200 mL of water to simulate the volume occupied by the butter. Twice a day, at early morning and at noon, the cans were opened, and 25 mL of the water were withdrawn, simulating the opening of the cans in real life and the increment of volume in the headspace due to gradual consumption of butter. During the whole assay, the containers were stored at 5°C.
Figure 2. Experimental set-up used to evaluate the effectiveness of the oxygen scavengers in removing oxygen from the butter containers adhesive labels (a) and sachets (b).
Figura 2. Diseño experimental usado para evaluar la efectividad de los absorbedores de oxígeno en la eliminación de oxígeno desde los envases de mantequilla, etiquetas adhesivas (a) y bolsitas (b).
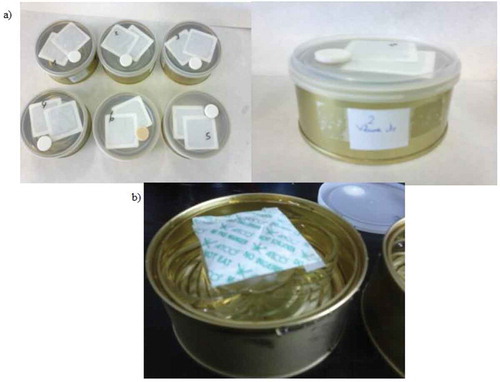
The assays were conducted with both types of plastic caps. Each experiment was made by duplicate or triplicate.
In the second series of trials, scavenger sachets were tested. Glass rings were placed inside the cans and the scavenger sachets were placed on them and thus avoiding the direct contact with water according the supplier’ recommendation ()). The experiments were also performed with both types of caps.
2.3. Equipments
In the above–mentioned assays, the residual oxygen was measured by using a gas analyser PBI Dansensor Check Mate 9900 O2/CO2 (Ringsted, Denmark). The gas sampling was made with a needle through the septum (Septa 20 MM TFE/SIL 0.130 FOR 20 mm SEAL). The oxygen concentration in the headspace of the container was measured each hour.
2.3.1. Differential scanning calorimetry
The thermo analytical curves were obtained in DSC module using Mettler Toledo equipment, operating under the following conditions: heating rate of 10°C/minute in an atmosphere of nitrogen (Ramp 10°C/min to 300°C). Polymer weight was 10 mg at a temperature range of 25–300°C.
2.3.2. Fourier transform infrared spectroscopy
FTIR was performed in Shimadzu equipment. Samples were analysed by attenuated total reflectance (ATR)-FTIR. The spectra focus on 4000–650 cm−1.
2.3.3. Oxygen transmission rate (OTR)
OTR was measured according to ASTM D3985:2010. “Standard test method for oxygen transmission rate through plastic film and sheeting using a coulometric sensor” in OX-TRAN 2/21MH equipment. (OTR was evaluated to 4 temperatures.
The test conditions were as follows: sample: flat area of adjustable closure cap and flat area of no adjustable cap; test temperature: 10°C, 20°C, 23°C, 30°C and 35°C and 0% RH; pressure: 760 mm Hg; sample gas was synthetic air: 21% oxygen and 0% RH; and tested area: 5 cm2.
3. Results and discussion
First of all, the plastic caps used in the study were characterized by DSC and FTIR. shows the DSC curves obtained for both types of caps, with and without adjustable closure, respectively. As can be seen in , first and second melting peaks are 102°C and 125°C which correspond to polyethylene (PE with different density) and the third melting peak at 164°C corresponds to polypropylene (PP). In , only a melting peak at 109°C was observed which correspond to PE.
Figure 3. (a) DSC measurement in adjustable closure cap and (b) DSC measurement in no adjustable closure cap.
Figura 3. (a) Medida DSC en tapa de cierre ajustable y (b) Medida DSC en tapa de cierre no ajustable.
