ABSTRACT
Kombucha is a beverage made by fermenting sugared tea using a symbiotic culture of bacteria and yeasts. Kombucha consumption has been associated with some health effects such as: the reduction of cholesterol levels and blood pressure, reduction of cancer propagation, the improvement of liver, the immune system, and gastrointestinal functions. The beneficial effects of kombucha are attributed to the presence of bioactive compounds that act synergistically. Bacteria contained in kombucha beverage belongs to the genus Acetobacter, Gluconobacter, and the yeasts of the genus Saccharomyces along with glucuronic acid, contribute to health protection. This review focuses on recent findings regarding beneficial effects of kombucha and discusses its chemical compounds, as well as the metabolites resulted by the fermentation process. Besides, some contraindications of kombucha consumption are also reviewed.
RESUMEN
La kombucha es una bebida hecha de té endulzado y fermentado utilizando un cultivo simbiótico de bacterias y levaduras. El consumo del kombucha se ha asociado con algunos efectos benéficos para la salud, entre ellos la reducción de los niveles de colesterol y la presión arterial, la disminución de la propagación del cáncer y el mejoramiento de las funciones hepática, inmunológica y gastrointestinal. Estos efectos benéficos han sido atribuidos a la presencia de compuestos bioactivos en la kombucha, los cuales actúan sinérgicamente. Las bacterias contenidas en esta bebida pertenecen a los géneros Acetobacter y Gluconobacter; a su vez, las levaduras del género Saccharomyces contribuyen, junto con el ácido glucurónico, a la protección de la salud. La presente revisión discute varios hallazgos recientes en torno a los efectos benéficos del kombucha, y examina sus compuestos químicos y los metabolitos que se producen durante el proceso de fermentación. Finalmente, se revisan algunas contraindicaciones relativas al consumo de kombucha.
PALABRAS CLAVE:
1. Introduction
All foods are essentially functional to a certain level because they provide the energy and nutrients needed to maintain life. However, there is evidence of the existence of bioactive food components that are not considered nutrients but can provide beneficial health effects (Crowe & Francis, Citation2013; Kaur & Singh, Citation2017; Shimizu, Citation2012; Tur & Bibiloni, Citation2016). Functional foods are those that have scientifically proven their beneficial effects in the organism, in one or more of its functions, providing optimal health and well-being, regardless of their nutritional value (Kaur & Singh, Citation2017).
These foods have a preventive function, due to the fact that they reduce risk factors that cause diseases. Shimizu (Citation2012) refers to functional foods called FOSHU (Food for Specified Health Uses), which were approved by Japan’s Consumer Affairs Agency, a leader in functional foods regulations. Some of these foods improve the intestinal microbiota, regulate nutrient absorption and/or reduce the risk of chronic non-communicable diseases (Crowe & Francis, Citation2013).
It has been reported that certain dietary factors, such as lactic acid bacteria, oligosaccharides, amino acids, and polyphenols, may be promising ingredients for the future development of functional foods (Shimizu, Citation2012). However, the bioavailability and efficacy of these compounds at levels that are scientifically achievable in typical eating patterns should be revised (Kaur & Singh, Citation2017). For a product to be considered as a functional food, it must meet the following requirements: being a food product, having scientific evidence that supports the benefit of the product, having measurable physiological effects and being consumed daily as part of a normal diet (Tur & Bibiloni, Citation2016).
2. Kombucha definition
Kombucha is the name of the beverage obtained from the fermentation of tea, mainly black tea (there are also other varieties that can be used as a base for its preparation, such as green and oolong tea, also known as blue tea); with added sugar as a substrate for fermentation. Although this beverage has originally been prepared using tea, it is possible to find variations made with infusions like mint, lemon balm or jasmine. The taste of the beverage is slightly acidic and slightly carbonated, which provides greater acceptance among consumers. Some metabolic products of Symbiotic Culture of Bacteria and Yeast (SCOBY), like acetic acid and other organic acids, posses antibacterial activity and prevents contamination of the drink by pathogenic bacteria (Watawana, Jayawardena, Gunawardhana, & Waisundara, Citation2015).
3. Preparation of kombucha
This beverage is prepared by fermenting sugared tea with a SCOBY (Jayabalan, Malbaša, Lončar, Vitas, & Sathishkumar, Citation2014). Its flavour is slightly sweet and sour at the same time, plus it may contain traces of carbon dioxide (Nummer, Citation2013).
The typical production of kombucha beverage is based on black, green or oolong tea. For its production, 5 g of tea leaves per litre of water may be used, then, sugar is added, which will serve as a substrate for tea fermenting bacteria and yeasts. Approximately 50 g of sugar per litre of water is enough. Before adding the SCOBY or a bit of prepared kombucha, the beverage should be at a temperature close to 20°C. It is extremely important to use sanitized utensils and work in clean areas while making kombucha, in order to have control over the growth of microorganisms and to prevent unwanted contamination (Watawana et al., Citation2015). Likewise, it is important to control pH levels during fermentation of kombucha, and preferably stop this process when a pH level of 4.2 is reached, since the overproduction of acetic acid may be counterproductive (Kovacevic et al., Citation2014). Other food safety methods include pasteurizing the final product to prevent overproduction of alcohol and carbon dioxide, as well as the addition of 0.1% of sodium benzoate and 0.1% of potassium sorbate as food preservatives, and finally, keeping it refrigerated (Watawana et al., Citation2015).
3.1. Fermentation
Kombucha fermentation period is typically known to require a minimum of 3 days to a maximum of 60 days, depending on cultural practices (Watawana et al., Citation2015). The fermentation of kombucha is carried out at room temperature, optimizing fermentation time. Sucrose is used as the main carbon source in a concentration of 5–20%, providing the media and nutrients necessary for microorganism development. A SCOBY or the resulting liquid at a 10% concentration from a previous fermentation may be used as starter culture for fermentation (Vīna, Semjonovs, Linde, & Patetko, Citation2013).
According to Vīna et al. (Citation2013), the variables of the fermentation process, such as time, temperature and sucrose concentration, will determine the final concentration of organic substances such as acids and pH. Organic acids produced during this fermentation process diminished the tea’s pH value, which leads to a lack of oxygen induced by the acidity. Due to this, the number of possible pathogenic microbial cells, if any, diminishes, resulting in a safe beverage for consumption, despite having a microbial origin (Watawana et al., Citation2015).
