ABSTRACT
Semitendinosus muscle marinated in 0.5% acid showed a significant decrease in the shear force. To explore the possible reasons responsible for tenderness of acid-treated beef, the changes in intramuscular connective tissue (IMCT) were taken into consideration. The thicknesses of primary perimysium (PP) and secondary perimysium (SP) decreased to 19.86 and 34.99 μm, respectively. Denaturations of IMCT were exhibited by decreased onset (To) and peak (Tp) temperature, and by the increase in collagen heat solubility. A significant increase in intensity ratios between 3 and 24 h group (p < 0.05) meant that the dissociation of decorin (DCN) enhanced gradually along with marinating time. Meanwhile, limited transitions between 310-helix, anti aggregated strands, and turn were observed, especially a significant decrease (p < 0.05) in turn. The dissociation of DCN and the changes in second structures could partially contribute to the initial denaturation of collagen and reduce TO and TP, resulting in the decrease in shear force.
RESUMEN
El presente estudio comprobó que el músculo semitendinoso marinado en 0,5% de ácido presenta una disminución significativa en la fuerza de corte. Para analizar las posibles razones que expliquen la ternura de la carne de res marinada en ácido, se analizaron los cambios detectados en el tejido conectivo intramuscular (IMCT), constatándose que el grosor del perimisio primario (PP) y el perimisio secundario (SP) disminuyó a 19.86 y 34.99 μm, respectivamente. Mediante la aparición disminuida (To), la temperatura máxima (TP) y el aumento de la solubilidad térmica del colágeno, se detectó la desnaturalización del IMCT. Por otra parte, el incremento significativo en las ratios de intensidad en el grupo de entre 3 h y 24 h (p < 0.05) indicó que la disociación de DCN mejoró gradualmente, así también el tiempo de marinado. A su vez, se observaron transiciones limitadas entre la hélice-310, los oligómeros antiagregantes y los giros, constatándose una disminución significativa (p < 0.05) en el giro. Ello lleva a concluir que la disociación de DCN y los cambios en las estructuras secundarias pueden contribuir parcialmente a la desnaturalización inicial del colágeno y reducir TO y TP, provocando la disminución en la fuerza de corte.
PALABRAS CLAVE:
1. Introduction
Meat tenderness was one of the most important quality attributes, which was related to meat texture (Chang, Wang, Zhou, Xu, & Li, Citation2010). The intramuscular connective tissue (IMCT), which is primarily composed of fibrillar collagens embedded in a matrix of proteoglycans (PGs), plays a significant role in meat texture (Nishimura, Citation2010).
There were several physical and chemical means of tenderizing meat (Ertbjerg, Larsen, & Moøller, Citation1999; Ke, Huang, Decker, & Hultin, Citation2009; Sawyer, Apple, & Johnson, Citation2008). Acidic marinating was widely used in the improvement of meat tenderness, involving the immersion of meat in an acidic solution of vinegar, wine, fruit juice, lactic acid, and so on (Chang et al., Citation2010; Han, Morton, Bekhit, & Sedcole, Citation2009; Naveena, Muthukumar, Sen, Babji, & Murthy, Citation2006). Malic acid (2,3-dihydroxybutanedioic acid) was an important additive in food industry. Nevertheless, to our knowledge, researches on the effect of tenderization marinated with malic acid were rare. The mechanism of IMCT weakening in solutions of weak organic acids is not thoroughly understood.
Thermal characteristics of IMCT, especially heat solubility and thermal transition temperature, were commonly considered to contribute to meat quality and texture properties. The heat solubility of collagen in beef increased by 12% after immersed with 3% sodium tripolyphosphate (STPP) and 3% sodium hexametaphosphate (SHMP) (Xu, Zhou, Peng, Zhao, & Yao, Citation2009). Aktaş and Kaya (Citation2001) explored that IMCT marinated in lactic and citric acid could significantly decrease its To and Tp. Similar results were also obtained by Chang et al. (Citation2010) in beef IMCT after marinated with organic acid and NaCl.