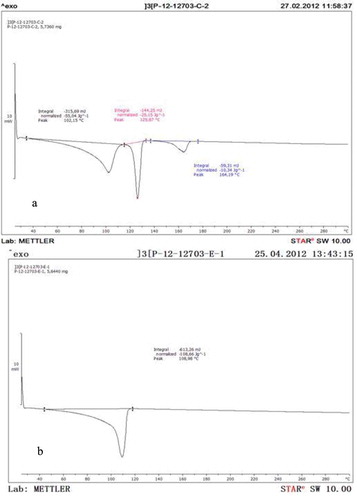
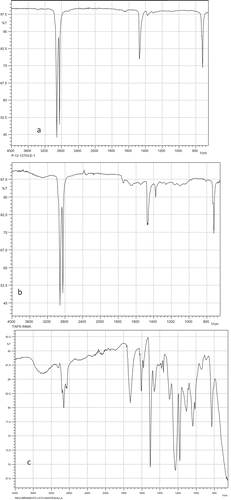
ATR-FTIR analysis () confirms PE and PP as plastic closure for adjustable cap and PE as plastic closure for no adjustable cap, respectively. For example, for PP and PE, the bands in the region around 2950 cm−1 correspond to methyl and methylene groups. The coating of the tin was also analysed by ATR-FTIR and the spectra obtained showed that the cans were coated with an epoxy. The peak around 3050 cm−1 corresponds to C–H stretching of epoxy ring.
Figure 4. (a) ATR-FTIR spectra in adjustable closure cap, (b) ATR-FTIR spectra in no adjustable closure cap and (c) ATR-FTIR spectra in tin coating.
Figura 4. (a) ATR-FTIR espectro en tapa con cierre ajustable, (b) ATR-FTIR espectro en tapa con cierre no ajustable y (c) ATR-FTIR espectro en el recubrimiento.
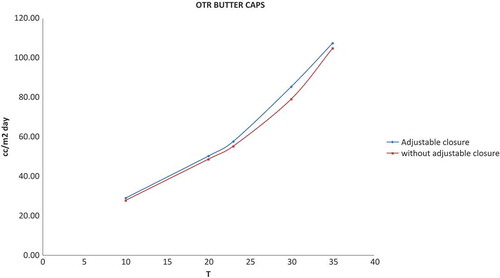
Moreover, oxygen barrier properties were analysed in both caps to evaluate oxygen transmission through the cap in the closed package. The results are presented in and the effect of temperature on the OTR is shown in . As can be inferred from the data, there are not differences between both types of caps because they have similar oxygen transmission rate.
Table 2. OTR results for adjustable and no closure adjustable cap.
Tabla 2. Resultados de velocidad de transmisión de oxígeno (OTR) para tapas con cierre ajustable y no ajustable.
Figure 5. OTR cap with adjustable closure and cap without adjustable closure vs. temperature.
Figura 5. Velocidad de transmisión de oxígeno en tapa con cierre ajustable y en tapa con cierre no ajustable frente a la temperatura.
Then, the efficiency of two oxygen scavengers, based on iron powder oxidation, to reduce the oxygen concentration from butter containers was evaluated.
Oxygen scavenger technology is a valuable and convenient tool for removing residual oxygen in packed foods and in this way maintains the quality and consequently extends the shelf-life of the products.
In the first series of experiments, two adhesive labels (Atco OS 200) were tested according the experimental procedure described earlier. shows that when the oxygen absorber labels (ATCO OS 200) were applied to butter containers with a cap without adjustable closure, an inadequate reduction of oxygen levels (>9%) was observed. On the contrary, when caps with adjustable closure were used, a oxygen concentration inside the package at a concentration lower than 3% was maintained for approximately 70 h (). This could be attributed to the type of closure but not to the permeability of the materials they are made of, if the results of OTR are taken into account.
Figure 6. Oxygen concentration in the headspace of the butter containers for samples containing two adhesive labels and no adjustable cap (a) and adjustable cap (b). Vertical lines indicate the opening of the container. Oxygen concentration in the headspace of the butter containers for samples containing two absorber sachets and no adjustable cap (c) and adjustable cap (d). Oxygen concentration in the headspace of the butter containers for samples containing adjustable caps and one adhesive label (e) and one absorber sachet (f).
