?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bioactive compounds are extracted from natural sources and they have beneficial effects on human health. Fruits and vegetables are rich in carotenoids, phenolic compounds, Vitamin C, among others. Extraction processes for these compounds depend on several factors such as the technique that is used, the raw material, and the organic solvent. Conventional techniques generally require large amounts of organic solvents, high energy expenditure, and are time consuming, which has generated interest in new technologies that are referred to as clean or green technologies. These can reduce or eliminate the use of toxic solvents, and thus preserve the natural environment and its resources. The aim of this review is to discuss recent techniques used to extract bioactive compounds from natural sources, in order to reduce the economic and ecological impact of these processes.
RESUMEN
Los compuestos bioactivos son extraídos de fuentes naturales y tienen efectos beneficiosos en la salud humana. Las frutas y verduras son ricas en carotenoides, compuestos fenólicos, vitamina C, así como en otras sustancias. Los procesos para extraer estos compuestos dependen de varios factores: la técnica utilizada, la materia prima y el solvente orgánico, entre otros. Además de ocupar mucho tiempo, las técnicas convencionales generalmente requieren grandes cantidades de solventes orgánicos y elevados gastos energéticos: ello ha generado interés en el uso de tecnologías nuevas, llamadas limpias o verdes. En tanto éstas pueden reducir o eliminar el uso de solventes tóxicos, permiten conservar el medio ambiente y sus recursos. El propósito de la presente revisión es examinar las técnicas empleadas recientemente para extraer compuestos bioactivos de fuentes naturales, con el fin de reducir el impacto económico y ecológico que conllevan estos procesos.
1. Introduction
Fruits and vegetables are fundamental foods for human health because they provide different flavors and are associated with improved quality of life. The consumption of vegetables and fruit is inversely associated with the development of cardiovascular diseases, and tends to be linked with protection from the major diet-related chronic diseases (Fardet & Boirie, Citation2014; da Silva, Barreira, & Oliveira, Citation2016).
Factors such as protection against diseases are related to the components of fruits and vegetables, particularly phenolic compounds, carotenoids, and vitamins, which are products of the secondary metabolism of plants (Sricharoen, Limchoowong, Techawongstien, & Chanthai, Citation2016). Bioactive compounds are found in foods, both of natural and synthetic origin, and they have specific metabolic or physiological actions provided that their safety for human consumption is proven (BRASIL, Citation2002). The levels of bioactive compounds in fruits depend on factors such as the cultivar, the growing conditions, storage, and transport conditions (Bennett et al., Citation2011).
Bioactive compounds can be used as food additives due to their antioxidant properties. Antioxidants are substances that reduce oxidative stress in foods. Synthetic antioxidants are widely used because of their stability and their widespread availability; however, they are related to mutagenic and carcinogenic effects and this has led to the search for antioxidants extracted from plant matrices (Soquetta et al., Citation2016).
The extraction of bioactive compounds depends on several factors, such as the extraction technique, raw materials, and the extraction solvent that are used (Tiwari, Citation2015). The techniques can be classified into conventional or non-conventional. Conventional techniques require the use of organic solvents, temperature, and agitation. Examples of this type of technique include Soxhlet, maceration, and hydrodistillation. Modern techniques, or non-conventional techniques, are green or clean techniques due to reduced use of energy and the implementation of organic solvent, which are beneficial in relation to the environment (Rodriguez Perez et al., Citation2015).
Many studies have discussed the use of green technologies in relation to food processing (Barba, Zhu, Koubaa, de Souza Sant’Ana, & Orlien, Citation2016; Boussetta; Vorobiev, Citation2014; Chemat et al., Citation2017a; Chemat et al., Citation2017b; Mustafa, Turner, Citation2011; Soliva-Fortuny, Balasa, Knorr, & Martín-Belloso, Citation2009). However, it was not possible to find a review in the literature which covers the various techniques in the same scientific paper, and which also suggests different extraction techniques according to the target biocompounds.
The aim of this paper was to discuss applications using non-conventional energy sources, such as supercritical extraction, pressurized liquid, assisted ultrasound, microwave, pulsed electric field, electric high-voltage discharges, and high hydrostatic pressure, in the extraction of bioactive compounds from fruit and vegetables.
2. Conventional techniques
The main conventional extraction techniques in relation to bioactive compounds are Soxhlet, maceration, and hydrodistillation.
The Soxhlet technique involves a small amount of dry sample, which is placed on the equipment where the solvent passes through. The process is performed repeatedly until the extraction is complete. This extraction system is optimized and the literature provides a vast amount of practical examples of favorable conditions (Cravotto et al., Citation2011; Xhaxhiu, Korpa, Mele, & Kota, Citation2013). However, this technique requires extensive extraction time and large amounts of solvent (Heleno et al., Citation2016).
Maceration consists of grinding the sample into smaller particles so as to increase the surface area for a good mixture with the solvent. The agitation in the maceration process makes extraction easier in two ways: by increasing the diffusion and by removing the concentrated solution from the surface of the sample. This process has been used for a long time to obtain essential oils and bioactive compounds (Azmir et al., Citation2013).
Ćujić et al. (Citation2016) used the conventional method to extract polyphenols from dried chokeberry (Aronia melanocarpa) fruit. The effects of various parameters in the extraction of total phenolics and anthocyanins were studied. The solvents, particle size, solid-solvent ratio, and extraction time were investigated as independent variables in two factor levels. The aforementioned study indicated that the steeping was effective and was a simple technique for the extraction of bioactive compounds from chokeberry fruit.
Hydrodistillation is performed with distilled water and is used to extract the volatile fraction in foods; this method usually takes 6–8 h and organic solvents are not involved. This technique involves three main physicochemical processes: hydrodiffusion, hydrolysis, and decomposition by heat. High temperatures during extraction can degrade compounds, which limits the use of this technique (Wu, Wang, Liu, Zou, & Chen, Citation2015). Hydrodistillation is a very complete process: volatile organic compounds and non-volatile organic compounds can be extracted and physically separated in one step. The volatile organic compounds are stripped from the matrix by azeotropic distillation; they are then condensed, collected, and separated in a Florentine flask. The soluble non-volatile organic compounds are extracted in the boiling water, which is in contact with the matrix inside the alembic. However, hydrodistillation consumes high levels of energy and is time consuming (Petigny et al., Citation2014).
The efficiency of conventional extraction methods depends on the choice of solvent and the polarity of the compound, since solvents of different polarities are needed for identification and isolation. The polarities of compounds vary and it is difficult to develop a single method for the efficient extraction of all compounds. A good solvent provides low toxicity, a low boiling point, quick mass transfer, preservative action, and the inability to make the complex extract dissociate. The yield and the amount of the extract obtained also depend on several other factors such as the type of extract, the temperature, and the extraction time (Silva, Rock-Santos and Duarte, Citation2016).
3. Green technologies
Security risks, such as the toxicity of solvents and the presence of solvent residues in the extracts, together with low yield, have stimulated the development of other extraction technologies, such as clean or green technologies, which can minimize or eliminate the use of organic solvents. These techniques are also known as cold extraction techniques, where the stability of the extracted compounds is not affected and the energy required for extraction is reduced (Tiwari, Citation2015).
According to Jacotet-Navarro et al. (Citation2016) the objective of these green extraction processes is to achieve a faster extraction rate, more effective energy use, increased mass and heat transfer, reduced equipment size, and a reduction in the number of processing steps.
The application of these technologies is also intended to preserve the natural environment and its resources (Mustafa & Turner, Citation2011; Silva et al., Citation2016).
Some authors have sought to define the main points or elementary principles of green chemistry. Basically, the following 12 factors need to be considered in the implementation of green chemistry: prevention (avoiding waste); economy of atoms (maximizing the incorporation of all the starting materials in the final product); the synthesis of less hazardous products (little or no toxicity in relation to human health); safe product design (which performs the desired function and at the same time is non-toxic); safer solvents and auxiliaries; the search for energy efficiency (reduced environmental and economic impacts); the use of raw materials from renewable sources; the prevention of the formation of derivatives; catalysis (reagent as selective as possible); design for degradation (innocuous degradation products which do not persist in the environment); real-time analysis for the prevention of pollution; and chemistry that is intrinsically safe in order to prevent accidents (Lenardão, Freitag, Dabdoub, Batista, & Silveira, Citation2003).
A number of new alternatives to conventional techniques have been proposed to extract target compounds from various matrices ().
Table 1. Green technologies for the extraction of bioactive compounds in fruits and vegetables.
Tabla 1. Tecnologías verdes para la extracción de compuestos bioactivos en frutas y verduras.
shows how the extraction of bioactive compounds using green technologies has been studied.
Table 2. Extraction of bioactive compounds through green technologies.
Tabla 2. Extracción de compuestos bioactivos mediante tecnologías verdes.
3.1. Supercritical fluid extraction
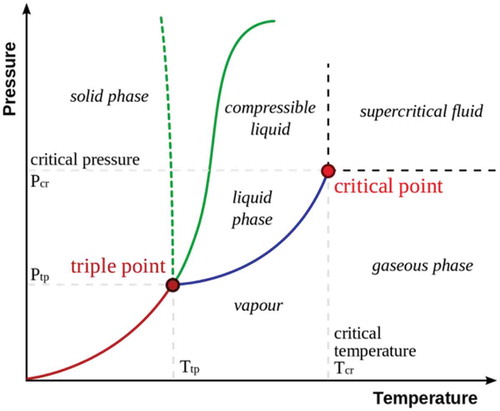
Supercritical extraction is characterized by changes in temperature and pressure, which transforms the gas in the supercritical fluid, where the gas and liquid phases are indistinguishable. As shown in , the critical temperature is considered to be the maximum temperature at which gas can be converted into liquid by increasing the pressure, and the critical pressure is the maximum pressure at which the liquid can be converted to gas by increasing the temperature.
