 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
It is first reported the elaboration and characterization of films (F2) containing 1% (w/v) citrus pectin, 0.2% (w/v) gellan gum, 0.5% (w/v) glycerol, CaCl2 5 mM, ethylenediaminetetraacetic acid (EDTA) 0.05 M and 90 (Arbitrary Units)/mL of an antimicrobial concentrated supernatant (ACS) from fermentation culture broths of the lactic acid bacterium, Streptococcus infantarius. The functional films inhibited the growth of Listeria monocytogenes, Escherichia coli and Staphylococcus aureus in “Barbacoa” medium in 7-day cultures at 35°C. “Barbacoa” is a highly appreciated Mexican meat product. In contrast, the control cultures exhibited bacterial-growths up to 107–109 (Colony-Forming Units)/g. An antimicrobial-activity synergy between ACS and EDTA was demonstrated. Some film-physical properties were modified by the EDTA-ACS incorporation [F2/control-film]: Young’s modulus (MPa), 1,394/707; elongation at break (%), 1.9/9.3; stress at break (MPa), 5.7/12.6; water vapor permeability (10–11 g m Pa−1 s−1 m−2), 3/20 and oxygen permeability (10–12 g m Pa−1 s−1 m−2), 1.9/1.2.
RESUMEN
Se reporta por primera vez la elaboración y caracterizaron de películas (F2) conteniendo 1% (p/v) pectina cítrica, 0.2% (p/v) goma gelana, 0.5% (p/v) glicerol, CaCl2 5 mM, ácido etilendiaminotetraacético (EDTA) 0.05 M, y 90 (Unidades Arbitrarias)/mL de sobrenadante concentrado de caldos de fermentación de la bacteria ácido láctica, Streptococcus infantarius, con actividad antimicrobiana (ACS). Las películas inhibieron el crecimiento de Listeria monocytogenes, Escherichia coli y Staphylococcus aureus en cultivos de 7 días a 35°C en medio “Barbacoa”. La Barbacoa es un producto cárnico Mexicano altamente apreciado. En contraste, los cultivos control sin película exhibieron crecimientos bacterianos hasta 107–109 (Unidades Formadoras de Colonias)/g. Se demostró un efecto antimicrobiano sinérgico entre ACS y EDTA. Algunas propiedades físico-mecánicas de las películas fueron afectadas por la inclusión de EDTA-ACS, como [F2/película control]: Módulo de Young (MPa), 1,394/707; Elongación a la ruptura (%), 1.9/9.3; Esfuerzo a la ruptura (MPa), 5.7/12.6; Permeabilidad al Vapor de Agua (10−11 g m Pa−1 s−1 m−2), 3/20, y Permeabilidad al Oxígeno (10−12 g m Pa−1 s−1 m−2), 1.9/1.2.
Introduction
The research concerning the packaging for the conservation of food products is of worldwide interest due to implications on food safety, biomaterials applications and sustainability, among others (Campos, Gerschenson, & Flores, Citation2010; Jabeen, Majid, Nayik, & Yildiz, Citation2015). Specifically, the research on the packaging of meat products encompasses studies concerning meat products from the minimal to the highly processed ones: fresh beef (Zinoviadou, Koutsoumanis, & Biliaderis, Citation2010); pork meat hamburgers (Vargas, Albors, & Chiralt, Citation2011) and fresh white shrimps (Meenatchisundaram et al., Citation2016) among others. Furthermore, the antimicrobial packaging materials can effectively control the growth of spoilage and pathogenic microorganisms in the surface of meat products; in this sense, one alternative is the use of edible biopolymer films enriched with bacteriocins (Pattanayaiying, H-Kittikun, & Cutter, Citation2015; Salmieri et al., Citation2014). Bacteriocins are natural antimicrobial peptides synthetized by one bacterium species that are active against other bacteria species. After rigorous evaluations, these antimicrobials can be used as safe additives in food products for human consumption. This is the case of both bacteriocins, Nisin and Colicin (FDA, Citation2000, Citation2016). In order to increase the antimicrobial spectrum of bacterium inhibition, bacteriocins are frequently used in combination with other substances, like the chelating agent, ethylenediaminetetraacetic acid (EDTA), which contributes to make more permeable the bacterium outer membranes, resulting in an effective antimicrobial activity against Gram negative bacteria as well (Vaara, Citation1992). Furthermore, some biopolymers, like pectins, also exhibit antimicrobial activities that can contribute to a more effective functional films for food packaging (Calce et al., Citation2014; Jindal, Kumar, Rana, & Tiwary, Citation2013).
The present article reports the main results concerning the elaboration and characterization of films of gellan gum mixed with citrus pectin, enriched with EDTA and antimicrobial concentrated supernatant (ACS) from fermentation culture broths of the lactic acid bacterium (LAB), Streptococcus infantarius, containing bacteriocin-like inhibitory substances (BLIS). The antimicrobial activity of the films was tested against Listeria monocytogenes, Escherichia coli and Staphylococcus aureus, growing in a medium based on Mexican “Barbacoa”, a highly appreciated meat product, that is usually prepared with lamb meat wrapped in agave leaves and cooked overnight in customized ovens (Natividad-Bonifacio et al., Citation2010). Also, some selected physical-mechanical properties of the films were determined (i.e. Young’s modulus, stress and elongation at break and water vapor and oxygen permeabilities). The development of bioconservation technologies for Mexican products, like “Barbacoa”, is of great interest for many reasons, including that concerning the food market (Rubio, Torres, Gutierrez, & Mendez, Citation2004).
