ABSTRACT
Unpasteurized artisanal cheeses are a source of pathogens for humans. We isolated lactic acid bacteria (LAB) from raw milk and from cheeses made with raw milk, and formulate starter cultures for producing an artisanal Mexican cheese known as ‘Queso Bola de Ocosingo’. We evaluated six combinations of strains as starter culture-treatments. The proximate composition of the cheeses, the sensorial evaluation, and the microbiological safety of cheeses made with pasteurized milk was evaluated. The use of starter culture helped to maintain the pathogen microbial total count of pasteurized cheese below the standard limit maximums. The sensory judges did not find differences among the cheese elaborated with unpasteurized milk and that elaborated with pasteurized milk and the starter culture added. The starter culture formulated with LAB derived from unpasteurized cheese can be used for producing ‘Bola de Ocosingo’ cheese which is sensory acceptable and microbiological safe.
RESUMEN
Los quesos artesanales no pasteurizados son una fuente de patógenos para los consumidores. Se aislaron y seleccionaron 23 bacterias lácticas (BAL) a partir de leche cruda y de quesos elaborados con leche cruda, y se formularon seis cultivos iniciadores (tratamientos) para la elaboración de Queso Bola de Ocosingo, un queso artesanal mexicano. Se evaluaron la composición proximal, la inocuidad microbiológica y las características sensoriales de los quesos. La combinación de 6 BAL usadas como cultivo iniciador ayudó a mantener las cuentas de microorganismos indicadores de contaminación en el queso pasteurizado por debajo de los límites máximos permitidos por la normativa local. Los panelistas no encontraron diferencias entre el queso elaborado con leche no pasteurizada y el elaborado con leche pasteurizada y añadido con el cultivo iniciador. El uso de este cultivo iniciador formulado con bacterias ácido lácticas obtenidas de queso no pasteurizado es útil para la producción de queso Bola de Ocosingo pasteurizado, el cual resulta sensorialmente aceptable y microbiológicamente inocuo.
1. Introduction
The cheese ‘Queso Bola de Ocosingo’ is artisanal and representative of the municipality of the same name (Ocosingo) in the Chiapas State, Mexico (González-Córdova et al., Citation2016). In 2004 the trademark registration for this dairy product was obtained in Mexico. Queso Bola de Ocosingo is unusual compared with other artisanal Mexican cheeses because it is formed from two cheeses, one bundled inside another. Its production process is peculiar and has been passed from generation to generation since the arrival of cattle livestock in the region. It is a matured cream cheese, covered with a double lining of spun curd. The maturation is realized in two stages, the first at room temperature (average 30°C) for 7 days, and the second at a temperature of 5°C for 14 days (González-Córdova et al., Citation2016). For processing, unpasteurized milk is used, the process is artisanal (handmade) so that the biochemical changes which occur during elaboration and maturation are a result of the action of the particular microorganisms and materials used, influencing the unique sensory characteristics of this product. Nevertheless, by this same process, these characteristics can become heterogeneous among different lots of production.
On the other hand, it has been documented that Mexican cheeses elaborated with unpasteurized milk can become a medium for transmission of pathogens of dire importance to public health (Guzman-Hernandez et al., Citation2016). In addition, there is a growing social interest in assuring food safety. This requires the producers of artisanal cheeses, including those of Queso Bola de Ocosingo cheese, to implement strategies for good manufacturing practices, which guarantee the destruction of possible pathogens that develop in raw milk. Examples of these measures are microfiltration and pasteurization of milk. Pasteurization is the primary choice because of its low cost and microbicide efficiency (Codex Alimentarius, Citation2011) however, upon implementing this procedure, the sensory characteristics of the product are greatly altered since this mainly eliminates the bacteria responsible for the production of metabolites which confer sui generis sensory characteristics upon the product, generally from a group of lactic acids (Frau, Font de Valdez, & Pece, Citation2014). To reestablish the original characteristics of the cheese, the use of starter cultures, with a capacity for producing the desired, safe metabolites for human consumption, is an appropriate alternative. Additionally, the use of starter cultures inhibits the development of undesirable microorganisms, producing compounds which do not allow for their growth (organic acids, bacteriocins, hydrogen peroxide, etc.).
Starter cultures generally consist of lactic acid bacteria (LAB), mainly of genus Lactococcus, Lactobacillus, and Leuconostoc. The isolation and selection of LAB, as well as its use on pasteurized milk, have helped the obtaining of cheeses with characteristics similar to those elaborated with unpasteurized milk. Widespread works have reported the use of these bacteria in the production of cheeses around the world (González, Fernández-Cuadrillero, Castro, Bernardo, & Tornadijo, Citation2015; Su, Yeon, Young, Hun, & Kim, Citation2016). Lactobacillus casei, Leuconostoc mesenteroides, Streptococcus thermophilus, Lactobacillus helveticus, and Lactobacillus lactis are examples of LAB species employed in cheeses of different origins and textures. The common thread among the bacteria employed as starter cultures is that they have been derived from the corresponding unpasteurized milk, that is to say, they are indigenous strains. However, to this date reports do not exist of the development of starter cultures which could be employed in the elaboration of the traditional Mexican cheese ‘Queso Bola de Ocosingo.’ From the preceding, the objective of this work was to evaluate the potential for indigenous lactic acid bacteria as a starter culture in the elaboration of ‘Queso Bola de Ocosingo’ cheese from pasteurized milk.