Meanwhile, some authors make a relation between PGs and meat quality (Nishimura, Hattori, & Takahashi, Citation1996; Dubost, Micol, Meunier, Lethias, & Listrat, Citation2013; Dubost, Micol, Picard, Lethias, Andueza, Bauchart & Listrat, Citation2013). Wang et al. (Citation2016) explored the negative relationship between decorin (the main PGs in striated muscle), heat solubility, and shear force. Nevertheless, the changes of decorin in IMCT after treated with organic acid were not clear. In the previous study, Wang et al. (Citation2013) explored the changes of secondary structure in the collagen incubated with the crude extracts from Alaska Pollock muscle. The α-helices in perimysia were partially transformed into β-sheets, while the β-turn and the random coil fractions were steady.
Thus in the present study, we intend to explain the decrease in shear force considering the changes of the IMCT. At the macro level, there were some concerns about the collagen heat solubility, thermodynamic property, and the microstructure. At the micro aspects, the present study aimed to explore the dissociation of DCN and the changes of secondary structure of collagen. These findings would help to explain the possible mechanism of the IMCT weakening that occurs in meat marinated with malic acid.
2. Materials and methods
2.1. Samples preparation
Semitendinosus muscle (SM) were collected from the carcasses (n = 3) of 2.5-year-old beef cattle 72 h post-mortem from Shaanxi Qinbao Animal Husbandry Development Co., Ltd. Each SM (1.5 kg) from each carcass was individually vacuum-packed and stored at −20°C until sampling. The samples were thawed overnight for 12 h at 4°C. The medial central portion of the muscle was used for analysis to avoid interference from the epimysium and obvious fat.
2.2. Marinating treatments
Beef SM (5 × 5 × 5 cm) were marinated in 0.5% (w/v) malic acid solution for about 3, 6, 12, and 24 h at 4°C. The ratio of sample to marinating solution was 1:5 (w/v). The raw meat (untreated) was used as control group. After marinating, the samples were washed with distilled water and surfaced-dried with paper towel and stored at 4°C until analyzed.
2.3. Measurement of shear force
The shear force was determined according to Wang et al. (Citation2016). Samples from each group were sealed in polyethylene bags and cooked in water bath (80°C) until the internal temperature of each sample reached 75°C. Muscle samples (n = 3) of about 1 cm × 1 cm were taken parallel to the fiber direction of the muscle avoiding visible fat. The shear force was measured using a digital meat tenderness meter (Model C-LM3B, Northeast Agricultural University, Harbin, China). Samples were sheared perpendicular to the fiber’s longitudinal axis. At least three samples from each SM specimen were sheared into 6–8 cuts (Wang et al., Citation2016).
2.4. Heat solubility of collagen
To estimate total collagen content, hydroxyproline content was measured in the method described by Xu et al. (Citation2009). Overall, 1.0 g sample was hydrolyzed in 6 mol/L HCl for 16 h at 110°C. The amount of hydroxyproline was determined and was converted to collagen content with a factor 7.25.
Heat solubility of collagen was determined by the procedure described by Nishimura, Hattori, and Takahashi (Citation1999). On the whole, 1.0 ± 0.01 g sample was suspended with Ringer’s solution and homogenized with a dispersion machine (T250, IKA Works, Guangzhou, China). The homogenated samples were heated in water bath (60°C, 70 min) and centrifuged (6000 × g, 20 min). The supernatant solution was decanted, and the pellet was suspended in the same solution and re-centrifuged. The two supernatants were combined, and the amount of soluble collagen was determined as described above. The amount of heat solubility of collagen was expressed as a percentage of the total amount of collagen.
2.5. Light microscopy
Histological analyses were executed by the method described by Chang et al. (Citation2010) with some modifications. In brief, 0.5 × 0.5 × 0.5 cm cubes were separated carefully. And then, the cubes were frozen in nitrogen and cut into 10-μm section, perpendicular to the orientation of muscle fiber. The procedure of staining was according to Li, Zhou, and Xu (Citation2010). After staining, the sections were examined using a light microscope (BX51, Olympus, Tokyo, Japan). The primary and secondary perimysia thickness were designated as the shortest distance between the two edges of the membrane described by Chang et al. (Citation2010) using Image-Pro Express 5.1 software. At least 10 measurement points were randomly selected from each photograph, and at least 5 photograph were chosen in each treatment.