3.2. SCOBY growth
The culture used for the kombucha fermentation has a variable microbiological composition according to its origin, the weather, geographical location and medium used for the fermentation process (Watawana et al., Citation2015). In kombucha beverage, “the most abundant prokaryotes in the symbiotic culture belong to the Acetobacter and Gluconobacter bacteria genus” (Jayabalan et al., Citation2014). These genus belong to the Acetobacteraceae family (), the bacteria are Gram-negative aerobic bacilli (Stasiak & Blazejak, Citation2009). They can be told apart by their ability to oxidize the acetate anion in carbon dioxide. The strains of the genus Acetobacter produce acetic acid from ethanol. This process is carried out by alcohol dehydrogenase and aldehyde dehydrogenase, enzymes that produce acetic acid, which enters the Krebs cycle obtaining water and carbon dioxide as end products (Teyssier & Hamdouche, Citation2016). The genus Gluconobacter is not capable of oxidizing acetate through the Krebs cycle, since it lacks the enzymes necessary for the oxidation process, like succinate dehydrogenase and alpha-ketoglutarate dehydrogenase, leading to accumulation of products, like gluconate, in the medium (Zoecklein, Fugeslang, Gump, & Nury, Citation1999).
Table 1. Microbiological compounds (bacteria and yeast species) contained in kombucha.
Tabla 1. Compuestos microbiológicos (especies de bacterias y levaduras) contenidos en la kombucha. Referencias: Marsh et al. (Citation2014); Vīna, Semjonovs, Linde, & Patetko (2013); Battikh, Chaieb, Bakhrouf and Ammar (Citation2011).
Moreover, different yeast species can be found in kombucha, which outnumbers the acetic acid bacteria (AAB) (Jayabalan, Malini, Sathishkumar, Swaminathan, & Yun, Citation2010). The enzyme invertase, derived from yeasts, catalyses the hydrolysis of sucrose to glucose and fructose, producing ethanol through the glycolysis pathway. On the other hand, Gluconobacter and Acetobacter bacteria use glucose to produce gluconic acid and ethanol to produce acetic acid (Jayabalan et al., Citation2014). The production of ethanol and acetic acid inhibits the growth of pathogenic bacteria in the kombucha (Dufrense & Farnworth, Citation1999).
The osmophilic yeast and bacteria that are inoculated in the beverage for fermentation are the ones responsible for the growth of what is known as tea fungus, which has the scientific name of Medusomyces gisevii. Using sucrose as a carbon source, the acetic acid bacteria of the tea produce a network of cellulose as a secondary metabolite of fermentation, mainly the bacteria Acetobacter xylinum. The symbiotic mass of bacteria and yeast adheres to the biofilm, forming a thick jelly-like membrane also called zooglea biofilm (Jayabalan et al., Citation2014).
The biofilm of microorganisms remains floating on the surface of the tea with an appearance very similar to a mushroom cap, which is why it usually receives that name (Watawana et al., Citation2015). Illana (Citation2007) mentioned that the growth of this consortium of bacteria and yeasts induces the addition of new thicker membranes that take the shape of their container and heightens the symbiotic effect between bacteria and yeast (). The cellulose membrane keeps the microorganisms on the surface, allowing enough oxygen availability for its development and protecting the microorganisms from UV rays (Suhartatik, Karyantina, Marsono, Rahayu, & Kuswanto, Citation2011).
Several factors play an important role in the concentration of kombucha constituents, one of them is temperature. According to the investigation by Fu, Yan, Cao, Xie, and Lin (Citation2014), keeping kombucha tea refrigerated at 4°C mildly decreases the content of acetic acid bacteria, from 9.3 × 106 CFU/mL to 3.4 × 106 CFU/mL during 14 days of storage; while the content of lactic acid bacteria decreases significantly, from approximately 23.5 × 106 CFU/mL to 2.7 × 103 CFU/mL during 8 days of storage. It has been reported that yeast has a positive impact on the survival of lactic acid bacteria at 30°C, but not at 12°C (Suharja, Henriksson, & Liu, Citation2012), which could mean that the low cooling temperature of 4°C may have limited the positive effect of yeasts over lactic acid bacteria, reducing its survival rate (Fu et al., Citation2014).
Marsh, O’Sullivan, Hill, Ross, and Cotter (Citation2014) reported a sequence analysis of multiple samples of kombucha, in order to provide the most in depth study of microflora to date and to observe the changes occurred in the microbial population during kombucha production. They extracted DNA from cellulosic pellicles from 5 different geographic locations at two fermentation times. Different profiles were detected among samples, however, the major bacteria genus present was Gluconacetobacter (>85%) and a prominent Lactobacillus population was also identified (up to 30%) with a number of sub-dominant genera that have not been detected previously on kombucha. Zygosaccharomyces genus was the yeast found at >95% in the fermented tea and other greater fungal diversity not previously identified.
Jayabalan et al. (Citation2010), analysed the microbiological and chemical composition of the kombucha fungus. Three samples of the tea fungus were used to evaluate its composition in different stages of fermentation, at 7, 14 and 21 days. Fibre and protein were the main components of the SCOBY. In regards to the proteins, a significantly large amount of amino acids was determined, the highest in concentration being the essential amino acids leucine and isoleucine (). In addition, an increase in all the components was observed over fermentation time. Likewise, minerals like sodium, potassium, and magnesium were found.
Table 2. Amino acid content (mg/g dry weight) of tea fungus (SCOBY) at different times of fermentationa.
Tabla 2. Contenido de aminoácidos (mg/g peso seco) de hongos de té (SCOBY) en distintos momentos de la fermentación. Referencia: Jayabalan et al. (Citation2010).
4. Chemical components of black tea and green tea
Tea comes from a leafy perennial crop, from the family Theaceae, known as Camellia sinensis, which was originally harvested in China. The young and tender leaves are used to make different varieties of tea, depending on the process to which it is subjected, resulting in black tea, green tea or oolong tea (González, Citation2003). For the production of black tea, the leaves are crushed and left exposed to high humidity, which causes an enzymatic oxidation by polyphenol oxidases (Valenzuela, Citation2004). To produce green tea, heating methods that inactivate enzymes using steam are utilized, which prevents fermentation (González, Citation2003). The oolong tea is produced by a partially fermented Chinese tea that is oxidized in the range from 10 to 70% (Chen et al., Citation2011) and is made by wilting fresh leaves by sun, then slightly bruising (Weerawatanakorn et al., Citation2015).
Tea has various components, like caffeine, alkaloids, amino acids, carbohydrates, proteins, chlorophyll, fluoride, aluminium, minerals and trace elements (National Cancer Institute, Citation2010). When the leaves are fresh, its flavonol or catechin content is very high. These flavonols, flavonoids derivatives, are characterized by their monomeric structure, and the ones commonly found in tea are epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG) and epigallocatechin gallate (EGCG) (Valenzuela, Citation2004).