2. Materials and methods
2.1. Samples and characterization
At summer of 2014, with sanitized instruments, 500 mL of unpasteurized milk and one cheese (500 g approximately) elaborated from unpasteurized milk were collected from each of the following small producers of cheese: 1) Quesos Laltic, 2) Quesos Santa Isabel, 3) Quesos Bulushbac and 4) Quesos Santa Rosa; all located in the municipality of Ocosingo, Chiapas, Mexico. Five samples (composite sample) were obtained from each producer site; in total 20 samples of unpasteurized milk and 20 cheese samples. The samples were transported to the laboratory at 4°C, where the following tests were performed: a) isolation of lactic acid bacteria; b) physicochemical analysis (milk temperature, density, alcohol stability, heat stability, acidity, fat, pH, and total solids) in accordance with methodologies described by AOAC (Citation2010); c) proximate analyses of the cheeses: moisture, ash, fat, protein (AOAC, Citation2010); and d) microbiological analysis of milk and cheese: aerobic mesophiles, total coliforms, fungi, and yeast, in accordance with methodology established by the analytic manual of bacteriology of the FDA (Citation2011).
2.2. Isolation of lactic acid bacteria
From the composite sample, in triplicate, 200 g of cheese or 200 mL of raw milk, respectively were aseptically collected and homogenized in 180 mL of a 2% sodium citrate solution pH 8.75 (Settanni et al., Citation2013). Serial dilutions to 10–8 were prepared (in triplicate) and 100 µL were plating on selective media for LAB. The MRS (BD DifcoTM Detroit, USA) agar incubated at 37°C and anaerobic conditions (achieved by injecting of CO2 into a sealed container) for Lactobacillus and the M17 (BD DifcoTM Detroit, USA) agar incubated at 25°C for Lactococcus for up to 5 days (Speranza, Bevilacqua, Corbo, Altieri, & Sinigaglia, Citation2015). The bacteria with different morphology were isolated and 23 strains were selected. Additionally, each strain was evaluated for catalase activity, peroxidase activity, microscopy morphology (Axiolab, Carl Zeiss Jena Germany), and Gram stain following the procedure described by Oberg et al. (Citation2016).
2.3. Acidifying capacity of LAB
For the isolated strains, their capacity to diminish the pH of pasteurized milk was evaluated by kinetics at temperatures of 30, 37, and 42°C. First, selected colonies were set in MRS broth for Lactobacillus or Elliker broth (BD DifcoTM Detroit, USA) for Lactococcus, and incubated at 37°C for 24 h or until reaching a concentration of 108 CFU mL−1. Following this, 500 µL of broth were collected from each of the strains and inoculated in flasks with 50 mL of pasteurized low-fat milk. They were incubated under agitation at 125 rpm and their pH was measured at 0, 1, 2, 3, 4, 5, 6, 12, and 24 h. The acidification continued until 72 h and, afterward, the milk was centrifuged at 12,000 g for 10 min in a Beckman GS-15R centrifuge, the supernatant was collected, pasteurized at 85°C for 5 min (to stop enzymatic activity), and used to determine lactic acid (Østergaard, Eklöw, & Dalgaard, Citation2014).
2.4. Compatibility among strains
To show the compatibility between the 23 selected strains, the method of diffusion in agar (Herreros et al., Citation2005) was used. Each of the strains was grown during 24 h in MRS or Elliker broth and later plating on MRS agar (each of the LAB, individually). One hour after inoculation at 30°C, five wells were cut into the agar plate with a sterile cork borer with 5 mm diameter. After this and separately, we added 50 µL of the supernatant free from cells of each study strain (previously grown during 24 h in MRS or Elliker broth). The dishes were incubated for 24 h at 37°C and the presence or absence of inhibition halos was noted around wells. The absence of halo denotes compatibility between the inoculated strain and the producer of the respective supernatant. Each plate assay was repeated until we completed 22 clashes for each of the strains.
2.5. Elaboration of the cheese
Based on the source of the strains (cheese or milk), the compatibility among them, and acidifying capacity, the combinations of bacteria shown in were established, obtaining in every case starter cultures of mixtures of strains to be added in the elaboration of the cheese. Starter cultures were grown in broths MRS or M17 at 30°C for 24 h. After 24 h, the broths were centrifuged (24,000 g for 5 min at 4°C in a Beckman GS-15R centrifuge) and the pellets were transferred to 10% skim milk, grown at 30°C for 24 h (to achieve cell concentration and eliminate the adaptation phase).
Table 1. Combination of LAB used as starter in the manufacture of ‘Bola de Ocosingo’ cheese.
Tabla 1. Combinación de BAL empleadas como cultivos iniciadores en la elaboración del queso Bola de Ocosingo
For elaboration of cheese, 20% (v v−1) of cream was added to 100 L of cow’s milk, which was pasteurized at 63°C for 30 min, afterwards 0.01% (p v−1) of CaCl2 was added, and finally 1% (v v−1) of the lactic acid bacteria culture (108 CFU mL−1) was added to be tested according to the treatment ().
The milk with the starter culture was maintained at 37°C for 2 h, rennet was subsequently added (Cuajamex®) in the proportion of 1:10,000 v v−1 and this was left to rest for 24 h. After this time, the curd was cut and put into crude cotton bags in order to drain them for 4 days until obtaining a semi-hard consistency; NaCl was added gradually by hand until totally salted (4–6%) changing the crude cotton cover every 24 h. It was matured under refrigeration (3–5°C) for 21 d. At the end of this time, it was shaped into the form of a ball, and a first lining was applied. To made the outer lining (forro), the milk was completely skimmed, then pasteurized and congealed under the same conditions as the creamed cheese. It was left to set for 50 min, the curd was cut and left under the crude cotton cover for 24 h until reaching acidity of 37° Dornic (when it formed strands). When the acidity was reached, the formed cheese was coated for the first time, and after 24 h the second lining was applied.
2.6. Microbiological quality of cheese
After 21 days of storage and maturation, the microbiological analyses were performed: aerobic mesophilic bacteria, total coliforms bacteria, fungi, and yeast, in accordance with the methodology established by the FDA (Citation2011).