2.6. IMCT preparation and marinating treatment
IMCT was prepared and purified by following the method of Aktaş & Kaya et al. (Citation2001) and Badii & Howell (Citation2003) with some modifications. Chopped fresh muscle (400 g) was homogenized with a pre-cooled 0.05 M NaCl solution (0.02 M phosphate buffer, pH = 6.1, and 1 mM PMSF) in a Waring blender. The dispersion was filtered through a size 25 mesh. This procedure was repeated until no muscle fibers were visible to the eye. The resulting connective tissue fibers were stirred into 500 mL of a 0.05 M NaCl solution containing 1 mM PMSF for 12 h in an ice bath. After centrifuged (3000 × g, 20 min, 4°C), the supernatant was discarded. The precipitate was homogenized again, and then, the resulting precipitate was stirred for 12 h and centrifuged. The precipitate was washed thoroughly with distilled water. The whole precipitate was divided into two groups. One part was stored at 0–4°C until differential scanning calorimetry (DSC) analysis, and the other part was freeze-dried and stored at −20°C until DCN dissociation experiment.
2.7. Thermal transition measurements by differential scanning calorimetry (DSC)
Overall, 0.5 ± 0.01 g purified IMCT were marinated in 0.5% (w/v) malic acid solution for about 12, 24, and 36 h in 4°C. The ratio of sample to marinating solution was same as the procedure in Section 2.2. The fresh IMCT was set as the control group. After marinating, the IMCT was washed and surfaced-dried, and stored at 4°C until DSC was analyzed.
In order to keep the samples, to have similar moisture, and to reduce the differences between each treatment (n = 3), all the DSC measurements were performed by the same person, who was advised to perform all the procedures (including washing and surfaced-dried) (Voutila, Ruusunen, & Puolanne, Citation2008). About 10 mg marinated and washed sample was placed in an aluminum DSC sample pan. The untreated IMCT was set as the control. The pans were hermetically sealed and heated from 30 to 90°C at 5°C/min by using DSC (Perkin-Elmer Diamond DSC) according to the method described by Aktaş & Kaya (Citation2001). An empty sample pan was used as a reference. DSC analyses were performed with triplicates.
2.8. Western blot analysis of the dissociation of DCN
To explore the dissociation of DCN in IMCT marinated in weak organic acid, western-blot was employed.
About 0.1 g freeze-dried IMCT were marinated in 0.5% (w/v) malic acid solution for about 6, 12, 24, and 36 h in 4°C. The ratio of sample to marinating solution was 1:5 (w/v). The freeze-dried IMCT marinated in ultra pure water (marinating time: 36 h) was set as the control group. After marinating, the supernatants were collected and concentrated. The protein concentrations were adjusted to approximately 0.25 mg/mL and the pH was adjusted to about 6.5. The protein in supernatant were fractionated by SDS-PAGE (running gel of 12% acrylamide) and transferred onto PVDF membranes (Bio-Rad Laboratories, Hercules, CA) in transfer buffer using a wet transfer apparatus (Bio Rad Laboratories) (Huang, Huang, Zhou, Xu, & Xue, Citation2011; Kishioka et al., Citation2008). The membranes were blocked for 1 h at 4°C with 5% Albumin from bovine serum (BSA) in Tris buffered saline with tween 20 (TBST). After blocking, the membranes were exposed to the following primary antibodies over night at 4°C: 1:1000 dilution of rabbit polyclonal anti-decorin antibody (sc-22753; Santa Cruz, CA). The membranes were washed (5 × 10 min) with TBST at room temperature and further incubated with secondary antibodies: 1:10,000 dilutions of peroxidase-conjugate goat anti-rabbit IgG (ZB-2301, ZSGB-BIO, Beijing). Finally, membranes were again washed, and blots were detected using enhanced chemiluminescence (ECL) reagent and scanned with Image Quant LAS4000 (GE, U.S.A.). The resulting images were analyzed using Quantity One software (Bio-Rad).
