?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pea protein isolate (PPI) prepared through isoelectric protein precipitation was heat-treated between 50°C and 100°C. The effect of heat treatment on the structural and functional properties of proteins was evaluated. Native gel electrophoresis showed the formation of protein aggregates (molecular weight ≫ 200 kDa) in the temperature range of 80–100°C. Intrinsic fluorescence data suggested that protein denaturation reached its highest level at pH 3.0. The formation of oil-in-water emulsions (1:5 oil:water volume ratio) was positively impacted by pH increase as evidenced by the decrease of minimum oil droplet size (d4,3) from 26 μm at pH 5.0 to 22 μm at pH 7.0 (P < 0.05). In contrast, heat pretreatment led to decreasing emulsion properties at pH 3.0 with d4,3 values increasing from 27 μm to 80 μm (P < 0.05). Regardless of pH applied, all heated PPI samples displayed low foaming properties.
RESUMEN
El aislado de proteína de arveja (PPI), preparado mediante precipitación isoeléctrica de proteínas, se sometió a un tratamiento térmico con temperaturas de entre 50 y 100°C. Posteriormente, se evaluó el efecto del tratamiento térmico en las propiedades estructurales y funcionales de las proteínas. La electroforesis nativa en gel reveló la formación de agregados proteicos (peso molecular ≫ 200 kDa) en el rango de temperatura de 80 a 100°C. Los datos obtenidos de la fluorescencia intrínseca sugieren que la desnaturalización de las proteínas alcanzó su nivel más elevado cuando el pH era 3.0. La formación de emulsiones de aceite en agua (ratio del volumen aceite:agua 1:5) se vio afectada positivamente por el aumento del pH, lo que se evidenció por la disminución del tamaño mínimo de la gota de aceite (d4,3), que pasó de 26 μm con pH de 5.0 a 22 μm con pH de 7.0 (P < 0.05). En contraste, el pretratamiento térmico produjo una disminución de las propiedades de emulsión cuando el pH era 3.0, pues los valores d4,3 aumentaron de 27 μm a 80 μm (P < 0.05). Independientemente del pH utilizado, todas las muestras de PPI calentadas presentaron limitadas propiedades espumantes.
Introduction
Currently, there has been an increasing demand for plant-based foods as a means of maintaining or improving human health as well as reducing environmental footprint, wastes, and demands in soil and water. The health benefits of plant-based foods can be attributed to the presence of unsaturated fatty acids, as compared to the mostly saturated lipids in animal-based foods. In order to expand the use of plant ingredients, one main issue to solve is to incorporate these into various formulations that meet the nutritional and organoleptic properties desirable to consumers. One of the most important nutrients in plants are proteins, which can be readily isolated and used as ingredients for the formulation of novel human foods. An increasingly popular example is the pea protein isolate (PPI), which provides a low-cost and nutritional protein-enriched product alternative to animal proteins, such as those in whey. Various pea seed products, including flours and protein-enriched products, have been used (albeit at low levels) in various food formulations, especially baked products, soups, sport drinks, and breakfast cereals (Marinangeli, Kassis, & Jones, Citation2009). However, the utilization of pea proteins as ingredients is still limited, due to the presence of insoluble proteins (Adebiyi & Aluko, Citation2011). Native pea globulins are salt-soluble proteins; the highest level of protein solubility (PS) is reached above pH 6.0 and below pH 4.0 (Adebiyi & Aluko, Citation2011). However, in case of an extraction procedure that could alter protein structure (by the use of strong acid/alkali, heating procedure, spray-drying), the level of insoluble protein is increased due to the formation of large aggregates (Fuhrmeister & Meuser, Citation2003; Shand, Ya, Pietrasik, & Wanasundara, Citation2007). The first condition to ensure satisfying functional properties is a low-denatured state, which enhances PS at the pH of interest.
Pea proteins consist of two major storage fractions, the 11S (legumin) and 7S (vicilin) subunits. The legumin is a hexameric protein of 350–400 kDa size while vicilin is trimeric and 150 kDa in size (Klassen & Nickerson, Citation2012; Mession, Assifaoui, Cayot, & Saurel, Citation2012). Previous works have shown the potential use of PPI as functional ingredients in foods, especially as emulsifiers, foaming agents, and gel-forming polypeptides, which could broaden utility in food (Adebiyi &; Aluko, Citation2011; Jiang, Zhu, Liu, & Xiong, Citation2014). For example, oil droplet size of emulsions formed with PPI was reduced when alkalinity of the aqueous phase increased from pH 4.0 to 9.0; in contrast, foam volume and foam stability (FS) were increased (Adebiyi & Aluko, Citation2011). PPI was also reported to have better emulsifying activity (smaller oil droplet size) at pH 3.0 and 7.0 when compared to pH 5.0 (Chang, Tu, Ghosh, & Nickerson, Citation2015; Liang & Tang, Citation2013). With respect to protein isolation method, the PPI produced by isoelectric protein precipitation formed smaller emulsion oil droplets than the salt-extracted PPI (Karaca, Low, & Nickerson, Citation2011). In contrast, PPI prepared by membrane ultrafiltration had better emulsifying and foaming properties than the one prepared by acid or heat precipitation (Fuhrmeister & Meuser, Citation2003). With regard to pea protein fractions, it was reported that whole albumin isolate had better foaming properties than the low-molecular weight (MW) fractions (Lu, Quillien, & Popineau, Citation2000). Meanwhile, a previous work showed that the emulsion capacity of pea globulin isolate was better at pH 3.0 and 7.0 when compared to pH 4.0 (Koyoro & Powers, Citation1987). Analysis of various pea seed flours has confirmed the superior ability of proteins to form and stabilize oil-in-water emulsions when compared to polysaccharides (Aluko, Mofolasayo, & Watts, Citation2009). A commercial PPI exhibited gelling abilities, though addition of NaCl was required for increased gel strength (Shand et al., Citation2007). An important finding was the extensive denaturation of proteins in commercial PPIs when compared to their laboratory-prepared counterparts. The existence of highly denatured proteins in commercial pea protein products was later confirmed (Osen, Toelstede, Wild, Eisner, & Schweiggert-Weisz, Citation2014). The pea products that contained denatured proteins were less soluble than the product with native protein. For protein network formation, a recent work suggested that the 11S proteins (legumins) exhibited higher gelation properties than the 7S proteins (vicilin), possibly attributable to the contribution of both non-covalent interactions and disulfide bonds. Note that the gelation properties of pea legumin were affected by the sulfhydryl/disulfide bonds contents, and also their relative accessibility/exposure on polypeptide chains upon denaturing heat treatment (Osen, Toelstede, Eisner, & Schweiggert‐Weisz, Citation2015). Moreover, the production of edible or biodegradable PPI films with desirable strength and resistance to puncture has also been accomplished (Kowalczyk, Gustaw, Świeca, & Baraniak, Citation2014).