2.7. Sensory evaluation
To evaluate the effect of the starter cultures on the sensory characteristic of cheese, two types of tests were conducted at different time. First, a test for acceptability was conducted with 80 consumers (52 women and 28 men with age range of 18–40 years old) were called together who normally eat cheese and who had at least tried ‘Queso Bola de Ocosingo’ cheese. They were given samples of 1 cm3 of each of the elaborated cheeses, identified with three-digit codes, and these consumers were asked to show their level of liking for each of the samples on a Hedonic Scale of 9 points, where 1 = dislike extremely and 9 = like extremely. With the results obtained from this test, the cheese which received the greatest level of satisfaction was selected. For the second test, the cheese selected was compared with the cheese elaborated from unpasteurized milk (the control), by means of a triangular test with a panel of eight expert judges (cheesemakers with more than 30 years of experience). The evaluation was conducted in a single session in a spacious, enclosed area with adequate lighting and a temperature of 25°C. For this test, the evaluators were each provided in triplicate two samples (portions of 1 cm3 of each cheese), one from each process of elaboration (coded with three-digit numbers) compared with the control sample, and they were asked to identify the two identical samples. In addition, the judges were asked to give a score for the attributes of external characteristics (shape of cheese, consistency, and color of the lining), characteristics of the curd (texture and color), aroma, flavor, and general acceptability. The highest score for a sample would be 20 points divided in the following way: external characteristics 3 points, characteristics of the curd 5 points, aroma 3 points, flavor 6 points, and acceptability 3 points. The results were graded by means of a points score (Esmerino et al., Citation2013).
2.8. Biochemical characterization of the LAB
The selected strains (from the best treatment) were cultivated in MRS or M17 broth. Subsequently following the recommendations of the manufacturer, the strains were characterized using the API50 CHL® (for Lactobacillus) and API20® STREP (for Lactococcus) systems.
2.9 Statistical analysis
The data obtained from the physicochemical variables were submitted to analysis of variance and subsequent comparison of means by the multiple range test of Duncan (α = 0.05). In the acceptability sensory test a non-parametric analysis of variance (test Kruskal Wallis α = 0.05) was carried out and subsequent comparison of means by the Dunn test with correction by Bonferroni; the data obtained from the triangular test was analyzed by a chi-square test with a −0.5 factor of correction for continuity (Lawless & Heymann, Citation2010). The analyses were realized using the statistical packages STATGRAPHIC PLUS for Windows and XL STAT v 2013.
3. Results and discussion
3.1. Physicochemical and microbiological characterization
The cheesemaking quality and performance depend to a great extent on the physicochemical and microbiological characteristics of the raw material used for the elaboration. shows the physic-chemical characteristics of the milk from the four sampling locations. In accordance with these parameters, all milk samples are within the criteria established to be considered as acceptable and without significant difference (p > 0.05) for the majority of analyzed parameters. It can be seen that results obtained for density, acidity, and fat are practically identical, which is unusual for samples of different origins, and which can be partly attributed to the fact that once obtained the milk is transferred to cheesemaking factories where it is immediately combined in order to obtain homogeneity. Other factors which can explain the numerical equality of almost all parameters is that the nutrition received by dairy cattle is identical in the region where the samples were gathered (Ocosingo, Chiapas, Mexico) and this is related directly to the type and quality of forage (grass called ‘Estrella’). Also, all cattle ranches possess very similar breeds of stock, such as European Swiss, American Swiss, and Zebu (which includes breeds of zebu: Indobrazil, Guzerat, Brahman, and Nerole). Also, all the samples presented heat stability and alcohol stability values of 90ºC and up to 85% ethanol, respectively.
Table 2. Physicochemical characteristics of raw fresh milk used in the manufactured of Bola de Ocosingo cheese.
Tabla 2. Características fisicoquímicas de la leche bronca empleada en la elaboración del queso Bola de Ocosingo
Similar to the findings for the milk, the cheeses, in spite of being elaborated by artisanal processes, possessed equal chemical composition (p > 0.05), explained in part by the homogeneity of components which were present in the samples of milk from distinct sites. Based on the moisture content without fat (MCWF), the Bola de Ocosingo cheese is characterized as being a firm or semi-firm cheese and by the process which it undergoes after elaboration is considered to be a matured cheese (Codex Alimentarius, Citation2011). In it is shown that by its fat content (average of 37.66% from the four samples), the queso bola de Ocosingo is classified as a semi-fat cheese according to Mexican Regulations (NMX-F-713-COFOCALEC-Citation2014; range 25–45%). This value is lower than cheeses produced with similar technology. As an example, the semi-hard cheese San Simón da Costa from Galicia, elaborated from pasteurized cow’s milk, with lactic cultures added, reports a fat content of 45% d.b. (González et al., Citation2015).
Table 3. Proximate composition (g 100 g−1) of ‘Bola de Ocosingo’ cheeses from several manufacturer´s elaborated with raw milk.
Tabla 3. Composición proximal (g 100 g−1) de quesos Bola de Ocosingo elaborados con leche sin pasteurizar obtenidos de diferentes productores artesanales.
