?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the odour-active volatile compounds present in a coffee aroma concentrate obtained from a soluble coffee extract in an industrial aroma recovery system based on distillation and evaporation were characterized by solid-phase microextraction coupled to gas chromatography with mass spectrometry and olfactometric detection technique. This analysis allowed the identification of 58 compounds, of which 15 pyrazines, 9 furans, 6 aldehydes, 4 ketones, 4 esters, 4 pyrroles, 4 sulphur compounds, 3 pyridines, 3 phenols, 3 alcohols, an oxazole, and a pyran are highlighted. From these families, the following compounds presented the highest sensory activities (%MF = 100): 2-methylbutanal, 2-methyl-2-butenal, 2-furancarboxaldehyde, furfural, and methyl phenyl acetate. These compounds can be considered as quality markers in the process control on the aroma recovery system.
RESUMEN
En este trabajo se realizó la caracterización de los compuestos volátiles olfativamente activos presentes un recuperado de aroma de café, obtenido a partir de un extracto de café soluble en un sistema industrial de recuperación de aromas basado en destilación y evaporación, mediante la técnica de microextracción en fase sólida acoplada a cromatografía de gases con detectores de espectrometría de masas y olfatometría SPME-GC-MS-O. El análisis permitió identificar 58 compuestos, dentro de los cuales se destacan 15 pirazinas, 9 furanos, 6 aldehídos, 4 cetonas, 4 esteres, 4 pirroles, 4 compuestos azufrados, 3 piridinas, 3 fenoles, 3 alcoholes, un oxazol y un pirano. De estas familias, los siguientes compuestos: 2-Methylbutanal, 2-Methyl-2-butenal, 2-Furancarboxaldehyde, Furfural y Methyl phenylacetate presentaron la mayor actividad sensorial (%MF=100). Estos compuestos pueden ser considerados como marcadores de la calidad en el control del proceso del sistema de recuperación de aromas.
RESUMEN
En este estudio se caracterizaron los compuestos volátiles responsables del aroma presentes en un concentrado de aroma de café obtenido de un extracto de café soluble empleando un sistema de recuperación de aromas basado en la destilación y la evaporación. Los compuestos volátiles fueron caracterizados mediante la microextracción en fase sólida vinculada a la cromatografía de gases con espectrometría de masas y la técnica de detección olfactométrica (SPME-GC-MS-O). Dicho análisis permitió identificar 58 compuestos, de los cuales se destacan 15 pirazinas, 9 furanos, 6 aldehídos, 4 cetonas, 4 ésteres, 4 pirroles, 4 compuestos de azufre, 3 piridinas, 3 fenoles, 3 alcoholes, 1 oxazol y 1 pirano. Los compuestos de estas familias que presentaron actividad sensorial más elevada (% MF = 100) fueron: 2-metibutanal, 2-metil-2-buten, 2- furancarboxaldehído, furfural y acetato de metil fenil, por lo que pueden ser considerados como marcadores de calidad en el proceso de control vinculado al sistema de recuperación de aromas.
Introduction
Coffee is the second-most commercialized product worldwide after oil. According to the International Coffee Organization (http://www.ico.org), 143.3 million sacks of coffee were commercialized in 2015, where 57.9% corresponded to coffee of the Arabica species. Of this, Colombia exported 14.6% of the green coffee and 0.7% of coffee roasted and instant. Colombian coffee is appreciated in the international market for having a high-quality cup, especially due to its sweet and fruity notes as well as a subtle acidity in the cup.
One of the most important challenges in the production of instant coffee is to achieve a cup with sensory attributes like those of a roasted coffee beverage. These attributes are the result of the interaction between more than 800 chemical substances present in the coffee, which can vary due to the species, geographical origin, and effect of the processing conditions along the entire production chain (Flament & Bessière-Thomas, Citation2002; Sunarharum, Williams, & Smyth, Citation2014).
Specifically, in the production of soluble coffee, it has been found that, compared with a roasted coffee beverage, losses above 70% of the volatile fraction can occur. This is responsible for the aroma profile of the product (R. J. Clarke & Macrae, Citation1985). Such a volatile fraction is defined by the presence of different families of odorant compounds, among which are ketones, aldehydes, furans, esters, pyrroles, pyridines, pyrazines, phenols, hydrocarbons, oxazoles, carboxylic acids, lactones, terpenes, amines, and sulphur compounds. These compounds are present in concentration ranges from ng/L up to mg/L and varying in composition throughout the complete processing of the grain (Flament & Bessière-Thomas, Citation2002).
