ABSTRACT
Due to the effect of interfering ion, the content of fluoride ion in tea brew, detected by ion-exchange chromatography, was markedly higher than ion-selective electrode. Ion-exclusion chromatography, a new method for the determination of fluoride ion in tea brew, was proposed and evaluated in this study. Two Metrosep organic acid columns in series resulted in better separation of fluoride ion than single column. The most suitable organic modifier for eluent and its percentage were checked, the optimal conditions of the mobile phase was 0.33 mM sulfuric acid solution containing 10.5% (v/v) acetone, at a flow rate of 0.5 mL/min. Satisfactory sensitivity and wide linearity range were obtained. The applicability of the method was demonstrated by the analysis of various tea samples in China, the accuracy of the results was proved by recovery tests, and the concordance with the values was obtained by ion-selective electrode.
RESUMEN
Debido al ion interferente, se detectó el contenido de iones de flúor en una infusión de té empleando cromatografía iónica de exclusión. Este método permitió que se registrara un nivel más elevado que utilizando el electrodo selectivo de iones. El presente estudio propuso y evaluó el método de cromatografía iónica de exclusión, un procedimiento nuevo empleado para determinar la presencia de iones de flúor en el té preparado. Cabe señalar que dos columnas Metrosep en serie de ácido orgánico produjeron una mejor separación de iones de flúor que una sola columna. Se determinó el modificador orgánico más adecuado y su porcentaje para el eluyente: las condiciones óptimas de la fase móvil están dadas por 0.33 mM solución de ácido sulfúrico con 10.5% (v/v) de acetona y un caudal de 0.5 mL/min. Ello permitió obtener una sensibilidad satisfactoria y un amplio rango de linealidad. La aplicabilidad del método fue demostrada a partir del análisis de varias muestras de té en China. La precisión de los resultados se demostró utilizando pruebas de recuperación, mientras que la concordancia con los valores se comprobó empleando un electrodo selectivo de iones.
PALABRAS CLAVE:
1. Introduction
Impact of fluoride on human health is still a controversial issue. A moderate amount of fluoride intake is widely recognized beneficial effect of reducing dental caries among children (Carey, Citation2014). However, the increase in fluoride concentrations can increase cases of dental fluorosis and skeletal fluorosis (Wang et al., Citation2012), as well as bone tumor occurrence among young boys (Bassin, Citation2001). The recent studies implied that excessive of fluoride intake would result in high concentration mental retardation on children’s intelligence (Kundu et al., Citation2015). Allergy and hypersensitivity to fluoride were also reported (Lamberg, Hausen, & Vartiainen, Citation1997).
Tea, rich in fluoride, is one of the most popular beverages in the world (Karak & Bhagat, Citation2010). According to statistics by Food and Agriculture Organization (FAO) of the United Nations, China holds a significant place among the world’s largest producer countries with a share of more than 30%. It has been found that fluorosis was prevalent mainly among the minority inhabitants in the west of China, and the prevalence of fluorosis provoked by drinking brick tea has attracted much more attentions (Fan et al., Citation2016). Precise measurement of fluoride content in tea would ensure intake safety and avoid fluoridation.
In most studies published to date, ion-exchange chromatography is often a method of choice in the analysis of fluoride content due to satisfactory sensitivity, wide linearity range, and usually short time of a single run. There are a few reports on the determination of fluoride content using this method (Han & Wu, Citation2010; Li et al., Citation2016; Lin, Wang, & Li, Citation2014; Yusenko, Kapsargin, & Nesterenko, Citation2014). However, the method still offered an important challenge when applied to complex matrix samples (Pytlakowska, Kita, Janoska, Połowniak, & Kozik, Citation2012). Fluoride ions in tea samples are usually accompanied by considerably larger amount of organic acids, which interfered with fluoride analysis (Gorgulu, Ozdemir, Kipcak, Piskin, & Derun, Citation2016; Kontozova-Deutsch, Krata, Deutsch, Bencs, & Grieken, Citation2008). Kumar et al. found that the peak for the fluoride was not well identify because of its closed retention time with interfering ions (Kumar, Narayan, & Hassarajani, Citation2008). Furthermore, in order to identify the fluoride peak, the standard addition method was applied to the tea brew; it was only an indirect method for analysis of fluoride ion as well as the increased risk of contamination (Schwalfenberg, Genuis, & Rodushkin, Citation2013). Hence, there is a need to develop a rapid, accurate, and direct method to detect the fluoride ion in complex matrix samples, such as tea.