The four samples of raw milk used in the elaboration of Bola de Ocosingo cheese showed an elevated microbial count (), principally a high number of total coliform colonies, with the principal reason for this being that the milk production sites show deficiencies in their sanitary practices and hygiene in milking, so also in the same are included the values of these parameters for the samples of Bola de Ocosingo cheese produced from unpasteurized milk. The sources of contamination and possible causes for the high microbial count in the cheese are, milk residue remaining on the surfaces of the apparatus used for milking and storage of milk, unclean udders or not cleaned before milking, and no rapid refrigeration of milk (Guzman-Hernandez et al., Citation2016). Furthermore, the presence of coliforms becomes an indicator of the degree of fecal contamination, in the case of raw milk, and is transformed into an evaluation of the degree of cleanliness of the hands of machine operators, the cleanliness and disinfection of the skin of the udder, among other factors (Guzman-Hernandez et al., Citation2016). These scenarios are the principal reason why regulations indicate that producers should pasteurize milk prior to the elaboration of cheese since in accord with the Official Mexican Regulations, the presence of coliform microorganisms is the indicator most related with inadequate hygienic practices and in the evaluation of the microbiological quality of the product (NOM-243-SSA1-Citation2010). Coliform bacteria are always present in milk, but their numbers should be reduced (<100 CFU mL−1) in hygienic conditions. The coliform values found in the milk samples, furthermore indicate inefficient transport and storage (temporary) practices for the milk, since it has been reported that the cooling milk (<10°C) diminishes the growth of this type of bacteria. The climatological conditions of the municipality of Ocosingo, Mexico (27°C on average) are a stimulating factor for the rapid growth. Eventhough the only cheesemaking process could reduce the number of coliforms, this is not enough for safe cheese production, since all unpasteurized cheese results are above the permitted maxima by local regulations ().
Table 4. Microbial total count of raw milk* (CFU mL−1), cheese make with raw milk** and, cheese manufacturer from pasteurized milka and added with starter cultureb (CFU g−1).
Tabla 4. Cuentas microbiológicas de la leche sin pasteurizar* (UFC mL−1), del queso elaborado con leche sin pasteurizar** y del queso elaborado con leche pasteurizada y cultivos iniciadores (UFC g−1).
3.2. Selection of lactic acid bacteria
In total, 56 colonies were selected and analyzed from all the samples, which were compared with each other, and based on the morphology of the colony and microscopic study this was reduced to a final collection of 23 strains (seven strains derived from samples of the Laltic company, six from Santa Rosa company, three from Santa Isabel company, and six from Bulushbac company). From the milk (Code L, ) 10 strains were selected, five in the MRS agar for Lactobacillus and five in the M17 agar for Lactococcus. From the Bola de Ocosingo cheese (Code Q, ) 13 strains were selected, eight in the MRS agar and five in the M17 agar. From the 23 strains, ten had presumed characteristics of the genus Lactobacillus and 13 of the genus Lactococcus. Similar results were reported by Centeno, Cepeda, and Rodriguez (Citation1996), who derived LAB from genus Lactobacillus and Lactococcus in M17 agar from samples of cheese and milk. The proportions reported by the authors were 55% Lactococcus and 32% Enterococci. Furthermore, when they used the medium chosen for LAB (MRS medium, pH 5.5), only 42% of the isolates corresponded to Lactobacilli and 40% to Lactococcus.
The LAB present in the milk depends on the hygienic quality of the milking. For example, Lactococcus spp. were the most encountered microorganisms in milk for the elaboration of cheese with small eyes, while Lactobacillus spp. were the most numerous in milk used for the elaboration of Swiss and Italian cheeses (Leroy & De Vuyst, Citation2004). In artisanal cheeses, the dominant lactic microorganisms in the first days of maturation are Lactococcus spp. and its prevalence can be explained by the elevated number of these bacteria in the milk. In post-maturation stages (30–45 days) the proportion of Lactococcus spp. y Lactobacillus spp. is inverted (Randazzo, Torriani, Akkermans, de Vos, & Vaughan, Citation2002).
3.3. Acidifying capacity of the strains and production of lactic acid
All the selected strains showed the same behavior in the acidification test (), regardless of the temperature evaluated (30, 37, and 42°C). This coincides with typical behavior reported for lactic acid bacteria (Østergaard et al., Citation2014). The values of pH obtained at 24 h (average of 4.7) are equivalent to the acidity of Queso de Bola cheese produced traditionally (4.6) which indicates these strains are adequate from this perspective for use in the process of fabrication of Bola de Ocosingo cheese.
Table 5. pH kinetic (37°C) and lactic acid production (of selected strains as starters) of LAB derived from raw milk (L) and ‘Bola de Ocosingo’ cheese (Q).
Tabla 5. Cinética del pH a 37°C y cantidad de ácido láctico producido de las BAL (seleccionadas como iniciadores) obtenidas de leche bronca (L) y del queso Bola de Ocosingo (Q).
Although all strains showed the same behavior in decreasing pH (), as a criterion for selection, the strains having the lowest values of pH were located. The strains 2L, 3L, 6L, 7L, 9L, 14Q, 16Q, 17Q, 18Q, 19Q, 20Q were selected to be used in the starter cultures. The decrease in pH is a result of the fermentation of lactose. The principal acid produced from the metabolism of the LAB is lactic acid, which, along with other metabolites produced by LAB (hydrogen peroxide, bacteriocins, peptides, etc.) has an important effect on other microorganisms considered pathogenic or undesirable. Aside from increasing the shelf life of the product, the presence of lactic acid has other effects such as gelation of protein, syneresis and formation of flavor and aroma, as its presence is associated with a smooth and agreeable acidic taste, which does not dominate over other aromatic components and rather enhances the flavor (Kieronczyk, Skeie, Langsrud, & Yvon, Citation2003). The inhibitory effect of the metabolites produced by the LAB is seen helped by other mechanisms of stress which in summary reduce the presence of pathogenic microorganisms. A reduction in water activity and the presence of additional salts in the process of elaboration of the cheese is cited as the most important (Bozoudi et al., Citation2015).
Individually, for the strains that showed the lowest values of pH, the content of lactic acid was determined (). The values obtained (0.84–1.38 g L−1) are slightly higher than those reported by other Lactobacillus (Pescuma, Hébert, Mozzi, & Font, Citation2010). Two strains derived from cheese presented high values in the production of lactic acid, these were 18Q and 20Q, and they were later identified as Lactobacillus helveticus and Lactococcus lactis ssp. cremoris, respectively ().
Table 6. Presumptive identification of LAB based on biochemical characterization used API® 50 CHL and API® 20 STREP.
