?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Whey is a by-product of cheese manufacture and contains proteins that can be recovered, but lactose presence limits its use. Proteins can be separated with aqueous two-phase extraction (ATPE), thus the objective was to evaluate this strategy to recuperate proteins from whey attending lactose elimination. An ATPE system with (NH4)2SO4 and polyethylene glycol 4000 (PEG4000) was studied at 25°C. As PEG4000 diminished the volume ratio between phases diminished and a biphasic system was maintained in absence of the polymer. Minimum protein recovering and lactose elimination were 54.5% and 62.1% with mixtures having 25% PEG4000 and 12.1% (NH4)2SO4, but values were 93.0% and 72.3%, respectively, with 0.1% PEG4000 and 34.7% (NH4)2SO4. Therefore, whey incorporated with (NH4)2SO4 in concentration higher than 34% and without polyethylene glycol can be used in ATPE systems to maximize lactose elimination and protein recovering and to concentrate proteins up to 2.45 times through a non-energy strategy.
RESUMEN
El suero es un subproducto de la manufactura de queso y contiene proteínas que pueden ser recuperadas, pero la presencia de lactosa limita su uso. Las proteínas pueden ser separadas con extracción acuosa en dos fases (ATPE), por lo que el objetivo fue evaluar esta estrategia para recuperar proteínas del suero atendiendo la eliminación de lactosa. Se estudió un sistema ATPE formado con (NH4)2SO4 y polietilenglicol 4000 (PEG4000) a 25°C. A medida que PEG4000 disminuyó se redujo la relación de volúmenes entre fases y en ausencia del polímero se mantuvo un sistema bifásico. El rendimiento mínimo de recuperación de proteínas y eliminación de lactosa fueron 54.5% y 62.1% con mezclas de 25% PEG4000 y 12.1% (NH4)2SO4, pero los valores fueron 93.0% y 72.3%, respectivamente, con 0.1% PEG4000 y 34.7% (NH4)2SO4. Por lo tanto, el suero incorporado con (NH4)2SO4 en concentración mayor a 34% y sin polietilenglicol puede ser usado en un sistema ATPE para maximizar la eliminación de lactosa y la recuperación de proteínas y para concentrar proteínas hasta 2.45 veces a través de una estrategia no energética.
PALABRAS CLAVE:
1. Introduction
Whey is a by-product of cheese manufacture (Perumalsamy & Murugesan, Citation2012). Depending on raw material quality, between 8 and 9 kg of whey can be obtained from every 10 kg of milk (Nath et al., Citation2015). Due to a high chemical oxygen demand, whey represents an industrial concern from an environmental point of view (Masotti, Cattaneo, Stuknytė, & De Noni, Citation2016; Nath et al., Citation2015). This product contains soluble proteins, vitamins, and mineral salts (Kreczmann et al., Citation2015), which makes it attractive for food preparation (Liutkevičius et al., Citation2016) or food supplementation (Masotti et al., Citation2016). The major proteins of whey are α-lactalbumin (αla), β-lactoglobulin (βlg), and bovine serum albumin (BSA) (Perumalsamy & Murugesan, Citation2012). Both, αla and βlg are excellent source of essential amino acids (Alcântara et al., Citation2011) and, in general, whey proteins possess high biological value, because they are almost totally absorbed by the digestive system (Kreczmann et al., Citation2015). However, whey contains also certain amounts of fat (Fagan, Castillo, O’Callaghan, Payne, & O’Donnell, Citation2009) that hinder recovery of proteins (Torkamani et al., Citation2016), and high concentration of lactose (Kalaivani & Regupathi, Citation2015), which has been identified as the main problem for the use of this by-product (De Souza et al., Citation2010), because many people around the world suffer from lactose intolerance (Jelen & Tossavainen, Citation2003).
Whey protein recovering has been commonly based on membrane separation and spray drying (Anandharamakrishnan, Rielly, & Stapley, Citation2007; Kreczmann et al., Citation2015); however, affectation by heat treatment can occur. The use of an aqueous two phase extraction (ATPE) system is an alternative that avoids thermal procedures and is feasible to separate proteins (Asenjo & Andrews, Citation2012; Glyk, Scheper, & Beutel, Citation2015). In ATPE systems, a liquid–liquid separation occurs through mixtures of two polymers or a polymer and a salt, which in certain concentrations produce a true solution in a single phase, but in others they cause formation of two immiscible phases, among which the biomolecules present in the mixture are separated (Raja, Murty, Thivaharan, Rajasekar, & Ramesh, Citation2011). ATPE has been studied even to separate proteins in selective form (Gai, Qu, Zhang, & Zhang, Citation2011; Nitsawang, Hatti-Kaul, & Kanasawud, Citation2006).
