 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Morchella protein hydrolysate (MPH) is produced from an edible and medicinal fungus, Morchella esculenta (L.), and exhibits antioxidant activity. In order to further improve the antioxidant activity of MPH, glycosylation of MPH was conducted by applying response surface methodology via the Maillard reaction (MR). Optimum glycosylation conditions were confirmed as: xylose/MPH mass ratio of 3.7:1, pH 11.80, and reaction time 60 min. The glycosylation of MPH under these optimized conditions was characterized by Fourier transform infrared spectroscopy, fluorescence spectroscopy, and scanning electron microscopy. In vitro antioxidant activity tests showed that glycosylated MPH had significantly enhanced antioxidant activities compared to the native MPH, heat-treated MPH, or a MPH–xylose mixture. Collectively, these results indicate that glycosylation by the MR can improve the antioxidant activity of MPH, making it a novel source of natural antioxidants with excellent developmental prospects as a functional food.
RESUMEN
La proteína hidrolizada de Morchella (MPH) se produce a partir de un hongo comestible y medicinal, Morchella esculenta (L.), constatándose que la misma posee actividad antioxidante. Con el fin de mejorar su actividad antioxidante se realizó la glicosilación de la MPH utilizando la metodología de superficies de respuesta mediante la reacción de Maillard. En este sentido, se verificó que las condiciones óptimas para la glicosilación fueran: relación de masa xilosa/MPH de 3.7:1, pH de 11.80 y tiempo de reacción de 60 min. Alcanzadas estas condiciones optimizadas, se caracterizó la glicosilación de la MPH empleando espectroscopía infrarroja transformada de Fourier, espectroscopía de fluorescencia y microscopía electrónica de barrido. Las pruebas de actividad antioxidante in vitro indicaron que, en comparación con la MPH nativa, la MPH tratada térmicamente o una mezcla de MPH-xilosa, la MPH glicosilada mostró un aumento significativo de sus actividades antioxidantes. En conjunto, estos resultados constatan que la glicosilación mediante la reacción de Maillard puede mejorar la actividad antioxidante de la MPH, convirtiéndola en una novedosa fuente de antioxidantes naturales que, además, exhibe excelentes posibilidades de ser desarrollada como alimento funcional.
1. Introduction
Morchella (Morchella esculenta) is a medicinal, edible, delicious, and sought-after wild mushroom rich in various nutrients, such as polysaccharides, proteins, vitamins, and trace elements. It can enhance the functioning of the immune system, and also contains anti-fatigue, antiviral, antioxidant, and antitumor growth properties (Ajmal, Akram, Ara, Akhund, & Nayyar, Citation2015; Meng et al., Citation2010). The protein content of Morchella is 32.7% (dry weight), comprising eight essential amino acids, making it a valuable source of protein (García-Pascual, Sanjuán, Melis, & Mulet, Citation2006; LeDuy, Kosaric, & Zajic, Citation1974). With the rapid advancement of submerged fermentation technology, the development of functional foods from Morchella has gained significant commercial interest. However, most of the existing studies have focused on utilization of the crude extract, such as aqueous-ethanol extracts (Nitha & Janardhanan, Citation2008), methanol extracts (Nitha, Meera, & Janardhanan, Citation2007), or polysaccharides (Li et al., Citation2013). Research on Morchella proteins is lacking.
In recent years, the antioxidant peptides from the enzymatic hydrolysis of proteins have received significant research attention. Studies have shown that these have a simple structure, possess good stability, are non-immunogenic, and are safe and healthy compounds with broad application potential in functional foods, food additives, pharmaceuticals, and cosmetics (Sarmadi & Ismail, Citation2010). However, the antioxidant activities of these peptides are not as high as those of synthetic antioxidants due to restrictions presented by the raw protein’s primary structure. Therefore, it is desirable to make further modifications to the native peptides in order to increase their antioxidant activity.
Due to its simplicity, low cost, and mild reaction conditions, the Maillard reaction (MR) is considered to be a method with significant potential for modifying proteins or peptides in the food industry (Liu, Ru, & Ding, Citation2012; Moisés, Corzomartinez, Villamiel, Javier, & Sanz, Citation2011). The MR is a widely used nonenzymatic browning reaction that occurs when foods are heated, and refers primarily to the complex reactions between carbonyl compounds (reducing saccharides) and amino compounds (amino acids, peptides, and proteins), which occur during food processing. It gives food a particular aroma, taste, and color, and plays an important role in the sensory quality of foods (Sun, Zhao, Cui, Zhao, & Bao, Citation2010; Zeng, Zhang, Guan, Zhang, & Sun, Citation2013). Maillard reaction products (MRPs) have antioxidant, anti-allergic, and anti-inflammatory properties (Chen & Kitts, Citation2011). In particular, their antioxidant activities make them potential substitutes for synthetic antioxidants (Vhangani & Van Wyk, Citation2016). The MR is influenced by many factors such as temperature, pH, reaction time, and the concentration and type of substrates (Yin, Sun, Zhang, & Hao, Citation2014). Among these, substrate type plays a leading role in the occurrence of MR and the characteristics of the resulting products. Protein hydrolysates, as a crude extract, are nourishing, nontoxic, and cost-effective. They possess diverse biological activities, are nonallergenic, and are easily digested and absorbed (Rao, Kamdar, & Labuza, Citation2013). They are suitable substrates for the MR; however, the MR of the saccharide–protein hydrolysate system has largely not been examined (Chawla, Chander, & Sharma, Citation2009).
Morchella protein hydrolysate (MPH) represents a potential source of protein and peptides that can undergo MR. It was anticipated that copolymerization of MPH with reducing saccharides would produce a functional product with high antioxidant activity, which could have wide application in the production of health-promoting foods. To address this, the present study sought to optimize the glycosylation of MPH in the MR using response surface methodology (RSM), and to examine the molecular characteristics of the glycosylated Morchella protein hydrolysates (MPHG) by Fourier transform infrared spectroscopy (FT-IR), fluorescence spectroscopy, and scanning electron microscopy (SEM). Furthermore, the in vitro antioxidant activities of the MPH and its glycosylated derivatives were determined using different model systems. The results of the present study may provide experimental evidence in support of the utilization of MPH and its glycosylated derivatives as highly effective natural antioxidants in health products and functional foods.
2. Materials and methods
2.1. Strain and liquid fermentation
Morchella strain ACCC 50537 was provided by the Agricultural Culture Collection of China. The strain was cultured on potato dextrose agar slants at 25°C for 7 days, and then stored at 4°C. It was subcultured every 2 months.
The fungal culture (0.5 cm2) was inoculated in a 250-mL culture flask with 100 mL culture media containing 100 g/L potato, 30 g/L dextrose, 5 g/L yeast extract, 1 g/L peptones, 1 g/L MgSO4.7H2O, and 1 g/L KH2PO4. The culture was incubated at 25°C for 3 days with shaking at 150 rpm. The fermented broth was filtered with a filter cloth, washed with distilled water, and freeze-dried to obtain the Morchella mycelia.
2.2. Morchella protein extraction and MPH preparation
The Morchella mycelia were homogenized, resuspended in NaOH solution (pH 12.0) at a ratio of 1:40 (w/v), and the proteins were extracted for 1 h in a 45°C water bath. The solution was centrifuged at 4000 g for 20 min, and the supernatant was adjusted to pH 4.1 with 2 mol/L HCl, followed by a further centrifugation at 4000 g for 20 min. After removal of the supernatant, the pellet was dissolved in a small amount of distilled water and freeze-dried to obtain the Morchella protein isolate (MPI). The protein content of the MPI was 63.03 ± 2.08% (w/w), as determined by the Bradford method (Bradford, Citation1976).
The MPI solution with a mass fraction of 5% was denatured at 100°C for 30 min and then cooled. Papain was added to the solution at an enzyme/protein (E/S) ratio of 2%, and the mixture was hydrolyzed in a shaking water bath at 45°C for 3 h at pH 6.0 using the pH-stat technique. At the end of hydrolysis, the enzyme was inactivated for 10 min in a boiling water bath, and the mixture was then centrifuged at 4000 g for 15 min. The supernatant was freeze-dried to obtain MPH.
2.3. MPH glycosylation
MPH (10 mL) with a mass fraction of 0.5% was placed in a screw-capped plastic centrifuge tube. The glycosylation reaction was conducted in a boiling water bath under a range of experimental conditions (saccharide type, pH, saccharide/MPH mass ratio, and reaction time), and then stopped by cooling in an ice bath to obtain MPHG for subsequent analysis.
2.3.1. Single factor experiment
Based on the glycosylation process, the effect of a single factor, such as saccharide type (xylose, glucose, lactose, and dextran-20), pH (9.0–13.0), saccharide/MPH mass ratio (1:2–4:1), and reaction time (0–100 min), on the glycosylation reaction was examined using total antioxidant activity (TAA) as an indicator.
2.3.2. Response surface experiment
Based on the previous single factor experiments, MPH glycosylation was optimized using Design-Expert software (version 8.06) based on the three-factor, three-level Box–Behnken design, where pH, saccharide/MPH mass ratio, and reaction time were the observed variables, and TAA was the response value. The experimental factors and design levels are shown in .
Table 1. Box–Behnken experimental design and results for glycation of Morchella protein hydrolysate (MPH).
Tabla 1. Diseño experimental Box–Behnken y resultados de la glicación de la proteína hidrolizada de Morchella (MPH).
2.4. Fourier transform infrared spectroscopy
Samples of native MPH (MPHN), heat-treated MPH (MPHH), MPH–xylose mixture (MPH-XM), MPHG, and native xylose (XYLN) were each mixed with potassium bromide powder at a concentration of 1:100, and then ground into powders in an agate mortar. The specimens were scanned at 400–4000 cm−1 with a spectral resolution of 4 cm−1 under transmission mode of a Nicolet 380 infrared spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) following compression molding.
2.5. Fluorescence spectroscopy
Solutions (0.05 mg/mL) of MPHN, MPHH, MPH-XM, and MPHG were prepared by mixing with 20 mM (pH 7.4) phosphate buffer. The fluorescence emission spectrum of each sample within the wavelength range of 300–400 nm was measured under a 291 nm excitation wavelength (5 nm slit) using a fluorescence spectrophotometer (F-4600, Hitachi, Japan).
2.6. Scanning electron microscopy
Freeze-dried samples were fixed onto a round sampling table with double-sided adhesive tape and scanned by a scanning electron microscope (ZEISS-EVO18, Germany) at an accelerating voltage of 20 kV under vacuum conditions. Images were recorded at appropriate magnification for structural analysis.
2.7. Antioxidant activity
2.7.1. Total antioxidant activity
TAA was determined in accordance with a previously published method (Salla, Sunkara, Ogutu, Walker, & Verghese, Citation2016). Briefly, 0.3 mL of a 1:10 diluted sample was added to a 3-mL ammonium molybdate reaction system containing 28 mM sodium phosphate, 0.6 M sulfuric acid, and 4 mM ammonium molybdate. The mixture was mixed well, sealed, and incubated at 95°C in a water bath for 90 min. After the sample was cooled, its absorbance was measured at 695 nm in a spectrophotometer (Model V-5000, Shanghai Yuanxi Instrument Co., Ltd., Shanghai, China). An equivalent volume of sample preparation mixture without MPH was used as the blank, and ascorbic acid (VC), at the same concentration as the samples, was used as a positive control.
2.7.2. Reducing power
The reducing power of the samples was determined using the method of Wang et al. (Citation2016), with minor modifications. Briefly, 1 mL of sample solution was mixed with 1.5 mL of phosphate buffer solution (PBS) (0.2 M, pH 6.6), followed by the addition of 2.5 mL of 1% potassium ferricyanide. After 20 min incubation at 50°C in a water bath, 2.5 mL of 10% trichloroacetic acid was added, and the mixture was then centrifuged at 2000 g for 10 min. The supernatant (2.5 mL) was mixed with 2.5 mL distilled water and 0.5 mL of 0.1% FeCl3, and the absorbance was read at 700 nm. As before, VC served as a positive control.
2.7.3. H2O2 scavenging activity
H2O2 scavenging activity was measured using the method of Al-Amiery, Al-Majedy, Kadhum, and Mohamad (Citation2015), with slight modifications. Briefly, 0.1 mL sample was mixed with 1 mL 40 mM H2O2 (prepared in 0.2 M pH 7.4 PBS), and PBS was added to bring the final volume up to 4 mL. The reaction was carried out at room temperature for 10 min in the dark, and sample absorbance was then measured at 230 nm. As before, VC served as a positive control. The following formula was used to calculate the H2O2 scavenging activity:
where A1 is the absorbance of the H2O2 and sample solution; A2 is the absorbance of the sample solution; and A3 is the absorbance of the H2O2 solution.
2.7.4. Nitrite scavenging activity
Nitrite scavenging activity was determined based on a previously reported method (Fu, Zhang, Guo, & Chen, Citation2014), with slight modifications. Briefly, 1.0 mL sample was mixed with 1.0 mL NaNO2 (5 μg/ml), and distilled water was used to bring the total volume up to 3 mL. The mixture was incubated at 37°C for 30 min and then 2 mL of 0.4% sulfanilic acid (prepared in 20% HCl) was added. The mixture was placed at room temperature for 5 min, followed by the addition of 1 mL 0.2% naphthyl ethylenediamine dihydrochloride. The mixture was placed at room temperature for another 15 min, and the sample absorbance was then read at 538 nm using distilled water as a blank. As before, VC served as a positive control. Nitrite scavenging activity was calculated according to the following formula:
where A0 and A1 represent the absorbance without and with sample present, respectively.
2.7.5. Free radical scavenging activity
The DPPH and ABTS free radical scavenging activity of the samples was measured using DPPH and ABTS as the free radical models, respectively (Zhuang, Tang, & Yuan, Citation2013). The superoxide anion free radical scavenging activity of the samples was determined by the pyrogallol autoxidation method (Liu et al., Citation2013). The free radical scavenging activities of the experimental samples were compared with that of positive control VC.
2.8. Statistical analysis
Data from the three experiments were expressed as mean ± standard deviation. Data from the response surface experiment were analyzed by the Design-Expert 8.06 software, whereas differences between groups in the other experiments were determined by the SPSS 19.0 software using an ANOVA-Tukey test. Statistical significance was determined at p < 0.01.
3. Results and discussion
3.1. Effect of different experimental factors on MPH glycosylation
The MR is a complex chemical reaction, and the extent of the reaction is influenced by the type and the nature of the chemical substrates, as well as the reaction parameters such as substrate concentration, pH, and reaction time (Jiang, Wang, Che, & Tian, Citation2014). Examination of the effects of sugar type and reaction time on MPH glycosylation at pH 11.0 and a saccharide/MPH mass ratio of 1:1 indicated that glycosylation could significantly enhance the TAA of MPH ()). Xylose showed the best glycosylation effect, followed by glucose, lactose, and dextran-20. This could be due to the shorter carbon chain of xylose, compared to hexose, disaccharides, and polysaccharides, which results in less steric hindrance and better reactivity (Huang et al., Citation2012). During the reaction, the overall TAA of different MRPs initially increased and then decreased as the reaction time increased, and TAA reached a maximum at 60 min when MPH was modified by xylose. This result is consistent with the findings of Yilmaz and Toledo (Citation2005), and demonstrates that the antioxidant activity of MRPs is not necessarily proportional to the reaction time.
Figure 1. Effect of reaction parameters on the total antioxidant of Morchella protein hydrolysate–saccharide copolymer. (a): saccharide type and reaction time (pH = 11.0; saccharide/MPH mass ratio = 1:1); (b): xylose/MPH mass ratio and pH (xylose as the substrate; reaction time = 60min). The error bars represent standard deviations of means that are based on three independent samples. MPH: Morchella protein hydrolysate.
Figura 1. Efecto de los parámetros de reacción en el total de antioxidantes de la proteína hidrolizada de Morchella–copolímero sacárido. (a): tipo de sacárido y tiempo de reacción (pH = 11.0; relación de masa sacárido/MPH = 1:1); (b): relación de masa xilosa/MPH y pH (empleando la xilosa como sustrato); tiempo de reacción = 60 min). Las barras de error representan las desviaciones estándar de las medias basadas en tres muestras independientes. MPH: proteína hidrolizada de Morchella.
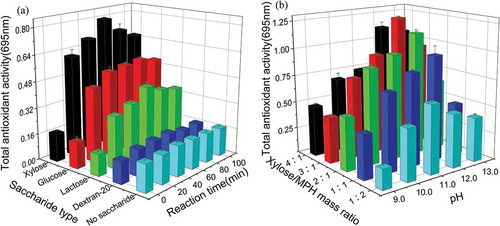
Examination of the effects of pH and xylose/MPH mass ratio on MPH glycosylation, using xylose as the substrate and a reaction time of 60 min, showed that the TAA of MRPs generally increased and then decreased as pH and mass ratio increased ()), the TAA of the MRPs was the highest at pH 12.0, and a 3:1 xylose/MPH mass ratio. The MR is essentially a base-catalyzed reaction that is facilitated by alkaline conditions (Vhangani & Van Wyk, Citation2016). However, excessively high pH can have a negative effect on the reaction (Dworschák & Carpenter, Citation1980). Research by Luo et al. (Citation2013) has shown that although higher proportions of xylose could improve the MR rate and the antioxidant activity of the reaction products, excessive xylose could reduce the antioxidant activity of MRPs. The results from the present study are consistent with these studies.
3.2. Optimization of MPH glycosylation
3.2.1. Model prediction and statistical analysis
The matrix design of the RSM and the corresponding results are shown in . The quadratic polynomial equation of TAA in terms of pH (X1), xylose/MPH mass ratio (X2), and reaction time (X3) was obtained by quadratic multinomial regression fitting, using the data in , and was
The results of variance analysis () showed that the regression model was highly significant (p < 0.0001), with no significant lack of fit (p = 0.39), and that the actual values and the predicted values were highly correlated (determination coefficient (R2) = 0.987, adjusted determination coefficient (Adj R2) = 0.970). The adequate precision was far greater than 4 (25.39) and the coefficient of variation was relatively small (CV was 4.67%), indicating that the model fitted well, had high reliability and accurately reflected the variation in the response values to each factor. Furthermore, the magnitude of the effects of the three independent variables on the response values was determined to be in the following order: xylose/MPH mass ratio (X2) > pH (X1) > reaction time (X3) (). It was also found that the model’s linear terms (X1, X2, X3), quadratic terms (X12 and X22), and interaction terms (X1 X2) all had a significant effect on the response values (p < 0.01), suggesting that the effects of the different factors on MPH glycosylation were not a simple linear relationship.
Table 2. Results of variance analysis for the response surface quadratic polynomial model.
Tabla 2. Resultados del análisis de varianza del modelo de polinomiales cuadráticos.
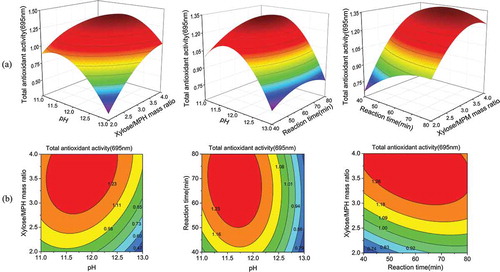
3.2.2. Response surface analysis and optimization
The effects of various factors and their interactions on the response values are shown by three-dimensional response surface and two-dimensional contour plots in . The presence of a maximum value on every curved surface indicates that the design of the response surface test was relatively accurate. The slopes of the curved surfaces ()) and the densities and shapes of the contour lines ()) demonstrate that xylose/MPH mass ratio and pH were the major influencing factors on MPH glycosylation among the three variables, while the effect of reaction time was relatively small. This finding was consistent with the results from the variance analysis shown in . Within the range of the selected experimental factors, regression model analysis by Design-Expert software showed that the optimal conditions for MPH glycosylation were as follows: pH 11.80, xylose/MPH mass ratio 3.69:1, and reaction time 61.46 min. Under these conditions, the TAA of the products could be up to 1.36. For operational convenience, the adjusted optimal reaction conditions were as follows: pH 11.80, xylose/MPH mass ratio 3.7:1, and 60 min reaction time. Under these optimal conditions, the TAA of the glycosylated MPH was determined to be 1.33 from three parallel experiments, which was consistent with the theoretical prediction, indicating that the model could be used to accurately predict the conditions of MPH glycosylation.
Figure 2. Response surface plots (a) and contour plots (b) showing the effects of pH (X1), xylose/MPH mass ratio (X2), and reaction time (X3, min) on the glycosylation of MPH. MPH: Morchella protein hydrolysate.
Figura 2. Mapas de superficies de respuesta (a) y mapas de contorno (b) que dan cuenta de los efectos de pH (X1), relación de masa xilosa/MPH (X2), y tiempo de reacción (X3, min) en la glicosilación de MPH. MPH: proteína hidrolizada de Morchella.
3.3. FT-IR analysis
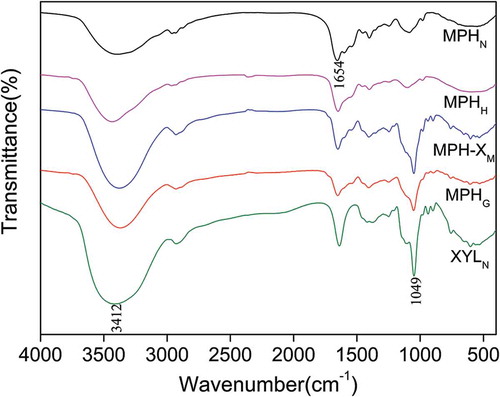
FT-IR is a commonly used method for analyzing protein and polypeptide secondary structures and food composition, and a powerful tool for identifying protein–saccharide polymers (Van et al., Citation2002; Wang, Bao, & Chen, Citation2013). The FT-IR spectra of MPHN, MPHH, MPH-XM, MPHG, and XYLN are shown in . The absorption peak near 1654cm−1 is attributed to the amide I band (C=O stretching vibration). MPHG molecules in this region were clearly modified by the MR because the intensity of the absorption peak was significantly lower than that of MPHN, MPHH, and MPH-XM, which is consistent with the findings of Hong, Meng, and Lu (Citation2015). The absorption peak at wavenumber 1180–953 cm−1 is the characteristic absorption peak of carbohydrate, belonging to C–C, C–O stretching vibrations and C–H bending vibrations. Most of the protein molecules showed very weak absorptions in this region (Gu et al., Citation2010). The absorption peak of MPHG was markedly greater in this region than those of MPHN and MPHH, but was significantly lower than those of MPH-XM and XYLN, indicating that covalent cross-linking had occurred between xylose and MPH during the MR (Wang et al., Citation2013). In addition, the wavenumber 3700–3000 cm−1 is the absorption peak of hydroxyl. The MPHG sample showed significantly increased absorption at this region compared to MPHN and MPHH, but significantly lower absorption compared to MPH-XM and XYLN, indicating that MPH was copolymerized with xylose (Geng et al., Citation2014). These observations indicated that graft copolymers had formed between MPHN and XYLN through covalent bonding during the MR.
Figure 3. Fourier transform infrared spectra of native Morchella protein hydrolysate (MPHN), heat-treated MPH (MPHH), MPH–xylose mixture (MPH-XM), glycosylated MPH (MPHG), and native xylose (XYLN) in compressed KBr pellets.
Figura 3. Espectros de infrarrojos por transformada de Fourier de proteína hidrolizada de Morchella nativa (MPHN), de MPH tratada térmicamente (MPHH), de una mezcla MPH–xilosa (MPH-XM), de MPH glicosilada (MPHG) y de xiclosa nativa (XYLN) en gránulos Kbr comprimidos.
3.4. Fluorescence spectra analysis
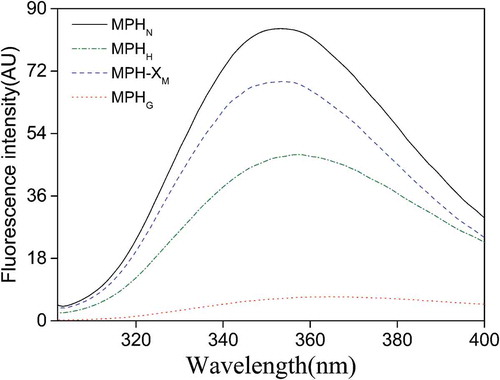
Tryptophan (Trp) and tyrosine (Tyr) residues in protein or protein hydrolysate are autofluorescent, and since they are extremely sensitive to the surrounding environment, the fluorescence spectra can be used for MR analysis. The fluorescence emission spectra of MPHN, MPHH, MPH-XM, and MPHG are shown in . Although the maximum fluorescent intensity of MPH-XM was slightly lower than that of MPHN, the maximum emission wavelength (Kmax) was not significantly different from that of MPHN. The maximum fluorescence intensities of both MPHH and MPHG were markedly reduced compared to MPHN, and the Kmax was slightly redshifted, but the magnitude change for MPHG was greater than for MPHH. These results are consistent with those of Huang et al. (Citation2012), and further confirm the occurrence of MR. The redshift of the fluorescence peaks indicates that the fluorescence emission groups are more exposed to the solvent, and results in an increase in the polarity of the microenvironment of these groups (Pallares, Vendrell, Aviles, & Ventura, Citation2004). The dramatic decrease in fluorescence intensity of the MPHG may be because of steric hindrance caused by the addition of carbohydrate, which could largely shield the signals from the fluorescence emission groups, and thereby reduce the fluorescence of the copolymers (Huang et al., Citation2012).
Figure 4. Intrinsic emission fluorescence spectra of native Morchella protein hydrolysate (MPHN), heat-treated MPH (MPHH), MPH–xylose mixture (MPH-XM), and glycosylated MPH (MPHG) dispersed in a phosphate buffer (20 mmol/L, pH 7.4) at a fixed hydrolysate concentration of 0.05 mg/mL. The excitation wavelength for emission spectra was set at 291 nm and the scanning wavelength ranged from 300 to 400 nm.
Figura 4. Espectro de emisiones fluorescentes intrínsecas de proteína hidrolizada de Morchella nativa (MPHN), de MPH tratada térmicamente (MPHH), de una mezcla MPH–xilosa (MPH-XM), de MPH glicosilada (MPHG) dispersada en un tampón fosfato (20 mmol/L, pH 7.4) en una concentración hidrolizada constante de 0.05 mg/mL. La onda de excitación para el espectro de emisiones se fijó en 291 nm y el barrido espectral cubrió de 300–400 nm.
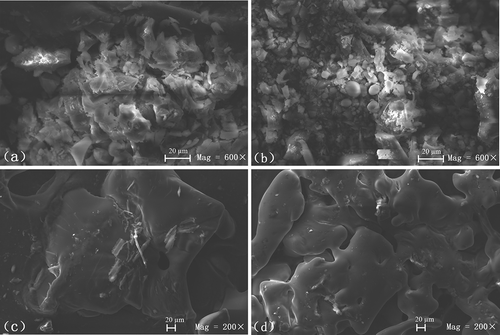
3.5. SEM analysis
SEM has been widely used to detect the effects of glycosylation on the microstructure of samples (Liu, Xu, Zhang, Zhao, & Ding, Citation2016; Moeckel, Duerasch, Weiz, Ruck, & Henle, Citation2016). Observations of MPHN, MPHH, MPH-XM, and MPHG by SEM are shown in . The structure of MPHN ()) was loose and irregular in shape, while the structure of MPHH ()) was mainly comprised of compact spherical micelles, indicating that heating had a particular effect on the conformation of MPH. The molecular morphology of MPH-XM ()) was significantly larger than that of MPHN, and was flat and compact in structure with a small amount of debris on the surface. The morphology of MPHG ((d)) was also flat and compact, similar to the structure of MPH-XM, and the volume was likewise significantly larger compared to MPHN. However, its surface was smooth and clean with obvious protrusions, indicating that MPHG was not a simple mixture of MPH and xylose, and that polymerization reactions were likely to have occurred between the two, which changed the microstructure of MPH. The mechanisms of MPH-xylose graft copolymer formation could be due to the breaking of intramolecular non-covalent bonds in MPH during MR (Niu, Jiang, Pan, & Zhai, Citation2011), paralleled by the covalent bonding of xylose molecules to MPH, which surrounded the MPH and extended the size of the molecule. As a result, the volume was increased and the structure became more compact, thereby leading to the formation of copolymers along with a unique microstructure.
Figure 5. Scanning electron micrographs of (a) native Morchella protein hydrolysate (MPHN), (b) heat-treated MPH (MPHH), (c) MPH–xylose mixture (MPH-XM), and (d) glycosylated MPH (MPHG).
Figura 5. Micrografías electrónicas de barrido de (a) de Morchella nativa (MPHN), (b) de MPH tratada térmicamente (MPHH), (c) de una mezcla MPH – xilosa (MPH-XM), y (d) de MPH glicosilada (MPHG).
3.6. In vitro antioxidant activity analysis
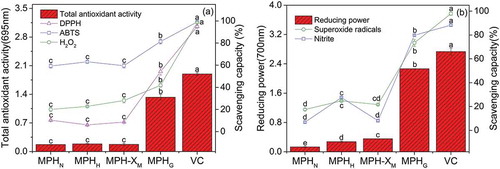
A variety of experimental models were used to analyze the in vitro antioxidant activities of MPHN, MPHH, MPH-XM, and MPHG (). Among the four molecules, MPHG exhibited the highest antioxidant activity, although this was less than that of the positive control (VC), at the same concentration. Its TAA and reducing power were 7.77 and 17.03 times those of MPHN, respectively. Moreover, its nitrite and H2O2 scavenging activities were 11.70 and 2.09 times those of MPHN, respectively, and its DPPH, ABTS, and superoxide anion radical scavenging activities were 426.29%, 37.33%, and 320.14% higher than those of MPHN, respectively. These findings indicate that MPH glycosylation by xylose through MR significantly increased the in vitro antioxidant activities of MPH, which is consistent with results of previous research (Wang et al., Citation2013). Although the reducing powers of MPHH and MPH-XM and the nitrite and superoxide anion free radical scavenging activities of MPHH were significantly enhanced compared to those of MPHN, these enhancements were significantly lower than those of MPHG (p < 0.01). According to a previously published study, reductone (an intermediate stage product), melanoidin, and heterocyclic compounds (advanced stage products) from the MR play important roles in antioxidation (Yin, Yang, Zhao, & Li, Citation2014), by breaking the free radical chain, chelating metal ions, providing hydrogen and electrons, and scavenging active oxygen (Kim & Lee, Citation2009; Nooshkam & Madadlou, Citation2016). The glycosylation of MPH conducted via MR in this study is a “natural” process, whereas other existing modification methods are artificial. Therefore, this study has revealed a promising means for improving the antioxidant activity of MPH, and provides experimental support for the development and application of MPH and its glycosylated derivatives as natural antioxidants in health products and functional foods.
Figure 6. Antioxidant activity of native Morchella protein hydrolysate (MPHN), heat-treated MPH (MPHH), MPH–xylose mixture (MPH-XM), glycosylated MPH (MPHG), and positive control ascorbic acid (VC). (a) Total antioxidant activity and scavenging capacities toward ABTS and DPPH radicals, and H2O2; (b) Reducing power and scavenging capacities toward nitrite and superoxide radicals. Values are presented as mean ± standard deviation (n = 3). Different letters (a–e) indicate significant differences (p < 0.01) among samples at the same concentration.
Figura 6. Actividad antioxidante de proteína hidrolizada de Morchella nativa (MPHN), de MPH tratada térmicamente (MPHH), de una mezcla MPH–xilosa (MPH-XM), de MPH glicosilada (MPHG) y del control positivo de ácido ascórbico (VC). (a) Actividad antioxidante total y capacidad de eliminación de los radicales ABTS y DPPH y H2O2; (b) Poder reductor y capacidad de eliminación de los radicales de nitrito y superóxido. Los valores figuran como la media ± la desviación estándar (n = 3). Las distintas letras (a–e) indican diferencias significativas (p < 0.01) entre las muestras sometidas a prueba con la misma concentración.
4. Conclusions
In this study, MPH glycosylation was successfully optimized by the response surface experiment, and the presence of structural modification of the MPH was confirmed by infrared spectroscopy, fluorescence spectroscopy, and SEM. In addition, changes in the antioxidant activities of MPH before and after glycosylation were examined using in vitro experimental models. The results of the response surface experiment showed that the optimal MPH glycosylation conditions were as follows: pH 11.80, xylose/MPH mass ratio of 3.7:1, and reaction time of 60 min. The in vitro antioxidation experiments demonstrated that the antioxidant activities of MPHG were significantly enhanced compared to those of MPHN, MPHH, and MPH-XM. These results demonstrate that the glycosylation of MPH through the MR is an effective way to improve its antioxidant activity. However, the present study has not considered whether the harmful advanced glycation end products are produced during the glycosylation of MPH, which remains to be done for further study.
Acknowledgments
This work was supported by the Doctorate Fellowship Foundation of Nanjing Forestry University, the Natural Science Foundation of Anhui Provincial Department of Education (KJ2017A515); the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYLX15_0916); and the Program of Key Discipline in Anhui Science and Technology University (No. AKZDXK2015B02).
Disclosure statement
No potential conflict of interest was reported by the authors
Additional information
Funding
References
- Ajmal, M., Akram, A., Ara, A., Akhund, S., & Nayyar, B. G. (2015). Morchella esculenta: An edible and health beneficial mushroom. Pakistan Journal of Food Sciences, 25(2), 71–78.
- Al-Amiery, A. A., Al-Majedy, Y. K., Kadhum, A. A. H., & Mohamad, A. B. (2015). Hydrogen peroxide scavenging activity of novel coumarins synthesized using different approaches. PloS One, 10(7), e0132175. doi:10.1371/journal.pone.0132175
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(12), 248–254. doi:10.1016/0003-2697(76)90527-3
- Chawla, S. P., Chander, R., & Sharma, A. (2009). Antioxidant properties of Maillard reaction products obtained by gamma-irradiation of whey proteins. Food Chemistry, 116(1), 122–128. doi:10.1016/j.foodchem.2009.01.097
- Chen, X. M., & Kitts, D. D. (2011). Antioxidant and anti-inflammatory activities of Maillard reaction products isolated from sugar–amino acid model systems. Journal of Agricultural & Food Chemistry, 59(20), 11294–11303. doi:10.1021/jf2031583
- Dworschák, E., & Carpenter, K. J. (1980). Nonenzyme browning and its effect on protein nutrition. Critical Reviews in Food Science and Nutrition, 13(1), 1–40. doi:10.1080/10408398009527283
- Fu, R., Zhang, Y., Guo, Y., & Chen, F. (2014). Antioxidant and tyrosinase inhibition activities of the ethanol-insoluble fraction of water extract of Sapium sebiferum (L.) Roxb. leaves. South African Journal of Botany, 93, 98–104. doi:10.1016/j.sajb.2014.04.003
- García-Pascual, P., Sanjuán, N., Melis, R., & Mulet, A. (2006). Morchella esculenta (morel) rehydration process modelling. Journal of Food Engineering, 72(4), 346–353. doi:10.1016/j.jfoodeng.2004.12.014
- Geng, X., Cui, B., Li, Y., Jin, W., An, Y., Zhou, B., … Li, B. (2014). Preparation and characterization of ovalbumin and carboxymethyl cellulose conjugates via glycosylation. Food Hydrocolloids, 37, 86–92. doi:10.1016/j.foodhyd.2013.10.027
- Gu, F. L., Jin, M. K., Abbas, S., Zhang, X. M., Xia, S. Q., & Chen, Z. X. (2010). Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–Glucose. Food Chemistry, 120(2), 505–511. doi:10.1016/j.foodchem.2009.10.044
- Hong, X., Meng, J., & Lu, R. R. (2015). Improvement of ACE inhibitory activity of casein hydrolysate by Maillard reaction with xylose. Journal of the Science of Food & Agriculture, 95(1), 66–71. doi:10.1002/jsfa.6682
- Huang, X., Tu, Z., Xiao, H., Wang, H., Zhang, L., Hu, Y. M., … Niu, P. P. (2012). Characteristics and antioxidant activities of ovalbumin glycated with different saccharides under heat moisture treatment. Food Research International, 48(2), 866–872. doi:10.1016/j.foodres.2012.06.036
- Jiang, Z., Wang, L., Che, H., & Tian, B. (2014). Effects of temperature and pH on angiotensin-I-converting enzyme inhibitory activity and physicochemical properties of bovine casein peptide in aqueous Maillard reaction system. LWT - Food Science and Technology, 59(1), 35–42. doi:10.1016/j.lwt.2014.06.013
- Kim, J. S., & Lee, Y. S. (2009). Antioxidant activity of Maillard reaction products derived from aqueous glucose/glycine, diglycine, and triglycine model systems as a function of heating time. Food Chemistry, 116(1), 227–232. doi:10.1016/j.foodchem.2009.02.038
- LeDuy, A., Kosaric, N., & Zajic, J. E. (1974). Morel mushroom mycelium growth in waste sulfite liquors as source of protein and flavouring. Canadian Institute of Food Science and Technology Journal, 7(1), 44–50. doi:10.1016/s0315-5463(74)73845-7
- Li, S., Sang, Y., Zhu, D., Yang, Y., Lei, Z., & Zhang, Z. (2013). Optimization of fermentation conditions for crude polysaccharides by Morchella esculenta using soybean curd residue. Industrial Crops & Products, 50(10), 666–672. doi:10.1016/j.indcrop.2013.07.034
- Liu, D., Sheng, J., Li, Z., Qi, H., Sun, Y., Duan, Y., & Zhang, W. F. (2013). Antioxidant activity of polysaccharide fractions extracted from Athyrium multidentatum (Doll.) Ching. International Journal of Biological Macromolecules, 56, 1–5. doi:10.1016/j.ijbiomac.2013.01.023
- Liu, J., Ru, Q., & Ding, Y. (2012). Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Research International, 49(1), 170–183. doi:10.1016/j.foodres.2012.07.034
- Liu, J., Xu, Q., Zhang, J., Zhao, P., & Ding, Y. (2016). Characterization of silver carp (Hypophthalmichthys molitrix) myosin protein glycated with konjac oligo-glucomannan. Food Hydrocolloids, 57, 114–121. doi:10.1016/j.foodhyd.2016.01.019
- Luo, Y., Ling, Y., Wang, X., Han, Y., Zeng, X., & Sun, R. (2013). Maillard reaction products from chitosan–xylan ionic liquid solution. Carbohydrate Polymers, 98(1), 835–841. doi:10.1016/j.carbpol.2013.06.023
- Meng, F., Liu, X., Jia, L., Song, Z., Deng, P., & Fan, K. (2010). Optimization for the production of exopolysaccharides from Morchella esculenta SO-02 in submerged culture and its antioxidant activities in vitro. Carbohydrate Polymers, 79(3), 700–704. doi:10.1016/j.carbpol.2009.09.032
- Moeckel, U., Duerasch, A., Weiz, A., Ruck, M., & Henle, T. (2016). Glycation reactions of casein micelles. Journal of Agricultural and Food Chemistry, 64(14), 2953–2961. doi:10.1021/acs.jafc.6b00472
- Moisés, L. J., Corzomartinez, M., Villamiel, M., Javier, M. F., & Sanz, Y. (2011). Maillard-type glycoconjugates from dairy proteins inhibit adhesion of Escherichia coli to mucin. Food Chemistry, 129(4), 1435–1443. doi:10.1016/j.foodchem.2011.05.102
- Nitha, B., & Janardhanan, K. K. (2008). Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food & Chemical Toxicology, 46(9), 3193–3199. doi:10.1016/j.fct.2008.07.007
- Nitha, B., Meera, C. R., & Janardhanan, K. K. (2007). Anti-inflammatory and antitumour activities of cultured mycelium of morel mushroom, Morchella esculenta. Current Science, 92(2), 235–239.
- Niu, L. Y., Jiang, S. T., Pan, L. J., & Zhai, Y. S. (2011). Characteristics and functional properties of wheat germ protein glycated with saccharides through Maillard reaction. International Journal of Food Science & Technology, 46(10), 2197–2203. doi:10.1111/j.1365-2621.2011.02737.x
- Nooshkam, M., & Madadlou, A. (2016). Maillard conjugation of lactulose with potentially bioactive peptides. Food Chemistry, 192, 831–836. doi:10.1016/j.foodchem.2015.07.094
- Pallares, I., Vendrell, J., Aviles, F. X., & Ventura, S. (2004). Amyloid fibril formation by a partially structured intermediate state of alpha-chymotrypsin. Journal of Molecular Biology, 342(1), 321–331. doi:10.1016/j.jmb.2004.06.089
- Rao, Q., Kamdar, A. K., & Labuza, T. P. (2013). Storage stability of food protein hydrolysates—a review. Critical Reviews in Food Science & Nutrition, 56(7), 1169–1192. doi:10.1080/10408398.2012.758085
- Salla, S., Sunkara, R., Ogutu, S., Walker, L. T., & Verghese, M. (2016). Antioxidant activity of papaya seed extracts against H2O2 induced oxidative stress in HepG2 cells. LWT - Food Science and Technology, 66, 293–297. doi:10.1016/j.lwt.2015.09.008
- Sarmadi, B. H., & Ismail, A. (2010). Antioxidative peptides from food proteins: A review. Peptides, 31(10), 1949–1956. doi:10.1016/j.peptides.2010.06.020
- Sun, W., Zhao, M., Cui, C., Zhao, Q., & Bao, Y. (2010). Effect of Maillard reaction products derived from the hydrolysate of mechanically deboned chicken residue on the antioxidant, textural and sensory properties of Cantonese sausages. Meat Science, 86(2), 276–282. doi:10.1016/j.meatsci.2010.04.014
- Van, D. V. C., Muresan, S., Gruppen, H., De Bont, D. B., Merck, K. B., & Voragen, A. G. (2002). FTIR spectra of whey and casein hydrolysates in relation to their functional properties. Journal of Agricultural & Food Chemistry, 50(24), 6943–6950. doi:10.1021/jf020387k
- Vhangani, L. N., & Van Wyk, J. (2016). Antioxidant activity of Maillard reaction products (MRPs) in a lipid-rich model system. Food Chemistry, 208, 301–308. doi:10.1016/j.foodchem.2016.03.100
- Wang, G. H., Chen, C. Y., Lin, C. P., Huang, C. L., Lin, C. H., Cheng, C. Y., & Chuang, Y. C. (2016). Tyrosinase inhibitory and antioxidant activities of three Bifidobacterium bifidum-fermented herb extracts. Industrial Crops & Products, 89, 376–382. doi:10.1016/j.indcrop.2016.05.037
- Wang, W. Q., Bao, Y. H., & Chen, Y. (2013). Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chemistry, 139(1–4), 355–361. doi:10.1016/j.foodchem.2013.01.072
- Yilmaz, Y., & Toledo, R. (2005). Antioxidant activity of water-soluble Maillard reaction products. Food Chemistry, 93(2), 273–278. doi:10.1016/j.foodchem.2004.09.043
- Yin, C., Yang, L., Zhao, H., & Li, C.-P. (2014). Improvement of antioxidant activity of egg white protein by phosphorylation and conjugation of epigallocatechin gallate. Food Research International, 64, 855–863. doi:10.1016/j.foodres.2014.08.020
- Yin, Z., Sun, Q., Zhang, X., & Hao, J. (2014). Optimised formation of blue Maillard reaction products of xylose and glycine model systems and associated antioxidant activity. Journal of the Science of Food & Agriculture, 94(7), 1332–1339. doi:10.1002/jsfa.6415
- Zeng, Y., Zhang, H., Guan, Y., Zhang, L., & Sun, Y. (2013). Comparative study on the effects of d-psicose and d-fructose in the Maillard reaction with β-lactoglobulin. Food Science & Biotechnology, 22(2), 341–346. doi:10.1007/s10068-013-0086-9
- Zhuang, H., Tang, N., & Yuan, Y. (2013). Purification and identification of antioxidant peptides from corn gluten meal. Journal of Functional Foods, 5(4), 1810–1821. doi:10.1016/j.jff.2013.08.013