2.9. Analysis of secondary structure
The marinating procedure was similar as the experiment executed in DSC section. The marinating time was set as 6, 12, 24, and 36 h. After marinating, the IMCT were washed with ultra pure water (3 × 20 s) and surfaced-dried with paper towel. The ATR-FTIR spectroscopy was obtained as the procedure described by Chadefaux, Le Hô, Bellot-Gurlet, & Reiche (Citation2009). Analyses were conducted in reflection mode based on a germanium (Ge) crystal. For each point, 64 scans were collected at spectral resolution of 2 cm−1. ATR-FTIR analyses were performed with triplicates. The shape of the amide I band (1600–1700 cm−1) in ATR-FTIR spectrometers was representative of the collagen secondary structure (Chadefaux et al., Citation2009). A curve-fitting treatment was carried out to estimate quantitatively the relative proportion of each component representing a type of secondary structure using the PeakFit software. The second derivative function and deconvolution were used to determine the number and positions of the bands corresponding to the different components in the amide I profile. According to bands decomposition, the amide I profile of collagen contained eight major components that could be linked, in analogy to protein models, with aromatic ring vibrations (1600–1610 cm−1), aggregated strands (1610–1628 cm−1), β-sheet (1625–1640 cm−1), random coil (1640–1648 cm−1), a-helix (1648–1660 cm−1), 310-helix (1660–1670 cm−1), anti-aggregated strands (1675–1695 cm−1), and turn (1680–1696 cm−1) (Chadefaux et al., Citation2009; Michael & Henry, Citation1995; Pelton & McLean, Citation2000). The amount of each secondary structure element was given in percentage terms, by dividing the area of one amide I band component by the area of the sum of all amide I band component areas.
2.10. Statistical analysis
The figures were designed by the Origin Pro SR4 (Microcal, Northampton, U.S.A.). The data were analyzed with the Statistical Analysis System (SAS Institute Inc., Cary, NC, U.S.A.). Means of each treatment were statistically analyzed by SAS and differences were compared using Duncan’s multiple-range test at significance level of 0.05.
3. Results and discussion
3.1. Shear force and collagen heat solubility
showed the effect of marinating time on shear force of beef SM. After 6 h marination, there was a significant decrease in shear force compared with control group (p < 0.05). Shear force reached about 43.2 N after 24 h marination, demonstrating that the SM tenderness was improved after marinated in malic acid. Similar results have been obtained by many researchers. Ke et al. (Citation2009) declared that citric acid was effective in both improving texture and inhibiting lipid oxidation. The results by Burke and Monahan (Citation2003) also indicated that the tenderness of shin beef could be improved using a citrus juice marinade. The main reason of tenderness improved was attributed to the solubilization of collagen (Burke & Monahan, Citation2003), the weakness of IMCT (Chang et al., Citation2010), and protein degradation (collagen and myofibrillar protein) caused by lysosomal cathepsins (Ertbjerg, Mielche, Larsen, & Moller, Citation1999).
Figure 1. Shear force and heat solubility of semitendinosus muscle. Note: The error bars indicate standard deviations. Different letters above the error bars significantly differ at p < 0.05.
Figura 1. Fuerza de corte y solubilidad térmica del músculo semitendinoso. Nota: Las barras de error indican la desviación estándar. Las distintas letras que se encuentran sobre las barras de error indican diferencias significativas a un nivel de p < 0.05.
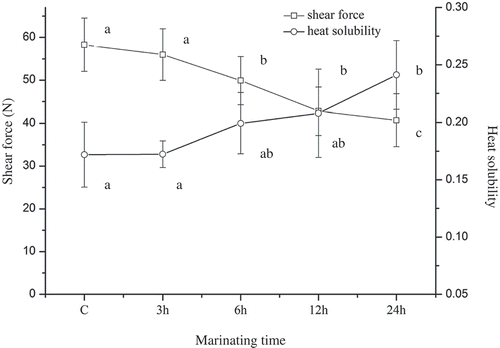
Meanwhile, collagen heat solubility increased gradually along with marinating time, from about 17.19% in control to 24.16% in 24 h marinated group. It indicated that more collagen would transform into gelatine during heating and the IMCT could not provide enough mechanical strength to maintain the integrity of the muscle. According to Burke and Monahan (Citation2003), the changes of collagen thermal properties attribute to peptide bond hydrolysis and low breakage of covalent cross-links in collagen. Researches by Ertbjerg, Mielche, et al. (Citation1999) and Burke et al. (2003) also showed that marination with acid resulted in an increase in cathepsin B and L activity and enhanced proteolytic attack by these enzymes.