It is important to consider that the oxidation process to which black tea is subjected induces a change in the monomeric structure of catechins, resulting in dimeric and polymeric flavonols, known as theaflavins and thearubigins. Therefore, green tea has a lower content of theaflavins and thearubigins due to the fact that it does not go through a fermentation process. For this very reason, teas have a different composition and thus its effect on health varies from one to another (Valenzuela, Citation2004). presents the flavonoid components of tea, both black and green tea. The benefits that have been attributed to tea, both black and green, are mainly due to its catechin content, which is polyphenol derivative. These substances act as potent antioxidants and protect against the development of diseases. The beneficial effects of tea mentioned below have been studied mainly in vitro, while others are based on clinical and epidemiological evidence (Valenzuela, Citation2004).
Table 3. Flavonoid components of black and green tea (dry weight, %).
Tabla 3. Componentes flavonoides de té negro y verde (peso seco, %). Referencia: González (Citation2003).
Tea catechins act as antioxidants, due to the fact that they are molecules with a high capacity to scavenge free radicals and even metals, which is also known as redox potential. This potential is measured by the capacity of a molecule to donate electrons or hydrogen atoms. When metals like iron and copper are found in a free state or not bound to proteins, they possess a pro-oxidant effect, which can damage lipids, proteins, and nucleic acids when they are oxidized. The chelating property of the antioxidants in tea, i.e. its combination with free metals, decreases their likelihood of damaging vital molecules that participate in physiological processes (Valenzuela, Citation2004).
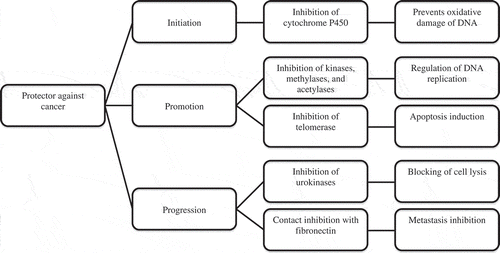
In addition, tea polyphenols have demonstrated great potential in protecting against the development of some types of cancer, by inhibiting enzymes and halting processes that result in the growth of cancer cells. Apoptosis induction of leukemic cells, and of stomach and colon cancer cells, has been observed (Illana, Citation2007). ECG and EGCG are capable of inhibiting kinase, methylase, and acetylase activity, determining processes in the appearance of tumour development should they not be controlled in a cell with DNA damage, for example by the action of an oxidant (Valenzuela, Citation2004).
The process where polyphenols of green tea assist in Phase II of liver detoxification of xenobiotics should be noted. This process requires the tripeptide glutathione (GSH), an endogenous antioxidant with the greatest potential, for the elimination of xenobiotics. GSH is conjugated by enzymes glutathione-S-transferases (GST), which are overexpressed due to green tea polyphenols. As a result, GSH increases, helping the liver in the elimination of xenobiotics, mainly of those that are carcinogenic. Another activity of tea components is their involvement in the initial, developmental, and progressive stages of cancerous diseases () (González, Citation2003).
Figure 1. Protective effect of polyphenols on cancer initiation, promotion, and progression. Adapted from:(Valenzuela, Citation2004).
Figura 1. Efecto protector atribuido a los polifenoles en la iniciación, promoción y progresión del cáncer. Adaptado de: Valenzuela (Citation2004).
Another relevant effect of tea polyphenols is their protection against the development of cardiovascular diseases (CVDs). By inhibiting the oxidation of low-density lipoproteins (LDL), they assist in the prevention of the development of atheroma. They may also be involved in cholesterol metabolism by inhibiting pancreatic lipase, thus decreasing cholesterol and triacylglycerol absorption. Finally, they promote smooth muscle relaxation, preventing high blood pressure induced by vasoconstrictors, such as thromboxanes (González, Citation2003).
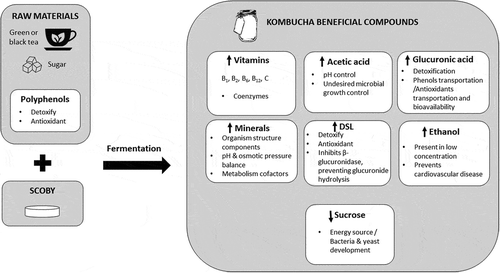
5. Chemical components of kombucha and their beneficial effects
Chemical assays of kombucha beverage have indicated the presence of a variety of compounds, including organic acids, mainly acetic, gluconic, and glucuronic acid (GlcUA), although citric, L-lactic, malic, tartaric, malonic, oxalic, succinic, pyruvic, and usnic acids may also be found; sugars (sucrose, glucose, and fructose), water soluble vitamins (B1, B2, B6, B12, C), amino acids, biogenic amines, purines, pigments, lipids, proteins, hydrolytic enzymes, ethanol, acetic acid bacteria and lactic acid bacteria, carbon dioxide, polyphenols, minerals (manganese, iron, nickel, copper, zinc, plumb, cobalt, chromium, and cadmium), anions (fluoride, chloride, bromide, iodide, nitrate, phosphate, and sulphate), D-saccharic acid-1,4-lactone (DSL), and metabolic products of yeasts and bacteria (Jayabalan, Malbaša, et al., Citation2014, Jayabalan, Malini, et al. Citation2010).
The presence and quantity of the chemical components are variable, mainly depending on the microorganisms of the symbiotic culture used for fermentation of kombucha, as well as fermentation time and temperature, sucrose content, and type of tea used, in addition to the analysis methods used for quantification. Beneficial metabolites produced in kombucha are synthetized in .
5.1. Vitamins
Regarding the vitamin content of this beverage, Bauer-Petrovska and Petrushevska-Tozi (Citation2000) analysed a brew of kombucha beverage prepared with 70 g of sucrose and 5 g/L of black tea, finding the following values of B vitamins: 74 mg/100 mL of vitamin B1, 52 mg/100 mL of vitamin B6 and 84 mg/100 mL of vitamin B12. Meanwhile, Malbaša, Lončar, Vitas, and Čanadanović-Brunet (Citation2011) reported that the content of vitamin B2 was 8.3 mg/100 mL, while the concentration of vitamin C constantly increased, reaching 28.98 mg/L on the tenth day of fermentation.
5.2. Minerals
Minerals are inorganic substances needed in small amounts for normal body functions and growth, as well as for the maintenance of its tissues. According to Bauer-Petrovska and Petrushevska-Tozi (Citation2000), copper, iron, manganese, nickel, and zinc are minerals that increased due to metabolic activity of kombucha. Mineral concentration was in a range of 0.004 μg/mL for cobalt and 0.462 μg/mL for manganese. Besides, traces of lead (0.005 μg/mL) were detected. It is worth noting that, according to the Agency for Toxic Substances and Disease Registry (ATSDR, Citation2007), toxic blood lead levels are 20 μg/dL for adults and 10 μg/dL for children, which is equivalent to 0.2 and 0.1 μg/mL, respectively. Kombucha tea has much lower concentrations, thus not representing a potential health risk. Additionally, Markowitz (Citation2011) mentioned that small amounts of lead in the blood of adults are not harmful.