Ion-exclusion chromatography is an excellent technique for determination of organic acids and weak retention anions based on the mechanism of Donnan exclusion, and no publication has been reported for determination of fluoride using ion-exclusion chromatography. In this work, ion-exclusion chromatography was adopted, after compared with ion-exchange chromatography, because it was possible to separate fluoride with organic acids directly using this method. Furthermore, the new method was validated and used for the analysis of fluoride content in various Chinese tea samples.
2. Materials and methods
2.1. Reagents
The standard solutions were bought from Institute for Reference Materials of State Environmental Protection Administration (Beijing, China) and stored at 4°C. Calibration solutions were prepared by diluting the stock standard solution to the required concentration just before used and prepared daily.
Acetone was High Performance Liquid Chromatography (HPLC) grade and all other reagents were reagent-grade. Water was obtained from Milli-Q (Millipore, Bedford, MA, USA) equipment. ProElut C18 cartridges were obtained from Dikma (Beijing, China).
2.2. Apparatus
The ion chromatographic instrument from Metrohm (Herisau, Switzerland) consisting of a 861 Advanced Compact IC with MSM (Metrohm Suppressor Module) suppressor and 853 CO2 Suppressor (MCS), a conductivity detector was used for the analysis. Fluoride separation was carried out in suppressor mode on two Metrosep organic acids’ analytical column (250 × 7.8 mm) connected in series with a Metrosep RP guard column. A solution of 20 mM LiCl.H2O was used as the regenerant. The volume of the sample injection loop was 50 μL. An isocratic elution was performed, using amobile phase of 0.33 mM sulfuric acid solution containing 10.5% (v/v) acetone, at a flow rate of 0.5 mL/min.
2.3. Sample preparation
Five kinds of teas (black, green, oolong, brick, and pu-er) were bought from market (Chengdu, Sichuan). The samples were dried at 103°C to constant weight. The 2 g dried teas were weighted and placed into a 250 mL flask with 100 mL of boiling deionised water for 10 min, the tea solution was centrifuged (4000 rpm, 20 min) and cooled to ambient temperature. Then, the supernatant was filtered through a disposable 0.2 μm membrane filter (Membrana, Germany) and a C18 cartridge by turns. C18 cartridge was first washed with 5 mL methanol and then with 10 mL deionised water. About 5 mL of the sample solution were loaded on the cartridge and allowed to flow at the rate of 1 mL/min. The first 3 mL elutes were rejected, and the following 2 mL were collected for direct injection into the ion chromatograph. This was done to ensure that there was no dilution of the sample solution during the pretreatment.
3. Results and discussion
3.1. Interfering ions in tea and the selection of ion-chromatography separation mechanism
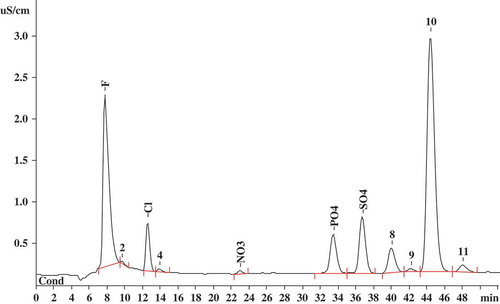
The main interfering ions of tea are the complex organic solutes in tea solutions. Thus, we could not achieve a separation between fluoride and interfering ions using ion-exchange chromatography. As shown in , the peaks of the different ingredients except for fluoride were well separated. The fluoride ion and some micromolecule organic acids ions were co-eluted, because of the approximate retention times. Although peak shape and recovery of fluoride ion were very good, the content of fluoride ion in teas by ion-exchange chromatography was markedly higher than ion-selective electrode, even over one or two orders of magnitude (). Some masked micromolecule organic acids ions in the peak of fluoride ion resulted in the huge differences of fluoride ion content. Therefore, it was concluded that ion-exchange chromatography separation mechanism could not fully eliminate influence of interfering ions. However, the advantage of ion-exclusion chromatography separation mechanisms that fluoride ion and micromolecule organic acid ions could be easily separated, attributed to Donnan exclusion and their, respectively, different pKa values. So, ion-exclusion chromatography could be used to accurately detect fluoride ion in tea.