3.2. Light microscopy examination and histological analysis
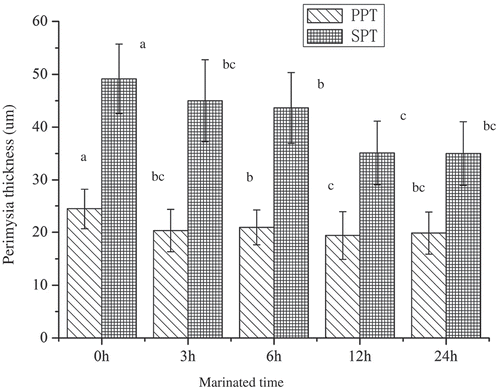
In and , malic acid caused microstructural changes of SM, which was mainly exhibited as the decrease in thickness of perimysia, partly due to the fracture of primary and secondary perimysia, and obfuscation of muscle fiber sections. showed that in marinated samples, the thicknesses of primary perimysium (PP) and secondary perimysium (SP) were lower than that of control samples, decreased to 19.86 and 34.99 μm, respectively. Moreover, a certain degree of collapse in PP could be observed after 12 and 24 h marinating. It could lead to the loss of the mechanical strengthening of ICMT and improve the tenderness. Chang et al. (Citation2010) also observed results using lactic and critic as marinated solution, which could be a reason for the reduction in shear force.
Figure 2. Histological changes of semitendinosus muscle by light microscopy (magnification: ×100). Note: Bar = 200 μm; (a) marinating time 0 h, control; (b) marinating time 3 h; (c) marinating time 6 h; (d) marinating time 12 h; €, marinating time 24 h; PP: primary perimysium; SP: secondary perimysium, MF: muscle fiber.
Figura 2. Cambios histológicos del músculo semitendinoso detectados por microscopía de luz (ampliación: ×100). Nota: Barra = 200 µm, (a), duración del marinado 0 h, control; (b), duración del marinado 3 h; (c), duración del marinado 6 h; (d), duración del marinado 12 h; E, duración del marinado 24 h; PP: perimisio primario; SP: perimisio secundario, MF: fibra muscular.
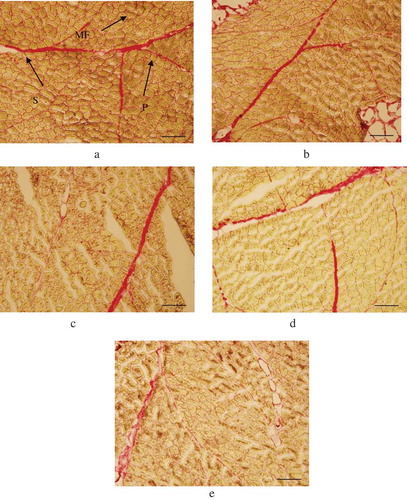
Figure 3. Perimysium thickness of semitendinosus muscle. Note: PPT: primary perimysium thickness; SPT: secondary perimysium thickness. Each value was expressed as the mean ± SD, different letters above the error bars significantly differ at p < 0.05.
Figura 3. Grosor del perimisio en el músculo semitendinoso. Nota: PPT: grosor del perimisio primario; SPT: grosor del perimisio secundario. Cada uno de los valores se expresa como la media ± DE; las distintas letras que se encuentran sobre las barras de error indican diferencias significativas a un nivel de p < 0.05.
