ABSTRACT
Chilean beekeeping is characterized by a variety of honey types, some unique due to a high participation of native plant species where nectar is collected by bees. For the first time flavonoids, phenolic compounds, and antioxidant capacity in honeys of species Azara integrifolia and Azara petiolaris, commonly known as corcolén honeys, have been determined. The results showed a high content of total phenol (between 48.79 and 153.30 mg GAE/100 g honey), while flavonoid content ranged between 8.80 and 12.33 mg QE/100 g honey. The ORAC-FL assay values varied between 2.15 and 7.74 µmol trolox/g honey, while ORAC-PGR assay values varied between 0.33 and 4.49 µmol trolox/g honey. Two phenolic acids (caffeic, coumaric acids) and three flavonoids (pinocembrin, chrysin, and luteolin) were identified by UHPLC-MS/MS in all honey samples. These results correlate with Azara sp. nectar contribution to the samples.
RESUMEN
La industria apícola chilena se caracteriza por diversidad de tipos de miel, algunas únicas en el mercado debido a la recolección de néctar desde especies florales nativas. En este estudio por primera vez se determinan los compuestos fenólicos, flavonoides y capacidad antioxidante de mieles monoflorales de las especies Azara petiolaris y Azara integrifolia, comúnmente conocidas como corcolén. Los resultado muestras un alto contenido de fenoles totales (entre 48.79 y 153.30 mg GAE/100 g de miel), mientras que el contenido de flavonoides se encontró entre 8.80 y 12.33 mg QE/100 g de miel. La capacidad antioxidante determinada mediante ORAC-FL fue entre 2.15 y 7.74 µmol trolox/g de miel; mientras que por ORAC-PGR se obtuvieron valores entre 0.33 y 4.49 µmol trolox/g de miel. Dos ácidos fenólicos (ácido caféico y ácido coumárico) y tres flavonoides (pinocembrina, crisina y luteolina) fueron identificados por UHPLC-MS/MS. Todos los resultados correlacionan con la contribución de polen de Azara sp. en las muestras de acuerdo al análisis melisopalinológico.
PALABRAS CLAVES:
Introduction
Chilean beekeeping products are characterized by a variety of honey types, with a high participation of native plant species. These give them unique qualities since the products take on the characteristics of plant species that produce them, that is their floral and geographical origin. Thus, it is possible to obtain bee products with unique and unrepeatable characteristics, especially when the hives are placed in areas of native vegetation (Bridi et al., Citation2015; Montenegro, Pizarro, et al., Citation2013).
Among the endemic species that produce monofloral honeys is quillay (Quillaja saponaria) (Montenegro, Salas, Peña, & Pizarro, Citation2009), which is the most emblematic honey plant species in Chile. Quillay is known for its abundant and extensive flowering, its capacity to generate large amounts of nectar that is very attractive to bees and its honey has also been scientifically shown to possess medicinal properties. Azara petiolaris and Azara integrifolia, both native Chilean species commonly known as corcolén, are dominant in the vegetation of central Chile, and have recently been identified as source of monofloral honeys Nevertheless there are no studies about their contribution to honey quality. (Montenegro et al., Citation2014).
The chemical composition of honey is variable and depends on geographicaland climatic conditions as well as the plant species that have been visited by the bees.
Some recognized features of honey are its antioxidant capacity and its antimicrobial activity, which are directly related to floral sources, post-harvest environmental conditions, and treatment (Montenegro, Santander, et al., Citation2013).
Polyphenols such as aromatic acids and flavonoids are responsible for antioxidant capacity since they have a chemical structure particularly suitable for exerting an antioxidant action because they act as free radical scavengers, neutralizing reactive oxygen species and chelating metal ions (Tenorio, Del Valle-Mondragón, & Pastelín-Hernández, Citation2006) Furthermore, numerous studies provide evidence on the beneficial effects of foods and beverages rich in phenolic compounds, mainly on cardiovascular diseases (Chua et al., Citation2013).