In the case of whey, mixtures based on polyethylene glycol (PEG) and potassium phosphate or sodium citrate have shown potential to separate αla in the polymeric phase and βlg in the saline one (Alcântara et al., Citation2011; Giraldo, Reis, & Minim, Citation2001; Kalaivani & Regupathi, Citation2015). On the other hand, Anandharamakrishnan, Raghavendra, Barhate, Hanumesh, and Raghavarao (Citation2005) showed that fat may be separated using also ATPE, which makes the method attractive to be used with whey. However, the case of lactose elimination through ATPE has not been studied, but based on works of Chethana, Nayak, and Raghavarao (Citation2007) with betalains, such component could be retained also in the saline phase of an ATPE system, but it would remain in mixture with βlg making difficult its purification. The present work is focused on the recuperation of whey proteins attending the elimination of lactose, in order to prepare a raw material for further fractioning of αla and βlg. Anandharamakrishnan et al. (Citation2005) studied the system PEG6000/ammonium sulfate (AS) and found that as the mixture was far away from the equilibrium monophasic/biphasic condition the recovery can be increased, although with small separation yields, but the study did not consider a clear evaluation of the volume ratio effect between phases. The AS is an auxiliary compound in protein quantification methods due to its ability to cause protein precipitation (Fujita et al., Citation1993; Jinn, Yeh, Chen, & Lin, Citation1989) and, due to this, the present work was conducted under the premise that a more in-depth study would be necessary to favor or rule out its use in ATPE systems oriented to separate proteins from whey, attending the lactose elimination. The development of ATPE systems is often supported by phase diagrams that provide data about the binodal curve that separates concentrations that produce a monophasic system from those that cause a biphasic one, and about concentrations of phase components in top and bottom phases, and phase volume ratios (Raja et al., Citation2011). Data corresponding to phase diagrams for the PEG/AS system are available in literature (González-Amado, Rodil, Arce, Soto, & Rodríguez, Citation2016) and this can facilitate an evaluation of using such salt in ATPE systems. The objective of the present work was to evaluate the use of an ATPE system based on PEG and AS to eliminate lactose and separate proteins from whey.
2. Materials and methods
2.1. Cheese whey
Basket cheese was made from whole milk produced by Holstein cattle with the method described by Lobato-Calleros, Lozano-Castañeda, and Vernon-Carter (Citation2009). Whey was separated and subjected to evaluation of total sugars, protein, and fat contents (see sub-section 2.5).
2.2. Phase binodal diagram
Data corresponding to phase diagrams for the mixture of PEG [HO-(CH2CH2O)n-CH2OH], poly(ethane-1,2-diol) 4000 (P; PEG4000) with AS (NH4)2SO4 were used. According to González-Amado et al. (Citation2016), the binodal curve for this system is represented by the Merchuk model (Merchuk, Andrews, & Asenjo, Citation1998) in the form of Equation (1), where and
are concentrations (%) of (NH4)2SO4 and PEG4000, and k1 (%), k2, (%−0.5), and k3 (%−3) are regression constants with values of 63.18, −0.3036, and 0.0006845 at 5°C, 69.02, −0.3388, and 0.0008589 at 20°C, and 81.88, −0.4486, and 0.001118 at 35°C, respectively. Lagrange polynomials (Burden & Faires, Citation2010) were applied and constants were interpolated to have values of 72.53%, −0.3671%−0.5, and 0.000936%−3, respectively, for a temperature of 25°C, which is representative of environmental conditions in many cheese production regions.
In order to identify limit operation conditions, solutions with 40% PEG4000 (Sigma-Aldrich, Co., Germany) and 40% ammonium sulfate (J. Bayer, Mexico) were prepared using deionized water as solvent. The cloud point method (Li, He, Liu, Li, & Liu, Citation2005) was used to identify points corresponding to the highest concentrations of (NH4)2SO4 (,
) and PEG4000 (
,
) on the binodal curve. A straight line with slope k4 (Equation 2) was fitted with such points and was plotted together with Equation (1), constituting the main tie line (TLmain) of the diagram.
Secondary tie lines (TLsec), parallel to TLmain, were constructed. To do that, the critical point (C), defined as the condition where a tie line have zero length (Raja et al., Citation2011), was located deriving Equation (1) and the result was matched with the slope k4 (Equation 3), from which the abscissa was obtained, whose substitution in Equation (1) allowed to obtain the ordinate
.
From the point C(,
) a perpendicular to TLmain, with slope −1/k4 (Riddle, Citation1995), was drawn. The intersection between both (denoted as D) was determined and five equidistant points (E, F, G, H, and I) were located on such perpendicular, through which TLsec were drawn. Equation (4) represents the secondary tie line passing through the point E(
,
).
The intersections of each TLsec with the binodal curve (Equation 1) were determined with a substitution procedure and the length (TLL, %) was determined for each line through the Pythagorean theorem (Raja et al., Citation2011).
2.3. Volume ratio analysis
The volume ratio (Vr) between top (VT) and bottom (VB) phases of ATPE systems was defined in the form of Equation (5) and was evaluated at the middle point of TLmain, at the point D, and at other six points of the same line, with PEG4000 concentration of 30%, 25%, 15%, 5%, 0.5%, and 0.1%.
The Vr value was correlated with PEG4000 and (NH4)2SO4 concentrations and the state that allowed for equitable volume ratio (Vr = 1) was determined. A similar procedure was performed with TLsec. All mixtures were prepared at 25°C with deionized water as solvent.
2.4. ATPE systems based on (NH4)2SO4-PEG4000-whey
Mixtures with 30%, 25%, 15%, 5%, 0.5%, and 0.1% PEG4000 were again prepared at 25°C, but with whey as solvent instead of water. Phases were evaluated in terms of Vr and protein and lactose concentrations. A partition coefficient (K) and separation yields (YT, YB) were determined for each component with Equations (6) and (7), where cT, cB, and c0 are concentrations in top (T) and bottom (B) phases, and in the original whey volume (V0), respectively.