It is a mass transfer operation, in which the convection in the supercritical solvent phase is generally the main transport mechanism (Silva & Martínez, Citation2014). The extraction is rapid, selective, and does not require further cleaning; furthermore, it can be performed on small amounts of sample (Oroian & Escriche, Citation2015). Another great advantage is the possibility of direct association with analytical chromatographic techniques such as gas chromatography (GC) and supercritical fluid chromatography (SFC) (Silva-Santos Rocha and Duarte, Citation2016).
This technique can be summarized in two steps: the solubilization of the chemical compounds present in the solid matrix, followed by their separation in the supercritical solvent. The solvent flows through the packed bed and solubilizes compounds that are present in the matrix. The solvent subsequently leaves the extractor and by a reduction in pressure and an increase in temperature it becomes a solvent-free extract (Silva et al., Citation2016).
Supercritical fluids exhibit desirable transport properties that enhance their ability to adapt. Compared to liquid solvent, which is used in conventional extraction processes, supercritical fluids have low viscosity, spreading more easily within the solid matrix, as well as low surface tension, which allows rapid penetration of the solvent into the solid and, consequently, increased extraction efficiency. Since density is related to solubility, by changing the extraction pressure the force of the fluid can be modified (Pouliot, Conway, & Leclerc, Citation2014).
Supercritical extraction is primarily used to isolate non-polar bioactive compounds (carotenoids and lipids) due to the fact that the solvents used in this technique are of this nature. One option for the extraction of polar compounds, such as flavonoids, is the addition of modifiers such as ethanol, methanol, water, and acetone (Herrero, Castro-Puyana, Mendiola, & Ibañez, Citation2013).
The solubility of the extracts depends on the density of the solvent. Thus, the following different supercritical fluids have been described in studies: CO2; propane; cooking gas (LPG); ethane; ethene; methanol; nitrous oxide; n-butene; n-pentene; sulfur hexafluoride; and water (Silva, Rocha- Santos and Duarte, Citation2016).
3.1.1. CO2 as a supercritical fluid
Carbon dioxide (CO2) is used as a supercritical fluid for three main reasons: it is harmless to human health and the environment, thereby respecting sustainability factors; its critical temperature is moderate (31.2 °C), which is a fundamental issue for the preservation of bioactive compounds in extracts; and the extract is preserved from contact with the air and light, where oxidation reactions may occur. Furthermore, it is also possible to modulate the power of CO2 in order to perform a selective extraction (Silva-Santos; Rocha and Duarte, Citation2016, Brunner, Citation2005).
The extraction of polar compounds, such as most phenolic compounds, requires the presence of polar solvents used as modifiers due to the non-polar nature of CO2 (Meneses, Caputo, Scognamiglio, Reverchon, & Adami, Citation2015; Oroian & Escriche, Citation2015; Solana Mirofci and Bertucco, Citation2016).
The use of CO2 is an alternative to the extraction of natural herbal antioxidants (Meneses et al., Citation2015; Vargas et al., Citation2013), fruits (Song et al., Citation2016; Viganó et al., Citation2016), vegetables (Bagheri et al., Citation2016), microorganisms (Guedes et al., Citation2013; Yen, Yang, Chen, & Chang, Citation2015), among others.
Cavalcanti, Albuquerque, and Meireles (Citation2015) conducted a study regarding the technical and economic feasibility of cupuaçu butter extraction using CO2 as the supercritical fluid. Cupuaçu butter obtained from extraction can be a valuable ingredient in foods, pharmaceuticals, and cosmetics, due to the high levels of alpha, gamma, and delta tocopherols that it contains, as well as high levels of unsaturated (oleic and linoleic) acids compared to saturated acids (palmitic and stearic). The optimum conditions in relation to the extraction kinetics, chemical composition, and manufacturing costs were 30–35 MPa and 50°C.
Supercritical CO2 has been commonly used for the extraction of bioactive compounds. Guedes et al. (Citation2013) used supercritical fluid to extract carotenoids and a, b and c chlorophylls from Microalga Scenedesmus obliquus for subsequent use in food processing. The highest yields were obtained at 250 bars. The most suitable temperature to obtain the highest yields for chlorophyll was 40°C and for carotenoids it was 60°C.
Malaman, Moraes, West, Ferreira, and Oliveira (Citation2011) used CO2 as the supercritical fluid to extract bioactive compounds in Brazilian cherry. The extractions were performed under different temperature and pressure conditions. The extracts showed high concentrations of phenolic compounds and the authors highlighted the flavor intensity, mainly in the extract which was performed at 50°C independent of pressure.
Sodeifian, Ghorbandoost, Sajadian, and Ardestani (Citation2015) performed a supercritical fluid extraction (SFE) with carbon dioxide to obtain oil from Pistacia khinjuk Stocks fruit. Response surface methodology (RSM) was used to optimize the process and to evaluate the effect of different operating parameters, including pressure (12–24 MPa), temperature (35–55°C), flow rate (2–6 g/min), and extraction time (0–300 min.) on the total oil yield. The relationship between the yield of P. khinjuk fruit oil and the extraction variables was determined by a second-order polynominal equation (Eq. 1) applying a central composite design (CCD).
Romo-Hualde, Yetano-Cunchillos, González-Ferrero, Sáiz-Abajo, and González-Navarro (Citation2012) studied various different parameters that can affect the extraction yield, using CO2 as the supercritical fluid. The highest yield of vitamin E (97%) and provitamin A (68.1%) in red pepper (Capsicum annum L.) was found at a temperature of 60°C, 24 Pa and using a particle size of 0.2–0.5 mm.
CO2 is also a suitable solvent for the extraction of bioactive compounds from biological substrates but it has limited ability to dissolve high molecular weight compounds such as carotenoids (Araus, del Valle, Robert, & Juan, Citation2012).
Babova, Occhipinti, Capuzzo, and Maffei (Citation2016) investigated the use of CO2 as supercritical fluid for the selective extraction of antioxidants from blueberry. The supercritical treatment allowed the selective extraction of the compounds.
3.1.2 Propane as a supercritical fluid
Extraction with propane has been shown to have significant benefits, even when compared to extraction with CO2, such as higher yields, higher solubility of non-polar compounds, selectivity, as well as shorter extraction times and reduced solvent consumption (Correa, Mesomo, Pianoski, Torres, & Corazza, Citation2016; Hamdan, Daood, Toth-Markus, & Illés, Citation2008).
Zanqui et al. (Citation2015) extracted flaxseed oil using n-propane as supercritical fluid under different pressures and temperatures. The yield was 28% at temperatures ranging from 30 to 45°C and pressures of 8–12 MPa.
da Silva et al. (Citation2015) evaluated the influence of temperature and pressure on perilla oil extraction using compressed n-propane gas. The experiments were performed using a temperature range of 40–80°C and pressures of 8–16 MPa at a constant flow rate of 1.0 cm3/min n-propane. The extraction yields showed satisfactory extraction and higher stability compared to the classical method.
Santos et al. (Citation2015) studied crambe seed (Crambe abyssinica) oil extraction using supercritical propane as solvent. The authors observed that temperature had a significant effect on the extraction, and the highest yield was obtained using 353°K and 16 MPa (32.8% in weight).
Pessoa et al. (Citation2015) studied the pequi pulp oil extraction process using supercritical propane at pressures of 5–15 MPa and temperatures ranging from 303.15 to 333.15°K. The highest yield (43% in weight) was for conditions of 15 MPa and 333.15°K.
Pederssetti et al. (Citation2011) investigated canola seed oil extraction using carbon dioxide and propane tablets as solvents. The extractions were performed at temperatures of 40, 50, 60, and 20°C and pressures 22.5 and 25 MPa for carbon, and temperatures of 30, 40, and 60°C and pressures of 8, 10, and 12 MPa for propane. The results indicated that the pressure and temperature were important variables for the efficiency of extraction. Extraction with propane proved much faster than with carbon dioxide.
Corso et al. (Citation2010) evaluated sesame seed oil extraction using carbon dioxide and propane tablets as solvents. The extractions were performed at temperatures of 313–333°K and pressures of 19–25 MPa for carbon dioxide and temperatures of 303–33°K and pressures of 2–12 MPa for propane. The results indicated that the solvent and the density were important variables in the extraction with CO2, while the temperature was most important in relation to propane. The extraction with propane was faster in than carbon dioxide; the extract characteristics were similar.
Nimet et al. (Citation2011) compared sunflower seed oil extraction using propane and CO2 as the supercritical fluid. The best yield was obtained used propane as a solvent, and the extracts showed high concentrations of vitamin E.
3.1.3 Compressed liquefied petroleum gas as supercritical fluid
Liquefied petroleum gas (LPG) is characterized by a mixture of propane and n-butane, and it has been used as fuel for heating appliances and for cooking food (Silva et al., Citation2015).