Today there is an increasing interest in the characterization of honey, since its differentiation can add value to unifloral honeys (Moujanni et al., Citation2017). The knowledge of the properties of Azara sp. honeys allows us to differentiate honeybee products. Therefore attention has especially been placed in chemical characterization, content of phenolic and flavonoids compounds and antioxidant capacity using Oxygen Radicals Absorbance Capacity (ORAC) assay using fluorescein (ORAC-FL) and pyrogallol red (ORAC-PGR) as probes.
Experimental
Melissopalinological assay
Honeys were obtained from the VI Region of Chile. The botanical origin of the honeys was determined according to Chilean Standard 2981 (Montenegro, Gómez, Díaz-Forestier, & Pizarro, Citation2008). Honey (10 g) was diluted in distilled water (10 mL), and then centrifuged at 2500 rpm for 5 min. The supernatant was eliminated and the sediment pollen was re-suspended in distilled water (0.1 mL). Optical microscopy was used to observe the pollen grains and these were identified using a pollen library. Only monofloral honeys, with a composition equal to or higher than 45% of a unique plant species were considered.
Total phenolic determination
The total phenolic content (TP) was determined according to the Folin-Ciocalteu (FC) method (Singleton, Orthofer, & Lamuela-Raventos, Citation1999). Honey (200 mg) was dissolved in Milli-Q water (4 mL). An aliquot (500 µL) was then mixed with FC reagent (1:10 v/v, 2.5 mL) and Na2CO3 solution (75 g/L; 2 mL) and incubated for 60 min at room temperature. The absorbance of the resulting blue solution was measured at 760 nm using an Agilent 8453 UV-visible spectrophotometer.
The effect of the polyvinylpolypyrrolidone (PVPP) process on the total phenolic content of the samples was measured (Bridi et al., Citation2014). The same honey dissolution (1.5 mL) was mixed with PVPP (150 mg), vortexed for 2 min and then centrifuged for 5 min at 10,000 rpm. The phenolic content in the supernatant was determined as previously described.
Flavonoid determination
Flavonoids content was estimated according to the aluminum chloride method (Woisky & Salatino, Citation1998). Honey (100mg) was dissolved in methanol (1,6 mL). An aliquot of the honey dissolution (500 µL) was mixed with 2% AlCl3 in methanol (500 µL) and incubated 60 min at room temperature before measuring the absorbance at 420 nm.
Oxygen radical absorbance capacity
The consumption of pyrogallol red (PGR, 5 µM), or fluoresceine (FL, 15 µM), associated to their incubation with AAPH (10 µM) at pH = 7.4 in presence of honey at appropriated dilution was estimated from absorbance (A) and fluorescence (F) measurements (López & Lissi, Citation2006). Values of (A/A0) or (F/F0) were plotted as a function of time. Integration of the area under the curve (AUC) was performed up to a time such that (A/A0) or (F/F0) reached a value of 0.2. Results are expressed as mM equivalents of Trolox per gram of honey (TE/g)
HPLC-DAD determination
An ethanolic extract using Amberlite XAD-2 obtained according to Montenegro and Ortega (Citation2011) was employed for the chromatographic analysis.
Phenolic separation was performed using a LaChrom Elite L-2130 binary pump coupled to a DAD L-2440 detector with a 20µL sample loop injector. A Hibar C18 column (5µm x 4.6mm x 150mm) with a guard column of the same material was used in a L-2300 oven at 35 °C, mobile phase A was KH2PO4 (10 mM, pH 2.6) and mobile phase B was acetonitrile (B), with a concentration gradient of 30–35% A (0–12 min) and 35% A (12–105 min) at a flow rate of 0.8 mL/min. The chromatographic elution of phenolic compounds was followed at 254, 290, 320, and 350 nm, and their UV spectra were recorded. Identification of phenolic components was evaluated by comparing their retention times and standard compound spectra.