Nevertheless, it is important to consider that because children are more susceptible to the effects of lead than adults, it would be advisable for children not to drink this beverage on a regular basis, to prevent a chronic exposure that could cause them lead poisoning. Meanwhile, Kumar, Narayan, and Hassarajani (Citation2008) established the presence of fluoride, chloride, bromide, iodide, nitrate, phosphate, and sulphate after seven days of fermentation of kombucha prepared with 100 g of sucrose and 5 g/L of black tea; being fluoride the anion with the highest concentration (3.2 mg/g).
5.3. Polyphenols
Polyphenols are active substances with more than one phenol structural unit per molecule. They represent the largest group of phytochemicals and they are the most abundant antioxidants present in the diet. Total intake of polyphenols can be up to 1 g/day (Scalbert, Johnson, & Saltmarsh, Citation2005). Moreover, they play a role in preventing several diseases related to oxidative stress, such as cancer, CVDs, and neurodegenerative diseases (Manach, Scalbert, Morand, Rémésy, & Jiménez, Citation2004). They modulate the activity of a variety of enzymes and cell receptors as a means of defence against oxidative stress caused by reactive oxygen species (Tsao, Citation2010). Main dietary polyphenol sources include fruits, vegetables, cereals, legumes, natural fruit juices, tea, coffee, and red wine (Scalbert et al., Citation2005).
The protective effect of kombucha beverage is mainly due to polyphenol activity, compounds produced during fermentation, and the synergistic effect of the different compounds found in the tea (Jayabalan, Subathradevi, Marimuthu, Sathishkumar, & Swaminathan, Citation2008). Total polyphenol content in kombucha tea shows a linear increase during fermentation time (Chu & Chen, Citation2006). As an example, both epicatechin (EC) and epigallocatechin (EGC) are found predominantly in the tea (Manach et al., Citation2004). A higher level of EC (~150%) was found on day 12 of fermentation of kombucha made with green tea, and of EGC (~115%) on the same day of one made with black tea (Jayabalan, Marimuthu, & Swaminathan, Citation2007).
A research conducted by Fu et al. (Citation2014) used different types of kombucha to compare free-radical scavenging abilities against 2,2-diphenyl-picrylhydrazyl (DPPH), hydroxyl radicals, and superoxide radicals. Different types of tea were used to prepare kombucha: low-cost green tea, black tea, and tea powder and fermentation process was 90 h. The results showed that kombucha made with green tea had the highest free-radical scavenging ability against DPPH, hydroxyl radicals, and superoxide anions. According to Chu and Chen (Citation2006), black tea reaches its maximum activity against DPPH radicals until day 15 of fermentation. Tea powder had greater antioxidant activity than black tea against DPPH and hydroxyl radicals, but not against superoxide radicals, upon which black tea had a greater activity than tea powder.
5.4. D-saccharic acid-1,4-lactone (DSL)
D-saccharic acid-1,4-lactone (DSL) is a component derived from D-glucaric acid (product of the GlcUA pathway), which has detoxifying and antioxidant properties (Bhattacharya, Gachhui, & Sil, Citation2012; ŻÓłtaszek, Hanausek, Kiliańska, & Walaszek, Citation2008). DSL content in kombucha has been found to range between 57.99 and 132.72 μg/mL, depending on the origin of the product. The highest DSL value was found on the eighth day of fermentation and diminished afterward. It was established that lactic acid bacteria had a positive effect on DSL production, in symbiosis with Gluconobacter genus bacteria (Yang et al., Citation2010).
5.5. Ethanol
According to Chen and Liu (Citation2000), ethanol concentration in kombucha increases with fermentation time, reaching an approximate maximum value of 5.5 g/L on the 20th day of fermentation, followed by a slow reduction.
The entire chemical composition of kombucha beverage, including residual sugar concentration, carbon dioxide, and organic acids, is what finally determines its flavour and depending on the fermentation time, different flavours will be obtained. It has been observed that kombucha tea with higher acetic acid concentration produces a more acid and astringent flavour, meanwhile, another with more gluconic acid produces a milder flavour. Therefore, by controlling fermentation conditions it is possible to obtain the desired quality in kombucha tea (Chen & Liu, Citation2000).
The French paradox is the observation of low coronary heart disease (CHD) death rates despite the high intake of dietary cholesterol and saturated fat. It was proposed by French epidemiologists in the 1980s, particularly for wine drinking in France, where a high intake of dietary cholesterol and saturated fat, daily consumption of wine, but low CHD death rates were observed. The French paradox states that moderate alcohol consumption has a protective effect against CHD, since it raises high-density lipoprotein (HDL) cholesterol concentrations (Ferrières, Citation2004). Since the daily consumption of ethanol in a lower concentration can protect the human body from CVDs, the consumption of kombucha, which has a low concentration of ethanol, could also have a role in preventing CVDs.
5.6. Acetic acid
Acetic acid bacteria in kombucha produce acetic acid when these act on ethanol from sucrose, which is metabolized into glucose and fructose (Spedding, Citation2015). Acetic acid is the chemical compound responsible for the acidic smell and taste of vinegar. Its name comes from the Latin acetum, which means vinegar (Bramforth, Citation2014). Acetic acid tends to slowly increase reaching 11 g/L at 30 days of fermentation, gradually diminishing until ending at 8 g/L at 60 days of fermentation. This decrease is due to its later utilization as a carbon source for bacteria when sugars in the tea are used up, or because of the decrease in ethanol metabolism by yeast due to low pH (Chen & Liu, Citation2000). If a different carbon source is used, such as molasses, the amount of acetic acid produced is considerably lower (Jayabalan et al., Citation2014).
Other important organic acids are contained in kombucha depending on the tea basis. Profiles of organic acids change during fermentation of green and black tea. Besides of acetic acid, lactic and citric acid are produced (Jayabalan et al., Citation2007). Gluconic acid, ethyl-gluconate, oxalic acid, saccharic acid, keto-gluconic, succinic and carbonic acids were considered to be present in kombucha. Some of those acids were proved to have in vitro antimicrobial activity and improve sleep (Greenwalt, Steinkraus, & Ledford, Citation2000; Sreeramulu, Zhu, & Knol, Citation2000). Kombucha is a high source of glucuronic acid, which has a detoxifying effect against drug, bilirubin, and chemicals, as well as pollutants and excess of steroid hormones (Nguyen, Nguyen, Nguyen, & Le, Citation2015). Knowing that glucuronic acid has specific benefits some of its specific properties are described below.