2.5. Response variables
Protein concentration was determined with the absorption method at 220 nm (Kamizake, Gonçalves, Zaia, & Zaia, Citation2003), with a Hach DR 5000 UV-Vis spectrophotometer (Hach, Mexico). Concentrations were expressed as mg equivalents of BSA per mL (mg mL−1) with the aid of a standard curve based on BSA in a range from 10 to 80 mg mL−1. Lactose concentration was quantified with the phenol-sulfuric method (Dubois, Gilles, Hamilton, Rebers, & Smith, Citation1956), with a spectrophotometer with a microplate reader (Biotek, Sinergy 2, BioTeck Instruments, U.S.A). A lactose standard curve in the range from 10 to 200 mg mL−1 was used to evaluate concentrations. In addition, fat content in the original whey was measured with a Milko Scan FT1 (Foss, Denmark).
2.6. Data analysis
The relationship between volume ratio (Vr), protein and lactose concentration, separation yields (YT, YB), and partition coefficient (K), with PEG4000 and (NH4)2SO4 concentrations, was analyzed. Data were submitted to regression routines and the determination coefficient (r2) was taken as indicator of the significance of change of behavior.
3. Results and discussion
3.1. Whey cheese composition
Whey had 1.02 (±0.01) % fat, 10.68 (±0.077) mg mL−1 protein, and 53.90 (±0.29) mg mL−1 lactose. Protein and lactose contents were of the same magnitude order as data reported by Kalaivani and Regupathi (Citation2015), who found values of 5.49 and 47.20 mg mL−1, respectively.
3.2. Phase binodal diagram
shows the binodal diagram obtained for the system (NH4)2SO4/PEG4000 with data of González-Amado et al. (Citation2016) corresponding to the binodal curve. PEG is one of the most used polymers in aqueous two phase systems and as molecular weight increases, the required quantity to form a biphasic system is lesser (Raja et al., Citation2011), but with higher molecular weight the system viscosity increases and may cause increment of the required time to achieve a complete separation, thus intermediate molecular weights are recommended (Regupathi, Srikanth, & Sindhu, Citation2011). In this regard, Jampani and Raghavarao (Citation2015) evaluated the recovering of anthocyanins from Brassica oleracea with ATPE systems using PEG with different molecular weights and found that the partition coefficient and the separation yield increased with an increment in molecular weight up to 4000 Da and decreased with further increments. For this reason, although phase diagrams were available with PEG4000 and PEG8000 (González-Amado et al., Citation2016), the present work was conducted using the former alternative. Points obtained with the cloud point method with the highest concentration of (NH4)2SO4 and PEG4000 had coordinates A(3.46, 35.25%) and B(34.78, 0.01%), on average (). The binodal curve of corresponds to a 25°C condition and was constructed through interpolation from data of González-Amado et al. (Citation2016). If the Equation (1) is subjected to a calculation of area under the binodal curve (AUC) with the Simpson’s 1/3 method (Burden & Faires, Citation2010), it is clear that a reduction in temperature induces an increase in AUC (), which indicates that the binodal curve is displaced to the right and suggests that with lower temperature higher concentrations of PEG4000 and (NH4)2SO4 are required to form a biphasic system. On the other hand, if the condition at 20°C of González-Amado et al. (Citation2016) is taken as reference, the binodal curve constructed at 25°C for the present work shows a difference of 5.49% in AUC, which constitutes the error that would be assumed if the system at 20°C were applied to the condition at 25°C.
Table 1. Areas under the binodal curve corresponding to data of González-Amado et al. (Citation2016) for the system (NH4)2SO4/PEG4000.
Cuadro 1. Áreas bajo la curva binodal correspondientes a datos de González-Amado et al. (Citation2016) para el sistema (NH4)2SO4/PEG4000.
Figure 1. (a) Phase diagram of the (NH4)2SO4/PEG4000 system at 25°C. Subscripts t and b correspond to top and bottom intersection points of tie lines with the binodal curve, respectively. (b) Relationship between volume ratio (Vr) and PEG4000 concentration for ATPE systems prepared with water and whey as solvent. MPm corresponds to the middle point of TLmain. The (NH4)2SO4 concentration in Et, Eb, Ft, Fb, Gt, Gb, Ht, Hb, It, and Ib was 9.64%, 18.38%, 8.10%, 21.65%, 6.93%, 24.90%, 5.69%, 28.25%, 4.52%, and 31.49%, respectively. An and Q: data of Anandharamakrishnan et al. (Citation2005).
Figura 1. (a) Diagrama de fases del sistema (NH4)2SO4/PEG4000 a 25°C. Los subíndices t y b corresponden a los puntos de intersección de las líneas de operación con la curva binodal. (b) Relación de volúmenes (Vr) y concentración de PEG4000 para sistemas ATPE preparados con agua y suero como disolvente. MPm corresponde al punto medio de TLmain. La concentración de (NH4)2SO4 en Et, Eb, Ft, Fb, Gt, Gb, Ht, Hb, It e Ib fue 9.64%, 18.38%, 8.10%, 21.65%, 6.93%, 24.90%, 5.69%, 28.25%, 4.52%, y 31.49%, respectivamente. An y Q: datos de Anandharamakrishnan et al. (Citation2005).