However, despite these advances in pea protein utilization, the functional properties of commercial or laboratory pea proteins could be enhanced by physical or chemical modifications. For example, structural modification of laboratory pea proteins with alkaline pH treatment led to improved emulsion properties in terms of smaller oil droplet size, increased oxidative stability, and higher emulsion stability, when compared to the untreated protein (Jiang et al., Citation2014). To date, there has been scarce information regarding the use of heat pretreatment as pea protein structure-modifying tool for improved functionality. Sufficient heat treatment induces breakup of the oligomer structure, denaturation of the subunits by polypeptide chains unfolding, and exposure of reactive groups (such as hydrophobic and sulfhydryl groups) previously buried in the core (He, Yuan, Wang, & Yang, Citation2016; Peyrano, Speroni, & Avanza, Citation2016; Tang & Ma, Citation2009). The denatured proteins reassociate predominantly via physical and nonspecific bonds into protein aggregates, displaying a wide range of MW and modified surface properties as compared to their native counterparts. The conditions of the heating process (pH, ionic strength, protein concentration, time/temperature) influence change in protein MW, hydrophilic/hydrophobic balance on protein surface, and thus solubility. In addition to the structure-modifying effects, protein functionality can also be enhanced by thermal treatment. For example, tensile strength of biodegradable protein-based films was enhanced by heat pretreatment to denature the proteins (Kowalczyk et al., Citation2014; Liu, Tellez-Garay, & Castell-Perez, Citation2004). In addition, a previous work showed that incorporation of heat-treated cowpea proteins into wheat flour formulations led to the production of bread containing softer crumb texture than bread with untreated proteins (Campbell, Euston, & Ahmed, Citation2016). Heat treatment, as a result of its ability to disrupt non-covalent bonds and induce sulfhydryl bond formation, is required to enhance protein gelation properties and film formation (He et al., Citation2016; Kowalczyk et al., Citation2014; Peyrano et al., Citation2016; Yin, Tang, Wen, & Yang, Citation2010). Therefore, the aim of this study was to determine the influence of laboratory heat treatment (50–100°C) on the structural and functional properties of a commercial PPI. Structural properties were determined using gel electrophoresis and intrinsic fluorescence, while functional properties were evaluated by emulsification and foam formation.
Materials and methods
Materials
PPI (dry weight basis) with ~82.0%, 0.3% lipids, 0.0% fiber, 4.0% ash, 0.7% starch, and 11.7% sugars (Aluko et al., Citation2009) was obtained from Nutri-Pea Limited (Portage la Prairie, MB, Canada). The PPI was produced by isoelectric pH precipitation at pH 4.5 of alkaline-extracted proteins and spray-dried (Nickel, Citation1981). For the thermal treatments, PPI slurries (2.5 g in 50-mL distilled water) were prepared in hermetically sealed tubes and held for 30 min in a water bath with temperature set at 50°C, 70°C, 80°C, 90°C, or 100°C. The samples were cooled to room temperature (by immersing in regular tap water), freeze-dried, and then stored at −20°C until analyzed for physicochemical and functional properties. The samples (10 mg) were solubilized by addition of 10 mL of 1 M NaOH, diluted with distilled water within the 40–100 μg mL−1 range and protein content determined by the modified Lowry method with bovine serum albumin as standard and water as blank (Markwell, Hass, Bieber, & Tolbert, Citation1978).
Polyacrylamide gel electrophoresis
Native-polyacrylamide gel electrophoresis (PAGE) and SDS-PAGE were performed on 10–15% precast gradient gels (GE Healthsciences, Montreal, PQ, Canada) using previously described methods (Aluko, Yada, Lencki, & Marangoni, Citation1997). For SDS-PAGE under reducing condition, the samples (10 mg mL−1) were prepared by mixing PPI with 0.1 M Tris-HCl buffer solution (pH 8.0) containing 10% (w/v) SDS, 0.5 g L−1 2-mercaptoethanol, and 1 mg L−1 bromophenol blue. The sample tubes were placed in boiling water for 5 min, cooled to room temperature, centrifuged (14,000 g for 20 min) and 1 μL of the supernatant was loaded onto the gradient gel. For native-PAGE, 10-mg mL−1 PPI dispersions were prepared in 0.1 M sodium phosphate buffer, pH 8.0, and then centrifuged (14,000 g for 10 min); 1 μL of the supernatant was loaded onto the gradient gel. Protein separation and staining were carried out using the Phastsystem Separation and Development electrophoresis unit (GE Healthsciences, Montreal, PQ, Canada) following the manufacturer’s instructions. Gels were stained with a solution that contained coomassie blue, methanol, acetic acid, and water while the destaining solution consisted of same solution without the coomassie blue (GE Healthsciences, Montreal, PQ, Canada).