UHPLC-MS/MS determination
An ABSciex triple Quad 4500 mass spectrometer equipped with an electrospray (TurboV) interface coupled to an Eksigent Ekspert Ultra LC100 with Ekspert Ultra LC100-XL autosampler system (AB/Sciex Concord, Ontario, Canada) was used in order to confirm the HPLC-DAD analysis. The chromatographic separation was carried out using gradient elution of 0.1% formic acid in water (A) and methanol (B) as the mobile phase. The gradient was programmed as follows: 0–1 min, 15% B; 1–17 min, 15–100% B; 17–21 min 100–100% B; 21–22 min, 100–15% B; 22–25 min, 15–15% B. The injection volume was 10 µL and the flow rate was kept at 0.5 mL/min. A LiChrospher 100 RP-18 endcapped column (125 mm x 4mm id, 5µm) (Merck,Darmstadt, Germany) was used with a controlled temperature of 50°C. Quantification was performed with calibration curves using commercially available standards. Parameters used for quantification are presented in .
Table 1. UHPLC – MS/MS parameters for targeted compound.
Tabla 1. Parámetros cromatográficos para el analisis de polifenoles por UHPLC-MS/MS.
Results and discussion
Pollen analysis is of great importance to authenticate honey origin and characteristics, since it provides information about geographical origin, honey extraction and possible contamination with dust or starch grains (Von Der Ohe, Persano Oddo, Piana, Morlot, & Martin, Citation2004). A melissopalinological assay was used to determine the plant species present in honey samples selected for this study. Honeys originated from two species: Azara integrifolia and Azara petiolaris. contains the pollen composition of samples used in this study. The highest value is attributed to the sample P3 with 83.6% of Azara petiolaris while sample P1 should be considered a multifloral honey since it only reaches 43.4% of Azara petiolaris in its composition.
Table 2. Pollen composition of Azara sp. honey samples.
Tabla 2. Composición polínica de mieles monoflorales de Azara sp.
Different species also have a contribution in the samples, mainly other Chilean natives’ species such as Lithraea caustica, Retanilla trinervia, Aristotelia chilensis, Escallonia pulverulenta, and Quillaja saponaria as well as introduced species such as Galega officinalis, Medicago sativa, Trifolium repens, and Brassica rapa.
In the case of Azara sp. honey samples an average of 99.5 mg GAE/100 g of honey was found. Among the values obtained () sample I1 presents the highest value for TP; (153.3 mg GAE/100 g of honey) while sample I3 presents the lowest value (48.8 mg GAE/100 g of honey). This result indicates that Azara sp. honey samples have a higher content of TP than quillay honey (54.5 mg GAE/100 g of honey) produced by honeybees from other Chilean endemic species (Muñoz, Copaja, Speisky, Peña, & Montenegro, Citation2007). Alzahrani et al. (Citation2012) showed values of total phenols of 89.9 ± 1.2 for the famous Manuka honey (Leptospermum scoparium), 62.7 ± 4.4 for black forest honey (Acacia sp.), and 50.3 ± 0.8 for wild carrot honey (Daucus carota). These results highlight the relevance of the poliphenolic content in corcolen honey.
Table 3. Determination of total phenols, real phenols, flavonoids, and antioxidant capacity for Azara sp. honey samples.
Tabla 3. Contenido de fenoles totales, fenoles reales, flavonoides y capacidad antioxidante de mieles monoflorales de Azara sp.
It should be noted that when examining TP content in honeys,the presence of non-phenolic compounds such as reductive sugars (fructose, glucose, and sucrose) and organic acids (ascorbic, citric, and tartaric acids) are capable of interfering with the assessment of phenolics in food matrixes by FC assay (Everette et al., Citation2010). To reduce overestimation a synthetic polymer PVPP, which adsorbs phenolic compounds by hydrogen bonding and hydrophobic interactions has been employed. Previous results showed that after employing three consecutive cycles of PVPP extraction, all polyphenols (including phenolic and cinnamic acid) had a percentage adsorption of 90% (Bridi et al., Citation2014). For Azara sp. honey samples, the percentage of interference found was between 45.2% (P3) and 71.6% (sample P2). The main contributor should be fructose and glucose content.