3.3. The analysis of IMCT by DSC
The thermal transition temperature of IMCT () was similar to that found by Aktaş & Kaya (Citation2001), Berge et al. (Citation2001), and Voutila et al. (Citation2008). In the 0 h group, namely control group, the TO and TP temperatures were about 60.63 and 65.38°C, respectively. TO temperature was decreased significantly to about 38.19°C (p < 0.05) after 12 h marinating. An unobvious change was observed in the TO temperature even in 36 h group. Meanwhile, the TP was around 41°C for marinated samples. As described by Chang et al. (Citation2010), To was considered to describe the least stable collagen, and the Tp was a measure of the average stability of collagen. According to theories provided by Aktaş and Kaya (Citation2001) and Aktaş (Citation2003), the decreases in thermal transition temperature were mainly attributed to the hydrolysis of a Schiff base, breakdown of unstable collagen cross-links (aldimine bonds), narrower hydrated layer in the presence of Ca2+ and charged interactions. Nevertheless, the changes in DCN and in the secondary structure of collagen have not been studied.
Figure 4. Dissociation of DCN. Note: (a) The immunoblots of actin as an internal reference: a, 36 h; b, 6 h; c, 12 h; d, 24 h; e, 36 h. (b) The immunoblots of decorin: a, 0 h; b, 6 h; c, 12 h; d, 24 h; e, 36 h. (c) The relative intensity of decorin immunoblots. Different letters over error bars significantly differ at p < 0.05.
Figura 4. Disociación de DCN. Nota: (a), los inmunoblots de actina como una referencia interna: a, 36 h; b, 6 h; c, 12 h; d, 24 h; e, 36 h. (b), los inmunoblots de decorina: a, 0 h; b, 6 h; c, 12 h; d, 24 h; e, 36 h. (c), Intensidad relativa de inmunoblots de decorina. Las distintas letras que se encuentran sobre las barras de error indican diferencias significativa a un nivel de p < 0.05.
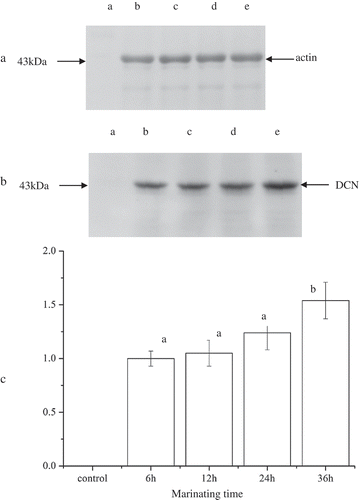
3.4. The dissociation of DCN in IMCT marinated in malic acid
DCN is the main PGs in striated muscle, which acts as a spacer during the lateral assembly of collagen molecular structure and is important in maintaining normal tissue function and mechanical properties (Eggen, Malmstrøm, & Kolset, Citation1994; Gillies & Lieber, Citation2011; Weber, Harrison, & Iozzo, Citation1996). shows the dissociation of DCN in IMCT after being marinated in malic acid. The amount of DCN dissociation was showed in intensity ratios, which was calculated as the intensity of the decorin band in each sample (4-B) over the intensity of the actin band in the internal designate densitometry standard (4-A). To make the results easy to be observed, the ultimate DCN dissociations were showed in the form of the normalized values. Considering there was almost no dissociation in control group, the intensity ratios of the 6 h group was set as normalized standard. It exhibited a significant difference between 12 and 24 h group (p < 0.05), which meant that the dissociation of DCN enhanced gradually along with marinating time. According to Weber et al. (Citation1996), the binding of decorin to one collagen triple helix within the gap zone and a second collagen molecule in a staggered arrangement would promote the formation of the correct fibril and prevent incorrect addition/fusion of the collagen. The dissociation of DCN might disturb the organization of collagen fibril. Meanwhile, decorin provides some attachment sites for glycosaminoglycan (GAGs). The dissociation of DCN meant the linkages of GAGs were weakened, which might have a negative effect on the properties of IMCT.
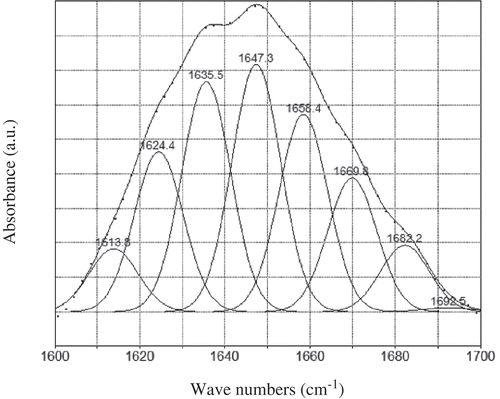
Figure 5. Curve-fitting analysis of amide I bands. Note: Solid line: experimental; dotted line: reconstructed. All the regression coefficients (R2) were over 0.999.