Silva et al. (Citation2015) evaluated the use of LPG to treat some enzymes high pressure to increase their catalytic power; LPG increased the processing speed.
Soares et al. (Citation2015) used CO2 and compressed LPG to obtain rice bran oil and concluded that the fatty acid profiles were similar. However, a kinetic analysis demonstrated that LPG decreased the mass of solvent/feed and extraction time. The authors concluded that LPG is promising, considering that reducing the analysis time reduces the energy required for the re-compression of the solvent.
Dal Prá et al. (Citation2016) published an article in which they described the use of solvents (including LGP) to extract biocompounds from palm (Elaeis guineensis).
Using LPG avoids the main recurring problem in terpene extraction and offers a series of advantages regarding ecological and technical issues. Therefore, this method offers a solution that can be applied for the recovery of terpenes in industrial processes and on a laboratory scale (Bier et al., Citation2016).
3.2. Extraction with pressurized liquid
This technique uses a separation process that involves the transfer of solutes from a solid matrix. Liquid solvents, at elevated temperatures and pressure, are used, which produces a reduction in the surface tension of the solvent, which in turn facilitates the penetration of the solvent into the pores of the matrix. The process disrupts the matrix, which increases the mass transfer of the analyte from the solvent sample (Garcia-Mendoza, Paula, Paviani, Cabral, & Martinez-Correa, Citation2015). The solvents are chosen based on the solubility characteristics of the desired solute. The versatility of pressurized solvents is excellent due to the physicochemical properties of the solvent, including the density, diffusivity, viscosity, and dielectric constant, which can be controlled by varying the temperature and the pressure of the extraction system (Pronyk & Mazza, Citation2009).
Extraction using pressurized liquid is an attractive technique because it allows rapid extraction and reduced solvent consumption; it has been successfully employed for the extraction of anthocyanins from various plants (Santos, Veggi, & Meireles, Citation2012).
Machado et al. (Citation2015) obtained an extract with antioxidant compounds from cranberry waste using extraction with pressurized liquid. They analyzed different solvents (water, acidified water:2.5, ethanol) and temperature (60, 80, and 100°C). The best condition was compared with conventional extraction (Soxhlet and maceration). The best results were obtained by extraction with pressurized liquid at a temperature of 100°C, using water and ethanol as solvent. The authors proved that the use of this technology is promising in terms of recovering bioactive compounds from fruit.
Xu et al. (Citation2016) used the pressurized water methodology to extract polysaccharides from gooseberry and to investigate the antioxidant activity. The optimum conditions were: 51 min, pressure of 1.6 MPa, and a temperature of 52°C. The study provided a new and efficient extraction method for polysaccharides from gooseberry.
Machado et al. (Citation2015) obtained extracts from blackberry residue using pressurized liquid extraction. The influence of the solvent (acidified water pH = 2.5 and ethanol + 50% water) was evaluated, as well as the temperature. The authors proved that this is a promising technique.
Bajer, Bajerová, Kremr, Eisner, and Ventura (Citation2015) applied the method of extraction with pressurized hot water in peppers to optimize the yield of bioactive compounds. An optimization study was performed using water as solvent at a constant pressure of 20 MPa, temperatures of 120–240°C and time from 5 to 60 min. The study was compared to the Soxhlet method and was approximately 113% relatively efficient in comparison. The authors emphasized that the only disadvantage of this method is that it requires sophisticated instrumentation because it requires greater application of pressure and extraction temperatures.
3.3. Ultrasound-assisted extraction
Ultrasound is a special kind of sound wave that ranges from 20 kHz to 100 MHz. Ultrasound-assisted extraction produces a phenomenon known as cavitation, which entails the production, growth and collapse of bubbles (Azmir et al., Citation2013). UAE is an effective extraction technique for a wide range of analytes from different types of samples. Ultrasounds have effects that accelerate heat and mass transfer via the disruption of plant cell walls, leading to improved release of the target compounds from several natural sources (Roselló-Soto et al., Citation2015).
Extraction using ultrasound involves two main types of physical phenomena; diffusion through the cell wall, and rinsing the cell content after breaking the walls. The temperature, pressure, frequency, and sonication time are all factors which regulate the action of ultrasound (Rajha et al., Citation2015; Vinatoru, Citation2001).
Ultrasound is relatively easy to use; it is versatile, flexible, and requires low investment compared with other extraction techniques. Ultrasound has been used to extract molecules and various biomaterials, including polysaccharides, essential oils, proteins, peptides, dyes, pigments, and bioactive compounds (Briones-Labarca et al., Citation2015; Tiwari, Citation2015).
This phenomenon can be indirect or direct. When ultrasound is applied directly to the medium without any barrier, such as a probe system, it provides an intensity that is approximately 100 times higher. For indirect sonication, such as using an ultrasonic water bath, the waves have to be transferred through the water until they reach the sample (Kek, Chin, & Yusof, Citation2013).
The application of ultrasound energy has been considered to be a promising alternative for the extraction of bioactive compounds; it increases the mass transfer coefficient, accelerates the kinetics, and increases the final yield (Riera et al., Citation2004).
Xu, Li, and Sun (Citation2015) reported on the impact of ultrasound-assisted extraction of natural antioxidants from the Eucommia oliver plant using distilled water as solvent. The use of ultrasound has improved the effectiveness of traditional treatments, providing higher yields and the selectivity of natural antioxidants.
D’Alessandro, Dimitrov and Nikov (Citation2014) validated the extraction of bioactive compounds from black chokeberry fruit. They studied the influence of the extraction time (0–240 min), the composition of the solvent (ethanol-water) and the levels of ultrasound energy (0–100 W). According to these authors, ultrasound is a suitable technology for the extraction of phenolic compounds (total anthocyanins and polyphenols).
Sivakumar, Ilanhtiraiyan, Ilayaraja, Ashly, and Hariharan (Citation2014) studied the influence of ultrasound in Avaram shell (Cassia auriculata) for the extraction of tannins. The results showed an improvement of 160% when using ultrasound at 100 W in comparison with magnetic stirring, suggesting that this was strongly connected to improved mass transfer in the leaching of tannins.
Khan, Abert-Vian, Fabiano-Tixier, Dangles, and Chemat (Citation2010) reported on the extraction of polyphenols, especially flavonoids, from orange peel (Citrus sinensis L.), using ethanol as a solvent of food grade. The best conditions were 40°C, power of 150 W and a ratio of 4:1 (ethanol: water). The authors found an increase in the yield of the compounds and antioxidant activity of the extracts obtained by ultrasound, and confirmed the suitability of this technique for preparing fruit extracts.
Rodríguez-Pérez, Quirantes-Piné, Fernández-Gutiérrez, and Segura-Carretero (Citation2015) used ultrasound to determine the best way to extract bioactive compounds from Moringa oleifera. They concluded that using ultrasound increased the yield of the extracts and the content of bioactive compounds.
Rabelo, Machado, Martínez, and Hubinger (Citation2016) used the ultrasound-assisted method to extract phenolic compounds from artichoke residues. The highest yields were observed for the extracts with high ethanol content (50%), ultrasonic power of 240 W, and 10 min. sonication.
Meullemiestre, Petitcolas, Maache-Rezzoug, Chemat, and Rezzoug (Citation2016) evaluated the impact of ultrasound on the extraction of phenolic compounds from waste pine. The optimum extraction conditions were 40°C, with ultrasonic intensity of 0.67 W/cm2, and a time of 43 min.
Corbin et al. (Citation2015) extracted phenolic compounds from pine seeds using ultrasound. The method was proven to be very effective for reducing the trapping of phenolic compounds. The optimal conditions were using supplemented water as solvent with 0.2 N sodium hydroxide, a 60 min. extraction time, 25°C temperature, and an ultrasonic frequency of 30 KHz. In comparison with conventional maceration this technique resulted in a 30% increase in the content of phenolic compounds.
de Paula et al. (Citation2016) used ultrasound-assisted extraction regarding bioactive compounds from dried fruit of Azadirachta indica A. Juss (Meliaceae). The results showed that the optimum conditions were a concentration of 75–80% ethanol, 30°C temperature and a material-solvent ratio of 0.55gmL−1. The authors suggest that ultrasound-assisted extraction is a more efficient extraction process because it is simple, fast, and inexpensive. Wang et al. (Citation2015) investigated the optimum conditions for the ultrasound-assisted extraction polysaccharides, as well as the antioxidant activity of pears (Pyrus sinkiangensis, subfamily Maloideae). The highest yield (5.16%) was obtained at 70°C, with a power of 230 W, and a water ratio of 13:1 mL/g. The technique was efficient and the authors concluded that this fruit has potential for applications in the food industry.
Hammi, Jdey, Abdelly, Majdoub, and Ksouri (Citation2015) also used ultrasound-assisted extraction regarding bioactive compounds from Zizyphus lotus fruit. The optimum operating conditions were as follows: 50% ethanol concentration, extraction time of 25 min., temperature of 63°C, and a solvent:material ratio of 67 mL/g. The authors’ conclusion was that this methodology was suitable for the extraction of bioactive compounds from fruit.
Chen, You, Abbasi, Fu, and Liu (Citation2015) used ultrasound to extract polysaccharides with antioxidant activity from black mulberry fruit. The most favorable conditions were with a water: material ratio of 40:25, a temperature of 69°C, time of 75 min., and ultrasound power of 190 W. The maximum yield was 3.13%, showing that the ultrasound technique was effective.