The content of flavonoids in Azara sp. honey ranged between 5.9 and 38.34 mg QE/100 g (). Muñoz et al. (Citation2007) conducted a study with 26 chilean honey samples, and found that the flavonoid content of samples varied from 0.014 to 13.80 mg QE/100 g of honey. However this study didn’t consider Azara sp. honeys. In general all of the samples´ flavonoid content were higher than in other Chilean honeys. The importance of the flavonoids content (FC) is that they play an essential role in protecting against oxidative damage phenomena, where anti-free radical properties are mainly directed toward the hydroxyl and superoxide free radicals and highly reactive species.
We studied the antioxidant capacity of the samples by the ORAC-PGR and the ORAC-FL methodologies. These indexes are related to the reactivity of the phenolic compounds toward free radicals generated in the AAPH thermolysis and this procedure has been widely used in the evaluation of the antioxidant capacity of polyphenol-rich foods (López & Lissi, Citation2006). The index values were between 0.33 and 4.49 µmol TE/g honey and between 2.15 and 7.74 µmol TE/g honey for ORAC-PGR and ORAC-FL respectively (). The ORAC index can be considered as a measure of the capacity of the sample to remove peroxyl and alkoxyl radicals (Dorta et al., Citation2015). In complex matrices, concentration, chemical structure, and possibly the interaction between the scavenger compounds present in the sample determine this index.
No significant difference between Azara integrifolia and Azara petiolaris honeys samples was found on the parameters studied, and in order to observe the influence of botanical origin on those parameters, all samples were grouped for the calculation of the correlation coefficients displayed in . It is observed that the analysis of correlation between total phenolic content with the pollen composition of Azara sp. in honey samples is very high and significant (0.84, p < 0.01) which indicates that the phenolic compounds are due to the presence of this plant species in the honey. Sample I1 showed the highest value of total phenols with 153.30 mg GAE/100 g of honey, while the lower value corresponds to sample I3; 48.79 mg GAE/100 g of honey.. In addition, the content of flavonoids correlates with the percentage of Azara sp. pollen (significant correlation 0.65, p < 0.007) which agrees with the correlation between total phenolics and botanical origin; for example sample P3 has the highest flavonoid content and a 83.6% of Azara sp. in their composition. A high and significant correlation for all parameters studied with ORAC-PGR was observed, meaning that most of the phenolic compounds and molecules with scavenger capacity belong to Azara sp. species. These correlations are quite interesting since just by means of the melissopalinological assay there could be evidence of the phenolics composition as well as the antioxidant capacity of monofloral Azara sp. honeys.
Table 4. Correlation values for parameters studied for Azara sp. honey samples.
Tabla 4. Correlación entre los parámetros estudiados en mieles monoflorales de Azara sp.
Polyphenols profile
The phenolic compound composition depends strongly on the plant species from which the nectar was collected (Biesaga & Pyrzyńska, Citation2013). Phenolic extracts of the honey were obtained using Amberlite XAD-2 columns for cleaning and concentrating the phenolic compounds (Montenegro & Ortega, Citation2011). An analysis by HPLC-DAD of the extract was used to obtain the chromatographic profile of different polyphenols; all samples exhibit a chemical profile similar to the one presented in . For a better characterization, an UHPLC-MS/MS methodology was developed in order to identify and quantify specific polyphenols in the honey samples, the results are shown in .
Table 5. Phenolic composition in Azara sp. honey samples by UHPLC-MS/MS.
Tabla 5. Contenido de polifenoles en muestras de miel monofloral de Azara sp. determinado por UHPLC-MS/MS.
Figure 1. Chromatographic profile of polyphenols in Azara sp. honey I2. Peaks: (1) caffeic acid, (2) coumaric acid, (3) abscisic acid, (4) luteolin, (5) apigenin, (6) pinocembrin, and (7) chrysin.
Figura 1. Perfil cromatográfico de polifenoles en muestra I2 de miel monofloral de Azara sp. Identificación: (1) ácido caféico, (2) ácido coumárico, (3) ácido abscisico, (4) luteolina, (5) apigenina, (6) pinocembrina, (7) crisina.