Figura 5. Análisis de ajuste de curva de bandas de amida I. Nota: Línea sólida: experimental; línea punteada: reconstruido. Todos los coeficientes de regresión (R2) superaron el valor de 0.999.
It was worth mentioning the theory of “thermally labile domains” provided by Miles and Ghelashvili (Citation1999) and Miles and Bailey (Citation2001). Thermal activation involved the partial uncoupling of the individual α-chains making up the triple helix. The unfolding occurred at a thermally labile domain unfolds first. Once the three α-chains in this thermally labile region were uncoupled, the whole structure unzips. The thermal activation of the denaturation of collagen was therefore governed by the properties of the thermally labile domain. In the known opinion,all three labile domains were located in the gap region of the fiber. Coincidentally, DCN was just bind to one collagen triple helix within the gap zone. It was inferred that the dissociation of DCN contributed to the initial denaturation of collagen.
3.5. ATR-FTIR
According to the band decomposition, the amine I band contained eight major components (). The position of the band components was fixed, whereas their bandwidths could be adjusted to perform the curve-fitting of the amine I profile. In , there were unobvious changes in most components. It was partially consistent with the result described by Hennessey, Johnson, Bahler, and Wood (Citation1982). Nevertheless, the content of 310-helix and anti-aggregated strands increased from 11.32 and 4.89 to about 12.26 and 5.71%, respectively, between the control group and 36 h treatment. A significant decrease (p < 0.05) was observed in turn between the control and the marinating group. These results indicated that combined with the dissociation of DCN, although there were limited transitions between 310-helix, anti-aggregated strands, and turn, these changes might attribute to the configuration change in collagen in IMCT.
Table 1. The thermal transition temperature of IMCT treated with different acid marinating time.
Tabla 1. La temperatura de transición térmica de IMCT tratada con diferente tiempo de marinado con ácido.
Table 2. The secondary structure of collagen in IMCT treated with different marinating time.
Tabla 2. La estructura secundaria de colágeno en IMCT con diferente tiempo de marinado.
Meanwhile, in mechanism “polymer-in-a-box” described by Miles and Ghelashvili (Citation1999), increased configuration of activation and decreased Gibbs free energy of activation were the probable reasons of the unstabilized structure in collagen fibers. The model here could provide a potential explanation in the present study. The dissociation of DCN and the changes in second structures could contribute to the unfold of the “thermally labile domains”, affect the configuration of collagen in IMCT, and be a supplement factor for the increased configuration of activation and reduced Gibbs free energy of activation, which would partially promote the initial denaturation of collagen and the decreased thermal transition temperature.
4. Conclusions
In conclusion, there was a significant decrease in shear force in SM marinated in 0.5% acid. Acid made the thicknesses of PP and SP lower, the collagen heat solubility increased, and TO and TP increased. The dissociation of DCN in marinated connective tissue and the limited transitions between 310-helix, anti-aggregated strands, and turn were expected to promote the initial denaturation of collagen, contribute to the denaturation of IMCT and result in the reduction in shear force.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aktaş, N. (2003). The effects of pH, NaCl and CaCl2 on thermal denaturation characteristics of intramuscular connective tissue. Thermochimica Acta, 407(1), 105–112.
- Aktaş, N., & Kaya, M. (2001). Influence of weak organic acids and salts on the denaturation characteristics of intramuscular connective tissue. A differential scanning calorimetry study. Meat Science, 58(4), 413–419.
- Badii, F., & Howell, N. K. (2003). Elucidation of the effect of formaldehyde and lipids on frozen stored cod collagen by FT-Raman spectroscopy and differential scanning calorimetry. Journal of Agricultural and Food Chemistry, 51(5), 1440–1446.