3.4. Microwave assisted extraction
Microwaves are electromagnetic fields in the range of 300 MHz to 300 GHz with two oscillating fields which are perpendicular, such as electric field and magnetic field frequencies. The solvent penetrates inside the solid matrix by diffusion and the solute is dissolved to reach a concentration that is limited by the solid’s characteristics (Angiolillo, Del Nobile and Conte, Citation2015).
Microwaves are a non-contact heat source that can provide more effective heating, accelerating the transfer of energy and reducing the thermal gradient. Several classes of compounds, such as essential oils, antioxidants, pigments, flavorings, and other organic compounds, can be efficiently separated using this method (Li et al., Citation2013).
According to Leadbeater (Citation2014), the use of microwave equipment is a flourishing technology because it is possible to have access to higher temperatures easily, safely, and in a reproducible manner; the reaction time can be reduced; the yield can be increased; and the purity can be improved, in comparison to conventional heating methods. This technique can be performed either with or without the addition of any solvent (Oroian & Escriche, Citation2015).
Simha, Mathew, and Ganesapillai (Citation2016) investigated the effectiveness of microwave-assisted extraction (MAE) to recover bioactive compounds from the pharmaceutically significant medicinal plants Cymbopogon citratus and Adathoda vasica.
Li et al. (Citation2013) provide an overview of the techniques that are available to extract bioactive compounds using microwaves without solvents. They showed that this can be an alternative to other techniques, with the advantages of reduced time, energy consumption, use of solvents, and CO2 emissions.
Grigoras, Lazar, and Elfakir (Citation2012) performed a comparative study of conventional methods; maceration; and extraction using pressurized liquid, ultrasound, and microwave. The microwave-assisted methodology provided the highest concentration of bioactive compounds in the apple extract.
Krishnan and Rajan (Citation2016) used the microwave-assisted technique to extract flavonoids from the Terminalia bellerica plant. The microwave equipment (R-219T (S)/(W), SHARP, Japan) was fitted with a timer to control the duration of the irradiation cycles. The maximum yield of flavonoids was 25.21 mg/g using water as solvent, with a ratio of 40 ml/g and a temperature of 100°C. The authors concluded that the extraction technique using microwaves was a suitable method for flavonoid extraction, recovering 82.74%, whereas the conventional method only recovered 63.75%.
Inoue, Tsubaki, Ogawa, Onishi, and Azuma (Citation2010) isolated hesperidin from the skin of Citrus unshiu fruit using microwave-assisted extraction. The microwave that was used was at 1 kW and 2.45 GHz of power. The optimal parameters were a temperature of 140°C and a time of 8 min. Under these conditions 86.8% (47.7 mg/g) of hesperidin was isolated. The process was efficient and it can be considered to be a simple application.
Thirugnanasambandham & Sivakumar, CitationIn Press optimized the extraction of betalains from pitaya (dragon fruit) using microwave-assisted extraction. The microwave that was used (VCX 400, Sonics) had a power of 100 W. The optimum conditions were: a temperature of 35°C, a sample weight of 20 g, and 8 min. treatment time. This extraction method was found to be efficient for bioactive compounds in fruits.
Simic et al. (Citation2016) optimized microwave-assisted extraction of phenolic compounds from chokeberries using response surface methodology. The parameters that were examined were: microwave power (300, 450, and 600 W), ethane concentrations (25%, 50%, and 75%) and extraction time (5, 10, and 15 min). The highest yield (420.1 equivalents mg gallic acid/100 g of plant material) was at a concentration of 53.6%, power of 300 W, and time of 5 min.
Seixas et al. (Citation2014) extracted pectin from passion fruit skin by heating that was induced by microwave. The results indicated that the exposure time and the microwave power significantly affected the yield of pectin with nitric and tartaric acid. The highest yield was obtained using the longest time (9 min) and the highest power (628 W). The method was efficient for the extraction of pectins in fruits.
Maran, Sivakumar, Thirugnanasambandham, and Sridhar (Citation2014) also extracted pectin from Citrullus lanatus shells. The parameters that were studied were the microwave power (160–480 W), irradiation time (60–180 s), and liquid-solid ratio (1:10–1: 30 g/ml). The results showed that all the process variables had a significant effect. The best yield (25.79%) was obtained with a power of 477 W, time of 128 s, and a solid:liquid ratio of 1:20, 20.3 g/ml. The extraction method was satisfactory.
Hydrodiffusion microwave is a green technology that does not use solvents, which results in increased permeability and fabric softening. It is an extraction method that is economic, efficient, and environmentally friendly (Oroian & Escriche, Citation2015).
Petigny et al. (Citation2014) presented a study from lab to pilot scale for the extraction and separation of volatile and non-volatile compounds from boldo leaves using microwave. The experimental conditions (microwave power and time of extraction) were optimized by using an experimental plan design. These authors concluded that the reduced cost of extraction provided by this proposed MAE method was based on reduced consumption of energy and time because hydrodistillation required 30 min to start the azeotropic distillation, whereas MAE only required 5 min. This proved the efficiency of heating energy delivered in the matrix. This extraction method, combined with this new microwave apparatus, indicates potential for industrial use for day-to-day operations; however, it could also be used in a process to create equipment using continuous extraction.
3.5. Pulsed electric field assisted extraction
The principle of pulsed electric field extraction is to induce the electroporation of the cell membrane, thereby increasing the extraction yield. An electric potential passes through the cell membrane and separates molecules according to their charge. This repulsion forms pores, increasing their permeability (Azmir et al., Citation2013; Rajha et al., Citation2015). Among the different applications of PEF, food preservation and the recovery of intracellular valuable compounds from plant food materials, food wastes, and by-products have been the most widely studied. PEF is a useful tool to selectively recover valuable compounds from different fruit and vegetable tissues from an economic and sustainable point of view, mainly due to its ability to soften and disrupt cell membranes, thus facilitating the release of intracellular compounds (Roselló-Soto et al., Citation2015).
Soliva-Fortuny et al. (Citation2009) studied the system typically used for the treatment of pumpable fluids, which consists of a PEF generation unit, which is in turn composed of a high voltage generator and a pulse generator, a treatment chamber, a suitable product handling system and a set of monitoring and controlling devices.
The effectiveness of the treatment depends on the process parameters, including the intensity field, input energy, pulse number, temperature, and material properties (Azmir et al., Citation2013).
Scientific studies and recent practice have shown that the pulsed electric field technique is compatible with the concept of green extraction techniques since it uses renewable plant resources and alternative solvents, such as water or agri-solvents (ethanol and methyl esters of fatty acids from vegetable oils), reduced energy consumption and unit operations, as well as producing extracts of high quality and purity (Parniakov et al., Citation2014).
The application of the pulsed electric field technique in water has been shown to improve the extraction of compounds from different raw materials, as well as increasing the rate of extracted compounds, reducing the temperature and reducing the level of solvents (Bousseta and Vorobiev, Citation2014).
This technique can facilitate the selective recovery of valuable compounds without deteriorating the treated matrix, thus favoring the separation and purification of subsequent stages (Barba et al., Citation2015b).
Electrically-pulsed and high-voltage discharges can be useful technologies for the recovery of food waste and by-products (Oroian & Escriche, Citation2015). Methods assisted by pulsed electrical energy can increase productivity and the quality of the extracted compounds, thus decreasing the time and temperature of the extraction operations (Parniakov et al., Citation2014).
Luengo, Alvarez and Raso (Citation2013) used the pulsed electric field method to extract of polyphenols and flavonoids from orange peel. A time of 60 μs (20 pulses of 3μs) resulted in the highest cell disintegration index. In comparison with the untreated sample, the yield of phenolic compounds using pulsed electric fields for 1, 3, 5, and 7 kV/cm increased by 20%, 129%, 153%, and 159%, as well as increasing antioxidant activity by 51%, 94%, 148%, and 192%, respectively. The results demonstrated the potential of the pulsed electric field technique as a method of extraction of bioactive compounds, reducing the extraction times and not requiring the use of organic solvents.
Xue and Farid (Citation2015) studied the effects of continuous treatment in relation to the extraction of bioactive compounds using pulsed electric field in the range of 12.4–38.4 kV/cm. The optimal yields were estimated at 38.4 kV/cm and a temperature of 85°C. The increased yield of bioactive compounds compared with conventional treatment was clear. The data obtained indicated that the permeabilization of the membrane, which assists the extraction of compounds, occurs within a short period of time.
Leong, Burritt and Okey (Citation2016) assessed the release of anthocyanins in grape juices after pulsed electric field treatment. The treatment chamber consisted of two stainless steel electrodes and the optimal variables were: a pulse of 20 mS, a frequency of 50 Hz and an electric field strength of 1.5 kV/cm. Compared with the untreated grape juice, PEF helped the release of anthocyanins, increased the content of bioactive compounds and vitamin C, as well as improving the antioxidant activity. The technique was shown to be efficient in providing a better phytochemical composition of the extracts, as well as the ability to protect cells from oxidative stress.
Segovia, Luengo, Corral-Pérez, Raso, and Almajano (Citation2015) reported on the extraction of polyphenols from Borago officinalis leaves using pulsed electric field treatment. The equipment used generated wave pulses with a maximum frequency of 300 Hz; the maximum output voltage was 30 kV and the current was 200 A. The polyphenol and ORAC values were increased between 1.3 and 6.6% and from 2.0 to 13.7%, respectively. The authors concluded that the procedure increased the antioxidant capacity of the extracts and reduced the extraction time.