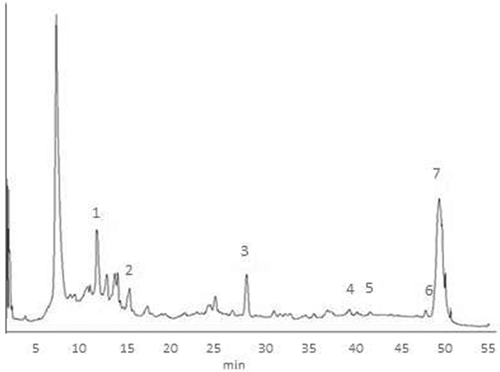
Two phenolic acids (caffeic and coumaric acids) and three flavonoids (pinocembrin, chrysin and luteolin) were identified among the samples; while syringic, sinapic and abscisic acids and quercetin and apigenin were found to be common among most of the samples; this shows that both Azara integrifolia and Azara petiolaris Chilean honey share similar chemical composition. The identification of propolis-derived compounds like pinocembrin and chrysin, could make an important contribution to the phenolic composition and antioxidant capacity in Azara sp. honey (Martos, Cossentini, Ferreres, & Tomás-Barberán, Citation1997). These results show that Azara sp. presents the same phenolic acids as Chilean quillay monofloral honey but differences in flavonoid content, since the latter shows the presence of caffeic acid, p-coumaric acid, gallic acid, chlorogenic acid, quercetin, rutin, and naringenin (Montenegro et al., Citation2009, Citation2014).
Conclusion
Our results indicate that Azara sp. honey samples exhibited high content of phenolics and flavonoids that correlate with their antioxidant capacity. Both Azara integrifolia and Azara petiolaris honeys shared similar chemical characteristics, including the presence of the specific phenolic acids and flavonoids (caffeic and coumaric acids, pinocembrin. chrysin and luteolin) which are related to the same geographical area.
All parameters studied correlate significantly with Azara sp. pollen composition, meaning that most of the phenolic compounds and molecules with scavenger capacity belong to Azara sp. species; this shows the importance of the melissopalinological assay as a tool for honey quality measurement.
Acknowledgments
We thank Project VRI Interdisciplina 31/2013, Project FIC Regional IDI 30126395-0, CONICYT for Beca Doctorado Nacional and Gastos operacionales Nº 21110822, PAI-CONICYT for Beca Tesis de Doctorado en la Empresa N° 781412002, Project FONDEQUIP EQM 150102 and Project RC 130006, CILIS, by Ministerio de Economía, Fomento y Turismo de Chile.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alzahrani, H. A., Boukraa, L., Bellik, Y., Abdellah, F., Bakhotmah, B. A., Kolayli, S., & Sahin, H. (2012). Evaluation of the antioxidant activity of three varieties of honey from different botanical and geographical origins. Global Journal of Health Science, 4, 191–196. doi:10.5539/gjhs.v4n6p191
- Biesaga, M., & Pyrzyńska, K. (2013). Stability of bioactive polyphenols from honey during different extraction methods. Food Chemistry, 136, 46–54. doi:10.1016/j.foodchem.2012.07.095
- Bridi, R., Montenegro, G., Nuñez-Quijada, G., Giordano, A., Morán-Romero, F., Jara-Pezoa, I., … López-Alarcón, C. (2015). International regulations of propolis quality: Required assays do not necessarily reflect their polyphenolic-related in vitro activities. Journal of Food Science, 80, C1188–C1195. doi:10.1111/1750-3841.12881
- Bridi, R., Troncoso, M. J., Folch-Cano, C., Fuentes, J., Speisky, H., & Lopez-Alarcón, C. (2014). A polyvinylpolypyrrolidone (PVPP)-Assisted Folin-Ciocalteau Assay to asses total phenol content of commercial Beverages. Food Analytical Methods, 7, 2075–2083. doi:10.1007/s12161-014-9856-0
- Chua, L., Rahaman, N., Adnan, N., & Tan, T. (2013). Antioxidant activity of three honey samples in relation with their biochemical components. Journal of Analytical Methods in Chemistry, 2013, 1–8. doi:10.1155/2013/313798