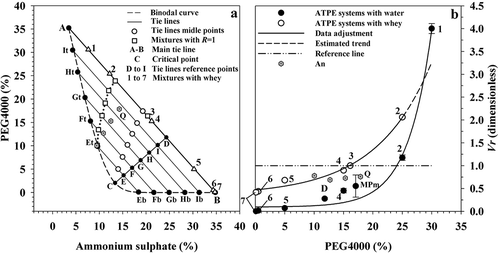
Points A and B delimited TLmain, which had slope k4 equal to −1.1252 (Equation 2). The application of Equation (3) allowed to obtain the critical point at C(13.35, 2.04%), from which a perpendicular to TLmain was drawn and the intersection between both was found at D(24.31, 11.78%). Along CD line, equidistant E to I points were located (), and through them the TLsec were plotted with lengths (TLL) 13.11, 20.42, 27.13, 33.93, 40.60, and 47.15% for TLE, TLF, TLG, TLH, TLI, and TLmain, respectively.
3.3. Volume ratio
Points 1, 2, and 4 to 7 in correspond to mixtures with PEG4000 (yP) at 30, 25, 15, 5, 0.5, and 0.1%, that showed volume ratio (Vr; Equation 5) equal to 4.00, 1.17, 0.45, 0.07, and 0.01, respectively, which means that such parameter decreased asymptotically to a value close to zero when yP diminished in water-based mixtures (), congruently (r2 = 0.9927) with Equation (8), where w represents Vr and k7, k8, and k9 are regression constants, with values equal to 0.0947 (dimensionless), 0.0025 (dimensionless), and 0.2453%−1, respectively.
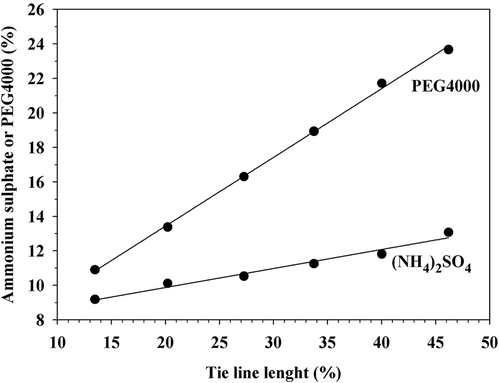
This behavior was expected, since in the absence of any of the components ((NH4)2SO4 or PEG4000) the ATPE system should tend to a single phase solution. On the other hand, in separation operations based on liquid–vapor equilibrium, the greater the difference between phase composition the easier the separation of components (McCabe, Smith, & Harriot, Citation2005). By analogy, in ATPE systems, with greater distance between the binodal curve and an operating condition, it is expected to have greater stability of the biphasic system, suggesting that maximum stability can be achieved with a mixture located on TLmain and particularly on point D, which is the most away from the binodal curve (). However, Vr had value of 0.2780 at such condition (), which means that the bottom phase was significantly greater than the top one. In a continuous operation, the separation of components based on ATPE can be implemented in a similar way to a continuous gravitational decantation (McCabe et al., Citation2005), and the phase extraction through overflows at the high and low regions of the equipment can be favored if the interface line is located at the middle of the system. However, based on Equation (8), the composition of mixtures with Vr = 1 on the main (TLmain) and also on secondary (TLsec) tie lines did not correspond to middle points, but to states located in the first upper third of tie lines when the system used water as solvent () and, as TLL was smaller, the condition for Vr = 1 was closer to the binodal curve, suggesting lower system stability. In addition, the relationship between TLL and concentration of (NH4)2SO4 and PEG4000 for Vr = 1 was linear (; Equations 9 and 10) and the required amounts of PEG4000 increased at a higher rate with TLL than those of (NH4)2SO4, which may be a disadvantage due to the polymer higher cost.
Figure 2. Relationship between tie line lengths and concentration of ammonium sulfate ((NH4)2SO4) and PEG4000 to obtain volume ratio Vr = 1.
Figura 2. Relación entre la longitud de las líneas de operación y la concentración de sulfato de amonio ((NH4)2SO4) y PEG4000 para obtener relación de volúmenes Vr = 1.
Based on these considerations and in order to evaluate the effect of PEG4000 concentration on whey protein purification, a study was made with mixtures located on the main tie line.
3.4. Protein and lactose separation
Mixtures for points 1, 2, and 4 to 7 of were prepared again, but whey was used as solvent instead of water. Mixture 1, which contained 30% PEG4000 and 7.5% ammonium sulfate, did not form a biphasic system, although it was located within the two-phase region in the binodal diagram prepared with water, suggesting that the equilibrium curve shifted to the right when whey was used as solvent. Systems 2 and 4–7 showed greater Vr than systems based on water at the same concentration of PEG4000 (). Similarly to the case of water-based systems, Vr decreased asymptotically and data fitted well (r2 = 0.9857) to Equation (8), but with values in constants k7, k8, and k9 equal to 0.3259 (dimensionless), 0.1236 (dimensionless), and 0.1053%−1, respectively. With this support, the whey-based mixture that allowed Vr = 1 had concentration of 20.01% (NH4)2SO4 and 16.13% PEG4000 (point 3; ). This condition contrasted with that identified when water was used as solvent (13.78, 23.63%, respectively; and was located near the middle point of TLmain, which strengthens the fact that the binodal curve shifted from the original. In this regard, Rito-Palomares and Hernandez (Citation1998) studied the effect of adding whey to an ATPE system formed by phosphate/PEG1000 with water. Authors chose six points near the monophasic–biphasic equilibrium curve and gradually added whey until a monophasic mixture was obtained. Results showed that the transition condition between phases shifted to the right at high concentration of PEG and this phenomenon may be due to presence of proteins, lactose, vitamins, and mineral salts in whey (Kreczmann et al., Citation2015), which alter the ionic balance. Also based on Equation (8), while systems prepared with water had Vr = 0 when =0, whey-based systems had Vr = 0.3259 at the same condition (). Although this result may seem unexpected, proteins are high molecular weight compounds formed by amino acids covalently linked together, forming long non-branched polymers (Nelson & Cox, Citation2008). In this regard, α-lactalbumin (αla) has 123 amino acid residues with molecular weight of 14,175 Da, while β-lactoglobulin (βlg) has 162 residues with molecular weight of 18,277 Da (Pihlanto, Citation2011). In this sense, when PEG4000 decreased, the role of the polymer in the two-phase system could be replaced by the polymeric characteristic of whey proteins and this explains that the biphasic structure of the system was maintained.