Intrinsic fluorescence
Protein stock solutions contained 10 mg mL−1 PPI in 0.1 mol L−1 NaH2PO4 and Na2HPO4 buffer, and adjusted to pH 3.0, 5.0, or 7.0 with 0.5 mol L−1 HCl. The protein dispersions were mixed at room temperature for 30 min followed by centrifugation (14,000 × g, 30 min, room temperature). The supernatants were diluted with water and protein contents determined by the modified Lowry method (Markwell et al., Citation1978) with bovine serum albumin as the standard and water as blank. Each stock solution was then diluted with the appropriate phosphate buffer to obtain a final 20-μg mL−1 protein concentration. After excitation at 295 nm, fluorescence emission spectra (300–500 nm) of the 20-μg mL−1 protein solutions were measured at 25°C in the FP-6300 spectrofluorimeter (Jasco, Tokyo, Japan). Fluorescence emission spectrum of the respective phosphate buffer was then subtracted from that of the sample to obtain the sample spectrum.
Protein solubility
PS was determined at pH 3.0–9.0 according to previously discussed protocols (Chao, Jung, & Aluko, Citation2018). Briefly, aqueous mixtures (1%, w/v, protein weight basis) were prepared and centrifuged at 10,000 g for 20 min followed by determination of the protein content in the supernatant using the modified Lowry method (Markwell et al., Citation1978). Total protein content was estimated by the Kjeldahl method (AOAC, Citation1990) using 5.7 as the nitrogen to protein conversion factor (Sosulski & Imafidon, Citation1990). PS was then calculated as follows:
Oil-in-water emulsion formation and measurement of oil droplet size
Protein mixtures (10, 25, and 50 mg mL−1) were prepared in 5 mL of 0.1 mol L−1 phosphate buffer pH 3.0, 5.0, or 7.0 followed by addition of 1 mL of canola oil (Aluko et al., Citation2009). Homogenization of the oil/water mixture was carried out at 20,000 rpm first for 1 min, and then second for another minute after a 5-s pause using a Polytron PT 3100 homogenizer (Kinematica AG, Lucerne, Switzerland). Emulsion oil droplet size (represented by the volume mean diameter, d4,3) was determined immediately after homogenization (time zero) in a Mastersizer 2000 (Malvern Instruments Ltd., Malvern, U.K.) using distilled water as the dispersant. The emulsions were allowed to stand at room temperature for 30 min and the d4,3 measured again. Emulsion stability (ES%) was calculated as follows (Equation 1):
Foam formation and measurement
To determine foaming capacity (FC), 5 mL protein slurries (10, 25, and 50 mg mL−1) were prepared with 0.1-mol L−1 phosphate buffer pH 3.0, 5.0, or 7.0 in graduated conical tubes followed by homogenization at 15,000 rpm for 1 min using a 20 mm shaft on the Polytron PT 3100 homogenizer (Aluko et al., Citation2009). FC was then measured as the increased (initial) volume of each sample and calculated as follows (Equation 2) according to the method of Fuhrmeister and Meuser (Citation2003):
where Vg is the total volume (mL) after homogenization while V1 is the sample solution volume (mL) prior to homogenization. FS was the volume measured after leaving the samples at room temperature for 30 min, which was then expressed as a percentage ratio of the initial volume
where V30 is the volume (mL) after 30 min while Vw is the total volume (mL) directly after homogenization.
Statistical analysis
Analyses of sample were done in triplicates and data analyzed using one-way ANOVA.
Significant differences (P < 0.05) between samples were determined by Duncan’s multiple-range tests using the Statistical Analysis System, version 9.2.
Results and discussion
Polyacrylamide gel electrophoresis
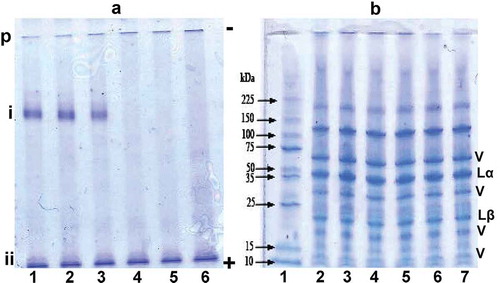
) presents electrophoretic patterns in non-denaturing conditions (i.e. native-PAGE) of heated pea proteins at different temperatures in the 50–100°C range. Two main protein bands are prominent in ) with i and ii bands representing extractable proteins that entered the gel. However, some of the proteins were too large to enter the gel and appeared at the gel origin (p). The i and ii bands are both present but the former was not detected in lanes corresponding to samples heated at 80°C, 90°C, and 100°C treatments. In contrast, the ii band appeared not to be affected by heat treatment. Therefore, the results are consistent with heat-induced protein aggregation, which is reflected as the protein that was too large to enter the gel. Similar native-PAGE results showing heat-induced aggregation and formation of large protein molecules that did not enter the gel have been reported for soy proteins (He et al., Citation2016). Addition of SDS and ME to the protein samples yielded several polypeptide bands ()), which confirms the oligomeric nature of the pea legumin (Lα + Lβ) and vicilin (V) as previously identified (Shand et al., Citation2007). The results are similar to those of previous reports on PPI polypeptide composition (Ribotta, Colombo, & Rosell, Citation2012; Shand et al., Citation2007). The >100 kDa polypeptides are probably polymerized products formed during commercial processing or as a result of the heat treatments (Shand et al., Citation2007). Heat treatment did not lead to any differences in the polypeptide composition, which could be attributed to the fact that only soluble proteins could be analyzed by gel electrophoresis. This is because at pH 8.0, there was only ~10% loss in PS as a result of heat treatment, especially at 90°C and 100°C. Previous works have also shown that heat treatment of cowpea protein isolate at 70°C and 90°C (Peyrano et al., Citation2016) or whey proteins at 72°C and 140°C (Patel, Singh, Anema, & Creamer, Citation2004) did not produce changes in the polypeptide patterns after SDS-ME PAGE analysis. However, some of the proteins extracted by the SDS-ME buffer were too large to enter the gel as shown by the band at the origin (top of gel), which corroborates the data from native-PAGE and is consistent with previous reports (Chihi, Mession, Sok, & Saurel, Citation2016; Mession, Sok, Assifaoui, & Saurel, Citation2013; Shand et al., Citation2007).