- Dorta, E., Fuentes-Lemus, E., Aspée, A., Atala, E., Speisky, H., Bridi, R., … López-Alarcón, C. (2015). The ORAC (oxygen radical absorbance capacity) index does not reflect the capacity of antioxidants to trap peroxyl radicals. RSC Advances, 5, 39899–39903. doi:10.1039/C5RA01645B
- Everette, J., Bryant, Q., Green, A., Abbey, Y., Wanguila, G., & Walker, R. (2010). Thorough study of reactivity of various compound classes toward the Folin-Ciocalteau reagent. Journal of Agricultural and Food Chemistry, 58, 8139–8147. doi:10.1021/jf1005935
- López, C., & Lissi, E. (2006). A novel and simple ORAC methodology based on the interaction of Pyrogallol Red with peroxyl radicals. Free Radical Research, 40(9), 979–985. doi:10.1080/10715760500481233
- Martos, I., Cossentini, M., Ferreres, F., & Tomás-Barberán, F. A. (1997). Flavonoid composition of Tunisian honeys and propolis. Journal of Agricultural and Food Chemistry, 45, 2824–2830. doi:10.1021/jf9609284
- Montenegro, G., Gómez, M., Díaz-Forestier, J., & Pizarro, R. (2008). Aplicación de la norma chilena oficial de denominación de origen botánico de la miel para la caracterización de la producción apícola. Ciencia e investigación agraria, 35(2), 181–190. doi:10.4067/S0718-16202008000200007
- Montenegro, G., & Mejías, E. (2014). Biological applications of honeys produced by Apis mellifera.. Biological Research, 46, 341–348. doi:10.4067/S0716-97602013000400005
- Montenegro, G., & Ortega, F. (2011). Uses of unifloral ulmo honey extract as a bactericide and a fungicide. WO/2011/057421.
- Montenegro, G., Pizarro, R., Mejias, E., & Rodriguez, S. (2013). Evaluación biológica de polen apícola de plantas nativas de Chile. Revista Internacional de botánica experimental, 82, 7–13.
- Montenegro, G., Salas, F., Peña, R. C., & Pizarro, R. (2009). Actividad antibacteriana y antifúngica de mieles monoflorales de Quillaja saponaria, especie endémica en Chile. Revista Internacional de Botánica Experimental, 78, 141–146.
- Montenegro, G., Santander, F., Jara, C., Núñez, G., & Fredes, C. (2013). Actividad antioxidante y antimicrobiana de mieles monoflorales de plantas nativas chilenas. Boletín Latinoamericano y del Caribe de plantas medicinales y aromáticas, 12(3), 257–268.
- Moujanni, A., Partida, L., Essamadi, K., Hernanz, D., Heredia, F. J., & Terrab, A. (2017). Physicochemical characterization of unique unifloral honey: Euphorbia resinífera. CyTA – Journal of Food, 16, 27–35. doi:10.1080/19476337.2017.1333529
- Muñoz, O., Copaja, S., Speisky, H., Peña, R., & Montenegro, G. (2007). Contenido de flavonoides y compuestos fenólicos de mieles chilenas e índice de antioxidante. Química nova, 4, 848–853. doi:10.1590/S0100-40422007000400017
- Singleton, V., Orthofer, R., & Lamuela-Raventos, R. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteau Reagent. Methods in Enzymology, 299, 152–178.
- Tenorio, F., Del Valle-Mondragón, L., & Pastelín-Hernández, G. (2006). Los flavonoides y el sistema cardiovascular. ¿Puede ser una alternativa terapéutica?. Archivos de cardiología de México, 76, 33–39.
- Von Der Ohe, W., Persano Oddo, L., Piana, M. L., Morlot, M., & Martin, P. (2004). Harmonized methods of melissopalynology. Apidologie, 35, S18–S25. doi:10.1051/apido:2004050
- Woisky, R., & Salatino, A. (1998). Analysis of propolis: Some parameters and procedures for chemical quality control. Journal of Apicultural Research, 37, 99–105. doi:10.1080/00218839.1998.11100961