5.7. Glucuronic acid (GlcUA)
GlcUA plays a role in xenobiotic liver detoxification, as it has the ability to combine with toxin molecules to favour their elimination from the organism, which makes it very important as an auxiliary on liver functions. It is as well involved in endobiotic elimination. One of this endobiotics is bilirubin, which is how GlcUA (by means of glucuronidation) prevents these pigment’s toxic effects and impedes inhibition of a variety of enzymes involved in protein and carbohydrate metabolism. Most of the bilirubin is excreted through bile and only a small portion of conjugated bilirubin is excreted through urine, which is why a high level of bilirubin in urine is an indicator of damage somewhere along the process of glucuronidation (Vīna, Linde, Patetko, & Semjonovs, Citation2013).
GlcUA also takes part in polyphenols’ increased bioavailability. Phenols conjugate with GlcUA, improving its transport and bioavailability. The UGT1A isoform of the glucuronosyltransferase family, located in the bowels, is the one that takes part in polyphenol glucuronidation. Polyphenols are secreted via bile into the duodenum, where they are subjected to the activity of β-glucuronidase (which promotes deglucuronidation, separating in this case the polyphenol from GlcUA by hydrolysing its glycosidic bond) and are then reabsorbed. This enterohepatic circulation can lead to a longer presence of polyphenols in the body, where they carry out its antioxidant activity preventing different diseases related to oxidative stress (Vīna et al., Citation2013).
Several steroid hormones and vitamin D derivatives, such as estrogens, androgens, glucocorticoids, mineralocorticoids, progestogens and cholecalciferol are essential for health. They regulate immune functions, decrease inflammatory response, balance extracellular fluid volume, among others (Cutolo et al., Citation2014). Deficiencies and/or excesses of steroid hormones have undesirable effects on health. Glucuronidation can prevent both scenarios: it prevents deficiencies by increasing steroid water solubility, therefore improving its transport and bioavailability; and prevents excesses by facilitating the elimination of excess steroids (Vīna, Linde, et al., Citation2013, Vīna, Semjonovs, et al., Citation2013).
As a constituent of glucuronidation, GlcUA is also important for biotransformation and protection of fatty acids from lipid peroxidation. Especially, polyunsaturated fatty acids, which are essential compounds for the body, essential components of cell membranes and precursors of eicosanoids. Polyunsaturated fatty acids are susceptible to react with reactive oxygen species, triggering chain reactions that damage the fatty acid molecule. Peroxidation has been considered a risk factor for the development of certain pathologies, such as atherosclerosis, kidney damage and Parkinson’s disease (Mylonas & Kouretas, Citation1999).
Kombucha consumption prevents polyunsaturated fatty acid oxidation in the human body, due to its GlcUA content that takes part in glucuronidation, increasing polyphenol bioavailability, which neutralizes free radicals that promote lipid peroxidation (Jayabalan et al., Citation2008).
GlcUA is necessary for many body functions since it is a constituent of various essential polysaccharides in the body, glycosaminoglycans (GAGs). GAGs are a series of compounds formed by dimers consisting of an amino sugar (D-Glucosamine or D-Galactosamine) and a uronic acid (D-glucuronic acid or L-iduronic acid), except for keratan sulphate which contains a galactose molecule instead of an acid. They are bound to sulphate groups in variable proportions and covalently bind to proteins, forming proteoglycans, except for hyaluronic acid which is not sulphated (Mylonas & Kouretas, Citation1999). Different GAGs are formed depending on the constituents of the dimer.
Glycosaminoglycans molecules are part of the extracellular matrix of all the body organs and they have multiple functions. GlcUA is part of the following GAGs: hyaluronic acid, chondroitin sulphate, heparin and dermatan sulphate. They all have structural functions, except for heparin, which is a non-structural GAG. Hyaluronic acid serves as a lubricant and shock absorber, and it is present in higher concentrations in the vitreous fluid in the eye, conjunctive tissue, synovial fluid of joints and cartilage (Frati-Munari, Citation2012; Vīna et al., Citation2013).
Chondroitin sulphate is mostly present in bones and cartilage, in the latter it binds to collagen and keeps fibres in a strong network; it also helps to prevent joint problems, being helpful in the relief of osteoarthritis. Heparin is an intracellular compound and a potent anticoagulant produced by mast cells. Dermatan sulphate is present in higher concentrations in the vascular endothelium, connective tissue, cartilage, skin, cornea, and bones. L-iduronic acid is the predominant uronic acid present in dermatan sulphate, and it is an epimer of D-glucuronic acid, although a variable amount of β-D-glucuronic acid is also present (Frati-Munari, Citation2012; Vīna et al., Citation2013).
Moreover, GlcUA is a precursor of L-ascorbic acid (vitamin C) in kombucha beverage, since it is synthesized from L-gulonic acid, which is involved in the metabolic pathway of GlcUA. Thus, GlcUA concentration in kombucha increases its antioxidant activity as well (Vīna et al., Citation2013).
Actually, there are published data describing the similarity of GlcUA contained in kombucha and the acid produced in the human body by metabolic pathways. Preliminary studies carried out by Jayabalan et al. (personal communication) did not find similarity. As previously discussed, the concentration and presence of GlcUA in kombucha tea is basically determined by the strains and species that acts by symbiosis. Meaning that the GlcUA concentration in kombucha is exhibited due to the specific SCOBY microorganisms.
6. Other reported beneficial effects of kombucha
Researchers and testimonials of individuals, which mention having consumed this drink, declare beneficial effects for human health. Aloulou et al. (Citation2012) evaluated the suppressing effect of α-amylase enzyme (secreted by intestinal epithelium and necessary for carbohydrate digestion) in diabetic rats (aloxan induced), which were administered 5 mL/kg of kombucha or black tea daily during 30 days. The results showed that the rats which drank kombucha had a better suppressing effect of α-amylase enzyme in pancreas and plasma, as well as postprandial glucose compared to those of the rats which drank black tea. Besides glucose metabolism disorders, pancreatic and plasma enzymatic changes were also evaluated.