Materials and methods
Biological specimens
The LAB, Streptococcus infantarius, originally isolated from Pozol, a traditional fermented food (Mendoza-Mendoza et al., Citation2013), was kindly provided by Dr. C. Wacher-Rodarte (School of Chemistry, UNAM, Mexico).
The bacterium indicators for testing the antimicrobial activity of the films were Listeria monocytogenes CFQ-103, Escherichia coli ATCC-25922 and Staphylococcus aureus ATCC-25923, kindly provided by Dr. G. Díaz-Ruiz (School of Chemistry, UNAM, Mexico).
All bacterial strains were conserved at −80°C in 2 mL-vials containing 1 mL of 24 h old-culture broths of each bacterium mixed with 20% v/v glycerol. For this purpose, S. infantarius was grown in De Man-Rogosa-Sharpe broth, MRS (BDTM DIFCO, France), although the other bacteria were grown in Brain Heart Infusion broth, BHI (Bioxon® México).
Antimicrobial additives
The ACS of S. infantarius was obtained according to Calderón-Aguirre et al. (Calderón-Aguirre et al., Citation2015). Briefly, reactivated S. infantarius-cells were cultured in MRS at 30°C for 6 h; then, the bacterial cells were removed by centrifugation. The supernatant was collected, adjusted to a pH of 6.5 and concentrated (60°C, 72 mbar) up to 40% of its initial volume. To inactive proteases, the ACS was heated at 110°C for 10 min; later, it was cooled and stored until use. The ACS exhibited an antimicrobial activity of 6,400 arbitrary units (AU)/mL, determined by the spot-on-the-lawn method (Nuñez, Tomillo, Gaya, & Medina, Citation1996), being this activity due to the presence of BLIS because its antimicrobial action was inhibited by proteases (i.e. Proteinase K (Invitrogen, U.S.A.); Peptidase and Trypsin (Sigma-Aldrich, U.S.A.) (Mimila-Méndez, Citation2017).
The chelating agent was EDTA disodium salt dehydrate (Sigma-Aldrich, U.S.A.).
Minimal inhibitory concentrations of the antimicrobial additives
The minimum inhibitory concentration (MIC) determination was performed by the broth microdilution method (CLSI, Citation2015). All combinations of ACS and EDTA at different concentrations (i.e. ACS: 0, 75, 90, 200 and 400 AU/mL; EDTA: 0, 1 × 10–3, 2 × 10–3, 4 × 10–3, 8 × 10–3, 0.02 and 0.05 M) were tested to find the MIC against L. monocytogenes, E. coli and S. aureus in BHI, with an initial inoculum of 4 × 104–7 × 104 Colony-Forming Units (CFU) per well, by triplicate, in microtiter plates (Corning® Costar®). The inoculated microdilution trays were incubated at 35°C for 20–24 h.
Film elaboration
Three film treatments (F1, F2 and F3) and the control (FC) were tested in this work. The film-forming solutions contained 1% (w/v) citrus pectin (GENU® Pectin, DE = 36%, CP-Kelco, U.S.A.), 0.2% (w/v) low acyl Gellan gum (Kelcogel, CP-Kelco, U.S.A.), 0.5% (w/v) glycerol (Química Meyer, Mexico) and CaCl2 (JT Baker, Mexico) 5 mM. The F1, F2, F3 and FC treatments contained the following EDTA (M)/ACS (AU/mL) ratios: 0.05/75, 0.05/90, 0.05/120 and 0/0, respectively. The biopolymers were dissolved separately in distilled water; later they were mixed together and heated at 60–65°C during 40 min; then, glycerol and CaCl2 were added and temperature was increased up to 75°C. Mixing continued another 30 min; then, the corresponding quantities of the antimicrobial additives were added into the mixture. Once the film-forming solutions were already prepared, they were poured into Teflon® casts (EKCO®, 39.5 × 27 × 2 cm) and dried in an oven (Shel-Lab, 1380FX) at 35°C during 17 h.
The obtained films were then conditioned into a desiccator during 48 h at a relative humidity (RH) of 50–55% and 23°C. The average thickness of the films were determined by measurements at five points each film with a micrometer to the nearest 0.0001 mm (Truper, Mexico)
Film effects on the growth of Listeria monocytogenes, Escherichia coli and Staphylococcus aureus in selective media
L. monocytogenes, E. coli and S. aureus were grown in BHI at 35°C for 24 h; then, decimal dilutions of each bacterium culture were done with isotonic salt solution (1% (w/v) NaCl). 200 µL-samples of convenient dilutions of each bacterium culture, containing 25 CFU, were taken and inoculated into Petri dishes (Interlux, 60 × 15 mm) containing selective media: Oxford (BDTM DIFCO, France) for L. monocytogenes, MacConkey (Sigma-Aldrich) for E. coli and Baird Parker (BDTM DIFCO, France) for S. aureus. After the surface spreading of the samples on the agar plates, the agar surfaces were aseptically covered with 6 cm-diameter circular films, previously sterilized with UV radiation during 24 h (12 h each side). The cultures were then incubated at 25°C during 30 days. The solid cultures with film treatments and controls without films (C) were tested by triplicate.