Since the 1980s, numerous investigations have been published related to the characterization of key compounds in the aroma, mainly of roasted coffees. These results differ between studies depending on the type, preparation of the evaluated coffee, and industrial processes used to produce the coffee (Sunarharum et al., Citation2014). In contrast, in the case of soluble coffee, there is very little information on the final composition of the aromatic compounds in the product, thus presenting an interesting topic to be explored in terms of the identification, extraction technologies, and interaction with the other components of the coffee.
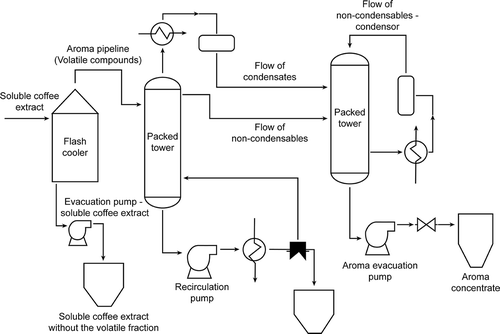
Given the importance of the aroma compounds in the cup quality, aroma recovery systems have been developed in order to separate the volatile fraction, concentrate it, and then reincorporate it into the coffee extracts, thus achieving better sensory attributes of instant coffee (Baggenstoss, Thomann, Perren, & Escher, Citation2010; R. Clarke & Vitzthum, Citation2008; Karlsson & Trägårdh, Citation1997; Khatachourian, Lascelles, & Rowan, Citation1991; Liu, Citation1986; Wylock, Eloundou Mballa, Heilporn, Debaste, & Fauconnier, Citation2015). One of the aroma recovery systems used in the coffee industry is based on the separation of the volatile compounds present in the coffee extract by a vaporization process in a flash cooler-type device, where the extract enters and, at reduced pressure and a specific temperature, the vaporization process occurs, thus allowing the vapour fraction to be enriched with volatile compounds of the coffee extract. Once the vapour phase, rich in volatile components, is separated, it is subjected to a rectification process by means of two packed towers to concentrate the component by approximately 200–300 times the original composition. No information on the composition of this type of concentrates has been reported in the literature, which is crucial to define its quality. Studies have been published in regards to strawberry aroma recovery processes with similar systems but not in regards to the coffee aroma (Kollmannsberger & Berger, Citation1994). Recent studies have used pervaporation aroma recovery systems, which are different from the distillation and partial condensation systems (Weschenfelder, Lantin, Viegas, De Castilhos, & Scheer, Citation2015).
The goal of the present study was to identify the sensory-active volatile compounds in coffee aroma concentrates obtained in the aroma recovery process by the headspace-solid-phase microextraction (HS-SPME) technique, followed by analysis by gas chromatography coupled to a mass spectrometer and an olfactometric detector (GC-MS-O). Identifying the sensory active compounds in the coffee concentrate will permit understand the role of these substances in the global aroma profile and find quality markers.
Materials and methods
Reagents
An alkane mixture (from C7 to C40) was provided by Supelco (Bellefonte, PA, USA). Analytical quality water was obtained from a Milli-Q® purification system from Millipore (Bedford, MA, USA). 2-Methylbutanal, 2-methoxyphenol, pyrazine, 3-methylbutanal, 2,5-dimethylpyrazine, 2,3-dimethylpyrazine, and 4-ethyl-2-methoxyphenol were provided by Wako (Richmond, VA, USA). 2-Furancarboxaldehyde, furfural, 2,3-pentenedione, methyl disulphide, 2-furanmethanol acetate, methylpyrazine, 2-butanone, and 2-furanmethanol were provided by Chem Service (West Chester, PA, USA). Pyridine and ethanol were provided by Merck (Bedford, MA, USA).