Tabla 6. Identificación preliminar de las BAL con base en las características bioquímicas mostradas usando los sistemas API® 50 CHL y API® 20 STREP.
3.4. Compatibility among strains
In spite of the great variety of substances which the LAB´s are capable of producing, including metabolites of oxygen (hydrogen peroxide), bacteriocins, acetaldehydes, and D-isomers of amino acids (Geria & Caridi, Citation2014), in our study all the strains displayed compatibility, that is to say no strain impeded the development of another in the test for growth in Petri dish, so that all combinations of starter cultures were feasible. If antagonism among lactic acid bacteria occurred, this could reduce their potential, such as proteolytic, acidifying capacity (Bozoudi et al., Citation2015) hence the importance of checking this trait.
3.5. Microbiological quality of cheese elaborated with pasteurized milk
With heat treatment (pasteurization) the initial microbial total count present in milk was reduced, both of aerobic mesophiles, total coliforms, as well as fungi and yeast that exceeded the permitted maximums in all samples of products unpasteurized (indicated with one and two asterisks, ). In the same , the average values are presented for counting of indicator microorganisms in cheeses matured for 21 d, from the five treatments where milk was pasteurized and starter cultures were added (for treatments see ). It can be seen that the microbial total count in Bola de Ocosingo cheese elaborated with pasteurized milk is found within the specifications of the Official Mexican Regulation. These values showed the efficiency of incorporating lactic cultures not only as starters for the process of fermentation but also as controls for possible contaminating microorganisms which could be introduced during the process of elaboration of the cheese. The rapid decrease in pH associated with the production of lactic acid () would be the principal explanations of the bacteriostatic effect of the strain combined. This could also be explained by a possible peptides production (Karahan et al., Citation2010), bacteriocins or homologous substances with bacteriocidal activity, which was reported in other food models (Balciunas et al., Citation2013).
In contrast with our results, Romero-Castillo, Leyva-Ruelas, Cruz-Castillo, and Santos-Moreno (Citation2009) reported that the pasteurization process in artisanal Mexican cheese (tropical cream cheese) is not efficient to make a safe cheese product. The preceding demonstrates the beneficial antimicrobial action of added the starter culture utilized in our study.
3.6. Sensory evaluation
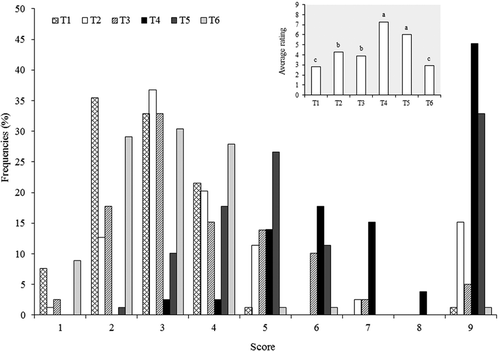
In the preference test conducted with 80 consumers, the greatest average score was achieved by treatments T4 and T5, being the average ratings of 7.24 and 6.06, respectively (). Both treatments resulted statistically equal between themselves (p > 0.05) and different from the remaining four treatments (p < 0.001). Similarly, the cheeses of treatments T4 and T5 had the highest frequency of receiving the highest score from the panelists (9 on the hedonic scale like extremely). 44% of the consumers gave the score of 9 to the cheese from treatment 4, followed in preference by the cheese from treatment 5 with 33.6% of the same level of pleasant reaction (). The other treatments had higher frequencies in the scores below 5 = it neither pleases nor displeases me. It is important to note that the cheese elaborated with pasteurized milk without the addition of starters (T1) received the lowest average score with a high frequency of scores less than 5, that is to say, the cheese was not pleasing to the consumers. Something similar happened with treatments T2, T3, and T6. This shows that the bacteria alone derived from the raw milk (T3) are scarcely contributing to the flavor and aroma which determine acceptability, and this can be due to several reasons: a) the synergic effect of all strains from the genus Lactobacillus could be aiding the production of precursor molecules of aroma and flavor which result in displeasure for the consumers; b) the catalytic strength of these strains is limited for production of precursors (Hannon, Kilcawley, Wilkinson, Delahunty, & Beresford, Citation2007); or c) the Lactobacillus which make up these treatments do not permit the development of other LAB (non-starters) which it is argued in some matured cheeses are responsible for the ultimate flavor (Hannon et al., Citation2003). Besides, the combination of all the strains (T2) did not improve the sensory characteristics of the pasteurized cheese, which could be due to the dominant action of LAB isolated from milk and thought to contribute to the production of non-pleasant flavor compounds (mainly bitterness); or, as Peláez and Requena (Citation2005) suggests, that the enzymatic activities of these LAB are not complementary. It is also possible that the greatest number of bacteria used (11 in T2) could obstruct the most desirable metabolism since this same phenomenon was observed in T6, where 8 strains were combined. The unusual result is that the treatment where all Lactococci bacteria were combined (T6), was not pleasing to the consumers since three of the strains of the most accepted treatment (T4) was from this genus.
Figure 1. Frequency of scores and average scores obtained in the preference test for the cheeses elaborated with pasteurized milk with different treatments. T1 = without starter cultures, T2 = with 11 strains of LAB, T3 = with five strains of LAB derived from milk, T4 = with six strains of LAB derived from cheese, T5 = with strains of the genus Lactobacillus, T6 = with strains of the genus Lactococcus. The scores run from 1 (dislike extremely) to 9 (like extremely). The letters in the average scoring (a–c) show significant differences between treatments (Dunn’s Test: p < 0.01).