Jaeger, Schulz, Lu, and Knorr (Citation2012) used the pulsed electric field technique in relation to apple juice. The yield increased according to the field intensity. The overall composition, polyphenol content and antioxidant capacity were similar to conventional treatments, but there was a reduction in process time.
Elez-Martinez and Martín-Belloso (Citation2007) subjected orange juice to high-intensity pulsed electric field treatment. The vitamin C content was higher than in conventionally pasteurized juices. This technique is effective in the recovery and protection of bioactive compounds.
In some cases, the application of electric fields at room temperature is not sufficient to damage the cells. The application of pulsed ohmic heating consists of increasing the temperature through ionic movements in the sequence for applying treatment (Barba et al., Citation2016).
3.6. High-voltage electrical discharges
In this technique, energy is introduced directly into an aqueous solution through a plasma channel formed by a current of high-voltage electrical discharge between two submerged electrodes (Barba et al., Citation2015). The intensity of the electric field is able to induce an avalanche of electrons that are responsible for starting the spread of the positive streamer for the negative electrode. Secondary phenomena, such as bubble cavitation, turbulence, and pressure shock waves, contribute to the improvement of cell damage, facilitating the release of compounds and the extraction of biomolecules from the cytoplasm of the cells (Rajha et al., Citation2015).
It is necessary to optimize the extraction parameters for each product. The nature of the raw materials significantly affects the efficiency of the treatment, as was demonstrated in the study by Barba et al. (Citation2015).
Authors who work with this type of apparatus have noted that during the electrical discharges cavitation bubbles are produced, which resembles the ultrasound technique (Vinatoru, Citation2001).
Boussetta et al. (Citation2011) applied the technique of high-voltage electrical discharge to extract phenolic antioxidants from grape pomace. The HVED experiments were performed in a laboratory treatment chamber connected to a pulsed high-voltage power supply. The technique increased the yield and improved the extraction kinetics. The best electrical treatment parameters were an 80 kJ/kg power source, an electrode aperture of 5 mm, and a liquid-solid ratio of 5.
In a comparative study between conventional techniques, pulsed electric field and high voltage discharge, Parniakov et al. (Citation2014) concluded that the latter showed better extraction efficiency to recover high value compounds. However, the electrical discharges can produce chemical electrolysis and free radicals, which can react with valuable compounds and antioxidants, reducing their beneficial properties.
3.7. High hydrostatic pressure
High hydrostatic pressure (HHP) has been developed as an alternative to thermal processes, with the aim of obtaining microbiologically safe food products and avoiding undesirable changes in the sensory, physicochemical, and nutritional properties of foods (Escobedo-Avellaneda et al., Citation2011). This technology operates under pressures generally ranging from 100 to 1,000 MPa (Briones-Labarca et al., Citation2015). It is widely known as an alternative to conventional heating treatments (Tao et al., Citation2016) and is considered to be a green technology. It is recognized by the Food and Drug Administration in the United States, since it only requires electric power and does not generate waste (Andrés et al., Citation2016).
The use of HHP improves mass transfer rates and increases the secondary metabolite diffusion according to phase transitions (Oroian & Escriche, Citation2015).
High pressures generate the deprotonation of the charged groups and disrupt hydrophobic bonds and salt bridges, resulting in changes in form and the denaturation of proteins. Additional solvent can enter the cells and further compounds can permeate the cell membrane, increasing the yield (Briones-Labarca et al., Citation2015).
In a comparative study between conventional, ultrasound and high hydrostatic pressure extraction techniques, Briones-Labarca et al. (Citation2015), found that the latter was more effective than the other techniques in the extraction of bioactive compounds from papaya (Vasconcellea pubescens).
George, Selvan, and Rastogi (Citation2016) studied high pressure treatment in the extraction of anthocyanins from apple. The high pressure treatment resulted in increased humidity and solid mass transfer due to the cell permeabilization, which was revealed via microstructure analysis. The authors also suggested that HHP resulted in greater infusion of the bioactive compounds compared with infusion at atmospheric pressure.
Andrés et al. (Citation2016) used the high pressure technique to isolate bean protein. The treatment was carried out at 70–90°C and at hydrostatic pressures of 200, 400 and 600 MPa. The functional properties were improved compared with the less energetic treatments (70°C and 200 MPa).
High hydrostatic pressure enhances the extraction of phenolic compounds from oak chips. The phenolic compounds and antioxidant activity of wine increased after processing in the presence of oak chips (Tao et al., Citation2016).
Andrés et al. (Citation2016) evaluated the physical and chemical properties, as well as the bioactive compounds from a soy smoothie treated with high hydrostatic pressure. They observed a higher antioxidant capacity using the latter technique.
3.8. Combination techniques
Rapid breakthroughs have occurred in the development and improvement of extraction methods. The combination of sample preparation and analytical techniques is a strategy that is primarily utilized to save energy and resources (Mustafa & Turner, Citation2011).
Extraction using ultrasound can be coupled with other techniques such as extraction heat-reflux, supercritical CO2, and microwave. Yang and Wei (Citation2015) developed an efficient method to extract bioactive compounds from Rabdosia rubescens by combining heat reflux extraction (conventional, solvent: ethanol) and ultrasound-assisted extraction (40 kHz frequency and power of 185 W, with agitation). The authors concluded that the combination of extraction techniques reduced the processing time and increased the yield of bioactive compounds.
Corrales, Toepfl, Butz, Knorr, and Tauscher (Citation2008) measured the extraction of anthocyanins from grape by-products using the methods of ultrasound, and a combination of pulsed electric field and high hydrostatic pressure. The authors concluded that the combination of effective extraction technologies and low-cost raw materials represents an environmental and economical alternative to conventional extraction methods, which require large amounts of organic solvents and long extraction times.
Garcia-Mendoza et al. (Citation2015) used two sequential steps for the extraction of bioactive compounds from mango skin (Mangifera indica L.). First, supercritical CO2 was used, followed by ethanol under pressure, both at 30 MPa and a temperature of 40°C. The results of this study demonstrated that a two-stage extraction process allows the recovery of bioactive compounds. The extracts showed significant antioxidant activity and potential application in the food industry.
Dias et al. (Citation2016) evaluated the effects of the extraction of bioactive compounds from red pepper (Capsicum baccatum L. var. Pendulum) through ultrasound and supercritical fluid techniques. The processes were tested at pressures of 15–25 MPa, temperatures of 40–60°C, ultrasound powers from 200 to 600 W, for 40–80 min. The authors found a higher content of phenolic compounds when using these techniques.
Parniakov, Barba, Grimi, Lebovka, and Vorobiev (Citation2016) used pulsed electric field and high voltage techniques to recover of bioactive compounds from mango. The techniques were performed using a 40-kV 10kA pulse generator (Polytechnic University Tomsk, Russian Federation) in the treatment chamber and two types of electrodes. The results of this study demonstrated the feasibility of a combination of the pulsed electric field and high voltage techniques to recover antioxidants and proteins from mango skin.
4. Conclusion
The extraction of bioactive compounds involves complex mechanisms and it can be accomplished by various techniques. Seeking to improve the extraction yields, reduce processing time and reduce environmental damage caused by toxic solvents, it has been proven that the replacement of conventional techniques by green technologies is promising. Studies have also suggested that a combination of methods can possibly improve these processes.
Acknowledgments
The authors wish to thank the Federal University of Santa Maria (UFSM) and the Coordination for the Improvement of Higher Level Personnel (CAPES), who funded this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alonso-Salces, R. M., Korta, E., Barranco, A., Berrueta, L. A., Gallo, B., & Vicente, F. (2001). Pressurized liquid extraction for the determination of polyphenols in apple. Journal of Chromatography A, 933(1–2), 37–43.
- Andrés, V., Mateo-Vivaracho, L., Guillamón, E., Villanueva, M. J., & Tenorio, M. D. (2016). High hydrostatic pressure treatment and storage of soy-smoothies: Colour, bioactive compounds and antioxidant capacity. LWT-Food Science and Technology, 69, 123–130.
- Angiolillo, L., Del Nobile, M. A., & Conte, A. (2015). The extraction of bioactive compounds from food residues using microwaves. Current Opinion in Food Science, 5, 93–98.
- Araus, K. A., del Valle, J. M., Robert, P. S., & Juan, C. (2012). Effect of triolein addition on the solubility of capsanthin in supercritical carbon dioxide. The Journal of Chemical Thermodynamics, 51, 190–194.
- Azmir, J., Zaidul, I. S. M., Rahman, M. M., Sharif, K. M., Mohamed, A., Sahena, F., & Omar, A. K. M. (2013). Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering, 117(4), 426–436.
- Babova, O., Occhipinti, A., Capuzzo, A., & Maffei, M. E. (2016). Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. The Journal of Supercritical Fluids, 107, 358–363.
- Bagheri, H., Yamini, Y., Safari, M., Asiabi, H., Karimi, M., & Heydari, A. (2016). Simultaneous determination of pyrethroids residues in fruit and vegetable samples via supercritical fluid extraction coupled with magnetic solid phase extraction followed by HPLC-UV. The Journal of Supercritical Fluids, 107, 571–580.