Figure 1. (a) Native-polyacrylamide gel electrophoresis (PAGE) and (b) SDS-PAGE (with mercaptoethanol) of pea protein isolate (PPI); lanes 1–6 represent untreated (25°C), 50°C, 70°C, 80°C, 90°C, and 100°C treatments, respectively. V = bands from vicilin proteins, Lα = legumin acidic subunit, and Lβ = legumin basic subunit.
Figura 1. (a) Native-PAGE y (b) SDS-PAGE (con mercaptoetanol) de aislado de proteína de arveja; filas 1–6 representan muestras sin tratar (25o C), 50o C, 70o C, 80o C, 90o C y 100o C, respectivamente. V = bandas de proteínas de vicilina, Lα = legumina subunidad ácida y Lβ = legumina subunidad básica.
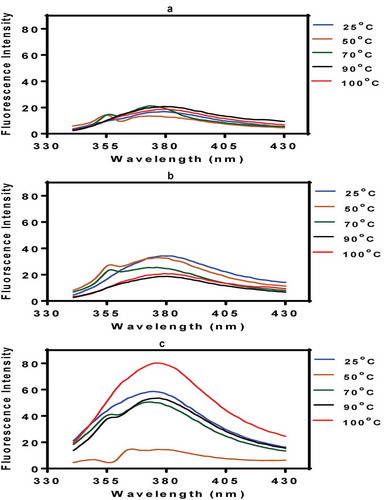
Intrinsic fluorescence emission
Fluorescence emission of soluble proteins is dependent mainly on the aromatic amino acids (phenylalanine, tyrosine, and tryptophan) and is a very sensitive tool for measuring protein structural changes (Schmid, Citation1989). The effect of heat treatment on fluorescence intensity (FI) of the PPI was dependent on environmental conditions, with least and most changes at pH 3.0 and 7.0, respectively (). At pH 3.0, the FI of the heat-treated proteins was very similar to that of the untreated PPI, which suggests minimal structural differences ()). In contrast, at pH 5.0, the 70–100°C pretreated samples had reduced FI when compared to the PPI treated at 50°C or the untreated (25°C) PPI ()). A plausible reason could be the exposed hydrophobic surfaces of the 70–100°C pretreated PPI, which would have increased protein–protein interactions at pH 5.0. Since the isoelectric point of pea proteins is at pH 4.5, it means that the lack of repulsive forces when combined with the high hydrophobicity led to increased protein–protein interactions of the 70–100°C pretreated PPI. The associated steric hindrance during protein–protein interactions could have led to reduced FI. However, at pH 7.0 the increased net charge would have limited protein–protein interactions and hence the increased FI values ()) when compared to pH 3.0 and 5.0. The 50°C treated PPI showed the least FI at pH 7.0, which reflect the lowest surface hydrophobicity. In contrast, the 70°C and 90°C pretreated cowpea protein isolates had reduced FI at pH 7.0 when compared to the untreated protein (Peyrano et al., Citation2016). It is possible that pea proteins used in this work had higher surface hydrophobicity, which would enhance protein–protein interactions as compared to the cowpea proteins. However, the higher FI at pH 7.0 for the 100°C pretreated PPI is similar to the data for zein, which also showed enhanced FI after pretreatment at 95°C (Sun, Dai, Liu, & Gao, Citation2016). Regarding the untreated protein sample, the wavelength of maximum emission for tryptophan in a hydrophobic pocket is usually ~330–350 nm (Schmid, Citation1989). Exposure of the tryptophan residues to a hydrophilic environment will result in a red shift to wavelengths >350 nm as observed for the PPI proteins. The samples had ~370 nm wavelength of maximum FI, which indicates extensive protein interactions as a result of the protein isolation method and the heat treatments. The results are similar to the reported value of 367 nm, which corresponds to the maximum emission wavelength for tryptophan as observed in pH-treated quinoa protein isolates (Abugoch, Romero, Tapia, Silva, & Rivera, Citation2008). Therefore, it is evident that heat treatment above a denaturing temperature led to protein unfolding, which exposed previously buried tryptophan residues to the hydrophilic environment.