Table 1. The mean content of fluoride ion (mg/g) in tea using ion-exchange chromatography and ion selective electrode.
Tabla 1. Contenido medio de iones de flúor (mg/g) en té, medido utilizando cromatografía de intercambio iónico y el electrodo selectivo de iones.
Figure 1. Chromatogram of the green tea sample solution showing different anionic mineral components peaks using a mixture of 3.2 mM Na2CO3 and 1.0 mM NaHCO3 as eluent, at a flow rate of 0.7 mL/min.
Figura 1. Cromatografía de la muestra de solución de té verde en la que se constatan distintos picos correspondientes a los componentes de minerales aniónicos, utilizando una mezcla de 3.2 mM NaHCO3 como eluyente, con un caudal de 0.7 mL/min.
3.2. Development of a new analytical method
The optimal conditions were selected for the analysis of the fluoride ion in tea brew. Two Metrosep organic acid columns in series resulted in better separation between fluoride ion and organic acids than a single column. The retention of an analysis was influenced by the eluent concentration (Albarran & Collins, Citation1987; Turkelson & Richards, Citation1978) and organic modifier (Tanaka & Fritz, Citation1986; Tanaka & Ishizuka, Citation1980) in the eluent.
Different concentrations (0–0.5 mM) of sulfuric acid solution as the eluent were tested, while sulfuric acid solution concentration increased, the resolution between fluoride ion and adjacent organic acids was improved, but recording time would increase. Considering resolution and recording time, 0.33 mM sulfuric acid solution was selected as the optimum concentration.
Methanol, acetone, and acetonitrile were considered organic modifiers, and the best result was obtained when acetone was used. When the percentage of acetone in the mobile phase increased, separation of fluoride and resolution also increased, as shown in . The suitable percentage of acetone was 10.5% (v/v), which resulted in the optimal resolution and symmetrical peak shape of fluoride ion.
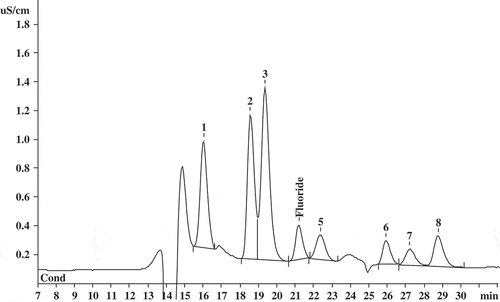
Figure 2. Chromatogram of the oolong tea sample solution showing fluoride and organic acid peaks using ion-exclusion chromatography.
Figura 2. Cromatografía iónica de exclusión de la muestra de solución de té oolong, en la que se constatan los picos de flúor y ácido orgánico.
The flow rate and column temperature were taken to optimise the chromatographic conditions. The flow rate (0.5–1.0) mL/min was investigated, the results showed that the resolution decreased along with the increased of flow rate, and the optimal conditions of a flow rate was 0.5 mL/min. In addition, the effect of column temperature was very small to separate the fluoride ion in the range of column temperature (25–30)°C .
3.3. Validation of the new analytical method
Linear range of calibration for fluoride ion, regression coefficient, the limit of detection (LOD) (S/N = 3), and the limit of quantification (LOQ) (S/N = 10) were evaluated using standard samples. Linearity was evaluated over five calibration points (from 0.02 to 100 μg/mL) with six measurements for each calibration point. The analyses exhibited good linearity over the evaluated range with correlation coefficient. The LOD and LOQ were considered the equivalent concentration of fluoride ion, when three times and 10 times baseline noise signal were produced on a detector. The obtained results are shown in .