Film effects on the growth of Listeria monocytogenes, Escherichia coli and Staphylococcus aureus in a meat product medium
L. monocytogenes, E. coli and S. aureus were grown in BHI at 35°C for 24 h; then, proper dilutions of each bacterium culture were done with isotonic salt solution to inoculate 100 CFU/plate by surface spreading on Petri dishes (Interlux, 60 × 15 mm) containing culture medium based on “Barbacoa”, a lamb meat product. The “Barbacoa”-medium contained 4.5% (w/v) Barbacoa (bought in a traditional market in Tulancingo, Hidalgo; México) and 1.5% (w/v) bacto-agar (BDTM DIFCO, France). A portion of 45 g of Barbacoa was thoroughly ground with a food processor (Oster® 3213, China), then mixed with 1 L of distilled water and 15 g of bacto-agar, maintaining constant agitation and heating, till boiling. Then the medium was sterilized (121°C for 2 h in an autoclave Tuttnauer, 3870ELV-D) and distributed into petri dishes until use.
The surface of “Barbacoa” plates, inoculated with bacteria, was covered with 6 cm-diameter circular films, previously sterilized with UV radiation during 24 h (12 h each side). The cultures were then incubated at 35°C during 7 days, taking samples at days 0, 1, 3, 5 and 7, by triplicate. Each sample (1 plate containing 5 mL of medium) was homogenized with 45 mL peptone water in a Seward Stomacher® 400 Circulator, at 300 rpm during 5 min. Viable cell counts were made involving decimal dilutions of the homogenate, then mixing 1 mL of diluted samples in BHI soft agar in plates (Interlux, 90 × 15 mm) to be incubated at 35°C during 24 h. Plates with F2 and FC films were tested, as well as bacterium inoculated plates without films (NF).
A bacterium growth curve was obtained for each experiment, being used to determine: (a) the initial viable cell count (X0; CFU/g); (b) the maximum bacterium concentration (Xmax; CFU/g); (c) the multiplication factor ((Xmax/X0), dimensionless) and (d) the maximum specific growth rate (µmax; h−1) calculated as the slope of the semi-logarithmic plot of the growth curve in the exponential growth phase (Liu, Citation2013).
Mechanical characterization of the films
Mechanical tests were done according to the ASTM D882-10 method (ASTM, Citation2010) in a Texture Analyser TA plus Lloyd. Film samples were cut into “dog-bone” shape (Type M-I tension test specimen) according to the specifications of the standard ASTM D638M-93 (ASTM, Citation1993). After conditioning the samples during 48 h at 50–55% RH and 25°C, they were gripped into the texture analyzer with an initial separation of 0.05 m. The tensile tests were carried out at a cross head speed of 1 mm/s. At least 30 replicates per treatment were carried out to obtain the stress-Hencky strain curves of the samples through the force-distance data. Specimens that failed at the grip contact point were discarded. Young’s modulus (EM; MPa) was determined through the slope of the linear region of the stress-strain curves. The ultimate mechanical properties of the films, stress (σT,max; MPa) and elongation at break (Emax; %) were determined in the rupture point (Calderón-Aguirre et al., Citation2015).
Water vapor permeability of the films
The water vapor permeability (WVP) of the films was determined with the ASTM E96-00 method (ASTM, Citation2000). Film disks, previously equilibrated at 53% RH and 25°C for 48 h, were mounted on permeation cells (aluminium cups) containing dried silica gel (0% RH); then cups were placed inside a cabinet that was equilibrated at 75 ± 2% RH and 23 ± 2°C during 24 h prior to WVP tests. The cups were weighed every hour during 8 h. The WVP was determined according to the procedure reported by Aguirre-Loredo, Rodríguez-Hernández & Chavarría-Hernández (Citation2014). Four determinations were done per treatment.
Oxygen permeability of the films
The oxygen permeability (PO2) of the films was determined in accordance with the ASTM D1434-82 method (ASTM, Citation1982) using a film-package permeability tester (Labthink VAC-V2, China). Films were conditioned at 50–55% RH during 48 h prior to be placed into the equipment chambers. The tests were performed at 25°C, using research-grade high-purity (99.998%) oxygen gas (34,161, INFRA® México). Four runs per treatment were carried out.
Statistical analysis
Data are presented as the mean ± standard deviation for each treatment. Results were analyzed for statistical significance using analysis of variance (ANOVA) followed by Tukey test (p < 0.05). Differences between pairs of means were assessed using t-test (p < 0.05) (SigmaPlot 12.5, SPSS Inc., USA).
Results and discussion
Antimicrobial activity of the films
First of all, the S. infantarius-ACS exhibited important activity against both Gram positive bacteria, L. monocytogenes and S. aureus, with no effects against the Gram negative bacterium, E. coli. The exhibited antimicrobial activity is due to the presence of BLIS involving molecules from 4 to 7 kDa of molecular weight, which can be inactivated by proteases (Mimila-Méndez, Citation2017); furthermore, the production of bacteriocins and BLIS by other bacterial strains which belong to the Streptococcus bovis/Streptococcus equinus complex has been reported. For example, S. bovis HC5 produces the bacteriocin, bovicin HC5, which exhibits important antilisterial activity (Mantovani & Russell, Citation2003). Besides, some of this bacterial strains have been isolated from traditional fermented dairy products (Jans et al., Citation2013).
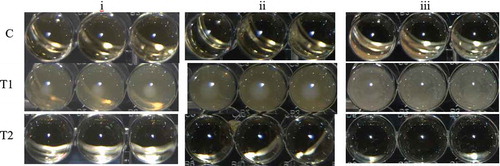
In the present work, in order to elaborate a film with antimicrobial activity against L. monocytogenes, S. aureus and E. coli, the metal chelator, EDTA, was added into the film-forming solutions to increase the bacterial sensitivity to the BLIS present in S. infantarius-ACS (Banin, Brady, & Greenberg, Citation2006). The determined MIC was a blend of ACS, 90 AU/mL, with EDTA, 0.05 M, which inhibited the growth of the three indicator bacteria ().