Figura 6. Concentración de oxígeno en el espacio de cabeza de los envases de mantequilla para muestras que contienen dos etiquetas adhesivas y tapa con cierre no ajustable (a) y tapa con cierre ajustable (b). Las líneas verticales indican la apertura del envase. Concentración de oxígeno en el espacio de cabeza de los recipientes de mantequilla para muestras que contienen dos absorbedores de oxígeno tipo bolsita y tapa con cierre no ajustable (c) y tapa con cierre ajustable (d). Concentración de oxígeno en el espacio de cabeza de los envases de mantequilla para muestras que contienen tapa con cierre ajustable y una etiqueta adhesiva (e) y un absorbedor de oxígeno tipo bolsita (f).
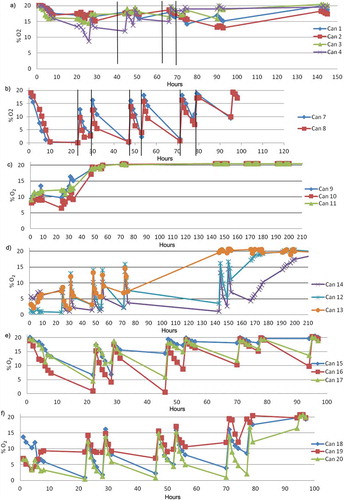
Secondly, tests were carried out with two oxygen absorber sachets (ATCO LH 200). As in the previous assay, the incorporation of the oxygen scavengers in the containers with cap without adjustable closure produces a less effective oxygen removal () than when the absorbers were in the containers with cap with adjustable closure ()). In this last case, the oxygen concentration inside the container remained below 3% during 150 h. It is interesting to note that these conditions would cover the expected butter consumption period.
On the other hand, when compared with adhesive labels, the sachets showed a higher oxygen-absorbing speed; like this, within the first hour, oxygen concentrations <5% were reached.
The following experiment was conducted with a single oxygen absorber. Caps with adjustable closure were selected due to the better results obtained previously.
The oxygen concentration in the containers with the adhesive label was initially 20% and was decreased until 3%. This concentration remained for 40–45 h ()). In the case of the containers stored with oxygen absorber sachets, oxygen content lower than 3% can be observed during 70–80 h ()). Thus, the absorber sachets provide an atmosphere low in oxygen for longer periods.
4. Conclusions
Briefly, the active systems have demonstrated effectiveness and utility in reducing oxygen levels from the butter containers and could be a good option to protect butter samples from oxidation. The absorber sachets have proved to be more efficient than the adhesive labels providing low oxygen concentrations longer. The lower oxygen concentrations achieved in the containers with adjustable closure suggest that the type of cap is an important factor to consider.