These enzymes “act on triacylglycerol to metabolize it into free fatty acids and monoacylglycerol”, an abnormal increase can be caused by pancreatic damage (Sastre, Sabater, & Aparisi, Citation2005). In the study, the rats administered aloxan, presented damage in pancreatic structure, more than that of the rats from the control group or those treated with kombucha. Aloxan increases reactive oxygen species (ROS), producing toxicity in pancreatic cells. An increase in pancreatic and plasmatic lipase concentration causes an increase in lipid absorption, which leads to an increase of triacylglycerol and low density proteins. The group treated with kombucha had a significant reduction of pancreatic and plasmatic lipase. The group treated with black tea had a decrease on both enzymes as well, but the decrease was lower than in the group consuming kombucha.
One more scientific support carried out by Kabiri, Setorki, and Ahangar (Citation2013), a study which determines the protective effects of kombucha beverage and silymarin (milk thistle) in rats with liver damage induced by thioacetamide (hepatic fibrosis related toxin). In the study, 36 rats were divided into 6 groups, where group 1 was designated as control group. Group 2 was integrated by rats injected with thioacetamide; group 3 included the rats injected with thioacetamide and later treated with kombucha (50 mL/during 3 weeks); group 4 included rats treated with kombucha (50 mL/during 3 weeks) and later injected with thioacetamide; group 5 included rats injected with thioacetamide and later treated with silymarin (200 mg/kg during 3 weeks); and group 6 included rats injected with thioacetamide and later treated with kombucha (50 mL/per rat) and silymarin (400 mg/kg) during 3 weeks.
The results showed that the group treated with silymarin had a significant descent in the previously mentioned parameters with exempt of bilirubin. This same situation happened with the group treated with kombucha tea and silymarin. The protective action of both foods is ought to their polyphenol component, which protect the liver against free radical formation which can produce hepatocyte malfunction and liver damage.
Deghrigue, Chriaa, Battikh, Abid, and Bakhrouf (Citation2013), evaluated kombucha’s antiproliferative properties, prepared with black or green tea, fermented during 12 days, on two human cancer cell lines (A549, lung cell carcinoma and Hep-2, epidermoid cell carcinoma). The cells were incubated in 96 microtitre plates for 24 h, and later were added kombucha beverage, previously centrifuged. Concentrations varied from 50–400 μg/mL, in order to determine IC50 (unit of measurement for a substance’s effectiveness to inhibit a biological process a 50% or greater).
The results showed that kombucha elaborated with green tea had a greater cytotoxic effect. The 50% of inhibition was attained at concentrations from 200 to 250 μg/mL on the cell lines A549 and Hep-2. On the other hand, kombucha beverage elaborated with black tea showed a moderate cytotoxic activity; the concentrations required to inhibit 50% of cellular growth were larger compared to that of green tea based kombucha, 386 μg/mL, and it only had an effect on Hep-2 cell lines.
Recently, Vázquez-Cabral et al. (Citation2017) used oak leaves infusions instead of black tea as a common substrate for preparing kombucha. They reported a significantly reduction on the levels of pro-inflammatory cytokines IL-6 and TNF-alpha. Besides, phytochemical compounds contained in the fermented beverage decreased oxidative stress.
Regarding on the increase in phenolic compounds and antioxidant activities of kombucha, Sun, Li, and Chen (Citation2015) elaborated the beverage with mixes in various ratios of sweetened black tea and wheatgrass juice. The highest antioxidant activity was obtained using a 1:1 (v/v) black tea decoction to wheat grass juice ratio and 3 days of fermentation. Under those processing conditions, this beverage produced various types of complementary phenolic acids with antioxidant effect, fact that was considered by the authors as an advantage over traditional kombucha beverage.
7. Contraindications of kombucha
Toxicity caused by kombucha has been suspected in several cases, reporting dizziness and nausea after consumption. Lead poisoning and gastrointestinal toxicity was found on two individuals after drinking the fermented beverage during a period of six months, however it was stated that the contaminant came from the enamel pigment on the vase containing the kombucha (Jayabalan et al., Citation2014).
Jayabalan et al. (Citation2014) described a case of acute kidney failure with lactic acidosis and hyperthermia after ingesting the beverage, as well as the presence of Bacillus anthrax, Penicillium and Aspergillus present in kombucha prepared under unhygienic conditions. Two cases were reported were metabolic acidosis was related to an excessive consumption of kombucha beverage (>12 oz. daily). It was later established that these cases suffered from certain conditions which made them vulnerable to developing acidosis (Nummer, Citation2013), as HIV and acute kidney failure (Sunghee Kole, Jones, Christensen, & Gladstein, Citation2009). According to the Centers for Disease Control and Prevention (CDC, Citation1994), the daily consumption of 4 oz. of kombucha does not present a risk for the consumer’s health.
As regards to pregnant women, it is contraindicated as a security parameter due to the possible heparin content (glycosaminoglycan component) in the tea, as it inhibits blood clotting system’s proteins and thins it, being harmful during the third trimester of pregnancy. It must be mentioned that authors claim heparin presence has not been proved in analysed samples; however, consuming the drink may favour its production in the organism, which is why caution must be taken (Rubio Delgado, Citation2015).
On the other hand, it has been proved the tea has potential to revert hepatic toxicity induced by CCl4 (carbon tetrachloride), a liquid which transforms into gas at room temperature. It comes from aerosol and certain refrigerants production and can cause adverse health effect and hepatic toxicity if recommended dose is exceeded (Agency for Toxic Substances and Disease Registry [ATSDR], Citation2005; Kovacevic et al., Citation2014). Four HIV positive patients reported secondary effects related to its consumption, such as allergic reaction, ictericia, nausea, vomit, neck pain and headache. However, kombucha cannot be declared toxic for human health due to the presented evidence not being substantial for the affirmation of its toxicity or the disease occurrence in previous studies (Jayabalan et al., Citation2014).
Some harmful effects of kombucha consumption have been described by several authors (Greenwalt et al., Citation2000). Organs internal lesions on rats after 12 weeks of kombucha consumption were reported and it was concluded that the susceptibility to toxicity depends on the specie (Ibrahim, Kwanashie, Njoku, & Olurinola, Citation1993). Developing of acidosis was described in individuals having severe pre-existing conditions. One of them increased the fermentation time from 7 to 14 days which produces a very acidic beverage (CDC, Citation1995). High acidity and microbial contamination of kombucha was reported as a warning for possible illness (Perry, Citation1995), and mycotoxigenic substances (as secondary metabolites) have health repercussions including toxic and carcinogenic effects.
6. Conclusions
Kombucha beverage is a source of bioactive components, such as polyphenols and glucuronic acid. The beneficial outcomes of kombucha consumption are attributed to the synergistic effect between these components, making it a drink with potential beneficial health properties (when elaborated under adequate sterile conditions). It is apparent that its consumption can protect against the development of CVDs, mainly due to its polyphenol content that inhibits the oxidation of LDL, regulates cholesterol metabolism, and prevents high blood pressure by promoting smooth muscle relaxation. GlcUA, one of its main components, plays a role in xenobiotic liver detoxification and endobiotic elimination, thus potentially enhancing liver functions. It must be emphasized that concentration of the drink’s active components will vary depending on the scoby and elaboration methods. Health effects on humans under controlled research are merited, because some contraindications have been reported.