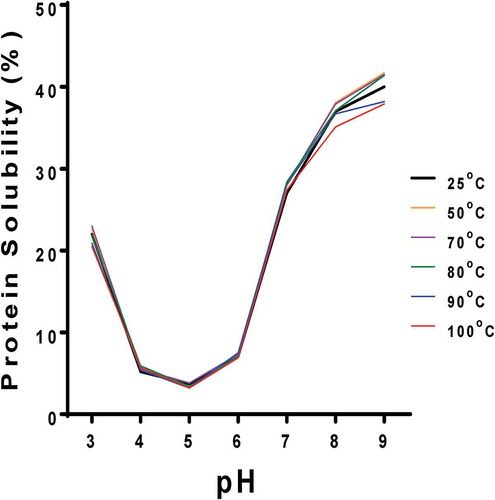
Protein solubility
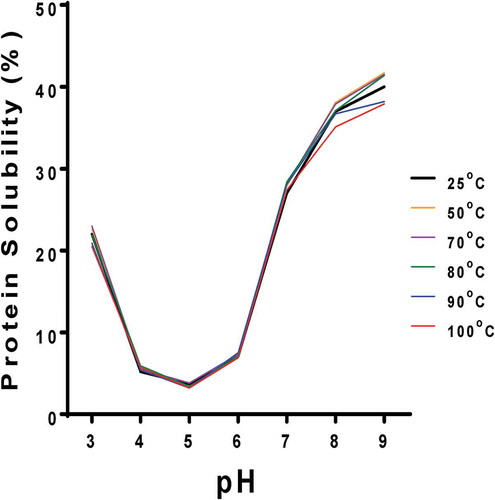
shows a PS pattern that is typical for plant storage proteins as evident in the minimal values at pH 4–6 with higher values above and below this range. The heat-treated samples had similar PS values as the unheated sample, except that the 100°C treatment led to solubility reductions at pH 7–9. The results suggest that the heat treatments did not cause excessive aggregation that would have produced lower interactions with water. Similar results were reported for heat-treated (70°C and 90°C) cowpea protein isolate (A10), which had similar PS as the unheated sample (Peyrano et al., Citation2016).
Effect of thermal pretreatment on emulsion formation and stability
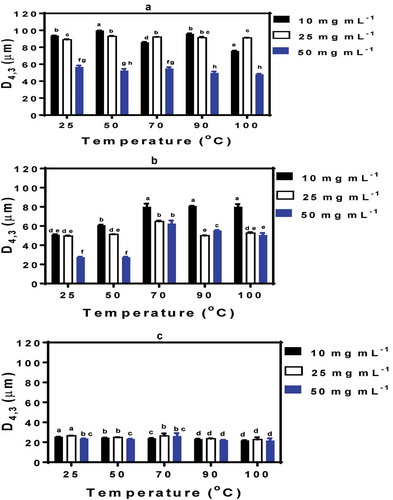
The particle size is an important index to represent oil-in-water emulsion quality since smaller oil droplet sizes are indicative of higher quality (larger surface area) and better emulsion-forming ability of the protein. In general, the average emulsion oil droplet size for heated and unheated proteins was in the order: pH 3.0 (48–100 μm) > pH 5.0 (27–80 μm) > pH 7.0 (22–27 μm), which indicates formation of significantly (P < 0.05) better emulsions at pH 7.0 (). The results are consistent with the intrinsic fluorescence data (), which showed increased exposure of hydrophobic groups (higher FI values) from pH 3.0–7.0, especially for heat-treated samples. Tang and Ma (Citation2009) have also suggested a close positive relationship between kidney bean protein isolate emulsifying activity and increased exposure of hydrophobic groups. The increased exposure of aromatic groups at pH 7.0 enhanced protein interactions with oil droplets. The interactions enabled higher oil droplet surface coverage by unfolded protein of increased surface hydrophobicity. This is consistent with the greater dependence of oil droplet size on protein concentration at pH 3.0 and 5.0 where the reduced protein interactions with the oil droplets benefitted from availability of more protein molecules. In contrast, at pH 7.0 the higher ability of protein to adsorb onto droplet surface resulted in lower protein concentration to stabilize the emulsion (thus smaller droplet size). A previous work has also reported pH-dependent increases in emulsifying activity index of soybean proteins with lowest value at pH 5.8 and highest at pH 8.0 (Liu et al., Citation2008). The results suggest a highly aggregated protein structure at pH 3.0 and 5.0 but not at pH 7.0 where increased net negative charge would have reduced protein–protein interactions accompanied by greater exposure of the hydrophobic groups. Heat-treated PPI produced significantly (P < 0.05) smaller emulsion oil droplet sizes at pH 3.0 and 7.0 but not at pH 5.0 when compared to the untreated (25°C) PPI (). The beneficial effect of temperature on oil droplet size at pH 3.0 and 7.0 may have been due to heat-induced structural changes and exposure of hydrophobic groups, which enhanced protein–oil interactions. Similar results have been reported for whey protein isolate where the oil droplet sizes of heat-treated protein were ~50% smaller than those of emulsions prepared with the untreated protein (Zhong, Wang, Hu, & Ikeda, Citation2013).
Figure 4. Oil droplet sizes of emulsions formed by untreated (25°C) and heat-treated PP I. (a) pH 3.0; (b) pH 5.0; (c) pH 7.0. Bars with different letters have significantly (P < 0.05) different mean values.
Figure 4. Tamaños de las gotas de aceite producidas por emulsiones formadas por aislados de proteí1;nas de arveja sin tratamiento (25° C) y con tratamiento tármico. (a) pH 3.0; (b) pH 5.0; (c) pH 7.0. Las barras con distintas letras tienen valores medios significativamente diferentes (P < 0.05)..