Acknowledgments
The study was financially supported by the Directorate General for Technology Transfer and Business Development (National R&D&I Plan 2008–2011; INNPACTO 2010 Call) of the Ministry of Economy and Competitiveness of the Spanish Government, ref no. IPT-060000-2010-014 “MIPFOOD” and by the “Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia”, ref. no. GRC 2014/012. R. Sendon is grateful to the “Parga Pondal” Program financed by “Consellería de Innovación e Industria, Xunta de Galicia” for her postdoctoral contract. The authors are also grateful to C. Casal, P. Blanco and G. Hermelo for their excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anthierens, T., Ragaert, P., Verbrugghe, S., Ouchchen, A., De Geest, B. G., Noseda, B., … Devlieghere, F. (2011). Use of endospore-forming bacteria as an active oxygen scavenger in plastic packaging materials. Innovative Food Science & Emerging Technologies, 12, 594–599. doi:10.1016/j.ifset.2011.06.008
- Antunez, P. D., Botero Omary, M., Rosentrater, K. A., Pascall, M., & Winstone, L. (2012). Effect of an oxygen scavenger on the stability of preservative-free flour tortillas. Journal of Food Science, 77(1), S1–S9. doi:10.1111/j.1750-3841.2011.02470.x
- Ayar, A., Özcan, M., Akgül, A., & Akin, N. (2001). Butter stability as affected by extracts of sage, rosemary and oregano. Journal of Food Lipids, 8, 15–25. doi:10.1111/j.1745-4522.2001.tb00180.x
- Barriuso, B., Astiasarán, I., & Ansorena, D. (2013). A review of analytical methods measuring lipid oxidation status in foods: A challenging task. European Food Research Technology, 236, 1–15. doi:10.1007/s00217-012-1866-9
- Berenzon, S., & Saguy, I. S. (1998). Oxygen absorbers for extension of crackers shelf-life. LWT Food Science and Technology, 31, 1–5. doi:10.1006/fstl.1997.0286
- Busolo, M. A., & Lagaron, J. M. (2012). Oxygen scavenging polyolefin nanocomposite films containing an iron modified kaolinite of interest in active food packaging applications. Innovative Food Science & Emerging Technologies, 16, 211–217. doi:10.1016/j.ifset.2012.06.008
- Byun, Y., Darby, D., Cooksey, K., Dawson, P., & Whiteside, S. (2011). Development of oxygen scavenging system containing a natural free radical scavenger and a transition metal. Food Chemistry, 124, 615–619. doi:10.1016/j.foodchem.2010.06.084
- Cecchi, T., Passamonti, P., & Cecchi, P. (2010). Study of the quality of extra virgin olive oil stored in PET bottles with or without an oxygen scavenger. Food Chemistry, 120, 730–735. doi:10.1016/j.foodchem.2009.11.001
- Charles, F., Sanchez, J., & Gontard, N. (2006). Absorption kinetics of oxygen and carbon dioxide scavengers as part of active modified atmosphere packaging. Journal of Food Engineering, 72, 1–7. doi:10.1016/j.jfoodeng.2004.11.006
- Coupland, J. N., & McClements, D. J. (1996). Lipid oxidation in food emulsions. Trends in Food Science & Technology, 7, 83–91. doi:10.1016/0924-2244(96)81302-1
- Dainelli, D., Gontard, N., Spyropoulos, D., Zondervan-van den Beuken, E., & Tobback, P. (2008). Active and intelligent food packaging: Legal aspects and safety concerns. Trends in Food Science & Technology, 19, S103–S112. doi:10.1016/j.tifs.2008.09.011
- de Kruijfy, N., Van Beest, M., Rijk, R., Sipiläinen-Malm, T., Paseiro Losada, P., & De Meulenaer, B. (2002). Active and intelligent packaging: Applications and regulatory aspects. Food Additives & Contaminants, 19(sup1), 144–162. doi:10.1080/02652030110072722
- Galdi, M. R., Nicolais, V., Di Maio, L., & Incarnato, L. (2008). Production of active PET films: Evaluation of scavenging activity. Packaging Technology and Science, 21, 257–268. doi:10.1002/pts.794
- Gill, C. O., & McGinnis, J. C. (1995). The use of oxygen scavengers to prevent the transient discolouration of ground beef packaged under controlled, oxygen-depleted atmospheres. Meat Science, 41(1), 19–27. doi:10.1016/0309-1740(94)00064-E