Acknowledgements
We acknowledge the support of Vanessa Anahí Cantú Hernández and Alejandra Karina González Ruiz. This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Agency for Toxic Substances and Disease Registry. (2005). Carbon tetrachloride. Retrieved July 12, 2017, from https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=35
- Agency for Toxic Substances and Disease Registry. (2007). Public health statement lead. U.S. Departament of Health and Human Services, Public Health Service. Retrieved June 11, 2017, from https://www.atsdr.cdc.gov/ToxProfiles/tp13-c1-b.pdf
- Aloulou, A., Hamden, K., Elloumi, D., Ali, M. B., Hargafi, K., Jaouadi, B., … Ammar, E. (2012). Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complementary and Alternative Medicine, 16, 12–63.
- Battikh, H., Chaieb, K., Bakhrouf, A., & Ammar, E. (2011). Antibacterial and antifungal activities of black and green kombucha teas. Journal of Food Biochemistry, 37, 231–236.
- Bauer-Petrovska, B., & Petrushevska-Tozi, L. (2000). Mineral and water-soluble vitamin contents in the kombucha drink. International Journal Food Sciences Technical 35, 201–205.
- Bhattacharya, S., Gachhui, R., & Sil, P. C. (2012). The prophylactic role of D-saccharic acid-1,4-lactone against hyperglycemia-induced hepatic apoptosis via inhibition of both extrinsic and intrinsic pathways in diabetic rats. Food & Function, 4, 283–296.
- Bramforth, C. W. (2014). Fermented beverages. Reference module in food science. Encyclopedia of agriculture and food systems. Kidlington, Oxford: Elsevier Inc.
- Centers for Disease Control and Prevention, CDC. (1995) Unexplained severe illness possibly associated with consumption of kombucha tea—Iowa. Morbidity and Mortality Weekly Report 44:892–900
- Chen, C., & Liu, B. Y. (2000). Changes in major components of tea fungus metabolites during prolonged fermentation. Journal of Applied Microbiology 89, 834–839.
- Chen, Y. L., Duan, J., Jiang, Y. M., Shi, J., Peng, L., Xue, S., & Kakuda, Y. (2011). Production, quality, and biological effects of oolong tea (Camellia sinensis). Food Reviews International, 27, 1–15.
- Chu, S., & Chen, C. (2006). Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chemistry, 98, 502–507.
- Crowe, K. M., & Francis, C. (2013). Position of the academy of nutrition and dietetics: Functional foods. Journal of the Academy of Nutrition and Dietetics, 113, 1096–1103.
- Cutolo, M., Paolino, S., Sulli, A., Smith, V., Pizzorni, C., & Seriolo, B. (2014). Vitamin D, steroid hormones, and autoimmunity. Annals NewYork Academic Sciences, 1317, 39–46.
- Deghrigue, M., Chriaa, J., Battikh, H., Abid, K., & Bakhrouf, A. (2013). Antiproliferative and antimicrobial activities of kombucha tea. Academic Journal, 7, 3466–3470.
- Dufrense, C., & Farnworth, E. (1999). Tea, Kombucha, and health: A review. Food Research International, 33, 409–421.
- Ferrières, J. (2004). The French paradox: Lessons for other countries. Heart, 90, 107–111.
- Frati-Munari, A. C. (2012). Glicosaminoglicanos en las enfermedades vasculares. Reviews Mex Angiol, 40, 89–99.
- Fu, C., Yan, F., Cao, Z., Xie, F., & Lin, J. (2014). Antioxidant activities of kombucha prepared from three different substrates and changes in content of probiotics during storage. Food Sciences Technology-Brazil, 34, 123–126.
- González, E. (2003). The chemo-preventive effect of tea and its components. Archivos Latinoamericanos De Nutrición, 53, 111–118.
- Greenwalt, C. J., Steinkraus, K. H., & Ledford, R. A. (2000). Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. Journal of Food Protection, 63, 976–981.
- Ibrahim, N. D. G., Kwanashie, H. O., Njoku, C. O., & Olurinola, P. E. (1993). Screening of ’Kargasok Tea’ IV: Studies of pathological effects in BALB/C mice and Wistar rats. Veterinary and Human Toxicology, 35, 399–402.
- Illana, C. (2007). El hongo kombucha. Boletín De La Sociedad Micológica De Madrid, 31, 269–272.
- Jayabalan, R., Malbaša, R. V., Lončar, E. S., Vitas, J. S., & Sathishkumar, M. (2014). A review on kombucha tea—Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Comprehensive Reviews in Food Science and Food Safety, 1, 538–550.
- Jayabalan, R., Malini, K., Sathishkumar, M., Swaminathan, K., & Yun, S. E. (2010). Biochemical characteristics of tea fungus produced during Kombucha fermentation. Food Science and Biotechnology, 19, 843–847.
- Jayabalan, R., Marimuthu, S., & Swaminathan, K. (2007). Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chemistry, 102, 392–398.
- Jayabalan, R., Subathradevi, P., Marimuthu, S., Sathishkumar, M., & Swaminathan, K. (2008). Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chemistry, 109, 227–234.
- Kabiri, N., Setorki, M., & Ahangar, M. (2013). Protective Effects of kombucha tea and silimarin against thioacetamide induced hepatic injuries in wistar rats. World Applied Sciences Journal, 27, 524–532.
- Kaur, N., & Singh, P. D. (2017). Deciphering the consumer behaviour facets of functional foods: A literature review. Appetite, 112, 167–187.
- Kovacevic, Z., Davidovic, G., Vuckovic-Filipovic, J., Janicijevic-Petrovic, M., Janicijevic, K., & Popovic, A. (2014). A toxic hepatitis caused the kombucha tea – Case report. Macedonian Journal of Medical Sciences, 7, 128–131.
- Kumar, S. D., Narayan, G., & Hassarajani, S. (2008). Determination of anionic minerals in black and kombucha tea using ion chromatography. Food Chemistry, 111, 784–788.
- Malbaša, R. V., Lončar, E. S., Vitas, J. S., & Čanadanović-Brunet, J. M. (2011). Influence of starter cultures on the antioxidant activity of kombucha beverage. Food Chemistry, 127, 1727–1731.