Samples
The samples analysed were obtained from the volatile fraction recovered from Colombian Arabica coffee extracts at a soluble solid concentration of approximately 10% obtained from roasted coffee grain, and the volatile fraction was separated in a partial aroma recovery system based on condensation and subsequent rectification. In total, samples from three production batches were evaluated. The flowchart in shows the industrial aroma recovery process. In this system, the coffee extract is subjected to a specific pressure and temperature in a flash cooler device that ensures the expansion and vaporization of the extract to separate the volatile compounds, thus enriching the vapour phase. The extract deprived of its volatile compounds is sent to the concentration process for the subsequent reincorporation of the aroma concentrate prior to drying. The gas flow is condensed in a first packed distillation tower to concentrate the volatile components; the most volatile fraction is taken to a second distillation packed tower along with the fraction (flow of non-condensables) that could not be evaporated in the first tower. This allows the concentration of most of the volatile components, which are condensed in the product called the aroma concentrate, the sample studied in this work.
Sensory panel training
For the olfactometric analysis of the different samples, a taster panel comprising 10 individuals (five men and five women) with ages between 22 and 45 years was trained. The training was carried out over 6 months in 2 h sessions per week. The methodology used was proposed by Vene (Vene, Seisonen, Koppel, Leitner, & Paalme, Citation2013). The tasters were trained to describe the odours of a coffee kit as perceived with the nose (Le nez du café®), which includes the 32 most important sensory descriptors present in the coffee, and then, they were trained with samples placed in dark containers marked with a three-digit code to avoid bias (d’Acampora Zellner, Dugo, Dugo, & Mondello, Citation2008). Finally, the panellists received training in olfactometry with a test solution prepared in the laboratory prior to performing the GC-O analysis of the aroma recovery samples to be characterized.
Analysis of the samples by GC-MS-O
The coffee extracts were diluted to 1% with a 6 M NaCl solution. From this solution, a 5-mL sample was taken in a standard vial for HS-SPME and left to stabilize for 1 min at 25°C with constant agitation (300 rpm). Subsequently, a divinylbenzene/carboxen/polydimethylsiloxane fibre of 2 cm–50/30 µm (Bellefonte, PA, USA) was placed in the sample headspace for 15 min under the same previously described conditions. At the end of the extraction process, the fibre was removed from the vial and taken to the GC-MS-O injection port for the thermal desorption of the analytes at a temperature of 270°C for 3 min.
The analyses were carried out in an Agilent 6890 gas chromatograph (Santa Clara, CA, USA) simultaneously coupled to a Gerstel ODP 2 temperature programmable sniffing port (Mülheim an der Ruhr, Germany) with an olfactometry detection system and a 5973 Network mass spectrometry detector with a quadruple analyser. The chromatograph had a standard split/splitless injector, and an SPME liner with a 0.75 mm internal diameter was used. The injection mode used was splitless at a temperature of 270°C with a pressure pulse of 62 kPa for 3 min. Helium was used (analytical grade 5.0) as the carrier gas at a constant flow of 1 mL/min during the entire analysis. At the output of the chromatographic column, the flow was divided into equal parts between the olfactometry detector and the mass spectrometer.
The chromatographic column used was a 50 m INNOWax column with a 0.25 mm inner diameter and 0.25 µm film thicknesses (Agilent J&W Scientific, Agilent, CA, USA). The initial temperature of the chromatographic oven was 50°C, which was held for 3 min; the temperature was then increased at a rate of 3°C/min up to 160°C and finally to 250°C at a rate of 25°C/min, where it was maintained for 22 min. The mass analyser temperature and the transfer line were kept at 250°C. The mass range selected as the mass/charge ratio was from 40 to 400. The temperature of the sniffing port was programmed by a linear heating ramp that began at 80°C and went up to 260°C at a rate of 3°C/min.
During each analysis, the judges evaluated the emanating odours. Each time an odour was recognized, the retention time was registered as well as the odour description and intensity on a scale of 0–3, where 0 = not detected; 1 = weak, odour hardly recognized; 2 = clear but not intense; and 3 = intense (Ferreira et al., Citation2009). Each injection was performed by three trained judges, and each judge performed the olfactometry during a period of 20 min. The coffee extracts were evaluated by 10 judges, and each judge performed the complete analysis for each sample in three different sessions.