Table 2. Results of validation of the analytical method evaluated with standard samples.
Tabla 2. Resultados de validación del método analítico evaluado con muestras estándar.
In order to verify their accuracy, we carried out recovery tests. The known amounts of the standard samples were added to preparation of tea brew at three concentration levels (from low to high). The recovery of fluoride ion was 93.8–104.5% (). In the case, the results showed that the proposed method presented good accuracy. Thus, the pretreatment procedure selectively removed the interfering components.
Table 3. Recovery studies of fluoride ion in tea brew.
Tabla 3. Estudio de recuperación del ion de flúor en té preparado.
3.4. Fluoride in tea samples and comparison with ion-selective electrode
Using this analytical method, several kinds of commercial tea samples were tested. The analysed tea samples belonged to three different varieties, non-fermented (green), fermented (black), and semi-fermented (oolong) teas. The contents of fluoride in dried teas are given in . An example of chromatogram obtained for oolong tea is shown in . As shown in , the fluoride contents of five tea samples are in good agreement with the values obtained using ion-selective electrode. The relative standard deviations of fluoride evaluated by replicate analysis (n = 6) were <5%. Tie Guan Yin sample (Chinese oolong tea), which is made by old and coarse tea leaves, contained relatively high amounts of fluoride.
Table 4. The mean content of fluoride ion (mg/g) in tea and comparison with ion-selective electrode.
Tabla 4. Contenido medio del ion de flúor (mg/g) en té y comparación con el electrodo selectivo de iones.
4. Conclusion
In this study, ion-exclusion chromatography was proposed for the analysis of fluoride ion in tea brew, after compared with ion-exchange chromatography. Using ion-exchange chromatography, some masked micromolecule organic acid ions in the peak of fluoride ion resulted in the huge inaccurate determination of fluoride ion content. Several advantages of using ion-exclusion chromatography were demonstrated. The most important one was that fluoride ion could be easily separated with micromolecule organic acids ions, because of Donnan exclusion and respective pKa values. Furthermore, samples could be injected directly after simple pretreatment. The lower detection limits and improved resolution were obtained using two Metrosep organic acid columns in series. The proposed method was successfully applied to the analysis of various tea samples in China. It could be adopted to analysis of complex matrix samples like tea.
Acknowledgments
This work was financially supported through the project (No. 2016YFF0202300).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Albarran, G., & Collins, C. H. (1987). Ion moderated partition chromatographic determination of carbon 14 calcium carbonate and carbon 14 barium carbonate self radiolysis products. Journal of Chromatography A, 395, 623–630. doi:10.1016/S0021-9673(01)94150-0
- Bassin, E. B. (2001). Association between fluoride in drinking water during growth and development and the incidence of osteosarcoma for children and adolescents ( Ph.D. Thesis). Harvard School of Dental Medicine, Boston, Massachusetts.