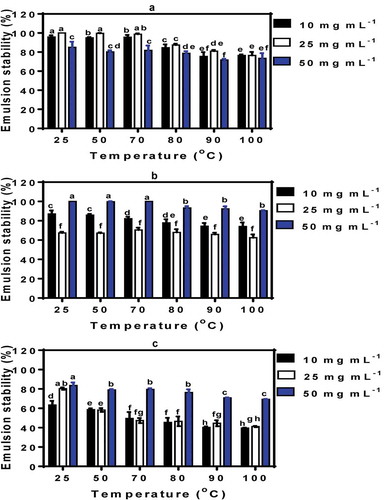
In contrast to emulsion oil droplet size, the ES was lowest at pH 7.0 followed by pH 5.0 and highest at pH 3.0 (). Therefore, at acidic pH and close to the isoelectric point of pea proteins, the reduced net charge probably led to formation of stronger interfacial membranes. However, at pH 7.0 the presence of several negative charges on the polypeptide chains led to formation of weak interfacial membranes, which would have enhanced oil droplet coalescence and hence reduced stability. The results are consistent with the protein concentration dependence of ES at pH 5.0 and 7.0 (P < 0.05). The results may be due to increased viscosity of the continuous phase as a result of increased protein concentration, hence reduced rate of oil droplet coalescence. The results are consistent with the increased ES that was observed for lentil protein-stabilized emulsions as protein concentration was increased (Joshi et al., Citation2012). Overall, heat pretreatment of PPI, especially at >70°C, led to significant (P < 0.05) reductions in ES at pH 3.0, 5.0, and 7.0; thus, pre-heat treatment did not improve emulsifying properties. The results suggest that high temperatures may have led to inability of PPI to form strong interfacial membranes and hence an increased tendency toward oil droplet coalescence.
Figure 5. Stability of emulsions formed by untreated (25°C) and heat-treated PP I. (a) pH 3.0; (b) pH 5.0; (c) pH 7.0. Bars with different letters have significantly (P < 0.05) different mean values.
Figura 5.Estabilidad de las emulsiones formadas por aislados de proteína de arveja sin tratamiento (25°C) y con tratamiento térmico. (a) pH 3.0; (b) pH 5.0; (c) pH 7.0. Las barras con distintas letras tienen valores medios significativamente diferentes (P < 0.05).
Effect of thermal pretreatment on foam formation and stability
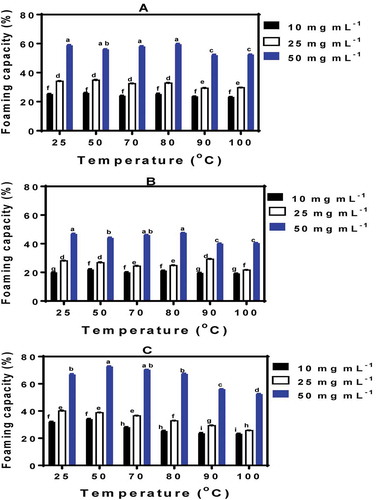
The ability of PPI to form foams was dependent (P < 0.05) on pH, protein concentration, and pretreatment temperature as shown in . FC was lowest at pH 5.0, which indicates that protein aggregation at the isoelectric point had a negative effect on ability to unfold and entrap air particles. In contrast, at pH 3.0 and 7.0 where the proteins would have gained net charges, the higher FC values may be due to higher level of protein unfolding at the air–water interface, which enhanced polypeptide flexibility. Since the FC values were higher at pH 7.0, the results suggest more net charge and greater protein flexibility when compared to pH 3.0 and 5.0. The significantly (P < 0.05) positive relationship between protein concentration and FC suggests that the increased availability of polypeptide chains contributed to efficiency of PPI as a foaming agent. However, foaming ability was significantly (P < 0.05) reduced at high temperatures because FC values were lowest for the 90°C- and 100°C-treated PPI. Similar decreases in foam overrun have been reported as a result of heat pretreatment of whey protein isolate (Nicorescu et al., Citation2011). The results suggest that emulsion properties are related to the hydrophilic/hydrophobic surface balance, whereas FC is correlated to exposure of polar groups onto the protein surface.
Figure 6. Foaming capacity of untreated (25°C) and heat-treated isolated PP I. (a) pH 3.0; (b) pH 5.0; (c) pH 7.0. Bars with different letters have significantly (P < 0.05) different mean values.
Figura 6.Capacidad espumante del aislado de proteína de arveja sin tratamiento (25°C) y con tratamiento tármico. (a) pH 3.0; (b) pH 5.0; (c) pH 7.0. Las barras con distintas letras tienen valores medios significativamente diferentes (P < 0.05).