Figura 1. Frecuencia de calificaciones y calificación promedio obtenida en la prueba de preferencia para los quesos elaborados con leche pasteurizada de diferentes tratamientos T1 = sin cultivos iniciadores, T2 = con 11 cepas de BAL, T3 = con 5 cepas de BAL aisladas de leche, T4 = con 6 cepas de BAL asiladas de queso, T5 = con cepas del género Lactobacillus, T6 = con cepas del género Lactococcus. Las calificaciones van de 1 (me desagrada mucho) a 9 (me gusta mucho). Las letras en la calificación promedio (a-c) muestran diferencia significativa entre tratamientos (prueba de Dunn; P < 0,01).
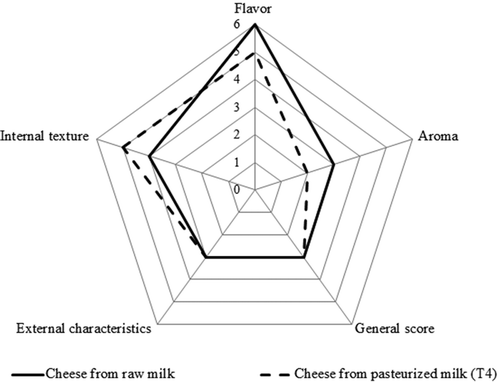
Based on preference results, the cheese from T4 was chosen and compared with Queso Bola de Ocosingo made in the traditional way (with raw/unpasteurized milk). For the triangular tests, we registered seven positive responses (from 24 tests performed, 8 judges x triplicate); which permitted the establishment of statistical equality (x2C = 0.094 versus x2T = 3.84) between the cheeses, thus judges could not discern among the cheese made from pasteurized milk (T4) and the cheese with unpasteurized milk. The point totals (five attributes) awarded by the judges for both samples (Control = 15.75; T4 = 15.25) did not present significant differences (p > 0.05). This result is interesting since the cheeses elaborated with pasteurized milk and with derived strains proved to be equal to those elaborated with raw milk. The general acceptability of both cheeses had the same numerical value (); while the other characteristics of the curd, including the flavor and aroma, were numerically very similar but statistically equal between the products of the two processes of production (). The characteristics evaluated by the expert panelists can be considered as all-important for the quality of the cheese, and their judgment is similar or even superior to that of trained panelists (Bittante et al., Citation2011). On the other hand, the fact that the experimental cheese (T4) was equivalent to the artisanal (which gave origin to the strains of this present study), indicates that the LAB employed as starters have a great potential for use on an industrial scale in the elaboration of ‘Queso Bola de Ocosingo’ cheese from pasteurized milk.
Figure 2. Scores given by expert panelists for five attributes of cheeses elaborated from unpasteurized milk and from pasteurized + starter cultures.
Figura 2. Puntuaciones otorgadas por panelistas expertos para cinco atributos de quesos elaborados con leche sin pasteurizar y con leche pasteurizada + cultivos iniciadores.
3.7. Biochemical presumptive identification of the LAB
The strains were characterized by means of biochemical tests (fermentation of carbohydrates). Two presumptive species of Lactobacillus delbrueckii subsp. lactis were identified, one species of Lactobacillus helveticus, one species of Lactococcus lactis subsp. lactis and two species of Lactococcus lactis subsp. cremoris (). The bacteria identified in the present study have been previously reported as starter cultures in other cheeses in other parts of the world. For example, the use of the strain CNRZ333 of Lactobacillus delbrueckii subsp. lactis has been reported in the elaboration of cheeses like Parmesan and Emmental where it is reported to give the texture and flavor characteristic of these cheeses (El Kafsi et al., Citation2014). In the same way, the profile of fermentation obtained by the strains classified as Lactobacillus helveticus is similar to other reports, who derived strains of Lactobacillus helveticus in samples of milk in the elaboration of Reggianito Argentino cheese (Candioti et al., Citation2002). This strain is greatly utilized as a starter culture in cheeses such as Parmesan, Romano, Provolone, and Mozzarella and as a supplemental culture for producing Cheddar, in the elaboration of Swiss cheeses such as Emmental, since the liberation of lactic acid in this type of cheese assists in its texture and eliminates bitterness during its maturation, as it possesses a great auto-catalytic and lipolytic capacity (Hannon et al., Citation2007). It is interesting the strain 18 Q (L. helveticus) was present in treatments T4 and T5, which received the best scores in the affective sensory tests () and T4 demonstrated equal scores with the cheese elaborated from unpasteurized milk (), which can be showing the great effect of this bacteria on the characteristics of Bola de Ocosingo cheese.
Lactococcus lactis subsp. lactis is a strain of great importance to the dairy industry; it is reported as responsible for the production of acetic acid from lactose, the hydrolysis of casein, and the fermentation of citric acid (Hannon et al., Citation2007). Its presence in Bola de Ocosingo cheese could contribute to the biosynthesis of final products of fermentation which have a significant influence in the direct or indirect form on the texture, flavor, and even hygienic quality of cheese by inhibiting the development of undesirable microorganisms (Gonzáles et al., Citation2003). It is commonly reported encountering strains of Lactococcus lactis subps. lactis in the first phases of cheese production (raw and curdled milk) and later during maturation. This microorganism establishing itself as the dominant bacteria during these phases may achieve elevated values in cheese. Something very similar occurs with Lactococcus lactis subsp. cremoris, which apart from bringing metabolites as acetic acid, pyruvic acid, propionic acid, butyric acid, etc., as a product of fermentation, is reported responsible for the production of acetoin (Mcsweeney & Sousa, Citation2000), highly desirable in dairy products for its characteristic aroma.