- Carey, C. M. (2014). Focus on fluorides: Updateon the use of fluoride for the prevention of dental caries. The Journal of Evidence-Based Dental Practice, 14, 95–102. doi:10.1016/j.jebdp.2014.02.004
- Fan, Z., Gao, Y., Wang, W., Gong, H., Guo, M., Zhao, S., … Sun, D. (2016). Prevalence of brick tea-type fluorosis in the Tibet autonomous region. Journal of Epidemiology, 26, 57–63. doi:10.2188/jea.JE20150037
- Gorgulu, T. Y., Ozdemir, O. D., Kipcak, A. S., Piskin, M. B., & Derun, E. M. (2016). The effect of lemon on the essential element concentrations of herbal and fruit teas. Applied Biological Chemistry, 59(3), 425–431. doi:10.1007/s13765-016-0161-z
- Han, Y., & Wu, C. (2010). Ion chromatography for rapid and sensitive determination of fluoride in milk after headspace single-drop microextraction with in situ generation of volatile hydrogen fluoride. Analytica Chimica Acta, 661, 161–166. doi:10.1016/j.aca.2009.12.018
- Karak, T., & Bhagat, R. M. (2010). Trace elements in tea leaves, made tea and tea infusion: A review. Food Research International, 43(9), 2234–2252. doi:10.1016/j.foodres.2010.08.010
- Kontozova-Deutsch, V., Krata, A., Deutsch, F., Bencs, L., & Grieken, R. V. (2008). Efficient separation of acetate and formate by ion chromatography: Application to air samples in a cultural heritage environment. Talanta, 75, 418. doi:10.1016/j.talanta.2007.11.025
- Kumar, S. D., Narayan, G., & Hassarajani, S. (2008). Determination of anionic minerals in black and kombucha tea using ion chromatography. Food Chemistry, 111, 784–788. doi:10.1016/j.foodchem.2008.05.012
- Kundu, H., Basavaraj, P., Singla, A., Gupta, R., Singh, K., & Jain, S. (2015). Effect of fluoride in drinking water on children’s intelligence in high and low fluoride areas of Delhi. Journal of Indian Association of Public Health Dentistry, 13, 116–121. doi:10.4103/2319-5932.159043
- Lamberg, M., Hausen, H., & Vartiainen, T. (1997). Community Dent Symptoms experienced during periods of actual and supposed water fluoridation. Oral Epidemiology, 25, 291–295. doi:10.1111/j.1600-0528.1997.tb00942.x
- Li, F., Feng, Z., Wang, C., Liang, Z., Gao, J., Wang, Q., & Hu, J. (2016). Simultaneous quantitative analysis of inorganic anions in commercial waste-oil biodiesel using suppressed ion exchange chromatography. Bulgarian Chemical Communications, 48, 30–35.
- Lin, L., Wang, H., & Li, R. (2014). Simultaneous determination of ions of fluoride, iodide, bromate radical and cyanide radical in cosmetics by ion chromatography. China Surfactant Detergent & Cosmetics, 2, 108–111.
- Michalski, R. (2006). Simultaneous determination of common inorganic anions in black and herbal tea by suppressed ion chromatography. Journal of Food Quality, 29, 607–616. doi:10.1111/jfq.2006.29.issue-6
- Pytlakowska, K., Kita, A., Janoska, P., Połowniak, M., & Kozik, V. (2012). Multi-element analysis of mineral and trace elements in medicinal herbs and their in fusions. Food Chemistry, 135(2), 494–501. doi:10.1016/j.foodchem.2012.05.002
- Schwalfenberg, G., Genuis, S. J., & Rodushkin, I. (2013). The benefits and risks of consuming brewed tea: Beware of toxic element contamination. Journal of Toxicology, 2013(1), 370460. doi:10.1155/2013/370460
- Tanaka, K., & Fritz, J. S. (1986). Separation of aliphatic carboxylic acids by ion-exclusion chromatography using a weak-acid eluent. Journal of Chromatography A, 361, 151–160. doi:10.1016/S0021-9673(01)86902-8
- Tanaka, K., & Ishizuka, T. (1980). Ion-exclusion chromatography of condensed phosphates on a cation-exchange resin. Journal of Chromatography A, 190, 77–83. doi:10.1016/S0021-9673(00)85513-2
- Turkelson, V. T., & Richards, M. (1978). Separation of the citric acid cycle acids by liquid chromatography. Analytical Chemistry, 50, 1420–1423. doi:10.1021/ac50033a012
- Wang, C., Gao, Y., Wang, W., Zhao, L., Zhang, W., Han, H., … Sun, D. (2012). A national cross-sectional study on effects of fluoride-safe water supply on the prevalence of fluorosis in China. BMJ Open, 2(5), e001564. doi:10.1136/bmjopen-2012-001564
- Yusenko, E. V., Kapsargin, F. P., & Nesterenko, P. N. (2014). Determination of fluoride ions in urinary stones by ion chromatography. Journal of Analytical Chemistry, 69, 474–479. doi:10.1134/S1061934814050128