Formation of relatively more stable foams occurred at pH 7.0 and 50 mg mL−1 protein concentration than at lower pH and protein levels (). A low FS value of a protein isolate results from a weak interfacial film of the adsorbed proteins. Therefore, at higher protein concentrations, more polypeptide chains are available to form the interfacial film, which could have led to increased strength and higher FS. But FS is also dependent on net protein charge, which can reduce interactions between entrapped air particles. Therefore, the higher FS at pH 7.0 could be due to reduced interactions between the entrapped air bubbles as a result of increased net charges on the interfacial protein membranes. FS was less mainly at pH 3.0 but not at pH 5.0 and 7.0. Reduced FS at pH 3.0 (10-mg mL−1 protein concentration) and pH 5.0 as a result of heat treatment is an indication that the protein molecules were unable to form strong interfacial films or did not possess adequate net charge, hence increased rupture of the entrapped air bubbles. However, the increased FS at pH 5.0 and 7.0, especially at 50-mg mL−1 protein concentration, suggests the presence of heat-induced polypeptides with increased net charge or formation of strong interfacial films through increased hydrogen bond-facilitated protein–protein interactions (Lawal, Adebowale, Ogunsanwo, Sosanwo, & Bankole, Citation2005). The results suggest reduced foam-forming ability by heat treatment of PPI. But once the foams were formed, the hydrophilic interfacial membranes reduced foam rupture tendency and hence better FS values of the emulsions formed by the unheated protein. In contrast, heat treatment led to reduced stability of foams formed by kidney bean protein isolate (Tang & Ma, Citation2009). Differences may be due to protein composition since the kidney bean protein isolate consisted mainly of vicilin while the pea protein used in this work contained both legumin and vicilin.
Figure 7. Foam stability of untreated (25°C) and heat-treated PPI. (a) pH 3.0; (b) pH 5.0; (c) pH 7.0. Bars with different letters have significantly (P < 0.05) different mean values.
Figura 7.Estabilidad de la espuma producida por el aislado de proteína de arveja sin tratamiento (25°C) y con tratamiento térmico. (a) pH 3.0; (b) pH 5.0; (c) pH 7.0. Las barras con distintas letras tienen valores medios significativamente diferentes (P < 0.05).
Conclusions
Thermal treatment up to 100°C did not modify the PPI soluble polypeptide composition as shown by SDS-PAGE but changes in protein conformations occurred as measured by intrinsic fluorescence and surface hydrophobicity. Parts of the protein were highly aggregated at 80–100°C and reflected as a blank band on the native-PAGE. Emulsion-forming ability was enhanced by heat pretreatment mainly at pH 7.0, which led to smaller-size emulsified oil droplets when compared to the untreated PPI. In contrast, oil droplet size increased at pH 5.0, suggesting the formation of heat-induced proteins with reduced ability to stabilize the oil:water interface. But the foaming ability was negatively impacted by heat pretreatment of PPI at pH 3.0, 5.0, and 7.0.
Acknowledgment
Operating (Discovery) and equipment (Research tools and instruments) research grants for this project were provided to Dr. R.E. Aluko by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abugoch, L. E., Romero, N., Tapia, C. A., Silva, J., & Rivera, M. (2008). Study of some physicochemical and functional properties of quinoa (Chenopodium quinoa Willd) protein isolates. Journal of Agricultural and Food Chemistry, 56, 4745–4750.
- Adebiyi, A. P., & Aluko, R. E. (2011). Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chemistry, 128, 902–908.
- Aluko, R. E., Mofolasayo, O. A., & Watts, B. M. (2009). Emulsifying and foaming properties of pea seed flours. Journal of Agricultural and Food Chemistry, 57, 9793–9800.
- Aluko, R. E., Yada, R. Y., Lencki, R. W., & Marangoni, A. G. (1997). Structural and functional properties of a partially purified cowpea globulin modified with protein kinase and glycopeptidase. Journal of Agricultural and Food Chemistry, 45, 2907–2913.
- AOAC. (1990). Official methods of analysis (15th ed). Washington, DC: Association of Official Analytical Chemists Inc.
- Campbell, L., Euston, S. R., & Ahmed, M. A. (2016). Effect of addition of thermally modified cowpea protein on sensory acceptability and textural properties of wheat bread and sponge cake. Food Chemistry, 194, 1230–1237.
- Chang, C., Tu, S., Ghosh, S., & Nickerson, M. T. (2015). Effect of pH on the inter-relationships between the physicochemical, interfacial and emulsifying properties for pea, soy, lentil and canola protein isolates. Food Research International, 77, 360–367.
- Chao, D., Jung, S., & Aluko, R. E. (2018). Physicochemical and functional properties of high pressure-treated isolated pea protein. Innovative Food Science & Emerging Technologies, 45, 179-185.
- Chihi, M.-L., Mession, J.-L., Sok, N., & Saurel, R. (2016). Heat-induced soluble protein aggregates from mixed pea globulins and β-lactoglobulin. Journal of Agricultural and Food Chemistry, 64, 2780–2791.
- Fuhrmeister, H., & Meuser, F. (2003). Impact of processing on functional properties of protein products from wrinkled peas. Journal of Food Engineering, 56, 119–129.
- He, X.-T., Yuan, D.-B., Wang, J.-M., & Yang, X.-Q. (2016). Thermal aggregation behavious of soy protein: Characteristics of different polypeptides and sub-units. Journal of the Science of Food and Agriculture, 96, 1121–1131.
- Jiang, J., Zhu, B., Liu, Y., & Xiong, Y. L. (2014). Interfacial structural role of pH-shifting processed pea protein in the oxidative stability of oil/water emulsions. Journal of Agricultural and Food Chemistry, 62, 1683–1691.
- Joshi, M., Adhikari, B., Aldred, P., Panozzo, J. F., Kasapis, S., & Barrow, C. J. (2012). Interfacial and emulsifying properties of lentil protein isolate. Food Chemistry, 134, 1343–1353.
- Karaca, A. C., Low, N., & Nickerson, M. (2011). Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Research International, 44, 2742–2750.
- Klassen, D. R., & Nickerson, M. T. (2012). Effect of pH on the formation of electrostatic complexes within admixtures of partially purified pea proteins (legumin and vicilin) and gum Arabic polysaccharides. Food Research International, 46, 167–176.