Figure 1. Minimal inhibitory concentrations (MIC) of blends of antimicrobial concentrated supernatant (ACS) of S. infantarius-fermentations, and ethylenediaminetetraacetic acid (EDTA) for: (i) Listeria monocytogenes, (ii) Escherichia coli, and (iii) Staphylococcus aureus inoculated in Brain Heart Infusion broth (BHI) and incubated at 35°C for 24 h. The highest bacterial growth occurred in the treatment T1 (ACS, 0 AU/mL; EDTA, 0 M) and the MIC for the three indicators occurred in the treatment T2 (ACS, 90 AU/mL; EDTA, 0.05 M). C was the abiotic control.
Figura 1. Concentración mínima inhibitoria (MIC) de mezclas de ACS y EDTA contra i) Listeria monocytogenes, ii) Escherichia coli, y iii) Staphylococcus aureus en BHI, incubadas a 35°C por 24 h. El mayor crecimiento bacterial ocurrió en T1 (ACS, 0 AU/mL; EDTA, 0 M) y la MIC para los tres indicadores ocurrió en T2 (ACS, 90 AU/mL; EDTA, 0.05 M). C fue el control abiótico.
There are reports of combinations of EDTA with antimicrobial agents, against both Gram positive and Gram negative bacteria. For example, Economou, Pournis, Ntzimani, and Savvaidis (Citation2009) reported the combination of 500–1,500 International Units of nisin with 50 mM EDTA, which affected the populations of the mesophilic bacteria, Pseudomonas sp., Brochothrix thermosphacta, lactic acid bacteria and enterobacteriaceae during the storage of fresh chicken meat. Sinigaglia, Bevilacqua, Corbo, Pati, and Del Nobile (Citation2008) used conditioning brines with 0.25 g/L lysozyme and 10–50 mM Na2-EDTA during the storage of mozzarella cheese, reporting a significant inhibition of coliforms and Pseudomonadaceae. Furthermore, Banin et al. (Citation2006) reported a synergic interaction of Gentamicin (10 mg/mL) with EDTA 50 mM, against P. aeruginosa.
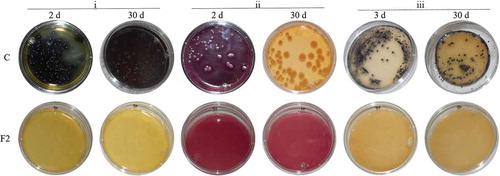
The antimicrobial activity-films were elaborated with a constant EDTA concentration, 0.05 M, testing three levels of ACS (i.e. 75, 90 and 120 AU/mL), in a complex biopolymer matrix of low-methoxyl pectin and deacetylated gellan gum, involving a gelation process greatly affected by the presence of calcium ions (Pérez-Campos, Chavarría-Hernández, Tecante, Ramírez-Gilly, & Rodríguez-Hernández, Citation2012; Thakur, Singh, & Handa, Citation1997). The presents the effects of the films on the growth of the three indicator bacteria in selective media. All bacteria grew well in control plates (C, bacterium inoculated media without films), where the counts were 105 CFU/plate and 78 CFU/plate for L. monocytogenes and E. coli after 2 days of incubation, respectively, although S. aureus exhibited 77 CFU/plate at the third day of incubation. In contrast, all plates with films (i.e. FC, F1, F2 and F3) did not exhibit any bacterial growth during a period of 30 days (). The antimicrobial activity exhibited by the FC films, which contained no ACS nor EDTA, would rely on the contents of gellan gum and pectin. It has been reported the antimicrobial properties of several carbohydrate polymers (i.e. karaya gum, chitosan, algal polysaccharides (Ramawat & Mérillon, Citation2015)); specifically, pectins extracted from both apple peel (pristine and modified samples) and Aegle marmelos fruit, have exhibited antimicrobial activity against E. coli and S. aureus (Calce et al., Citation2014) and Bacillus cereus and E. coli (Jindal et al., Citation2013), respectively. Therefore, the bacterial growth inhibition exhibited by the FC films would be attributed to the pectin contents, being this antimicrobial activity associated to the uronic acid contents in the biopolymer (Jindal et al., Citation2013); furthermore, the pectic oligosaccharides have also been proposed as prebiotics with valuable antimicrobial properties (Gullón et al., Citation2013).
Figure 2. Aspect of the growth of the indicator bacteria in selective media at 2 or 3, and 30 days of incubation at 25°C. (i) Listeria monocytogenes in Oxford medium; (ii) Escherichia coli in MacConkey agar, and (iii) Staphylococcus aureus in Baird Parker medium. Bacteria grew well in control plates (C) (bacterium-inoculated culture media with no films). No bacterial growth was recorded in plates with films F2 (base biopolymer film containing gellan gum and pectin, antimicrobial concentrated supernatant (ACS) of S. infantarius-fermentations, 90 AU/mL, and ethylenediaminetetraacetic acid (EDTA), 0.05 M). The bacterial growth was also inhibited in F1, F3 and FC film treatments (not shown).