Among the most interesting characteristics of ATPE systems applied to purify proteins is the possibility of allowing a separation, in selective form, of one protein among a group, through partition between phases (Gai et al., Citation2011). In fact, Alcântara et al. (Citation2011) and Kalaivani and Regupathi (Citation2015) demonstrated the feasibility of separation of αla from βlg with this type of strategy. However, if the objective is to maximize the separation of all proteins, the results of the present work suggest that the use of a mixture of whey and (NH4)2SO4 in amounts required to form a biphasic system, could develop a non-energy strategy of protein concentration. In this regard, as PEG4000 concentration (yP) decreased, the protein concentration increased in the polymeric phase and decreased in the saline one (). In the first case, the behavior was congruent (r2 = 0.9821) with a logarithmic model with the form of Equation (11), where v represents protein concentration in the top phase (%) and constants k10, k11, and k12 had values equal to 32.2469 mg mL−1, 7.5125 mg mL−1, and 2.1430%, respectively. In contrast, data of the saline phase fitted well (r2 = 0.9895) to an exponential model with the form of Equation (8), where k7, k8, and k9 had values equal to 0.3367 mg mL−1, 1.0062 mg mL−1, and 0.0778%−1, respectively.
Figure 3. Effect of PEG4000 concentration on protein (a) and lactose (b) concentrations, separation yield (c), and partition coefficients (d) in ATPE systems. Circled numbers identify the ATPE systems signaled in .
Figura 3. Efecto de la concentración de PEG4000 sobre la concentración de proteína (a) y lactosa (b), rendimiento de separación (c) y coeficientes de partición (d) en sistemas ATPE. Los números encerrados en círculos identifican los sistemas ATPE señalizados en la .
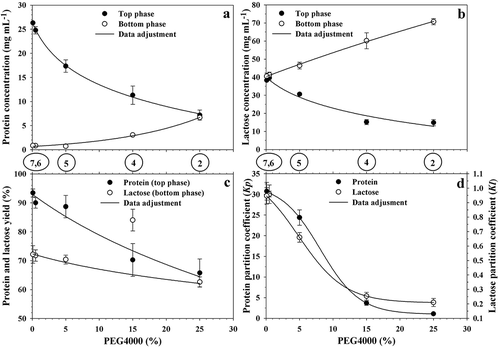
However, it should be considered that Vr decreased with PEG4000 concentration (), causing that the separation yield of protein (YT, Equation 7) increased in the upper phase as yP decreased (), in congruent form with Equation (11), where v represents YT, k10 = 206.5252%, k11 = 37.0026%, and k12 = 21.5045%, so that when the mixture had 25% PEG4000 and 12.1% (NH4)2SO4, YT was 54.45%, but with 0.1% PEG4000 and 34.7% (NH4)2SO4, YT increased to 92.99%, indicating high protein separation efficiency. The AS is an auxiliary compound in protein quantification methods due to its ability to cause protein precipitation (Fujita et al., Citation1993; Jinn et al., Citation1989). As a result, as the saline phase volume increased, a phase with high protein content was formed in the polymeric region. In system 2, which had the highest Vr value among the evaluated mixtures (), the protein concentration was similar between phases, whereas in system 7, where Vr had the smallest value, the greatest difference in protein concentration between phases occurred (). The protein concentration in the original whey was 10.68 mg mL−1, while value in the upper phase of system 7 was 26.18 mg mL−1, which means that protein was concentrated 2.45 times by a non-energy method formed by the ATPE system. Anandharamakrishnan et al. (Citation2005) studied the system PEG6000/(NH4)2SO4 and found that as the mixture corresponded to a higher tie line length the protein separation yield (YT) increased. shows the mixtures prepared by these authors, where maximum YT was 24.6%, which was obtained with a mixture having 17.88% PEG6000 and 14.26% (NH4)2SO4 that produced a volume ratio (Vr) equal to 0.76 (point Q, . Although the corresponding diagram could be slightly displaced to the left in relation to that prepared with PEG4000 (González-Amado et al., Citation2016), results of the present work suggest that Vr and PEG6000 concentration were not small enough and that of (NH4)2SO4 was not high enough to obtain high protein recovering, because all mixtures showed Vr around 0.74. On the other hand, protein recovering and protein fractioning have been frequently studied using potassium phosphate in the ATPE system (Alcântara et al., Citation2011; Giraldo et al., Citation2001). In fact, Anandharamakrishnan et al. (Citation2005) studied the ATPE system formed with PEG6000 and potassium phosphate and showed that with Vr equal to 13.5 it can be possible to have protein recovering from whey of 85%, although target compounds still remain much diluted, because the polymeric phase would be too large and the operation could be expensive due to the cost of the polymer.