- Kowalczyk, D., Gustaw, W., Świeca, M., & Baraniak, B. (2014). A study on the mechanical properties of pea protein isolate films. Journal of Food Processing and Preservation, 38, 1726–1736.
- Koyoro, H., & Powers, J. R. (1987). Functional properties of pea globulin fractions. Cereal Chemistry, 64, 97–101.
- Lawal, O. S., Adebowale, K. O., Ogunsanwo, B. M., Sosanwo, O. A., & Bankole, S. A. (2005). On the functional properties of globulin and albumin protein fractions and flour of African locust bean (Parkia biglobossa). Food Chemistry, 92, 681–691.
- Liang, H.-N., & Tang, C.-H. (2013). pH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocolloids, 33, 309–319.
- Liu, C., Wang, X., Ma, H., Zhang, Z., Gao, W., & Xiao, L. (2008). Functional properties of protein isolates from soybeans stored under various conditions. Food Chemistry, 111, 29–37.
- Liu, C. C., Tellez-Garay, A. M., & Castell-Perez, M. E. (2004). Physical and mechanical properties of peanut protein films. LWT – Food Science and Technology, 37, 731–738.
- Lu, B.-Y., Quillien, L., & Popineau, Y. (2000). Foaming and emulsifying properties of pea albumin fractions and partial characterisation of surface-active components. Journal of the Science of Food and Agriculture, 80, 1964–1972.
- Marinangeli, C. P. F., Kassis, A. N., & Jones, P. J. H. (2009). Glycemic responses and sensory characteristics of whole yellow pea flour added to novel functional foods. Journal of Food Science, 74, S385–S389.
- Markwell, M. A., Hass, S. M., Bieber, L. L., & Tolbert, N. E. (1978). A modification of the Lowry procedure to simplify protein determination in membrane and in protein samples. Analytical Biochemistry, 87, 206–210.
- Mession, J.-L., Assifaoui, A., Cayot, P., & Saurel, R. (2012). Effect of pea proteins extraction and vicilin/legumin fractionation on the phase behavior in admixture with alginate. Food Hydrocolloids, 29, 336–346.
- Mession, J.-L., Sok, N., Assifaoui, A., & Saurel, R. (2013). Thermal denaturation of pea globulins (Pisum sativa L.)- molecular interactions leading to heat-induced protein aggregation. Journal of Agricultural and Food Chemistry, 61, 1196–1204.
- Nickel, G. B. (1981). Process for preparing products from legumes, Canadian Patent 1104871.
- Nicorescu, I., Vial, C., Talansier, E., Lechevalier, V., Loisel, C., Della Valle, D., … Legrand, J. (2011). Comparative effect of thermal treatment on the physicochemical properties of whey and egg white protein foams. Food Hydrocolloids, 25, 797–808.
- Osen, R., Toelstede, S., Eisner, P., & Schweiggert‐Weisz, U. (2015). Effect of high moisture extrusion cooking on protein–Protein interactions of pea (Pisum sativum L.) protein isolates. International Journal of Food Science and Technology, 50, 1390–1396.
- Osen, R., Toelstede, S., Wild, F., Eisner, P., & Schweiggert-Weisz, U. (2014). High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. Journal of Food Engineering, 127, 67–74.
- Patel, H. A., Singh, H., Anema, S. G., & Creamer, L. (2004). Effects of heat and high hydrostatic pressure treatments on the aggregation of whey proteins in whey protein concentrate solutions. Food New Zealand, 4, 29–35.
- Peyrano, F., Speroni, F., & Avanza, M. V. (2016). Physicochemical and functional properties of cowpea protein isolates treated with temperature or high hydrostatic pressure. Innovative Food Science and Emerging Technologies, 33, 38–46.
- Ribotta, P. D., Colombo, A., & Rosell, C. M. (2012). Enzymatic modifications of pea protein and its application in protein-cassava and corn starch gels. Food Hydrocolloids, 27, 185–190.
- Schmid, F. X. (1989). Spectral methods of characterizing protein conformation and conformational changes. In T. E. Creighton (Eds.). Protein structure. A practical approach (pp. 251–285). Oxford: IRL Press.
- Shand, P. J., Ya, H., Pietrasik, Z., & Wanasundara, P. K. J. P. D. (2007). Physicochemical and textural properties of heat-induced pea protein isolate gels. Food Chemistry, 102, 1119–1130.
- Sosulski, F. W., & Imafidon, G. I. (1990). Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. Journal Of Agricultural And Food Chemistry, 38, 1351–1356.
- Sun, C., Dai, L., Liu, F., & Gao, Y. (2016). Simultaneous treatment of heat and high pressure homogenization of zein in ethanol-water solution: Physical, structural, thermal and morphological characteristics. Innovative Food Science and Emerging Technologies, 34, 161–170.
- Tang, C.-H., & Ma, C.-Y. (2009). Heat-induced modifications in the functional and structural properties of vicilin-rich protein isolate from kidney (Phaseolus vulgaris L.) bean. Food Chemistry, 115, 859–866.
- Yin, S.-W., Tang, C.-T., Wen, Q.-B., & Yang, X.-Q. (2010). Functional and conformational properties of phaseolin (Phaseolus vulgaris L.) and kidney bean protein isolate: A comparative study. Journal of the Science of Food and Agriculture, 90, 599–607.
- Zhong, Q., Wang, W., Hu, Z., & Ikeda, S. (2013). Sequential preheating and transglutaminase pretreatments improve stability of whey protein isolate at pH 7.0 during thermal sterilization. Food Hydrocolloids, 31, 306–316.