4. Conclusion
From the ‘Queso Bola de Ocosingo’ cheese elaborated with raw milk and raw milk, we isolate 23 autochthones bacteria with presumptive characteristics as lactic acid bacteria. Six of these strains were characterized through biochemical tests (API® 50 CHL and API® 20 STREP) as Lactobacillus delbrueckii ssp. lactis (14Q and 16Q; ID = 91.0 and 96.5%, respectively), Lactobacillus helveticus (18Q; ID = 97.9%), Lactococcus lactis ssp. cremoris (17Q and 20Q; ID = 97.2 and 97.2%, respectively) and Lactococcus lactis ssp. lactis (19Q; ID = 98.0%). When these LAB´s were employed as a starter culture in the elaboration of ‘Queso Bola de Ocosingo’ cheese using pasteurized milk, the sensory acceptability was similar to those of the cheese elaborated from raw milk. Furthermore, the cheese made with this starter culture was microbiological safety, with levels of coliforms as well as molds and yeast below of limits established by Mexican standards.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Balciunas, E. M., Castillo-Martinez, F. A., Todorov, S. D., de Melo-Franco, B. D. G., Converti, A., & de Souza-Oliveira, R. P. (2013). Novel biotechnological applications of bacteriocins: A review. Food Control, 32, 134–142. doi:10.1016/j.foodcont.2012.11.025
- Bittante, G., Cologna, N., Cecchinato, A., De Marchi, M., Penasa, M., Tiezzi, F., … Gasperi, F. (2011). Monitoring of sensory attributes used in the quality payment system of Trentingrana cheese. Journal of Dairy Science, 94, 5699–5709. doi:10.3168/jds.2011-4319
- Bozoudi, D., Kotzamanidis, C., Hatzikamari, M., Tzanetakis, N., Menexes, G., & Litopoulou-Tzanetakia, E. (2015). A comparison for acid production, proteolysis, autolysis and inhibitory properties of lactic acid bacteria from fresh and mature Feta PDO Greek cheese, made at three different mountainous areas. International Journal of Food Microbiology, 200, 87–96. doi:10.1016/j.ijfoodmicro.2015.02.008
- Candioti, M. C., Hynes, E., Quiberoni, A., Palma, S. B., Sabbag, N., & Zalazar, C. A. (2002). Reggianito Argentino cheese: Influence of Lactobacillus helveticus strains from natural whey cultures on cheese making and ripening processes. International Dairy Journal, 12, 923–931. doi:10.1016/S0958-6946(02)00115-2
- Centeno, J. A., Cepeda, A., & Rodriguez, J. L. (1996). Lactic Acid Bacteria isolated from Arzúa Cheese cow’s milk cheese. International Dairy Journal, 6, 65–78. doi:10.1016/0958-6946(96)80001-X
- CODEX ALIMENTARIUS. (2011). Milk and milk products (2nd ed.). Rome: World Health Organization, Food and Agriculture Organization of the United Nations. ISBN: 978-92-5-105837-4.
- El Kafsi, H., Binesse, J., Loux, V., Buratti, J., Boudebbouze, S., Dervyn, R., … Van de Guchte, M. (2014). Lactobacillus delbrueckii ssp. lactis and ssp. bulgaricus: A chronicle of evolution in action. BMC Genomics, 15, 407. doi:10.1186/1471-2164-15-407
- Esmerino, E. A., Cruz, A. G., Pereira, E. P. R., Rodrigues, J. B., Faria, J. A. F., & Bolini, H. M. A. (2013). The influence of sweeteners in probiotic Petit Suisse cheese in concentrations equivalent to that of sucrose. Journal of Dairy Science, 96, 5512–5521. doi:10.3168/jds.2013-6616
- FDA. (2011). Bacteriological analytical manual. Food and Drugs Administration. Chapters 3, 4 and 18. Silver Spring, MD. Retrieved from http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm2006949.htm
- Frau, F., Font de Valdez, G., & Pece, N. (2014). Effect of pasteurization temperature, starter culture, and incubation temperature on the physicochemical properties, yield, rheology, and sensory characteristics of spreadable goat cheese. Journal of Food Processing, Article ID 705746, 8 pages.
- Geria, M., & Caridi, A. (2014). Methods to assess lactic acid bacteria diversity and compatibility in food: Review. Acta Alimentaria, 43, 96–104. doi:10.1556/AAlim.43.2014.1.10
- Gonzáles, J., Mas, M., Tabla, R., Moriche, J., Roa, I., Rebollo, J., & Cáceres, P. (2003). Autochthonous starter effect on the microbilogical, physicochemical and sensorial characteristics of ibores goat´s milk cheese. Le Lait, 82, 192–202.
- González, L., Fernández-Cuadrillero, A., Castro, J. M., Bernardo, A., & Tornadijo, M. E. (2015). Selection of lactic acid bacteria isolated from San Simón da costa cheese (PDO) in order to develop an autochthonous starter culture. Advances in Microbiology, 5, 748–759. doi:10.4236/aim.2015.511079
- González-Córdova, A. F., Yescas, C., Ortiz-Estrada, A. M., De La Rosa-Alcaraz, M. L., Hernández-Mendoza, A., & Vallejo-Cordoba, B. (2016). Invited review: Artisanal Mexican cheeses. Journal of Dairy Science, 99, 3250–3262. doi:10.3168/jds.2015-10103
- Guzman-Hernandez, R., Contreras-Rodriguez, A., Hernandez-Velez, R., Perez-Martinez, I., Lopez-Merino, A., Zaidi, M. B., & Estrada-Garcia, T. (2016). Mexican unpasteurised fresh cheeses are contaminated with Salmonella spp., non-O157 Shiga toxin producing Escherichia coli and potential uropathogenic E. coli strains: A public health risk. International Journal of Food Microbiology, 237, 10–16. doi:10.1016/j.ijfoodmicro.2016.08.018
- Hannon, J. A., Kilcawley, K. N., Wilkinson, M. C., Delahunty, C. M., & Beresford, T. P. (2007). Flavour precursor development in Cheddar cheese due to lactococcal starters and the presence and lysis of Lactobacillus helveticus. International Dairy Journal, 17, 316–327. doi:10.1016/j.idairyj.2006.03.001
- Hannon, J. A., Wilkinson, M. G., Delahunty, C. M., Wallace, J. M., Morrissey, P. A., & Beresford, T. P. (2003). Use of autolytic starter systems to accelerate the ripening of Cheddar cheese. International Dairy Journal, 13, 313–323. doi:10.1016/S0958-6946(02)00178-4
- Herreros, M. A., Sandoval, H., González, L., Castro, J. M., Fresno, J. M., & Tornadijo, M. E. (2005). Antimicrobial activity and antibiotic resistance of lactic acid bacteria isolated from Armada cheese (a Spanish goats’ milk cheese). Food Microbiology, 22, 455–459. doi:10.1016/j.fm.2004.11.007
- Karahan, G., Başyiğit, K., Kart, A., Şanlıdere, A., Öner, Z., Aydemir, S., … Harsa, Ş. (2010). Genotypic identification of some lactic acid bacteria by amplified fragment length polymorphism analysis and investigation of their potential usage as starter culture combinations in Beyaz cheese manufacture. Journal of Dairy Science, 93, 1–11. doi:10.3168/jds.2008-1801
- Kieronczyk, A., Skeie, S., Langsrud, T., & Yvon, M. (2003). Cooperation between Lactococcus lactis and nonstarter lactobacilli in the formation of cheese aroma from amino acids. Applied and Environmental Microbiology, 69, 734–739. doi:10.1128/AEM.69.2.734-739.2003
- Lawless, H. T., & Heymann, H. (2010). Sensory evaluation of food principles and practices (2nd ed.). New York, NY: Springer. ISBN: 978-1-4419-6487-8.
- Leroy, F., & De Vuyst, L. (2004). Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science & Technology, 15, 67–78. doi:10.1016/j.tifs.2003.09.004
- Mcsweeney, P. L. H., & Sousa, M. J. (2000). Biochemical pathways for the production of flavor compounds in cheeses during ripening: A rewiew. Le Lait, 80, 293–324. doi:10.1051/lait:2000127
- NMX-F-713-COFOCALEC-2014. (2014). Sistema producto leche- alimentos-lácteos-queso y queso suero- denominaciones, especificaciones y métodos de prueba. In Spanish. Retrieved from http://www.economia-nmx.gob.mx/normasmx/detallenorma.nmx?clave=NMX-F-713-COFOCALEC-2014.
- NOM-243-SSA1-2010. (2010). Productos y servicios. Leche, fórmula láctea, producto lácteo combinado y derivados lácteos. Disposiciones y especificaciones sanitarias. Métodos de prueba. In Spanish. Retrieved from http://dof.gob.mx/nota_detalle_popup.php?codigo=5160755.
- Oberg, C. J., Oberg, T. S., Culumber, M. D., Ortakci, F., Broadbent, J. R., & McMahon, D. J. (2016). Lactobacillus wasatchensis sp. nov., a non-starter lactic acid bacteria isolated from aged Cheddar cheese. International Journal of Systematic and Evolutionary Microbiology, 66, 158–164. doi:10.1099/ijsem.0.000689
- Østergaard, N. B., Eklöw, A., & Dalgaard, P. (2014). Modelling the effect of lactic acid bacteria from starter- and aroma culture on growth of Listeria monocytogenes in cottage cheese. International Journal of Food Microbiology, 188, 15–25. doi:10.1016/j.ijfoodmicro.2014.07.012
- Peláez, C., & Requena, T. (2005). Exploiting the potential of bacteria in the cheese ecosystem. International Dairy Journal, 15, 831–844. doi:10.1016/j.idairyj.2004.12.001
- Pescuma, M., Hébert, E. M., Mozzi, F., & Font, V. G. (2010). Functional fermented whey-based beverage using lactic acid bacteria. International Journal of Food Microbiology, 141, 73–81. doi:10.1016/j.ijfoodmicro.2010.04.011
- Randazzo, C. L., Torriani, S., Akkermans, A. D. L., de Vos, W. M., & Vaughan, E. E. (2002). Diversity, dynamics, and activity of bacterial communities during production of an artisanal Sicilian cheese as evaluated by 16s rRNA analysis. Applied and Environmental Microbiology, 68, 1882–1892. doi:10.1128/AEM.68.4.1882-1892.2002
- Romero-Castillo, P. A., Leyva-Ruelas, G., Cruz-Castillo, J. G., & Santos-Moreno, A. (2009). Evaluación de la calidad sanitaria de quesos crema tropical mexicano de la región de Tonalá, Chiapas. Revista Mexicana de Ingeniería Química, 8, 111–119. In Spanish.
- Settanni, L., Gaglio, R., Guarcello, R., Francesca, N., Carpino, S., Sannino, C., & Todaro, M. (2013). Selected lactic acid bacteria as a hurdle to the microbial spoilage of cheese: Application on a traditional raw ewes’ milk cheese. International Dairy Journal, 32, 126–132. doi:10.1016/j.idairyj.2013.04.010
- Speranza, B., Bevilacqua, A., Corbo, M. R., Altieri, C., & Sinigaglia, M. (2015). Selection of autochthonous strains as promising starter cultures for Fior di Latte, a traditional cheese of southern Italy. Journal of the Science of Food and Agriculture, 95, 88–97. doi:10.1002/jsfa.2015.95.issue-1
- Su, O. N., Yeon, J. J., Young, L. J., Hun, K. S., & Kim, Y. (2016). Characterization of the microbial diversity and chemical composition of Gouda cheese made by potential probiotic strains as an adjunct starter culture. Journal of Agricultural and Food Chemistry, 64, 7357–7366. doi:10.1021/acs.jafc.6b02689
- AOAC. (2010). Chapter 33, Dairy Products. In W. Horwitz & G. Latimer (Eds.), Official methods of analysis of association of official analytical chemists (18th ed., 3rd revision). Gaithersburg, MD: Author. ISBN: 9-780935584-80-6.