- Bajer, T., Bajerová, P., Kremr, D., Eisner, A., & Ventura, K. (2015). Central composite design of pressurised hot water extraction process for extracting capsaicinoids from chili peppers. Journal of Food Composition and Analysis, 40, 32–38.
- Barba, F. J., Boussetta, N., & Vorobiev, E. (2015). Emerging technologies for the recovery of isothiocyanates, protein and phenolic compounds from rapeseed and rapeseed press-cake: Effect of high voltage electrical discharges. Innovative Food Science & Emerging Technologies, 31, 67–72.
- Barba, F. J., Zhu, Z., Koubaa, M., de Souza Sant’Ana, A., & Orlien, V. (2016). Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends in Food Science & Technology, 49, 96–109.
- Bennett, L. E., Jegasothy, H., Konczak, I., Frank, D., Sudharmarajan, S., & Clingeleffer, P. R. (2011). Total polyphenolics and anti-oxidant properties of selected dried fruits and relationships to drying conditions. Journal of Functional Foods, 3(2), 115–124.
- Bier, M. C. J., Medeiros, A. B. P., de Oliveira, J. S., Côcco, L. C., da Luz Costa, J., de Carvalho, J. C., & Soccol, C. R. (2016). Liquefied gas extraction: A new method for the recovery of terpenoids from agroindustrial and forest wastes. The Journal of Supercritical Fluids, 110, 97–102.
- Boussetta, N., Lesaint, O., & Vorobiev, E. (2013). A study of mechanisms involved during the extraction of polyphenols from grape seeds by pulsed electrical discharges. Innovative Food Science & Emerging Technologies, 19, 124–132.
- Boussetta, N., & Vorobiev, E. (2014). Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chimie, 17(3), 197–203.
- Boussetta, N., Vorobiev, E., Deloison, V., Pochez, F., Falcimaigne-Cordin, A., & Lanoisellé, J.-L. (2011). Valorisation of grape pomace by the extraction of phenolic antioxidants: Application of high voltage electrical discharges. Food Chemistry, 128(2), 364–370.
- Brasil. (2002). Agência Nacional de Vigilância Sanitária. Resolução RDC nº2, de 02 de janeiro de 2002. Aprova o regulamento técnico das substâncias bioativas e probióticos isolados com alegação de propriedade funcional e ou de saúde. Brasília, DF: ANVISA.
- Brianceau, S., Turk, M., Vitrac, X., & Vorobiev, E. (2016). High voltage electric discharges assisted extraction of phenolic compounds from grape stems: Effect of processing parameters on flavan-3-ols, flavonols and stilbenes recovery. Innovative Food Science & Emerging Technologies, 35, 67–74.
- Briones-Labarca, V., Plaza-Morales, M., Giovagnoli-Vicuña, C., & Jamett, F. (2015). High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT-Food Science and Technology, 60(1), 525–534.
- Brunner, G. (2005). Supercritical fluids: Technology and application to food processing. Journal of Food Engineering, 67, 21–33.
- Cavalcanti, R. N., Albuquerque, C. L., & Meireles, M. A. A. (2015). Supercritical CO 2 extraction of cupuassu butter from defatted seed residue: Experimental data, mathematical modeling and cost of manufacturing. Food and Bioproducts Processing, 97, 48–62.
- Chemat, F., Rombaut, N., Meullemiestre, A., Turk, M., Perino, S., Fabiano-Tixier, A.-S., & Abert-Vian, M. (2017a). Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innovative Food Science & Emerging Technologies, 41, 357–377.
- Chemat, F., Rombaut, N., Sicaire, A.-G., Meullemiestre, A., Fabiano-Tixier, A.-S., & Abert-Vian, M. (2017b). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry, 34, 540–560.
- Chen, C., You, L.-J., Abbasi, A. M., Fu, X., & Liu, R. H. (2015). Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydrate Polymers, 130, 122–132.
- Corbin, C., Fidel, T., Leclerc, E. A., Barakzoy, E., Sagot, N., Falguiéres, A., & Lainé, E. (2015). Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrasonics Sonochemistry, 26, 176–185.
- Corrales, M., Toepfl, S., Butz, P., Knorr, D., & Tauscher, B. (2008). Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innovative Food Science & Emerging Technologies, 9(1), 85–91.
- Correa, M., Mesomo, M. C., Pianoski, K. E., Torres, Y. R., & Corazza, M. L. (2016). Extraction of inflorescences of Musa paradisiaca L. using supercritical CO 2 and compressed propane. The Journal of Supercritical Fluids, 113, 128–135.
- Corso, M. P., Fagundes-Klen, M. R., Silva, E. A., Cardozo Filho, L., Santos, J. N., Freitas, L. S., & Dariva, C. (2010). Extraction of sesame seed (Sesamun indicum L.) oil using compressed propane and supercritical carbon dioxide. The Journal of Supercritical Fluids, 52(1), 56–61.
- Cravotto, G., Bicchi, C., Mantegna, S., Binello, A., Tomao, V., & Chemat, F. (2011). Extraction of kiwi seed oil: Soxhlet versus four different non-conventional techniques. Natural Product Research, 25(10), 974–981.
- Ćujić, N., Šavikin, K., Janković, T., Pljevljakušić, D., Zdunić, G., & Ibrić, S. (2016). Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chemistry, 194, 135–142.
- D’Alessandro, L. G., Dimitrov, K., Vauchel, P., & Nikov, I. (2014). Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chemical Engineering Research and Design, 92(10), 1818–1826.
- da Silva, B. V., Barreira, J. C., & Oliveira, M. B. P. (2016). Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends in Food Science & Technology, 50, 144–158.
- da Silva, C. M., Zanqui, A. B., Gohara, A. K., de Souza, A. H. P., Cardozo-Filho, L., Visentainer, J. V., … Matsushita, M. (2015). Compressed n-propane extraction of lipids and bioactive compounds from Perilla (Perilla frutescens). The Journal of Supercritical Fluids, 102, 1–8.
- Dal Prá, V., Soares, J. F., Monego, D. L., Vendruscolo, R. G., Freire, D. M. G., Alexandri, M., … da Rosa, M. B. (2016). Extraction of bioactive compounds from palm (Elaeis guineensis) pressed fiber using different compressed fluids. The Journal of Supercritical Fluids, 112, 51–56.
- de Paula, J. A. M., Brito, L. F., Caetano, K. L. F. N., de Morais Rodrigues, M. C., Borges, L. L., & da Conceição, E. C. (2016). Ultrasound-assisted extraction of azadirachtin from dried entire fruits of Azadirachta indica A. Juss (Meliaceae) and its determination by a validated HPLC-PDA method. Talanta, 149, 77–84.
- Dias, A. L. B., Sergio, C. S. A., Santos, P., Barbero, G. F., Rezende, C. A., & Martínez, J. (2016). Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. var. pendulum). Ultrasonics Sonochemistry, 31, 284–294.
- Elez-Martínez, P., & Martín-Belloso, O. (2007). Effects of high intensity pulsed electric field processing conditions on vitamin C and antioxidant capacity of orange juice and gazpacho, a cold vegetable soup. Food Chemistry, 102(1), 201–209.
- Escobedo-Avellaneda, Z., Moure, M. P., Chotyakul, N., Torres, J. A., Welti-Chanes, J., & Lamela, C. P. (2011). Benefits and limitations of food processing by high-pressure technologies: Effects on functional compounds and abiotic contaminants Beneficios y limitaciones del procesamiento de alimentos por tecnologías de alta presión: Efectos en componentes funcionales y contaminantes abióticos. CyTA-Journal of Food, 9(4), 351–364.
- Fardet, A., & Boirie, Y. (2014). Associations between food and beverage groups and major diet-related chronic diseases: an exhaustive review of pooled/meta-analyses and systematic reviews. Nutrition Reviews, 72(12), 741–762.
- Ferrari, G., Maresca, P., & Ciccarone, R. (2011). The effects of high hydrostatic pressure on the polyphenols and anthocyanins in red fruit products. Procedia Food Science, 1, 847–853.
- Garcia-Castello, E. M., Rodriguez-Lopez, A. D., Mayor, L., Ballesteros, R., Conidi, C., & Cassano, A. (2015). Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Science and Technology, 64(2), 1114–1122.
- Garcia-Mendoza, M. P., Paula, J. T., Paviani, L. C., Cabral, F. A., & Martinez-Correa, H. A. (2015). Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT-Food Science and Technology, 62(1), 131–137.
- George, J. M., Selvan, T. S., & Rastogi, N. K. (2016). High-pressure-assisted infusion of bioactive compounds in apple slices. Innovative Food Science & Emerging Technologies, 33, 100–107.
- Grigoras, E. D., Lazar, G., & Elfakir, C. (2012). Bioactive compounds extraction from pomace of four apple varieties. J Eng Stud Res, 18, 96–103.
- Guedes, A. C., Gião, M. S., Matias, A. A., Nunes, A. V., Pintado, M. E., Duarte, C. M., & Malcata, F. X. (2013). Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. Journal of Food Engineering, 116(2), 478–482.
- Hamdan, S., Daood, H. G., Toth-Markus, M., & Illés, V. (2008). Extraction of cardamom oil by supercritical carbon dioxide and sub-critical propane. The Journal of Supercritical Fluids, 44, 25–30.