Figura 2. Aspecto del crecimiento bacteriano en medio selectivo a los 2 o 3, y 30 días de incubación a 25°C. i) Listeria monocytogenes en medio Oxford; ii) Escherichia coli en agar MacConkey, y iii) Staphylococcus aureus en medio Baird Parker. Las bacterias crecieron bien en las cajas control (C) (medio de cultivo inoculado y sin película). Las cajas con película F2 (película biopolimérica base con gelana y pectina, ASC, 90 AU/mL, y EDTA, 0.05 M) no exhibieron crecimiento bacteriano, siendo el mismo resultado para los tratamientos F1, F3 y FC (no mostrados)
The presents the effects of the films on the growth of L. monocytogenes, E. coli and S. aureus in the “barbacoa” medium at 35°C. The three bacteria grew well in “barbacoa” medium plates with no films (, triangle symbols) involving maximum specific growth rates, µmax, from 3.21 day−1 (≡0.13 h−1) for L. monocytogenes, to 5.57 day−1 (≡0.23 h−1) for S. aureus, with multiplication factors (Xmax/X0) from 1 × 105 times for L. monocytogenes, to 8.3 × 106 times for S. aureus (). The “barbacoa” medium (3.4% (w/v) muscle and 1% (w/v) total fat (Rubio et al., Citation2004)), supported vigorous bacterial growths with increases in the bacterial concentrations up to 5–6 log cycles. In analogous studies, Mansur, Park, and Oh (Citation2016) reported the growth of S. aureus from 103 to 109 CFU/g in ham at 35°C, although Shekarforoush, Basiri, Ebrahimnejad, and Hosseinzadeh (Citation2015) recorded the growth of E. coli from 104 to 108.8 CFU/g and L. monocytogenes from 105 to 108.5 CFU/g, in barbecue chicken at 20°C.
Table 1. Growth parameters of Listeria monocytogenes, Escherichia coli and Staphylococcus aureus, in “barbacoa” medium at 35°C.
Tabla 1. Parámetros del crecimiento de Listeria monocytogenes, Escherichia coli y Staphylococcus aureus, en medio “barbacoa” a 35°C.
Figure 3. Growth kinetics of the indicator bacteria (CFU/g) at 35°C in “Barbacoa” medium. (i) Listeria monocytogenes; (ii) Escherichia coli, and (iii) Staphylococcus aureus. Treatments were bacterial inoculated plates with: (![]()
 ) FC films (base biopolymer films containing pectin and gellan gum), and (
) FC films (base biopolymer films containing pectin and gellan gum), and ( ) F2 film (base biopolymer films, plus ACS, 90 AU/mL, and EDTA, 0.05 M). Runs were done by triplicate. Error bars are standard deviations, and same letters indicate no statistical differences on the basis of Tukey’s means comparison, p < 0.05.
) F2 film (base biopolymer films, plus ACS, 90 AU/mL, and EDTA, 0.05 M). Runs were done by triplicate. Error bars are standard deviations, and same letters indicate no statistical differences on the basis of Tukey’s means comparison, p < 0.05.Figura 3. Cinética de crecimiento de los indicadores (UFC/g) a 35°C en medio “Barbacoa”. i) Listeria monocytogenes; ii) Escherichia coli, y iii) Staphylococcus aureus. Los tratamientos fueron medios inoculados con bacterias y: (![]()
 ) recubiertos con FC (películas biopoliméricas base, contendiendo pectina y gelana), y (
) recubiertos con FC (películas biopoliméricas base, contendiendo pectina y gelana), y (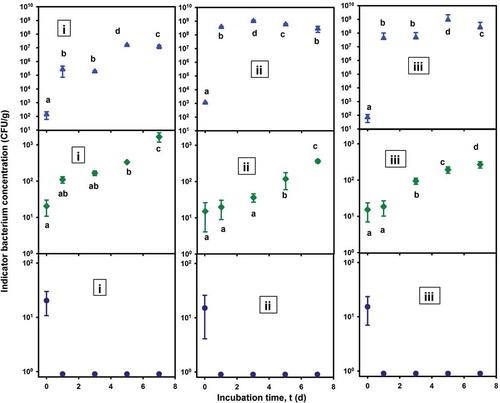
On the other hand, the FC films partially inhibited the growth of the three bacteria inoculated in “barbacoa” medium (, diamond symbols), involving low µmax values (). In fact, the achieved bacterial concentrations were from 2.67 × 102 CFU/g, for S. aureus, to 1.6 × 103 CFU/g, for L. monocytogenes, associated with multiplication factors from 1.8 × 101 to 8.3 × 101 times, respectively, involving increases in the bacterial concentrations of only 1 log cycle during the experiments. The partial antimicrobial activity exhibited by the FC films would be attributed to the presence of pectins (Jindal et al., Citation2013).
Furthermore, an outstanding antimicrobial activity was exhibited by the F2 films against the three tested bacteria which did not grow during the cultures; even more, from the day 1 of experiments, the viable cell counts were minor than 1 CFU/g (, circle symbols). This bacterial inhibition would imply a synergy of the individual antimicrobial-activity contributions of ACS, EDTA and pectins, present in the bioactive F2 films. In an analogous study, Sivarooban, Hettiarachchy, and Johnson (Citation2008) reported a maximum antimicrobial activity of soy protein films enriched with grape seed extract (1%), nisin (10,000 IU/g) and EDTA (0.16% w/w), which reduced the populations of L. monocytogenes, E. coli and Salmonella typhimurium, in 3, 2 and 1 log (CFU/mL), respectively, in contact periods of 1 h at 25°C. In other study concerning the antimicrobial activity of whey-protein-isolate films enriched with nisin (6,000 IU/g), EDTA (1.6 mg/mL) and malic acid (1%), Gadang, Hettiarachchy, Johnson, and Owens (Citation2008) reported an E. coli O157:H7-growth inhibition of 4.6 log cycles in turkey Frankfurters inoculated with 6.15 log (CFU/g), after a storage of 28 days at 4°C.