- Manach, C., Scalbert, A., Morand, C., Rémésy, C., & Jiménez, L. (2004). Polyphenols: Food sources and bioavailability. The American Journal of Clinical Nutrition, 79, 727–747.
- Markowitz, M. (2011). Lead poisoning. Nelson textbook of pediatrics. Philadelphia, PA: Elsevier Saunders.
- Marsh, A. J., O’Sullivan, O., Hill, C., Ross, R. P., & Cotter, P. D. (2014). Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiology, 38, 171–178.
- Mylonas, C., & Kouretas, D. (1999). Lipid peroxidation and tissue damage. In Vivo, 13, 295–309.
- National Cancer Institute. (2010). Tea and cancer prevention. Retrieved June 11, 2017, from https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/tea-fact-sheet
- Nguyen, N. K., Nguyen, P. B., Nguyen, H. T., & Le, P. H. (2015). Screening the optimal ratio of symbiosis between isolated yeast and acetic acid bacteria strain from traditional kombucha for high-level production of glucuronic acid. LWT- Food Sciences Technological, 64, 1149–1155.
- Nummer, B. A. (2013). Kombucha brewing under the food and drug administration model Food Code: Risk analysis and processing guidance. Journal of Environmental Health, 76, 8–11.
- Perry, N. (1995). Culture shock. Emergency Medical Services, 24, 35–36.
- Rubio Delgado, A. (2015) Té de Kombucha y sus beneficios para el sistema digestivo (Dissertation). Universidad Particular Equinoccial
- Sastre, J., Sabater, L., & Aparisi, L. (2005). Fisiología de la secreción pancreática. Gastroenterol Hepatol, 28, 3–9.
- Scalbert, A., Johnson, I. T., & Saltmarsh, M. (2005). Polyphenols: Antioxidants and beyond. The American Journal of Clinical Nutrition, 81, 215S–217S.
- Shimizu, M. (2012). Functional food in Japan: Current status and future of gut-modulating food. Journal Food Drug Analysis, 20, 213–216.
- Spedding, G. (2015) So what is kombucha? An alcoholic or a non-alcoholic beverage? A brief selected literature review and personal reflection. Brewing and Distilling Analytical Services, LLC. BDAS, LLC White Paper No. 2. Retrieved June 11, 2017, from http://alcbevtesting.com/wp-content/uploads/2015/06/WhatIsKombucha_BDASLLC_WPSPNo2_Oct-4-2015.pdf.
- Sreeramulu, G., Zhu, Y., & Knol, W. (2000). Kombucha fermentation and its antimicrobial activity. Journal of Agricultural and Food Chemistry, 48, 2589–2594.
- Stasiak, L., & Blazejak, S. (2009). Acetic Acid Bacteria, perspectives of application in biotechnology, a review. Polish Journal of Food and Nutrition Sciences, 59, 17–23.
- Suharja, A. S., Henriksson, A., & Liu, S. Q. (2012). Impact of saccharomyces cerevisiae on viability of probiotic Lactobacillus rhamnosus in fermented milk under ambient conditions. Journal of Food Processing and Preservation, 10, 1–12.
- Suhartatik, N., Karyantina, M., Marsono, Y., Rahayu, E. S., & Kuswanto, K. R. (2011) Kombucha as anti hypercholesterolemic agent (in Vitro Study using SD rats). Proceedings of the 3rd International Conference of Indonesian Society for Lactic Acid Bacteria (3rd IC-ISLAB): Better Life with Lactic Acid Bacteria: Exploring Novel Functions of Lactic Acid Bacteria, Yogyakarta, Indonesia
- Sun, T. Y., Li, J. S., & Chen, C. (2015). Effects of blending wheatgrass juice on enhancing phenolic compounds and antioxidant activities of traditional kombucha beverage. Journal Food Drug Analysis, 23, 709–718.
- Sunghee Kole, A., Jones, H. D., Christensen, R., & Gladstein, J. (2009). A case of Kombucha tea toxicity. Journal of Intensive Care Medicine, 24, 205–207.
- Teyssier, C., & Hamdouche, Y. (2016). Acetic acid bacteria: Prospectives applications in food biotechnology. Northwestern, FL: CRC Press.
- Tsao, R. (2010). Chemistry and biochemistry of dietary polyphenols. Nutrients, 2, 1231–1246.
- Tur, J. A., & Bibiloni, M. M. (2016). Functional foods. Reference module in food science. Encyclopedia of food and health. Kidlington, Oxford: Elsevier B.V. doi:10.1016/B978-0-12-384947-2.00340-8
- Valenzuela, A. (2004). El consumo de té y la salud: Características y propiedades benéficas de esta bebida milenaria. Revista Chilena De Nutrición, 31, 72–82.
- Vázquez-Cabral, B. D., Larrosa-Pérez, M., Gallegos-Infante, J. A., Moreno-Jiménez, M. R., González-Laredo, R. F., Rutiaga-Quiñones, J. G., & Gamboa-Gómez, C. I. (2017). Oak kombucha protects against oxidative stress and inflammatory processes. Chem-Biol Interact, 272, 1–9.
- Vīna, I., Linde, R., Patetko, A., & Semjonovs, P. (2013). Glucuronic acid from fermented beverages: Biochemical functions in humans and its role in health protection. International Journa of Recent Research and Applied Studies, 14, 17–25.
- Vīna, I., Semjonovs, P., Linde, R., & Patetko, A. (2013). Glucuronic acid containing fermented functional beverages produced by natural yeasts and bacteria associations. International Journal of Recent Research and Applied Studies, 14, 17–25.
- Watawana, M. I., Jayawardena, N., Gunawardhana, C. B., & Waisundara, V. Y. (2015). Health, wellness, and safety aspects of the consumption of kombucha. Journal Chem-NY, 1, 1–11.
- Weerawatanakorn, M., Hung, W. L., Pan, M. H., Li, S., Li, D., Wan, X., & Ho, C. T. (2015). Chemistry and beneficial effects of oolong tea and theasinensins. Food Science and Human Wellness, 4, 133–146.
- Yang, Z., Zhou, F., Ji, B., Luo, Y., Yang, L., & Li, T. (2010). Symbiosis between microorganisms from kombucha and kefir: Potential significance to the enhancement of kombucha function. Applied Biochemistry and Biotechnology, 160, 446–455.
- Zoecklein, W., Fugeslang, K., Gump, B., & Nury, F. (1999). Wine analysis and production. Nueva York, USA: Aspen Publishers.
- ŻÓłtaszek, R., Hanausek, M., Kiliańska, Z., & Walaszek, Z. (2008). The biological role of D -glucaric acid and its derivatives: Potential use in medicine. Postepy Higieny I Medycyny Doswiadczalnej, 62, 451–462.