- Karpińska, M., Borowski, J., & Danowska-Oziewicz, M. (2001). The use of natural antioxidants in ready-to-serve food. Food Chemistry, 72, 5–9. doi:10.1016/S0308-8146(00)00171-0
- Kerry, J. P., O’Grady, M. N., & Hogan, S. A. (2006). Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Science, 74, 113–130. doi:10.1016/j.meatsci.2006.04.024
- López-Cervantes, J., Sánchez-Machado, D. I., Pastorelli, S., Rijk, R., & Paseiro-Losada, P. (2003). Evaluating the migration of ingredients from active packaging and development of dedicated methods: A study of two iron-based oxygen absorbers. Food Additives and Contaminants, 20(3), 291–299. doi:10.1080/0265203021000060878
- López-Rubio, A., Almenar, E., Hernandez-Muñoz, P., Lagarón, J. M., Catalá, R., & Gavara, R. (2004). Overview of active polymer-based packaging technologies for food applications. Food Reviews International, 20(4), 357–387. doi:10.1081/FRI-200033462
- Mallia, S., Escher, F., & Schlichtherle-Cerny, H. (2008). Aroma-active compounds of butter: A review. European Food Research Technology, 226, 315–325. doi:10.1007/s00217-006-0555-y
- Mallia, S., Piccinali, P., Rehberger, B., Badertscher, R., Escher, F., & Schlichtherle-Cerny, H. (2008). Determination of storage stability of butter enriched with unsaturated fatty acids/conjugated linoleic acids (UFA/CLA) using instrumental and sensory methods. International Dairy Journal, 18, 983–993. doi:10.1016/j.idairyj.2008.05.007
- Mills, A. (2005). Oxygen indicators and intelligent inks for packaging food. Chemical Society Reviews, 34, 1003–1011. doi:10.1039/B503997P
- Mohan, C. O., Ravishankar, C. N., & Srinivasagopal, T. K. (2008). Effect of O2 scavenger on the shelf-life of catfish (Pangasius sutchi) steaks during chilled storage. Journal of the Science of Food and Agriculture, 88, 442–448. doi:10.1002/jsfa.3105
- Mu, H., Gao, H., Chen, H., Tao, F., Fang, X., & Ge, L. (2013). A nanosised oxygen scavenger: Preparation and antioxidant application to roasted sunflower seeds and walnuts. Food Chemistry, 136, 245–250. doi:10.1016/j.foodchem.2012.07.121
- Ozdemir, M., & Floros, J. D. (2004). Active food packaging technologies. Critical Reviews in Food Science and Nutrition, 44, 185–193. doi:10.1080/10408690490441578
- Panseri, S., Soncin, S., Chiesa, L. M., & Biondi, P. A. (2011). A headspace solid-phase microextraction gas-chromatographic mass-spectrometric method (HS-SPME–GC/MS) to quantify hexanal in butter during storage as marker of lipid oxidation. Food Chemistry, 127, 886–889. doi:10.1016/j.foodchem.2010.12.150
- Pastorelli, S., Valzacchi, S., Rodriguez, A., & Simoneau, C. (2006). Solid-phase microextraction method for the determination of hexanal in hazelnuts as an indicator of the interaction of active packaging materials with food aroma compounds. Food Additives and Contaminants, 23(11), 1236–1241. doi:10.1080/02652030600778744
- Restuccia, D., Spizzirri, U. G., Parisi, O. I., Cirillo, G., Curcio, M., Iemma, F., … Picci, N. (2010). New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control, 21, 1425–1435. doi:10.1016/j.foodcont.2010.04.028
- Sängerlaub, S., Gibis, D., Kirchhoff, E., Tittjung, M., Schmid, M., & Müller, K. (2013). Compensation of pinhole defects in food packages by application of iron-based oxygen scavenging multilayer films. Packaging Technology and Science, 26, 17–30. doi:10.1002/pts.1962
- Suppakul, P., Miltz, J., Sonneveld, K., & Bigger, S. W. (2003). Active packaging technologies with an emphasis on antimicrobial packaging and its applications. Journal of Food Science, 68(2), 408–420. doi:10.1111/j.1365-2621.2003.tb05687.x
- Vermeiren, L., Devlieghere, F., van Beest, M., de Kruijf, N., & Debevere, J. (1999). Developments in the active packaging of foods. Trends in Food Science & Technology, 10, 77–86. doi:10.1016/S0924-2244(99)00032-1