Mechanical characterization
The selected physical-mechanical properties of both bioactive (F2) and control (FC) films are shown in the . The mechanical parameters were statistically significant different (p < 0.001). The incorporation of EDTA and ACS to the films led to increase the Young’s modulus (EM), F2 showed more resistance to axial deformation than FC. Accordingly, F2 was almost five times less extensible than FC, and the stress value at the end of the stretching of the FC was higher than that of the F2 film. The high stiffness of the bioactive film can be attributed to the development of a heterogeneous film structure, presumably due to the formation of crystals composed of calcium and H2EDTA−2 ions and water molecules (Zabel, Poznyak, & Pawlowski, Citation2006), which could be formed during either drying or storage process of the films. This effect was not expected, CaCl2 was added to film-forming solution to promote gellan and pectin gelation and yield biopolymer matrices with higher degree of cross-linking and therefore, less gas-permeable films. High values of mechanical properties have been also reported for other bioactive films; for instance, pectin-polylactic acid-nisin films (EM = 2,590 MPa, %Emax = 3%) (Jin, Liu, Zhang, & Hicks, Citation2009), chitosan-eugenol and chitosan-cinnamon essential oil (EM = 1,660–1,460 MPa, %Emax = 6–8%) (Valencia-Sullca et al., Citation2016) and antibacterial gelatin films reinforced with metallic nanoparticles (EM = 2.30–2.63 GPa, %Emax = 8.3–9%) (Shankar, Jaiswal, Selvakannan, Ham, & Rhim, Citation2016).
Table 2. Selected physical-mechanical properties of bioactive (F2) and control (FC) films. Mean values ± standard deviation.
Tabla 2. Selección de propiedades físico-mecánicas de las películas bioactiva (F2) y control (FC). Promedio ± desviación estándar.
Water vapor permeability
The presents the values of WVP of the FC and F2 films. The results obtained were statistically significant different (p < 0.001). The WVP was affected by the incorporation of antimicrobial additives, mainly EDTA, F2 film was near 85% less WVP than FC. This decrease could be explained by the structural modifications arose in the F2 network by the development of crystal aggregates of EDTA-calcium. This assumption is based on the observations reported in studies concerning films containing nanoparticles (nanoclays, nanofibers, nanowhiskers (Sanchez-Garcia, Lopez-Rubio, & Lagaron, Citation2010)). These studies have attributed the reduction in WVP of nanocomposite films to the high nanodispersion of the particles across the matrix, the high crystallinity and the good interfacial adhesion in the nanobiocomposites. However, it has been also reported that at high concentration of nanoparticles, the WVP increases due to the formation of filler agglomeration, which usually results in the creation of preferential paths for the permeants to diffuse faster. Thus, the good dispersion of fillers into the biopolymer matrix as well as the amount and nature of the plasticizers could be relevant aspects to consider to enhance the water barrier properties of the films.
Furthermore, in the present study, the films did confer a dehydration-protection to the tested agar media, due to its WVP properties. The agar plates with no films were spontaneously dried during the experiments; in contrast, the agar plates with films remained without apparent signs of desiccation (). This properties are important for the conservation of processed foods.
Oxygen permeability
The oxygen permeability (PO2) of the films was not affected by the presence of EDTA and ACS (). There was not a statistically significant difference between the oxygen permeability of the two films, F2 and FC (p = 0.358). These values were lower than the corresponding WVP ones. The hydrophilic nature of the pectin-gellan films, their low plasticization and microstructural organization, provided a good barrier to oxygen diffusion and this could have enhanced the antimicrobial activity of the films.
Conclusions
It is reported the elaboration and characterization of functional films containing citrus pectin, gellan gum, glycerol, CaCl2, EDTA and ACS from fermentation culture broths of S. infantarius. The bioactive films exhibited inhibitory effects against L. monocytogenes, E. coli and S. aureus, inoculated in both selective media (Oxford, MacConkey and Baird Parker, respectively) and in “Barbacoa” medium (this last to mimic “Barbacoa”, an important Mexican meat product). The recorded antimicrobial activity would be attributed to a synergic interaction of ACS, EDTA and Pectin. On the other hand, the mechanical properties of the bioactive films were influenced by the EDTA contents, giving stronger and less extensible films than the controls with no EDTA; these effects might be attributed to the formation of EDTA-calcium crystals into the biopolymer matrix. These crystals also enhanced the water barrier properties of the bioactive films. Notwithstanding more research must be done to improve the mechanical properties of the films without affecting the antimicrobial activity.
Acknowledgments
LTG acknowledged CONACyT MSc. Scholarship. The authors thanked Dr. C Wacher and Dr. G. Ruiz for providing the bacterial strains and for helpful discussions. This work was supported by the CONACyT-México under Grants INFRA 2014, No. 230138; INFRA 2015, No. 254437, and INFRA 2016, No. 269805; and PRODEP-SEP-México under Grants Red CAs 2016/2017 “Diseño y Caracterización de Películas Alimentarias a base de Biopolímeros y Antimicrobianos Naturales”, and Nuevos PTC-2015/2016-Dr. MRLC.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aguirre-Loredo, R. Y., Rodríguez-Hernández, A. I., & Chavarría-Hernández, N. (2014). Physical properties of emulsified films based on chitosan and oleic acid. CyTA - Journal of Food, 12(4), 305–312. doi:10.1080/19476337.2013.853207
- ASTM. (1982). D1434-82. Standard test method for determining gas permeability characteristics of plastic film and sheeting. Philadelphia, PA: American Society for Testing and Materials.