shows the case of lactose behavior. As PEG4000 concentration decreased, the lactose concentration increased in the polymeric phase and decreased in the saline one, which seems to be an opposite effect to the desired goal of reducing lactose in the protein extract. The behavior was logarithmic, congruent with Equation (11), where v represents lactose concentration and k10, k11, and k12, had values equal to 59.4740 mg mL−1, 13.8419 mg mL−1, and 4.0770% in the case of top phase (r2 = 0.9535), and 455.5747 mg mL−1, 112.9901 mg mL−1, and 80.5589%, in the bottom one (r2 = 0.9698), respectively. However, as the polymeric phase volume decreased and that of the saline phase increased with reduction of PEG4000 concentration, the lactose separation yield in the saline phase increased (), which fitted well (r2 = 0.9686) to Equation (11), with k10 = 101.1726, k11 = 10.5688, and k12 = 15.2788%, from which the separation of lactose in the lower phase (YB, Equation 7) went from 62.11% with 25% PEG4000 to 72.29% with 0.1% of the polymer. The initial whey had lactose concentration of 53.90 mg mL−1 and, although at the almost total absence condition of PEG4000 (system 7, the upper phase of the ATPE system recorded 39.69% lactose (), the analysis of separation yield showed that, of the total, more than 70% of the disaccharide was retained by the lower phase (). This suggested that the best condition for separating whey proteins is an ATPE system without PEG, formed only with the addition of AS, because it allows the concentration of protein and the elimination of lactose in high percentage.
The partition coefficient K (Equation 6) had similar behavior for both protein and lactose (). The tendency was sigmoidal type, according to Equation (12), where regression constants k13 (dimensionless), k14 (dimensionless), k15 (%), and k16 (%) had values of 0.9735, 31.5051, 8.1000, and 2.9243 for the protein case (r2 = 0.9928), and 0.2070, 0.9240, 4.8594, and 3.4524 for the lactose one (r2 = 0.9895), respectively.
A value greater than unity in K means the number of times that a compound concentration is higher in the polymeric phase than in the saline one, whereas a number lesser than unity means that the saline part has higher concentration than the polymeric. In the case of protein, the partition ranged from 1.07 to 30.56 in the transition from 25.0 to 0.1% PEG4000, whereas for lactose the variation went from 0.21 to 0.95, indicating that the protein was mainly concentrated in the polymeric phase and the lactose in the saline one. On the other hand, whey contains fat that, although normally is present in small quantities (Fagan et al., Citation2009; Kreczmann et al., Citation2015), it has been identified as a factor that can cause fouling that hinders the recovery or proteins with processes that use ultrafiltration with membranes (Torkamani et al., Citation2016). In this regard and although fat elimination was not studied in the present work, Anandharamakrishnan et al. (Citation2005) showed that it can be retained within the saline phase of the ATPE system and would be separated at the same time of lactose. Authors explained that the presence of salts reduces the solubility and dispersibility of fat and this forms big droplets in salt rich phase.
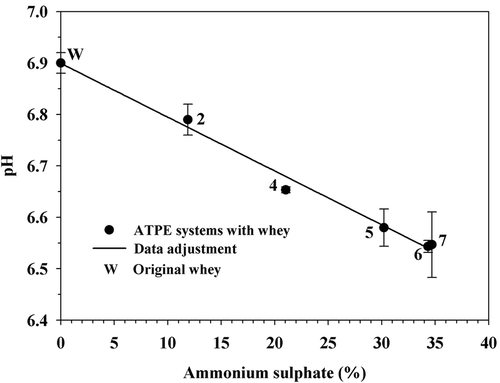
3.5. pH variation
The pH of the original whey was 6.90 (±0.02) and decreased linearly (r2 = 0.9502) at a rate of 0.0105%−1 as the concentration of (NH4)2SO4 increased and that of PEG4000 decreased in ATPE systems (). However, the difference between the original whey and the system with the highest concentration of (NH4)2SO4 was only 0.36 units, even though this compound forms solutions with acid pH (Benavides & Rito-Palomares, Citation2008). Hill, Irvine, and Bullock (Citation1985) reported a whey buffering capacity when pH was within 5.6 and 7.0, due to the content of phosphate salts, which may explain the observed behavior. The basket cheese whey contains β-lactoglobulin, α-lactalbumin, and BSA in approximate amounts of 60.0%, 30.0%, and 6.0% (Boaglio, Bassani, Picó, & Nerli, Citation2006), in relation to total protein content, with isoelectric points of 5.2–5.4, 4.7–5.1, and 4.9–5.1, respectively (Capezio, Romanini, Picó, & Nerli, Citation2005). The pH of systems of the present work was far more than one unit from the isoelectric point for the proteins contained in whey, which may be beneficial, because the solubility of such compounds is not modified to a great extent.