- Hammi, K. M., Jdey, A., Abdelly, C., Majdoub, H., & Ksouri, R. (2015). Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chemistry, 184, 80–89.
- Heleno, S. A., Diz, P., Prieto, M. A., Barros, L., Rodrigues, A., Barreiro, M. F., & Ferreira, I. C. (2016). Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chemistry, 197, 1054–1063.
- Herrero, M., Castro-Puyana, M., Mendiola, J. A., & Ibañez, E. (2013). Compressed fluids for the extraction of bioactive compounds. TrAC Trends in Analytical Chemistry, 43, 67–83.
- Hiranvarachat, B., & Devahastin, S. (2014). Enhancement of microwave-assisted extraction via intermittent radiation: Extraction of carotenoids from carrot peels. Journal of Food Engineering, 126, 17–26.
- Inoue, T., Tsubaki, S., Ogawa, K., Onishi, K., & Azuma, J.-I. (2010). Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chemistry, 123(2), 542–547.
- Jacotet-Navarro, M., Rombaut, N., Deslis, S., Fabiano-Tixier, A.-S., Pierre, F.-X., Bily, A., & Chemat, F. (2016). Towards a “dry” bio-refinery without solvents or added water using microwaves and ultrasound for total valorization of fruit and vegetable by-products. Green Chemistry, 18(10), 3106–3115.
- Jaeger, H., Schulz, M., Lu, P., & Knorr, D. (2012). Adjustment of milling, mash electroporation and pressing for the development of a PEF assisted juice production in industrial scale. Innovative Food Science & Emerging Technologies, 14, 46–60.
- Kek, S. P., Chin, N. L., & Yusof, Y. A. (2013). Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food and Bioproducts Processing, 91(4), 495–506.
- Khan, M. K., Abert-Vian, M., Fabiano-Tixier, A.-S., Dangles, O., & Chemat, F. (2010). Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chemistry, 119(2), 851–858.
- Krishnan, R. Y., & Rajan, K. S. (2016). Microwave assisted extraction of flavonoids from Terminalia bellerica: Study of kinetics and thermodynamics. Separation and Purification Technology, 157, 169–178.
- Leadbeater, N. E. (2014). Organic synthesis using microwave heating. reference module in chemistry, molecular sciences and chemical engineering. comprehensive organic synthesis II (2nd ed., Vol. 9, pp. 234–286). Retrieved from https://doi.org/10.1016/B978-0-08-097742-3.00920-4
- Lenardão, E. J., Freitag, R. A., Dabdoub, M. J., Batista, A. C. F., & Silveira, C. D. C. (2003). Green chemistry: The 12 principles of green chemistry and it insertion in the teach and research activities. Química Nova, 26(1), 123–129.
- Leong, S. Y., Burritt, D. J., & Oey, I. (2016). Evaluation of the anthocyanin release and health-promoting properties of Pinot Noir grape juices after pulsed electric fields. Food Chemistry, 196, 833–841.
- Li, Y., Fabiano-Tixier, A. S., Vian, M. A., & Chemat, F. (2013). Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. TrAC Trends in Analytical Chemistry, 47, 1–11.
- Luengo, E., Álvarez, I., & Raso, J. (2013). Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innovative Food Science & Emerging Technologies, 17, 79–84.
- Machado, A. P. D. F., Pasquel-Reátegui, J. L., Barbero, G. F., & Martínez, J. (2015). Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Research International, 77, 675–683.
- Malaman, F. S., Moraes, L. A. B., West, C., Ferreira, N. J., & Oliveira, A. L. (2011). Supercritical fluid extracts from the Brazilian cherry (Eugenia uniflora L.): Relationship between the extracted compounds and the characteristic flavour intensity of the fruit. Food Chemistry, 124(1), 85–92.
- Manna, L., Bugnone, C. A., & Banchero, M. (2015). Valorization of hazelnut, coffee and grape wastes through supercritical fluid extraction of triglycerides and polyphenols. The Journal of Supercritical Fluids, 104, 204–211.
- Maran, J. P., Sivakumar, V., Thirugnanasambandham, K., & Sridhar, R. (2014). Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydrate Polymers, 101, 786–791.
- Meneses, M. A., Caputo, G., Scognamiglio, M., Reverchon, E., & Adami, R. (2015). Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. Journal of Food Engineering, 163, 45–53.
- Meullemiestre, A., Petitcolas, E., Maache-Rezzoug, Z., Chemat, F., & Rezzoug, S. A. (2016). Impact of ultrasound on solid–Liquid extraction of phenolic compounds from maritime pine sawdust waste. Kinetics, optimization and large scale experiments. Ultrasonics Sonochemistry, 28, 230–239.
- Moussa-Ayoub, T. E., Jaeger, H., Youssef, K., Knorr, D., El-Samahy, S., Kroh, L. W., & Rohn, S. (2016). Technological characteristics and selected bioactive compounds of Opuntia dillenii cactus fruit juice following the impact of pulsed electric field pre-treatment. Food Chemistry, 210, 249–261.
- Mustafa, A., & Turner, C. (2011). Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Analytica Chimica Acta, 703(1), 8–18.
- Nayak, B., Dahmoune, F., Moussi, K., Remini, H., Dairi, S., Aoun, O., & Khodir, M. (2015). Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chemistry, 187, 507–516.
- Nimet, G., da Silva, E. A., Palú, F., Dariva, C., dos Santos Freitas, L., Neto, A. M., & Cardozo Filho, L. (2011). Extraction of sunflower (Heliantus annuus L.) oil with supercritical CO2 and subcritical propane: Experimental and modeling. Chemical Engineering Journal, 168(1), 262–268.
- Oliveira, A. L., Destandau, E., Fougère, L., & Lafosse, M. (2014). Isolation by pressurised fluid extraction (PFE) and identification using CPC and HPLC/ESI/MS of phenolic compounds from Brazilian cherry seeds (Eugenia uniflora L.). Food Chemistry, 145, 522–529.
- Oroian, M., & Escriche, I. (2015). Antioxidants: Characterization, natural sources, extraction and analysis. Food Research International, 74, 10–36.
- Parniakov, O., Barba, F. J., Grimi, N., Lebovka, N., & Vorobiev, E. (2014). Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Research International, 65, 337–343.
- Parniakov, O., Barba, F. J., Grimi, N., Lebovka, N., & Vorobiev, E. (2016). Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chemistry, 192, 842–848.
- Parniakov, O, Lebovka, N. I, Van Hecke, E, & Vorobiev, E. (2014). Pulsed electric field assisted pressure extraction and solvent extraction from mushroom (agaricus bisporus). Food And Bioprocess Technology, 7(1), 174-183.
- Pederssetti, M. M., Palú, F., Da Silva, E. A., Rohling, J. H., Cardozo-Filho, L., & Dariva, C. (2011). Extraction of canola seed (Brassica napus) oil using compressed propane and supercritical carbon dioxide. Journal of Food Engineering, 102(2), 189–196.
- Pessoa, A. S., Podestá, R., Block, J. M., Franceschi, E., Dariva, C., & Lanza, M. (2015). Extraction of pequi (Caryocar coriaceum) pulp oil using subcritical propane: Determination of process yield and fatty acid profile. The Journal of Supercritical Fluids, 101, 95–103.
- Petigny, L., Périno, S., Minuti, M., Visinoni, F., Wajsman, J., & Chemat, F. (2014). Simultaneous microwave extraction and separation of volatile and non-volatile organic compounds of boldo leaves. From lab to industrial scale. International Journal of Molecular Sciences, 15(5), 7183–7198.
- Pouliot, Y., Conway, V., & Leclerc, P. L. (Eds.). (2014, April 11). Separation and concentration technologies In Food processing: principles and applications (2nd ed., pp. 33–60). doi:10.1002/9781118846315.ch3
- Pradal, D., Vauchel, P., Decossin, S., Dhulster, P., & Dimitrov, K. (2016). Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimization. Ultrasonics Sonochemistry, 32, 137–146.
- Pronyk, C., & Mazza, G. (2009). Design and scale-up of pressurized fluid extractors for food and bioproducts. Journal of Food Engineering, 95(2), 215–226.
- Rabelo, R. S., Machado, M. T., Martínez, J., & Hubinger, M. D. (2016). Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. Journal of Food Engineering, 178, 170–180.
- Rajha, H. N., Boussetta, N., Louka, N., Maroun, R. G., & Vorobiev, E. (2015). Effect of alternative physical pretreatments (pulsed electric field, high voltage electrical discharges and ultrasound) on the dead-end ultrafiltration of vine-shoot extracts. Separation and Purification Technology, 146, 243–251.
- Riera, E., Golas, Y., Blanco, A., Gallego, J. A., Blasco, M., & Mulet, A. (2004). Mass transfer enhancement in supercritical fluids extraction by means of power ultrasound. Ultrasonics Sonochemistry, 11(3–4), 241–244.
- Rodríguez-Pérez, C., Quirantes-Piné, R., Fernández-Gutiérrez, A., & Segura-Carretero, A. (2015). Optimization of extraction method to obtain a phenolic compounds-rich extract from moringa oleifera lam leaves. Industrial Crops And Products, 66, 246-254.
- Rodríguez-Pérez, C., Quirantes-Piné, R., Fernández-Gutiérrez, A., & Segura-Carretero, A. (2015). Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Industrial Crops and Products, 66, 246–254.