- ASTM. (1993). D638M-93. Standard test method for tensile properties of plastics (Metric). Philadelphia, PA: American Society for Testing and Materials.
- ASTM. (2000). E96-00. Standard test methods for water vapor transmission of materials. Philadelphia, PA: American Society for Testing and Materials.
- ASTM. (2010). D882-10. Standard test method for tensile properties of thin plastic sheeting. Philadelphia, PA: American Society for Testing and Materials.
- Banin, E., Brady, K. M., & Greenberg, E. P. (2006). Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Applied and Environmental Microbiology, 72(3), 2064–2069. doi:10.1128/aem.72.3.2064-2069.2006
- Calce, E., Mignogna, E., Bugatti, V., Galdiero, M., Vittoria, V., & De Luca, S. (2014). Pectin functionalized with natural fatty acids as antimicrobial agent. International Journal of Biological Macromolecules, 68, 28–32. doi:10.1016/j.ijbiomac.2014.04.011
- Calderón-Aguirre, Á.-G., Chavarría-Hernández, N., Mendoza-Mendoza, B., Vargas-Torres, A., García-Hernández, E., & A.-I, R.-H. (2015). Antilisterial activity and physical-mechanical properties of bioactive caseinate films. CyTA - Journal of Food, 13(4), 483–490. doi:10.1080/19476337.2014.1003200
- Campos, C. A., Gerschenson, L. N., & Flores, S. K. (2010). Development of edible films and coatings with antimicrobial activity. Food and Bioprocess Technology, 4(6), 849–875. doi:10.1007/s11947-010-0434-1
- CLSI. (2015). M07-A10. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard, 10th ed. Wayne, PA: Clinical and Laboratory Standards Institute.
- Economou, T., Pournis, N., Ntzimani, A., & Savvaidis, I. N. (2009). Nisin–EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chemistry, 114(4), 1470–1476. doi:10.1016/j.foodchem.2008.11.036
- FDA. (2000). GRAS Nisin. Food and Drug Administration. Retrieved from https://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm266587.pdf
- FDA. (2016). GRAS Notice No. 676. Food and Drug Administration. Retrieved from http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm
- Gadang, V. P., Hettiarachchy, N. S., Johnson, M. G., & Owens, C. (2008). Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a Turkey frankfurter system. Journal of Food Science, 73(8), M389–394. doi:10.1111/j.1750-3841.2008.00899.x
- Gullón, B., Gómez, B., Martínez-Sabajanes, M., Yáñez, R., Parajó, J. C., & Alonso, J. L. (2013). Pectic oligosaccharides: Manufacture and functional properties. Trends in Food Science & Technology, 30(2), 153–161. doi:10.1016/j.tifs.2013.01.006
- Jabeen, N., Majid, I., Nayik, G. A., & Yildiz, F. (2015). Bioplastics and food packaging. A Review. Cogent Food & Agriculture, 1(1). doi:10.1080/23311932.2015.1117749
- Jans, C., Follador, R., Hochstrasser, M., Lacroix, C., Meile, L., & Stevens, M. J. A. (2013). Comparative genome analysis of Streptococcus infantarius subsp. infantarius CJ18, an African fermented camel milk isolate with adaptations to dairy environment. BMC Genomics, 14(1), 200.
- Jin, T., Liu, L., Zhang, H., & Hicks, K. (2009). Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes. International Journal of Food Science & Technology, 44(2), 322–329. doi:10.1111/j.1365-2621.2008.01719.x
- Jindal, M., Kumar, V., Rana, V., & Tiwary, A. K. (2013). Aegle marmelos fruit pectin for food and pharmaceuticals: Physico-chemical, rheological and functional performance. Carbohydrate Polymers, 93(2), 386–394. doi:10.1016/j.carbpol.2012.12.012
- Liu, S. (2013). Bioprocess engineering. Kinetics, biosystems, sustainability, and reactor design. Spain: Elsevier.
- Mansur, A. R., Park, J. H., & Oh, D. H. (2016). Predictive model for growth of staphylococcus aureus on raw pork, ham, and sausage. Journal of Food Protection, 79(1), 132–137. doi:10.4315/0362-028X.JFP-15-227
- Mantovani, H. C., & Russell, J. B. (2003). Inhibition of Listeria monocytogenes by bovicin HC5, a bacteriocin produced by Streptococcus bovis HC5. International Journal of Food Microbiology, 89(1), 77–83. doi:10.1016/S0168-1605(03)00110-7
- Meenatchisundaram, S., Chandrasekar, C. M., Udayasoorian, L. P., Kavindapadi Rajasekaran, R., Kesavan, R. K., Srinivasan, B., & Muthusamy, S. (2016). Effect of spice-incorporated starch edible film wrapping on shelf life of white shrimps stored at different temperatures. Journal of the Science of Food and Agriculture, 96(12), 4268–4275. doi:10.1002/jsfa.7638
- Mendoza-Mendoza, B., Rodríguez-Hernández, A. I., Vargas-Torres, A., Díaz-Ruiz, G., Montiel, R., Ramos-Aboites, H. E., … Chavarría-Hernández, N. (2013). Characterization of the effects on the growth kinetics of Listeria monocytogenes in solid culture in contact with caseinate base edible films added with antilisterial activity from Streptococcus sp. ABMX isolated from Pozol, an indigenous Mexican beverage. International Food Research Journal, 20(5), 2917–2925.
- Mimila-Méndez, K. (2017). Caracterización de sustancias similares a bacteriocinas producidas por Streptococcus infantarius con potencial aplicación biotecnológica. (Maestría en Ciencia de los Alimentos). Tulancingo de Bravo, Hgo, México: Universidad Autónoma del Estado de Hidalgo.
- Natividad-Bonifacio, I., Vazquez-Quinones, C. R., Rodas-Suarez, O. R., Fernandez, F. J., Rodriguez-Solis, E., Quinones-Ramirez, E. I., & Vazquez-Salinas, C. (2010). Detection of Clostridium perfringens in yearling lamb meat (barbacoa), head, and gut tacos from public markets in Mexico city. International Journal of Environmental Health Research, 20(3), 213–217. doi:10.1080/09603120903511101
- Nuñez, M., Tomillo, J., Gaya, P., & Medina, M. (1996). Bacteriocin quantification by the critical dilution method: A comparison af arbitrary units with diameter and area of the zone of growth inhibition. Milchwissenschaft, 51(1), 7–10.
- Pattanayaiying, R., H-Kittikun, A., & Cutter, C. N. (2015). Incorporation of nisin Z and lauric arginate into pullulan films to inhibit foodborne pathogens associated with fresh and ready-to-eat muscle foods. International Journal of Food Microbiology, 207, 77–82. doi:10.1016/j.ijfoodmicro.2015.04.045
- Pérez-Campos, S. J., Chavarría-Hernández, N., Tecante, A., Ramírez-Gilly, M., & Rodríguez-Hernández, A. I. (2012). Gelation and microstructure of dilute gellan solutions with calcium ions. Food Hydrocolloids, 28(2), 291–300. doi:10.1016/j.foodhyd.2012.01.008
- Ramawat, K.-G., & Mérillon, J.-M. (Eds.). (2015). Polysaccharides. Bioactivity and biotechnology. Switzerland: Springer International Publishing.
- Rubio, M. S., Torres, N., Gutierrez, J., & Mendez, R. D. (2004). Composition and sensory evaluation of lamb carcasses used for the traditional Mexican lamb dish, “barbacoa”. Meat Science, 67(2), 359–364. doi:10.1016/j.meatsci.2003.10.022
- Salmieri, S., Islam, F., Khan, R. A., Hossain, F. M., Ibrahim, H. M. M., Miao, C., … Lacroix, M. (2014). Antimicrobial nanocomposite films made of poly(lactic acid)-cellulose nanocrystals (PLA-CNC) in food applications: Part A—Effect of nisin release on the inactivation of Listeria monocytogenes in ham. Cellulose, 21, 1837–1850.
- Sanchez-Garcia, M. D., Lopez-Rubio, A., & Lagaron, J. M. (2010). Natural micro and nanobiocomposites with enhanced barrier properties and novel functionalities for food biopackaging applications. Trends in Food Science & Technology, 21(11), 528–536. doi:10.1016/j.tifs.2010.07.008
- Shankar, S., Jaiswal, L., Selvakannan, P. R., Ham, K. S., & Rhim, J. W. (2016). Gelatin-based dissolvable antibacterial films reinforced with metallic nanoparticles. RSC Advances, 6(71), 67340–67352. doi:10.1039/c6ra10620j
- Shekarforoush, S. S., Basiri, S., Ebrahimnejad, H., & Hosseinzadeh, S. (2015). Effect of chitosan on spoilage bacteria, Escherichia coli and Listeria monocytogenes in cured chicken meat. International Journal of Biological Macromolecules, 76, 303–309. doi:10.1016/j.ijbiomac.2015.02.033
- Sinigaglia, M., Bevilacqua, A., Corbo, M. R., Pati, S., & Del Nobile, M. A. (2008). Use of active compounds for prolonging the shelf life of mozzarella cheese. International Dairy Journal, 18(6), 624–630. doi:10.1016/j.idairyj.2007.11.022
- Sivarooban, T., Hettiarachchy, N. S., & Johnson, M. G. (2008). Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Research International, 41(8), 781–785. doi:10.1016/j.foodres.2008.04.007
- Thakur, B. R., Singh, R. K., & Handa, A. K. (1997). Chemistry and uses of pectin–A review. Critical Reviews in Food Science and Nutrition, 37(1), 47–73. doi:10.1080/10408399709527767
- Vaara, M. (1992). Agents that increase the permeability of the outer membrane. Microbiological Reviews, 56(3), 395–411.
- Valencia-Sullca, C., Jiménez, M., Jiménez, A., Atarés, L., Vargas, M., & Chiralt, A. (2016). Influence of liposome encapsulated essential oils on properties of chitosan films. Polymer International, 65(8), 979–987. doi:10.1002/pi.5143
- Vargas, M., Albors, A., & Chiralt, A. (2011). Application of chitosan-sunflower oil edible films to pork meat hamburgers. Procedia Food Science, 1, 39–43. doi:10.1016/j.profoo.2011.09.007
- Zabel, M., Poznyak, A. L., & Pawlowski, V. I. (2006). Crystal structure of calcium dihydroethylenediaminetetraacetate(2-) dihydrate Ca(H2Edta)·2H2O. Journal of Structural Chemistry, 47(3), 581–584. doi:10.1007/s10947-006-0341-5
- Zinoviadou, K. G., Koutsoumanis, K. P., & Biliaderis, C. G. (2010). Physical and thermo-mechanical properties of whey protein isolate films containing antimicrobials, and their effect against spoilage flora of fresh beef. Food Hydrocolloids, 24(1), 49–59. doi:10.1016/j.foodhyd.2009.08.003