Calculation of the linear retention indices (LRI)
To perform the calculation of the experimental retention indices, a standard that contained a series of linear alkanes from C7 to C40 was analysed under the same chromatographic conditions as the analysed samples. The LRI was calculated for each compound that presented a sensory response (Guiochon, Citation1964).
Identification of compounds
The identification of the sensory-active compounds was carried out with the following criteria: comparison with the reference mass spectrum of the NIST Mass Spectral Library 2014; comparison with the LRI reported in the literature in a polar phase and by olfactometry through comparison of the olfactory perception description carried out by the judges with respect to the reference olfactory description of each compound. The criterion for accepting the identification of a compound was the correct correspondence of the mass spectrum, the retention index, and the olfactory description with the reference data reported for each compound. When available, pure standards were injected for comparison with the identified compounds. This way, the active aromatic compounds positively identified.
Modified frequency
The data obtained from the olfactometry are expressed in terms of the modified frequency (MF), which was calculated with the model proposed by Dravnieks & ASTM Committee E-18 on Sensory Evaluation of Materials and Products. Section E-18.04.12 on Odor Profiling (Citation1985) as
where F(%) is the detection frequency of an aromatic attribute expressed as a percentage and I(%) is the average intensity, also expressed as a percentage (Dravnieks & ASTM Committee E-18 on Sensory Evaluation of Materials and Products. Section E-18.04.12 on Odor Profiling Citation1985).
Results and discussion
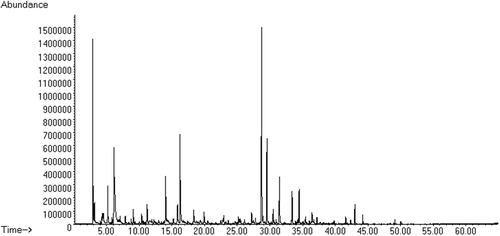
The general chromatographic profile of the studied coffee extracts is shown in In this study, 58 sensory-active volatile compounds present in the three studied sample batches were identified. These identified compounds are classified by families, and a total of 3 alcohols, 6 aldehydes, 4 esters, 9 furans, 4 ketones, 1 lactone, 1 oxazole, 3 phenols, 1 pyran, 16 pyrazines, 3 pyridines, 4 pyrroles, and 4 sulphur compounds were obtained. Each of the compounds is listed in with the identification parameters described in the methodology. To evaluate the sensory impact of the odour zones identified by GC-O, the MF values were determined considering the intensities and the detection frequencies reported by the judges.
Table 1. Aroma compounds identified in the coffee aroma concentrate.
Tabla 1. Compuestos de aroma identificados en el concentrado de aromas de café
Figure 2. Chromatogram of the coffee aroma concentrate extract by SPME-GC-MS.
Figura 2. Cromatograma del concentrado de aromas de café por SPME-GC-MS.
The identified compounds can be classified into four groups according to the MF values found. First, a group of five compounds with high sensory scores are highlighted with MF values of 100%, out of which compounds 1, 3, and 4 have been reported as aroma impact compounds of roasted coffee beverages (R. Clarke & Vitzthum, Citation2008), while compounds 2 and 5, although not cited as being of high sensory impact, have been previously found in coffee beverages (Flament & Bessière-Thomas, Citation2002).
In the second group of compounds, with MF values in a range between 70% and 100% are compounds 6–13. These compounds presented aromatic descriptors including coffee, sweet, honey, hazelnut, and onion, and due to their high MF values, they could be important in the aroma of the recovery sample.
In the third group of compounds with MF values between 30% and 70% are compounds 14–34. All of these compounds have been previously reported in roasted coffee (R. Clarke & Vitzthum, Citation2008; Sunarharum et al., Citation2014); these compounds mainly present aromatic notes than can be classified as roasted notes, such as coffee, cocoa, and dried fruits. In this group of compounds with significant scores, 50% are compounds from the pyrazine family, which indicates that this family of compounds could be important in the formation of the aroma of the recovery sample.
Finally, the fourth group of compounds 35–58 had MF values below 30%, and although they are reported in roasted coffee and their mass spectra were obtained, they can be considered compounds of low incidence in the formation of the global aroma of the product due to their low frequencies and intensities in the studied samples. Some authors even consider them as noise in the odour regions that present these low MF values (Ferreira et al., Citation2009).