- Berge, P., Ertbjerg, P., Larsen, L. M., Astruc, T., Vignon, X., & Møller, A. J. (2001). Tenderization of beef by lactic acid injected at different times post mortem. Meat Science, 57(4), 347–357.
- Burke, R., & Monahan, F. (2003). The tenderisation of shin beef using a citrus juice marinade. Meat Science, 63(2), 161–168.
- Chadefaux, C., Le Hô, A.-S., Bellot-Gurlet, L., & Reiche, I. (2009). Curve-fitting Micro-ATR-FTIR studies of the amide I and II bands of type I collagen in archaeological bone materials. e-PRESERVATION Science, 6, 129–137.
- Chang, H. J., Wang, Q., Zhou, G. H., Xu, X. L., & Li, C. B. (2010). Influence of weak organic acids and sodium chloride marination on characteristics of connective tissue collagen and textural properties of beef semitendinosus muscle. Journal of Texture Studies, 41(3), 279–301.
- Dubost, A., Micol, D., Meunier, B., Lethias, C., & Listrat, A. (2013). Relationships between structural characteristics of bovine intramuscular connective tissue assessed by image analysis and collagen and proteoglycan content. Meat Science, 93(3), 378–386.
- Dubost, A., Micol, D., Picard, B., Lethias, C., Andueza, D., Bauchart, D., & Listrat, A. (2013). Structural and biochemical characteristics of bovine intramuscular connective tissue and beef quality. Meat Science, 95(3), 555–561.
- Eggen, K. H., Malmstrøm, A., & Kolset, S. O. (1994). Decorin and a large dermatan sulfate proteoglycan in bovine striated muscle. Biochimica Et Biophysica Acta (Bba)-Protein Structure and Molecular Enzymology, 1204(2), 287–297.
- Ertbjerg, P., Larsen, L. M., & Moøller, A. J. (1999). Effect of prerigor lactic acid treatment on lysosomal enzyme release in bovine muscle. Journal of the Science of Food and Agriculture, 79(1), 95–100.
- Ertbjerg, P., Mielche, M. M., Larsen, L. M., & Moller, A. (1999). Relationship between proteolytic changes and tenderness in prerigor lactic acid marinated beef. Journal of the Science of Food and Agriculture, 79(7), 970–978.
- Gillies, A. R., & Lieber, R. L. (2011). Structure and function of the skeletal muscle extracellular matrix. Muscle & Nerve, 44(3), 318–331.
- Han, J., Morton, J., Bekhit, A., & Sedcole, J. (2009). Pre-rigor infusion with kiwifruit juice improves lamb tenderness. Meat Science, 82(3), 324–330.
- Hennessey, J. P., Jr., Johnson, W. C., Jr., Bahler, C., & Wood, H. G. (1982). Subunit interactions of transcarboxylase as studied by circular dichroism. Biochemistry, 21(4), 642–646.
- Huang, F., Huang, M., Zhou, G., Xu, X., & Xue, M. (2011). In vitro proteolysis of myofibrillar proteins from beef skeletal muscle by caspase-3 and caspase-6. Journal of Agricultural and Food Chemistry, 59(17), 9658–9663.
- Ke, S., Huang, Y., Decker, E. A., & Hultin, H. O. (2009). Impact of citric acid on the tenderness, microstructure and oxidative stability of beef muscle. Meat Science, 82(1), 113–118.
- Kishioka, Y., Thomas, M., Wakamatsu, J. I., Hattori, A., Sharma, M., Kambadur, R., & Nishimura, T. (2008). Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. Journal of Cellular Physiology, 215(3), 856–867.
- Li, C. B., Zhou, G. H., & Xu, X. L. (2010). Dynamical changes of beef intramuscular connective tissue and muscle fiber during heating and their effects on beef shear force. Food and Bioprocess Technology, 3(4), 521–527.
- Michael, J., & Henry, H. M. (1995). The use and misuse of FTIR spectroscopy in the determination of protein structure. Critical Reviews in Biochemistry and Molecular Biology, 30(2), 95–120.
- Miles, C., & Bailey, A. (2001). Thermally labile domains in the collagen molecule. Micron, 32(3), 325–332.
- