ABSTRACT
Methyl ketones can be formed by the metabolism of intermediate products of β-oxidation by enzymes. In this article, the effect of soluble saccharides on the activity of key enzymes involved in methyl ketone synthesis in L. lactis was studied by detecting the relationship between soluble saccharides and enzyme activity. The results showed that glucose, fructose, sucrose, lactose and galactose could promote the growth of L. lactis. Glucose induced the activity of enoyl-coenzyme A (CoA) hydratase but inhibited the activity of β-hydroxyacyl-CoA dehydrogenase. Fructose induced the activities of acyl-CoA dehydrogenase and enoyl-CoA hydratase while maintaining the activity of β-hydroxyacyl-CoA dehydrogenase. Sucrose induced the activities of enoyl-CoA hydratase and β-hydroxyacyl-CoA dehydrogenase and maintained the activity of thiolase. Lactose also induced enoyl-CoA hydratase activity. Galactose induced enoyl-CoA hydratase activity and maintained thiolase activity. Glucose, lactose, fructose and galactose induced thioesterase activity. The data showed fructose and lactose could inhibit thiolase activity and induce thioesterase activity, which would be beneficial to methyl ketone synthesis, so fructose and lactose were the best soluble saccharides, followed by galactose, glucose and sucrose. A positive and significant correlation was found between thioesterase activity and enoyl-CoA hydratase activity (p < 0.01). In summary, the β-oxidation system of L. lactis is related to sugar metabolism.
RESUMEN
Las metil cetonas pueden ser formadas por la acción de enzimas en el metabolismo de productos intermedios resultantes de la β-oxidación. El presente estudio examinó el efecto que provocan los sacáridos solubles en la actividad de enzimas clave involucradas en la síntesis de metil cetona en L. lactis, a fin de determinar la relación entre la presencia de sacáridos solubles y la actividad enzimática. Los resultados mostraron que tanto la glucosa, como la fructosa, sacarosa, lactosa y galactosa pueden promover el crecimiento de L. lactis. La glucosa induce la actividad de la enoil-CoA hidratasa pero inhibe la actividad de la β-hidroxiacil-CoA deshidrogenasa. La fructosa estimula las actividades de la acil-CoA deshidrogenasa y la enoil-CoA hidratasa, y mantiene la actividad de la β-hidroxiacil-CoA deshidrogenasa. La sacarosa acelera las actividades de la enoil-CoA hidratasa y la β-hidroxiacil-CoA deshidrogenasa y mantiene la actividad de la tiolasa. La lactosa también induce la actividad de la enoil-CoA hidratasa. La galactosa estimula la actividad de la enoil-CoA hidratasa y mantiene la actividad de la tiolasa. La glucosa, lactosa, fructosa y galactosa aceleran la actividad de la tioesterasa. Los datos demuestran que la fructosa y la lactosa pueden inhibir la actividad de la tiolasa e inducir la actividad de la tioesterasa, lo cual puede favorecer la síntesis de metil cetona. Por lo tanto, la fructosa y la lactosa fueron los mejores sacáridos solubles, seguidos por la galactosa, glucosa y sacarosa. Asimismo, se constató la existencia de una correlación positiva y significativa entre la actividad de la tioesterasa y la enoil-CoA hidratasa (p<0.01). Por lo que se concluye que el sistema de β-oxidación de L. lactis se encuentra relacionado con el metabolismo del azúcar.
1. Introduction
Lactococcus lactis is widely used to process fermented dairy products (Shi, Li, Gao, & Fu, Citation2016) and L. lactis plays an important role in flavor production (Brandsma, Kraats, Abee, Zwietering, & Meijer, Citation2012), especially in the production of methyl ketones (Hannon, Kilcawley, Wilkinson, Delahunty, & Beresford, Citation2007; Li & Ma, Citation2013). Methyl ketones synthesis is related to the incomplete β-oxidation pathway that is completed by the action of the enzymes acyl-CoA dehydrogenase, enoyl-CoA hydratase, hydroxyacyl-CoA dehydrogenase, thiolase and thioesterase. β-Oxidation of the released fatty acid is thought to end with the formation of β-ketoacyl-CoA esters. Subsequently, β-ketoacyl-CoA esters are broken down into β-ketoacids under the action of thioesterase, and then decarboxylation of keto acids form methyl ketones (Engelvin, Feron, Perrin, Mollé, & Talon, Citation2000). In most organisms, β-oxidation is linked to sugar metabolism pathways, the citric acid cycle or oxidative phosphorylation, that can change the concentration of acetyl-CoA and NADH (the reduced form of nicotinamide-adenine dinucleotide) and then affect the β-oxidation potential; however, this strong link has been rarely studied.
L. lactis is a heterotrophic microorganism that uses soluble saccharides (carbon source) as its energy source (Burgain et al., Citation2014), specifically soluble saccharides that are important nutrients for living cells. Research shows that soluble saccharides can affect the growth, enzyme activity and products of L. lactis (Buňková, Buňka, Pollaková, Podešvová, & Dráb, Citation2011). On the other hand, a close relationship between the metabolism of soluble saccharides and β-oxidation pathways has been observed. This article focuses on the effect of soluble saccharides on the activity of key enzymes in methyl ketone synthesis pathways of L. lactis.
2. Materials and methods
2.1. Strains
L. lactis subsp. lactis was grown in M17 liquid broth medium in an Erlenmeyer flask without agitation at 37°C for 18h. Glucose, fructose, lactose, sucrose and galactose were separately used as carbon sources in M17 broth media. The cell density was measured using a spectrophotometer (TU-1800 Pgeneral Instrument Co. Ltd., Beijing, China) at 600 nm.
2.2. Preparation of cell-free extracts (CFEs)
CFEs were obtained as described by Engelvin et al. (Citation2000) with some modifications. Relevant carbon sources (2% W/V) were added into M17 medium before sterilization; after inoculation, cells were collected by high speed centrifugation (4°C, 5000 ×g for 10 min). The collected cells were washed with K2HPO4/KH2PO4 (100 mmol/L, pH 7.5) and then suspended in HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid, Aladdin Chemistry Co., Shanghai, China) buffer (20 mmol/L, pH 7.5) containing 1 mmol/L EDTA (Aladdin Chemistry Co.) and 1 mmol/L PMSF (phenyl methyl sulfonyl fluoride, Aladdin Chemistry Co.). The collected cells were sonicated (30 cycles of 10 s on and 10 s off at 300 W) by an ultrasonic homogenizer (JY92-II, Ningbo Scientz Biotechnology Co., Ltd., Zhejiang, China) and then centrifuged (4°C, 5000 ×g for 10 min). The resulting supernatant was the crude CFE. The CFE protein concentration was determined using an Enhanced BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, China).
2.3. Acyl-CoA dehydrogenase activity analysis
Acyl-CoA dehydrogenase activity was determined according to the procedure reported by Baltazar and Feron (Baltazar, Dickinson, & Ratledge, Citation1999; Feron, Blin-Perrin, Krasniewski, Mauvais, & Lherminier, Citation2005). Enzyme activity was measured by monitoring the reduction of DCPIP (2,6-dichlorophenolindophenol) at 600 nm. Enzyme activity was determined in a final volume of 1 mL HEPES/KOH buffer (50 mmol/L, pH 8.0). The assay mixture also contained 100 μmol of DCPIP, 50 μmol of phenazine metosulfate (Aladdin Chemistry Co.), 72 nmol of acyl CoA and 200 μg of CFE protein. The assay was initiated by the addition of CFE protein. The absorption coefficient was 21,500 M−l cm−l.
2.4. Enoyl-CoA hydratase, β-hydroxyacyl-CoA dehydrogenase and thiolase activity analysis
The analyses of the enoyl-CoA hydratase (crotonase), β-hydroxyacyl-CoA dehydrogenase and thiolase activities were performed by spectrophotometric measurements as performed by Binstock and Schulz (Citation1981). The enoyl-CoA hydratase, β-hydroxyacyl-CoA dehydrogenase and thiolase were determined by recording the decrease in absorbance at 263, 340 and 303 nm, respectively. The assay was initiated by the addition of CFE protein.
The assay mixture used to determine the enoyl-CoA hydratase activity consisted of the following: 0.2 mol/L potassium phosphate (pH 8), 0.2 mg/mL bovine serum albumin (Aladdin Chemistry Co.) and 30 μmol/L crotonyl-CoA (Trilithium salt, Sigma-Aldrich Co., Shanghai, China). The extinction coefficient was 6700 M−1 cm−1.
The activity assay system of β-hydroxyacyl-CoA dehydrogenase was as follows: 0.1 mol/L potassium phosphate (pH 7), 0.2 mg/mL bovine serum albumin, 0.1 mmol/L NADH (Aladdin Chemistry Co.), and 30 μmol/L acetoacetyl-CoA (sodium salt hydrate, Sigma-Aldrich Co.). The extinction coefficient was 6220 M−1 cm−1.
The activity assay system of 3-ketoacyl-CoA thiolase was as follows: 0.1 mol/L HEPES (pH 8.1), 25 mmol/L MgC12, 0.2 mg/mL bovine serum albumin, 2 mmol/L mercaptoethanol, 5% (v/v) glycerol, 0.1 mmol/L CoASH (Trilithium salt, Sigma-Aldrich Co.), and 30 μmol/L acetoacetyl-CoA. The extinction coefficient was 12,000 M−1 cm−1.
2.5. Thioesterase activity analysis
Thioesterase activity was assayed by measuring the absorbance increase at 412 nm (εDTNB(412) = 13,600 M−1 cm−1). The enzyme activity was determined in a final volume of 1 mL of K2HPO4/KH2PO4 (300 mmol/L, pH 8.0), The assay mixture also contained 250 nmol of DTNB (Aladdin Chemistry Co.), 72 nmol of palmitoyl-CoA (Sigma-Aldrich Co.) and 280 μg of CFE protein. The assay was initiated by the addition of CFE protein. The enzyme activity is expressed as nmol palmitoyl-CoA deacylated mg−1 protein min−1 (Engelvin et al., Citation2000).
2.6 The effect of soluble saccharides on the growth of L. lactis
L. lactis (4%, v/v) was inoculated in liquid broth medium containing separately glucose, fructose, lactose, sucrose and galactose. The mixture was cultured without agitation at 37°C for 18h. The concentration of each sugar was 0.5% (w/v) or 2% (w/v). The cell density was measured at 600 nm after they were cultured for 18 h. The sample without additional carbon source was control. The experiment was repeated three times.
2.7. Effect of soluble saccharides on enzyme activity
L. lactis cultures were inoculated without agitation at 37°C for 18 h in medium containing separately 2% (w/v) glucose, 2% (w/v) fructose, 2% (w/v) lactose, 2% (w/v) sucrose and 2% (w/v) galactose to a final 4% L. lactis mixture. After inoculation, relevant CFEs were prepared to analyze the enzyme activity using a spectrophotometer. The experiment was repeated three times.
2.8. Statistical analysis
All statistical analyses were performed using SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA). All assays were repeated in triplicate. The data were expressed as the mean values ± SD and were compared using an analysis of variance (ANOVA), as appropriate.
3. Results and discussion
3.1. The effect of soluble saccharides on the growth of L. lactis
Generally, the effect of carbon sources on growth is consistent with their effects on enzyme synthesis. Therefore, the maximum enzyme activity should occur during the most active phase of growth. L. lactis was inoculated at 4% (order of magnitude was 109 cfu/mL) in medium containing separately glucose, fructose, lactose, sucrose and galactose. The cell density of L. lactis was measured at 600 nm after they were cultured for 18 h. The effect of soluble saccharides on the growth of L. lactis was shown in .
Figure 1. Effect of soluble saccharides on the growth of L. lactis.
Lowercase letters indicates significantly different between 5 g/L soluble saccharides; capital letters represents significantly different between 20 g/L soluble saccharides (p < 0.05).
Figura 1. Efecto de sacáridos solubles en el crecimiento de L. lactis.
Nota: Las letras minúsculas indican la existencia de diferencias significativas entre sacáridos solubles a 5 g/L; las letras mayúsculas representan diferencias significativas entre sacáridos solubles a 20 g/L (p < 0.05).
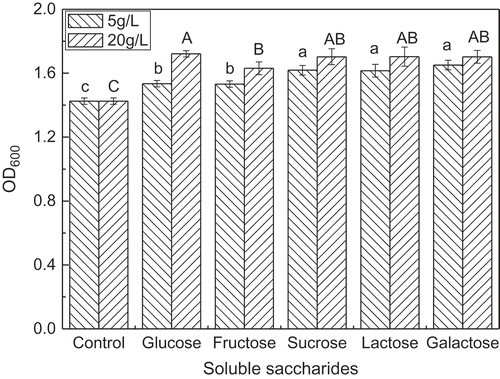
showed that glucose, fructose, sucrose, lactose and galactose promoted the growth of L. lactis (p < 0.05). When the concentration of the soluble saccharide was 20 g/L, there was significant difference in the growth of L. lactis between glucose and fructose (p < 0.05); however, there was no significant difference between glucose and other soluble saccharides, and there was also no significant difference among fructose, sucrose, lactose or galactose (p > 0.05). Glucose is one of the best carbon sources for stimulating the growth of L. lactis (). This result confirmed the data from previous studies. The growth of the recombinant L. lactis NZ9000 was markedly improved when using glucose as a carbon source, but sucrose and lactose were less efficient carbon sources than glucose (Ibrahim, Rahman, Mohamad, & Rahim, Citation2010). The maximal cell density of L. lactis IO-1 at 600 nm using glucose as a carbon source exceeded that of xylose (Machii et al., Citation2013). Previous researches had shown that glucose supported rapid growth, while galactose or lactose supported significantly slower rates of growth (Garrigues, Loubiere, Lindley, & Cocaign-Bousquet, Citation1997). The maximum specific growth rate of L. lactis IL1403 did not depend on initial glucose concentration (2–10 g/L). Meanwile, the shortest lag-phase was observed at glucose concentration 20 g/L, the longest was observed at 100 g/L (Kabanova, Kazarjan, Stulova, & Vilu, Citation2009; Kabanova, Stulova, & Vilu, Citation2013). However, several studies have reported inconsistent results: lactose is more effective for L. lactis G5 growth than fructose, galactose, sucrose and glucose (Kimoto-Nira, Suzuki, Sasaki, Kobayashi, & Mizumachi, Citation2010).
Lactococci rely on glycolysis and substrate-level phosphorylation to generate compounds that serve as energy donors for solute transport and growth (Stuart, Chou, & Weimer, Citation1999), so soluble saccharides could promote the growth of L. lactis (this study). L. lactis preferred a certain kind of sugar as their carbon source, which can be explained by the partial use of the sugar as alternative external electron acceptor, thereby enhancing the energy yield for growth during fermentation (Vrancken, Rimaux, De Vuyst, & Leroy, Citation2008). The difference between the effects of these sugars may be related to different subspecies, different soluble saccharides concentrations and other components in the culture media.
3.2. The effect of soluble saccharides on the enzyme activity of the β-oxidation pathway
Each β-oxidation cycle of sequence of four reactions results in the removal of two carbon atoms from the fatty acyl residues in the form of acetyl-CoA. The key enzymes of β-oxidation are acyl-CoA dehydrogenase, enoyl-CoA hydratase, β-hydroxyacyl-CoA dehydrogenase and thiolase (Schulz, Citation1991). Single soluble saccharide (2%, w/v) was added separately into M17 medium, and the resulting mixtures were incubated with 4% (v/v) L. lactis at 37°C for 18 h . After incubation, the cells were collected, and CFEs were prepared. The activities of acyl-CoA dehydrogenase, enoyl-CoA hydratase, β-hydroxyacyl-CoA dehydrogenase and thiolase of L. lactis were shown in .
Table 1. Effect of soluble saccharides on enzyme activity of β-oxidation pathway (nmol/(mg∙min)).
Tabla 1. Efecto de sacáridos solubles en la actividad enzimática de la ruta de β-oxidación (nmol/(mg∙min)).
As seen in , glucose induced enoyl-CoA hydratase activity but inhibited the activity of β-hydroxyacyl-CoA dehydrogenase. The result was not inconsistent with the results obtained in Escherichia coli. Glucose inhibited acyl-CoA dehydrogenase, enoyl-CoA hydratase, β-hydroxyacyl-CoA dehydrogenase and thiolase activities in E. coli (Weeks, Shapiro, Burns, & Wakil, Citation1969). Sucrose induced enoyl-CoA hydratase and β-hydroxyacyl-CoA dehydrogenase activities and maintained the activity of thiolase. Lactose induced enoyl-CoA hydratase activity. Galactose induced enoyl-CoA hydratase activity and maintained the activity of thiolase. Fructose induced acyl-CoA dehydrogenase and enoyl-CoA hydratase activities while maintaining β-hydroxyacyl-CoA dehydrogenase activity. As shown in , all the soluble saccharides maintained or inhibited thiolase activity.
The relationship between glucose metabolism and the β-oxidation of fatty acids had been studied in bacteria, yeast and mitochondria. Acetyl-CoA formed by β-oxidation system in Candida tropicalis was condensed by the action of malate synthase with glyoxylate, which is derived from isocitrate by isocitrate lyase reaction (Kawamoto, Nozaki, Tanaka, & Fukui, Citation1978). Acetyl-CoA formed in yeast peroxisomes might be transported to mitochondria for the operation of the tricarboxylic acid cycle and the glyoxylate cycle (Ueda, Yamanoi, Morikawa, Okada, & Tanaka, Citation1985). The oxidation of fatty acids in the mitochondria may be considered to depend on the interaction of two metabolic cycles, a β-oxidation cycle and the citric acid cycle (Bremer & Wojtczak, Citation1972). Clostridia could convert glucose to butyrate and butanol and Escherichia coli activated pathway of β-oxidation, gains the capacity to transform glucose into butyrate and butanol (Seregina, Shakulov, Debabov, & Mironov, Citation2010). So sugar metabolism could impact on β-oxidation of bacteria, like E. coli (Weeks et al., Citation1969) and L. lactis (in this study), but the mechanism of their association was unclear. In general, many monosaccharides enter central metabolism via the glycolytic pathway until pyruvate (Cocaign-Bousquet, Even, Lindley, & Loubière, Citation2002), the major products derived from pyruvate (acetyl-coA, lactate and mixed acid fermentation products) could affect β-oxidation system. In L. lactis, glucose is converted to pyruvate through the glycolytic pathway, sucrose is mediated by a sucrose-specific Phosphotransferase system (PTS), and the resulting sucrose 6-phosphate is hydrolyzed yielding glucose 6-phosphate and fructose that has to be phosphorylated to enter glycolysis (Neves, Pool, Kok, Kuipers, & Santos, Citation2005). Lactose is hydrolyzed into glucose and galactose-6-phosphate, which are concurrently metabolised to lactic acid (De Vos & Hugenholtz, Citation2004; Gadaga, Mutukumira, & Narvhus, Citation2001).
3.3. The effect of soluble saccharides on thioesterase activity
Acyl-CoA thioesterases control an important step in lipid utilization by catalyzing the breakdown of fatty acyl-CoA into free fatty acids (Moffat et al., Citation2014) and the CoA molecule (Hung et al., Citation2014). Acyl-CoA thioesterases have the potential to modulate the levels of fatty acid oxidation substrates and their intermediates and, consequently, to modulate fatty acid oxidation (Moffat et al., Citation2014). The results of the effects of soluble saccharides on thioesterase activity in L. lactis were shown in .
Table 2. Effect of soluble saccharides on thioesterase activity.
Tabla 2. Efecto de sacáridos solubles en la actividad de la tioesterasa.
Soluble saccharides could affect thioesterase activity. Glucose, lactose, fructose and galactose significantly induced thioesterase activity (p < 0.05), but the effect of sucrose was relatively weak (). There was no significant difference between the effects of fructose and lactose on thioesterase activity, but there was a significantly higher thioesterase activity with these sugars than with other soluble saccharides. Fructose and lactose were the best soluble saccharides for increasing thioesterase activity, followed by galactose, glucose and sucrose.
3.4. Correlation analysis of enzyme activity
A correlation analysis can be used to evaluate two closely correlated variables. To analyze the enzyme activity, a correlation coefficient is calculated by performing a correlation analysis and is used to understand the correlation between the enzyme activities. The Pearson correlation analysis between thioesterase activity and acyl-CoA dehydrogenase activity, enoyl-CoA hydratase activity, β-hydroxyacyl-CoA dehydrogenase activity or thiolase activity was analyzed, and the results were shown in .
Table 3. Pearson correlation analysis between enzyme activity.
Tabla 3. Análisis de correlación de Pearson entre actividades enzimáticas.
From , we observed no significant correlation between the activities of four enzymes (acyl-CoA dehydrogenase, enoyl-CoA hydratase, β-hydroxyacyl-CoA dehydrogenase and thiolase) at 99% significant level. In contrast, a significant and positive correlation was found between thioesterase activity and enoyl-CoA hydratase activity (p < 0.01).
ANOVA is a method of dividing the variability into identifiable sources of variation and quantifying the associated degrees of freedom in an experiment (Muthukrishnan & Davim, Citation2009). Data obtained from the ANOVA performed on the thioesterase and enoyl-CoA hydratase activities were shown in .
Table 4. ANOVA between the thioesterase and enoyl-CoA hydratase activities.
Tabla 4. ANOVA entre las actividades de la tioesterasa y la enoil-CoA hidratasa.
showed the results of the ANOVA. This analysis was carried out for a significance level of 1%, i.e. for a level of confidence of 99%. The ANOVA showed a statistically significant correlation between thioesterase activity and enoyl-CoA hydratase activity (p < 0.01).
4. Conclusions
In this study, the effect of soluble saccharides on the enzyme activities involved in methyl ketone synthesis in L. lactis was analyzed. The results showed that glucose, fructose, sucrose, lactose and galactose promoted the growth of L. lactis. In addition, the data showed fructose and lactose could inhibited thiolase activity and induced thioesterase activity, which would be beneficial to methyl ketone synthesis, so fructose and lactose was the best soluble saccharides for enriching the levels of methyl ketone precursors. In summary, the β-oxidation system of L. lactis is related to sugar metabolism, and soluble saccharides may regulate methyl ketone synthesis under the changing activity of related enzymes.
Acknowledgments
This study was supported by the National Natural Science Foundation of China [No. 31401513], the Postdoctoral Research Fund of Heilongjiang Province [No. LBH-Z15021] and ‘‘Young Talents’’ Project of Northeast Agricultural University [No. 14QC41].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Baltazar, M. F., Dickinson, F. M., & Ratledge, C. (1999). Oxidation of medium-chain acyl-CoA esters by extracts of Aspergillus niger: Enzymology and characterization of intermediates by HPLC. Microbiology, 145(1), 271–278. doi:10.1099/13500872-145-1-271
- Binstock, J. F., & Schulz, H. (1981). Fatty acid oxidation complex from Escherichia coli. Methods in Enzymology, 71, 403–411. doi:10.1016/0076-6879(81)71051-6
- Brandsma, J. B., Kraats, I. V. D., Abee, T., Zwietering, M. H., & Meijer, W. C. (2012). Arginine metabolism in sugar deprived Lactococcus lactis enhances survival and cellular activity, while supporting flavour production. Food Microbiology, 29(1), 27–32. doi:10.1016/j.fm.2011.08.012
- Bremer, J., & Wojtczak, A. B. (1972). Factors controlling the rate of fatty acid β-oxidation in rat liver mitochondria. Biochimica Et Biophysica Acta (Bba)-Lipids and Lipid Metabolism, 280(4), 515–530. doi:10.1016/0005-2760(72)90131-2
- Buňková, L., Buňka, F., Pollaková, E., Podešvová, T., & Dráb, V. (2011). The effect of lactose, NaCl and an aero/anaerobic environment on the tyrosine decarboxylaseactivity of Lactococcus lactis subsp. cremoris and Lactococcus lactis subsp. lactis. International Journal of Food Microbiology, 147(2), 112–119. doi:10.1016/j.ijfoodmicro.2011.03.017
- Burgain, J., Scher, J., Francius, G., Borges, F., Corgneau, M., Revol-Junelles, A. M., … Gaiani, C. (2014). Lactic acid bacteria in dairy food: Surface characterization and interactions with food matrix components. Advances in Colloid and Interface Science, 213, 21–35. doi:10.1016/j.cis.2014.09.005
- Cocaign-Bousquet, M., Even, S., Lindley, N. D., & Loubière, P. (2002). Anaerobic sugar catabolism in Lactococcus lactis: Genetic regulation and enzyme control over pathway flux. Applied Microbiology and Biotechnology, 60(1–2), 24–32. doi:10.1007/s00253-002-1065-x
- De Vos, W. M., & Hugenholtz, J. (2004). Engineering metabolic highways in Lactococci and other lactic acid bacteria. Trends in Biotechnology, 22(2), 72–79. doi:10.1016/j.tibtech.2003.11.011
- Engelvin, G., Feron, G., Perrin, C., Mollé, D., & Talon, R. (2000). Identification of β-oxidation and thioesterase activities in Staphylococcus carnosus 833 strain. FEMS Microbiology Letters, 190(1), 115–120. doi:10.1111/j.1574-6968.2000.tb09272.x
- Feron, G., Blin-Perrin, C., Krasniewski, I., Mauvais, G. V., & Lherminier, J. (2005). Metabolism of fatty acid in yeast: Characterisation of β-oxidation and ultrastructural changes in the genus Sporidiobolu ssp. cultivated on ricinoleic acid methyl ester. FEMS Microbiology Letters, 250(1), 63–69. doi:10.1016/j.femsle.2005.06.045
- Gadaga, T. H., Mutukumira, A. N., & Narvhus, J. A. (2001). Growth characteristics of Candida kefyr and two strains of Lactococcus lactis subsp. lactis isolated from Zimbabwean naturally fermented milk. International Journal of Food Microbiology, 70(1–2), 11–19. doi:10.1016/S0168-1605(01)00501-3
- Garrigues, C., Loubiere, P., Lindley, N. D., & Cocaign-Bousquet, M. (1997). Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: Predominant role of the NADH/NAD+ ratio. Journal of Bacteriology, 179(17), 5282–5287. doi:10.1128/jb.179.17.5282-5287.1997
- Hannon, J. A., Kilcawley, K. N., Wilkinson, M. G., Delahunty, C. M., & Beresford, T. P. (2007). Flavour precursor development in Cheddar cheese due to lactococcal starters and the presence and lysis of Lactobacillus helveticus. International Dairy Journal, 17(4), 316–327. doi:10.1016/j.idairyj.2006.03.001
- Hung, Y.-H., Chan, Y.-S., Chang, Y.-S., Lee, K.-T., Hsu, H.-P., Yen, M.-C., … Lai, M.-D. (2014). Fatty acid metabolic enzyme acyl-CoA thioesterase 8 promotes the development of hepatocellular carcinoma. Oncology Reports, 31(6), 2797–2803. doi:10.3892/or.2014.3155
- Ibrahim, S. B., Rahman, N. A., Mohamad, R., & Rahim, R. A. (2010). Effects of agitation speed, temperature, carbon and nitrogen sources on the growth of recombinant Lactococcus lactis NZ9000 carrying domain 1 of aerolysin gene. African Journal of Biotechnology, 9(33), 5392–5398. doi:10.5897/AJB10.149
- Kabanova, N., Kazarjan, A., Stulova, I., & Vilu, R. (2009). Microcalorimetric study of growth of Lactococcus lactis IL1403 at different glucose concentrations in broth. Thermochimica Acta, 496(1–2), 87–92. doi:10.1016/j.tca.2009.07.003
- Kabanova, N., Stulova, I., & Vilu, R. (2013). Microcalorimetric study of growth of Lactococcus lactis IL1403 at low glucose concentration in liquids and solid agar gels. Thermochimica Acta, 559, 69–75. doi:10.1016/j.tca.2013.02.013
- Kawamoto, S., Nozaki, C., Tanaka, A., & Fukui, S. (1978). Fatty acid beta-oxidation system in microbodies of n-alkane-grown Candida tropicalis. European Journal of Biochemistry, 83(2), 609–613. doi:10.1111/j.1432-1033.1978.tb12130.x
- Kimoto-Nira, H., Suzuki, C., Sasaki, K., Kobayashi, M., & Mizumachi, K. (2010). Survival of a Lactococcus lactis strain varies with its carbohydrate preference under in vitro conditions simulated gastrointestinal tract. International Journal of Food Microbiology, 143(3), 226–229. doi:10.1016/j.ijfoodmicro.2010.07.033
- Li, L., & Ma, Y. (2013). Effect of fatty acids on the β-oxidation system and thioesterase of Lactococcus lactis subspecies lactis. Journal of Dairy Science, 96(4), 2003–2010. doi:10.3168/jds.2012-5996
- Machii, M., Watanabe, S., Zendo, T., Chibazakura, T., Sonomoto, K., Shimizu-Kadota, M., & Yoshikawa, H. (2013). Chemically defined media and auxotrophy of the prolific L-lactic acid producer Lactococcus lactis IO-1. Journal of Bioscience and Bioengineering, 115(5), 481–484. doi:10.1016/j.jbiosc.2012.11.024
- Moffat, C., Bhatia, L., Nguyen, T., Lynch, P., Wang, M., Wang, D., … Seifert, E. L. (2014). Acyl-CoA thioesterase-2 facilitates mitochondrial fatty acid oxidation in the liver. Journal of Lipid Research, 55(12), 2458–2470. doi:10.1194/jlr.M046961
- Muthukrishnan, N., & Davim, J. P. (2009). Optimization of machining parameters of Al/SiC-MMC with ANOVA and ANN analysis. Journal of Materials Processing Technology, 209(1), 225–232. doi:10.1016/j.jmatprotec.2008.01.041
- Neves, A. R., Pool, W. A., Kok, J., Kuipers, O. P., & Santos, H. (2005). Overview on sugar metabolism and its control in Lactococcus lactis – the input from in vivo NMR. FEMS Microbiology Reviews, 29(3), 531–554. doi:10.1016/j.fmrre.2005.04.005
- Schulz, H. (1991). Beta oxidation of fatty acids. Biochimica Et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 1081(2), 109–120. doi:10.1016/0005-2760(91)90015-A
- Seregina, T. A., Shakulov, R. S., Debabov, V. G., & Mironov, A. S. (2010). Construction of a butyrate-producing E. coli strain without the use of heterologous genes. Applied Biochemistry and Microbiology, 46(8), 745–754. doi:10.1134/S000368381008003X
- Shi, W., Li, Y., Gao, X., & Fu, R. (2016). Improvement of the respiration efficiency of Lactococcus lactis by decreasing the culture pH. Biotechnology Letters, 38(3), 495–501. doi:10.1007/s10529-015-1999-6
- Stuart, M. R., Chou, L. S., & Weimer, B. C. (1999). Influence of carbohydrate starvation and arginine on culturability and amino acid utilization of Lactococcus lactis subsp. lactis. Applied and Environmental Microbiology, 65(2), 665–673.
- Ueda, M., Yamanoi, K., Morikawa, T., Okada, H., & Tanaka, A. (1985). Peroxisomal localization of enzymes related to fatty acid β-oxidation in an n-alkane-grown yeast Candida tropicalis. Agricultural and Biological Chemistry, 49(6), 1821–1828. doi:10.1080/00021369.1985.10866984
- Vrancken, G., Rimaux, T., De Vuyst, L., & Leroy, F. (2008). Kinetic analysis of growth and sugar consumption by Lactobacillus fermentum IMDO 130101 reveals adaptation to the acidic sourdough ecosystem. International Journal of Food Microbiology, 128(1), 58–66. doi:10.1016/j.ijfoodmicro.2008.08.001
- Weeks, G., Shapiro, M., Burns, R. O., & Wakil, S. J. (1969). Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. Journal of Bacteriology, 97(2), 827–836.