4. Conclusions
The ATPE system formed with (NH4)2SO4/PEG4000 allowed the separation of proteins and lactose from whey. The use of whey as dissolvent of the forming ATPE components caused displacement of the binodal curve in relation to the case that used water as dissolvent. The volume ratio (Vr) in the ATPE system affected partition between phases and separation yields of proteins and lactose. As PEG concentration diminished and that of (NH4)2SO4 increased the separation yield of proteins and lactose increased, thus small Vr values are recommended to maximize target biomolecules separation. Even in conditions of absence of PEG4000 of a biphasic system was still formed, which was attributed to the polymeric structure of proteins. The handling of whey incorporated with AS can be used as a non-energy strategy of concentration and purification of proteins of this by-product.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alcântara, L. A. P., Minim, L. A., Minim, V. P. R., Bonomo, R. C. F., Da Silva, L. H. M., & Da Silva, M. D. C. H. (2011). Application of the response surface methodology for optimization of whey protein partitioning in PEG/phosphate aqueous two-phase system. Journal of Chromatography B, 879, 1881–1885. doi:10.1016/j.jchromb.2011.05.007
- Anandharamakrishnan, C., Raghavendra, S. N., Barhate, R. S., Hanumesh, U., & Raghavarao, K. S. M. S. (2005). Aqueous two-phase extraction for recovery of proteins from cheese whey. Food and Bioproducts Processing, 83, 191–197. doi:10.1205/fbp.03403
- Anandharamakrishnan, C., Rielly, C. D., & Stapley, A. G. F. (2007). Effects of process variables on the denaturation of whey proteins during spray drying. Drying Technology, 25, 799–807. doi:10.1080/07373930701370175
- Asenjo, J. A., & Andrews, B. A. (2012). Aqueous two-phase systems for protein separation: Phase separation and applications. Journal of Chromatography A, 1238, 1–10. doi:10.1016/j.chroma.2012.03.049
- Benavides, J., & Rito-Palomares, M. (2008). Generic application of polyethylene glycol – Salt aqueous two phase systems for the development of processes to biological products primary recovery. Revista Mexicana De Ingeniería Química, 7, 99–111.
- Boaglio, A., Bassani, G., Picó, G., & Nerli, B. (2006). Features of the milk whey protein partitioning in polyethylene glycol-sodium citrate aqueous two-phase systems with the goal of isolating human alpha-1 antitrypsin expressed in bovine milk. Journal of Chromatography B, 837, 18–23. doi:10.1016/j.jchromb.2006.03.049
- Burden, R. L., & Faires, J. D. (2010). Numerical analysis (9th ed. ed.). Boston, MA: Brooks/Cole, Cengage Learning.
- Capezio, L., Romanini, D., Picó, G. A., & Nerli, B. (2005). Partition of whey milk proteins in aqueous two-phase systems of polyethylene glycol–Phosphate as a starting point to isolate proteins expressed in transgenic milk. Journal of Chromatography B, 819, 25–31. doi:10.1016/j.jchromb.2005.01.020
- Chethana, S., Nayak, C. A., & Raghavarao, K. S. M. S. (2007). Aqueous two phase extraction for purification and concentration of betalains. Journal of Food Engineering, 81, 679–687. doi:10.1016/j.jfoodeng.2006.12.021
- De Souza, R. R., Bergamasco, R., Costa, S. C., Feng, X., Faria, S. H. B., & Gimenes, M. L. (2010). Recovery and purification of lactose from whey. Chemical Engineering and Processing: Process Intensification, 49, 1137–1143. doi:10.1016/j.cep.2010.08.015
- Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356. doi:10.1021/ac60111a017
- Fagan, C. C., Castillo, M., O’Callaghan, D. J., Payne, F. A., & O’Donnell, C. P. (2009). Visible-near infrared spectroscopy sensor for predicting curd and whey composition during cheese processing. Sensory and Instrumentation for Food Quality, 3, 62–69. doi:10.1007/s11694-009-9073-5
- Fujita, M., Nomura, K., Hong, K., Ito, Y., Asada, A., & Nishimuro, S. (1993). Purification and characterization of a strong fibrinolytic enzyme (Nattokinase) in the vegetable cheese natto, a popular soybean fermented food in japan. Biochemical and Biophysical Research Communications, 197, 1340–1347. doi:10.1006/bbrc.1993.2624
- Gai, Q., Qu, F., Zhang, T., & Zhang, Y. (2011). Integration of carboxyl modified magnetic particles and aqueous two-phase extraction for selective separation of proteins. Talanta, 85, 304–309. doi:10.1016/j.talanta.2011.03.055
- Giraldo, Z. A. D., Reis, C. J. S., & Minim, L. A. (2001). Partitional coefficients of α-lactalbumin and β-lactoglobulin in aqueous two phase systems: Influence of molecular mass. Ciencia Y Tecnología Alimentaria, 3, 149–155. doi:10.1080/11358120109487722
- Glyk, A., Scheper, T., & Beutel, S. (2015). PEG–Salt aqueous two-phase systems: An attractive and versatile liquid–Liquid extraction technology for the downstream processing of proteins and enzymes. Applied Microbiology and Biotechnology, 99, 6599–6616. doi:10.1007/s00253-015-6779-7
- González-Amado, M., Rodil, E., Arce, A., Soto, A., & Rodríguez, O. (2016). The effect of temperature on polyethylene glycol (4000 or 8000)–(Sodium or ammonium) sulfate aqueous two phase systems. Fluid Phase Equilibria, 428, 95–101. doi:10.1016/j.fluid.2016.06.019
- Hill, A. R., Irvine, D. M., & Bullock, D. H. (1985). Buffer capacity of cheese wheys. Journal of Food Science, 50, 733–738. doi:10.1111/j.1365-2621.1985.tb13784.x
- Jampani, C., & Raghavarao, K. S. M. S. (2015). Differential partitioning for purification of anthocyanins from Brassica oleracea L. Separation and Purification Technology, 151, 57–65. doi:10.1016/j.seppur.2015.07.030
- Jelen, P., & Tossavainen, O. (2003). Low lactose and lactose-free milk and dairy products – Prospects, technologies and applications. Australian Journal of Dairy Technology, 58, 161–165.
- Jinn, T. L., Yeh, Y. C., Chen, Y. M., & Lin, C. Y. (1989). Stabilization of soluble proteins in vitro by heat shock proteins-enriched ammonium sulfate fraction from soybean seedlings. Plant and Cell Physiology, 30, 463–469. doi:10.1093/oxfordjournals.pcp.a077764
- Kalaivani, S., & Regupathi, I. (2015). Synergistic extraction of α-lactalbumin and β-lactoglobulin from acid whey using aqueous biphasic system: Process evaluation and optimization. Separation and Purification Technology, 146, 301–310. doi:10.1016/j.seppur.2015.03.057
- Kamizake, N. K. K., Gonçalves, M. M., Zaia, C. T. B. V., & Zaia, D. A. M. (2003). Determination of total proteins in cow milk powder samples: A comparative study between the Kjeldahl method and spectrophotometric methods. Journal of Food Composition and Analysis, 16, 507–516. doi:10.1016/S0889-1575(03)00004-8
- Kreczmann, B., Alonso, A., Liloia, M., Zamboni, E., Cerutti, R., Baroni, D., & Poluján, D. (2015). Procesamiento del lactosuero: Elaboración de lactosa y aprovechamiento de proteínas. Tecnología Láctea Latinoamericana, 87, 44–49.
- Li, S., He, C., Liu, H., Li, K., & Liu, F. (2005). Ionic liquid-based aqueous two-phase system, a sample pretreatment procedure prior to high-performance liquid chromatography of opium alkaloids. Journal of Chromatography B, 826, 58–62. doi:10.1016/j.jchromb.2005.08.005
- Liutkevičius, A., Speičienė, V., Kaminskas, A., Jablonskienė, V., Alenčikienė, G., Mieželienė, A., … Garmienė, G. (2016). Development of a functional whey beverage, containing calcium, vitamin D, and prebiotic dietary fiber, and its influence on human health. CyTA – Journal of Food, 14, 309–316. doi:10.1080/19476337.2015.1108366
- Lobato-Calleros, C., Lozano-Castañeda, I., & Vernon-Carter, E. J. (2009). Texture and microstructure of low-fat and low-cholesterol panela type cheeses: Different methodologies. Ingeniería Agrícola Y Biosistemas, 1, 39–48. doi:10.5154/r.inagbi.2009.05.009
- Masotti, F., Cattaneo, S., Stuknytė, M., & De Noni, I. (2016). An analytical approach to reveal the addition of heat-denatured whey proteins in lab-scale cheese making. Food Control, 63, 28–33. doi:10.1016/j.foodcont.2015.11.016
- McCabe, W. L., Smith, J. C., & Harriot, P. (2005). Unit operations of chemical engineering (7th ed.). New York, NY: McGraw-Hill Co. Inc.
- Merchuk, J. C., Andrews, B. A., & Asenjo, J. A. (1998). Aqueous two-phase systems for protein separation: Studies on phase inversion. Journal of Chromatography B: Biomedical Sciences and Applications, 711, 285–293. doi:10.1016/S0378-4347(97)00594-X
- Nath, A., Verasztó, B., Basak, S., Koris, A., Kovács, Z., & Vatai, G. (2015). Synthesis of lactose-derived nutraceuticals from dairy waste whey - a review. Food and Bioprocess Technology, 9, 16–48. doi:10.1007/s11947-015-1572-2
- Nelson, D. L., & Cox, M. M. (2008). Lehninger principles of biochemistry (5tn ed. ed.). New York, NY: W.H. Freeman and Company.
- Nitsawang, S., Hatti-Kaul, R., & Kanasawud, P. (2006). Purification of papain from Carica papaya latex: Aqueous two-phase extraction versus two-step salt precipitation. Enzyme and Microbial Technology, 39, 1103–1107. doi:10.1016/j.enzmictec.2006.02.013
- Perumalsamy, M., & Murugesan, T. (2012). Extraction of cheese whey proteins (α-lactalbumin and β-lactoglobulin) from dairy effluents using environmentally benign aqueous biphasic system. International Journal of Chemical and Environmental Engineering, 3, 50–54.
- Pihlanto, A. (2011). Whey proteins and peptides. Nutrafoods, 10, 29–42. doi:10.1007/bf03223386
- Raja, S., Murty, V. R., Thivaharan, V., Rajasekar, V., & Ramesh, V. (2011). Aqueous two phase systems for the recovery of biomolecules – A review. Science and Technology, 1, 7–16. doi:10.5923/j.scit.20110101.02
- Regupathi, I., Srikanth, C. K., & Sindhu, N. (2011). Liquid-liquid equilibrium of poly(ethylene glycol) 2000 + diammonium hydrogen citrate + water system at different temperatures. Journal of Chemical & Engineering Data, 56, 3643–3650. doi:10.1021/je200485x
- Riddle, D. F. (1995). Analytic geometry (6th ed.). Boston, MA: Cengage Learning, Inc.
- Rito-Palomares, M., & Hernandez, M. (1998). Influence of system and process parameters on partitioning of cheese whey proteins in aqueous two-phase systems. Journal of Chromatography B: Biomedical Sciences and Applications, 711, 81–90. doi:10.1016/S0378-4347(98)00011-5
- Torkamani, A. E., Juliano, P., Fagan, P., Jiménez-Flores, R., Ajlouni, S., & Singh, T. K. (2016). Effect of ultrasound-enhanced fat separation on whey powder phospholipid composition and stability. Journal of Dairy Science, 99, 4169–4177. doi:10.3168/jds.2015-10422