- Romo-Hualde, A., Yetano-Cunchillos, A. I., González-Ferrero, C., Sáiz-Abajo, M. J., & González-Navarro, C. J. (2012). Supercritical fluid extraction and microencapsulation of bioactive compounds from red pepper (Capsicum annum L.) by-products. Food Chemistry, 133(3), 1045–1049.
- Roselló-Soto, E., Koubaa, M., Moubarik, A., Lopes, R. P., Saraiva, J. A., Boussetta, N., … Barba, F. J. (2015). Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends in Food Science & Technology, 45(2), 296–310.
- Santos, D. T., Veggi, P. C., & Meireles, M. A. A. (2012). Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. Journal of Food Engineering, 108(3), 444–452.
- Santos, K. A., Bariccatti, R. A., Cardozo-Filho, L., Schneider, R., Palú, F., da Silva, C., & da Silva, E. A. (2015). Extraction of crambe seed oil using subcritical propane: Kinetics, characterization and modeling. The Journal of Supercritical Fluids, 104, 54–61.
- Segovia, F. J., Luengo, E., Corral-Pérez, J. J., Raso, J., & Almajano, M. P. (2015). Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Industrial Crops and Products, 65, 390–396.
- Seixas, F. L., Fukuda, D. L., Turbiani, F. R., Garcia, P. S., Carmen, L. D. O., Jagadevan, S., & Gimenes, M. L. (2014). Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating. Food Hydrocolloids, 38, 186–192.
- Silva, J. R., Cantelli, K. C., Soares, M. B., Tres, M. V., Oliveira, D., Meireles, M. A. A., … Mazutti, M. A. (2015). Enzymatic hydrolysis of non-treated sugarcane bagasse using pressurized liquefied petroleum gas with and without ultrasound assistance. Renewable Energy, 83, 674–679.
- Silva, L. P. S., & Martínez, J. (2014). Mathematical modeling of mass transfer in supercritical fluid extraction of oleoresin from red pepper. Journal of Food Engineering, 133, 30–39.
- Silva, R. P. F. F., Rocha-Santos, T. A. P., & Duarte, A. C. (2016). Supercritical fluid extraction of bioactive compounds. Trac Trends in Analytical Chemistry, 76, 40–51.
- Simha, P., Mathew, M., & Ganesapillai, M. (2016). Empirical modeling of drying kinetics and microwave assisted extraction of bioactive compounds from Adathoda vasica and Cymbopogon citratus. Alexandria Engineering Journal, 55(1), 141–150.
- Simić, V. M., Rajković, K. M., Stojičević, S. S., Veličković, D. T., Nikolić, N. Č., Lazić, M. L., & Karabegović, I. T. (2016). Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Separation and Purification Technology, 160, 89–97.
- Sivakumar, V., Ilanhtiraiyan, S., Ilayaraja, K., Ashly, A., & Hariharan, S. (2014). Influence of ultrasound on Avaram bark (Cassia auriculata) tannin extraction and tanning. Chemical Engineering Research and Design, 92(10), 1827–1833.
- Soares, J. F., Dal Prá, V., de Souza, M., Lunelli, F. C., Abaide, E., da Silva, J. R., & Mazutti, M. A. (2015). Extraction of rice bran oil using supercritical CO2 and compressed liquefied petroleum gas. Journal of Food Engineering, 170, 58–63.
- Sodeifian, G., Ghorbandoost, S., Sajadian, S. A., & Ardestani, N. S. (2015). Extraction of oil from Pistacia khinjuk using supercritical carbon dioxide: Experimental and modeling. The Journal of Supercritical Fluids, 110, 265–274.
- Solana, M., Mirofci, S., & Bertucco, A. (2016). Production of phenolic and glucosinolate extracts from rocket salad by supercritical fluid extraction: Process design and cost benefits analysis. Journal of Food Engineering, 168, 35–41.
- Soliva-Fortuny, R., Balasa, A., Knorr, D., & Martín-Belloso, O. (2009). Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends in Food Science & Technology, 20(11–12), 544–556.
- Song, S. M., Ham, Y. M., Ko, Y. J., Ko, E. Y., Oh, D. J., Kim, C. S., & Yoon, W. J. (2016). Anti-inflammatory activities of the products of supercritical fluid extraction from Litsea japonica fruit in RAW 264.7 cells. Journal of Functional Foods, 22, 44–51.
- Soquetta, M. B., Stefanello, F. S., da Mota Huerta, K., Monteiro, S. S., da Rosa, C. S., & Terra, N. N. (2016). Characterization of physiochemical and microbiological properties, and bioactive compounds, of flour made from the skin and bagasse of kiwi fruit (Actinidia deliciosa). Food Chemistry, 199, 471–478.
- Sricharoen, P., Limchoowong, N., Techawongstien, S., & Chanthai, S. (2016). A novel extraction method for β-carotene and other carotenoids in fruit juices using air-assisted, low-density solvent-based liquid–Liquid microextraction and solidified floating organic droplets. Food Chemistry, 203, 386–393.
- Strati, I. F., Gogou, E., & Oreopoulou, V. (2015). Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food and Bioproducts Processing, 94, 668–674.
- Tao, Y., Sun, D.-W., Górecki, A., Błaszczak, W., Lamparski, G., Amarowicz, R., & Jeliński, T. (2016). A preliminary study about the influence of high hydrostatic pressure processing in parallel with oak chip maceration on the physicochemical and sensory properties of a young red wine. Food Chemistry, 194, 545–554.
- Thirugnanasambandham, K., & Sivakumar, V. (In Press). Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. Journal of the Saudi Society of Agricultural Sciences, 16(1), 41–48.
- Tiwari, B. K. (2015). Ultrasound: A clean, green extraction technology. TrAC Trends in Analytical Chemistry, 71, 100–109.
- Vallverdú-Queralt, A., Odriozola-Serrano, I., Oms-Oliu, G., Lamuela-Raventós, R. M., Elez-Martínez, P., & Martín-Belloso, O. (2013). Impact of high-intensity pulsed electric fields on carotenoids profile of tomato juice made of moderate-intensity pulsed electric field-treated tomatoes. Food Chemistry, 141(3), 3131–3138.
- Vargas, R. M. F., Barroso, M. S. T., Neto, R. G., Scopel, R., Falcão, M. A., da Silva, C. F., & Cassel, E. (2013). Natural products obtained by subcritical and supercritical fluid extraction from Achyrocline satureioides (Lam) DC using CO2. Industrial Crops and Products, 50, 430–435.
- Viganó, J., Coutinho, J. P., Souza, D. S., Baroni, N. A., Godoy, H. T., Macedo, J. A., & Martínez, J. (2016). Exploring the selectivity of supercritical CO 2 to obtain nonpolar fractions of passion fruit bagasse extracts. The Journal of Supercritical Fluids, 110, 1–10.
- Vinatoru, M. (2001). An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrasonics Sonochemistry, 8(3), 303–313.
- Wang, Y., Wang, F., Ma, X., Sun, S., Leng, F., Zhang, W., & Wang, X. (2015). Extraction, purification, characterization and antioxidant activity of polysaccharides from Piteguo fruit. Industrial Crops and Products, 77, 467–475.
- Wu, C., Wang, F., Liu, J., Zou, Y., & Chen, X. (2015). A comparison of volatile fractions obtained from Lonicera macranthoides via different extraction processes: Ultrasound, microwave, Soxhlet extraction, hydrodistillation, and cold maceration. Integrative Medicine Research, 4(3), 171–177.
- Xhaxhiu, K., Korpa, A., Mele, A., & Kota, T. (2013). Ultrasonic and soxhlet extraction characteristics of the orange peel from “Moro” Cultivars grown in Albania. Journal of Essential Oil Bearing Plants, 16(4), 421–428.
- Xu, J.-K., Li, M.-F., & Sun, R.-C. (2015). Identifying the impact of ultrasound-assisted extraction on polysaccharides and natural antioxidants from Eucommia ulmoides Oliver. Process Biochemistry, 50(3), 473–481.
- Xu, Y., Cai, F., Yu, Z., Zhang, L., Li, X., Yang, Y., & Liu, G. (2016). Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chemistry, 194, 650–658.
- Xue, D., & Farid, M. M. (2015). Pulsed electric field extraction of valuable compounds from white button mushroom (Agaricus bisporus). Innovative Food Science & Emerging Technologies, 29, 178–186.
- Yang, Y.-C., & Wei, M.-C. (2015). Kinetic and characterization studies for three bioactive compounds extracted from Rabdosia rubescens using ultrasound. Food and Bioproducts Processing, 94, 101–113.
- Yen, H.-W., Yang, S.-C., Chen, C.-H., & Chang, J. S. (2015). Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresource Technology, 184, 291–296.
- Zaghdoudi, K., Framboisier, X., Frochot, C., Vanderesse, R., Barth, D., Kalthoum-Cherif, J., … Guiavarc’h, Y. (2016). Response surface methodology applied to Supercritical Fluid Extraction (SFE) of carotenoids from Persimmon (Diospyros kaki L.). Food Chemistry, 208, 209–219.
- Zanqui, A. B., de Morais, D. R., da Silva, C. M., Santos, J. M., Gomes, S. T. M., Visentainer, J. V., … Matsushita, M. (2015). Subcritical extraction of flaxseed oil with n-propane: Composition and purity. Food Chemistry, 188, 452–458.