Conclusions
The HS-SPME-GC-MS-O technique allowed, for the first time, the identification of sensory-active compounds present in an aroma recovery sample obtained from a flash industrial system used for the improvement of the sensory profile of soluble coffee products. Among the 58 volatile compounds identified in the samples, 34 compounds presented MF values above 30%, indicating that these are the compounds that presented the highest olfactory activity in the recovery sample, within which the key compounds in the formation of the aroma of the product would be expected to be found. The volatile compounds that presented higher olfactory activities (%MF = 100%) correspond to 2-methylbutanal, 2-methyl-2-butenal, 2-furancarboxaldehyde, furfural, and methyl phenyl acetate, which presented fruity, old crop, almond, sweet, and honey odour descriptors.
These results will allow quality markers of the aroma recoveries to be established through qualitative analysis of these components in the samples, followed by reconstitution/omission sensory tests that allow the role of these compounds in aroma formation and their correlation with the sensory quality of aromatized soluble coffees and derived products to be determined.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Baggenstoss, J., Thomann, D., Perren, R., & Escher, F. (2010). Aroma recovery from roasted coffee by wet grinding. Journal of Food Science, 75(9), C697–C702.
- Clarke, R., & Vitzthum, O. G. (2008). Coffee: Recent developments. Oxford, UK: Blackwell Science Ltd.
- Clarke, R. J., & Macrae, R. (1985). Coffee: Chemistry. Netherlands: Springer Netherlands.
- d’Acampora Zellner, B., Dugo, P., Dugo, G., & Mondello, L. (2008). Gas chromatography–Olfactometry in food flavour analysis. Journal of Chromatography A, 1186(1), 123–143.
- Dravnieks, A. & ASTM Committee E-18 on Sensory Evaluation of Materials and Products. Section E-18.04.12 on Odor Profiling. (1985). Philadelphia, PA: ASTM.
- Ferreira, V., San Juan, F., Escudero, A., Culleré, L., Fernández-Zurbano, P., Saenz-Navajas, M. P., & Cacho, J. (2009). Modeling quality of premium spanish red wines from gas chromatography−olfactometry data. Journal of Agricultural and Food Chemistry, 57(16), 7490–7498.
- Flament, I., & Bessière-Thomas, Y. (2002). Coffee flavor chemistry. West Sussex, England: John Wiley & Sons Ltd.
- Guiochon, G. (1964). Retention indices in programmed temperature gas chromatography. Analytical Chemistry, 36(3), 661–663.
- Karlsson, H. O. E., & Trägårdh, G. (1997). Aroma recovery during beverage processing. Journal of Food Engineering, 34(2), 159–178.
- Khatachourian, E. L., Lascelles, L. G. J., & Rowan, W. C. O. G. F. C. (1991). Process for the recovery of volatile coffee constituents. London, UK: Patent and Trademark Office. U.S. Patent No. EP0227263(A1).
- Kollmannsberger, H., & Berger, R. G. (1994). Industrial recovery of strawberry flavour — Fractional distillation versus plate condensation. Zeitschrift Für Lebensmittel-Untersuchung Und Forschung, 198(6), 491–494.
- Liu, R. T. (1986). Process for improving the flavor and aroma of instant coffee. Washington, DC: U.S. Patent and Trademark Office. U.S. Patent No. US 4606921 A.
- Sunarharum, W. B., Williams, D. J., & Smyth, H. E. (2014). Complexity of coffee flavor: A compositional and sensory perspective. Food Research International, 62, 315–325.
- Vene, K., Seisonen, S., Koppel, K., Leitner, E., & Paalme, T. (2013). A method for GC–olfactometry panel training. Chemosensory Perception, 6(4), 179–189.
- Weschenfelder, T. A., Lantin, P., Viegas, M. C., De Castilhos, F., & Scheer, A. D. P. (2015). Concentration of aroma compounds from an industrial solution of soluble coffee by pervaporation process. Journal of Food Engineering, 159, 57–65.
- Wylock, C., Eloundou Mballa, P. P., Heilporn, C., Debaste, F., & Fauconnier, M. L. (2015). Review on the potential technologies for aromas recovery from food industry flue gas. Trends in Food Science & Technology, 46(1), 68–74.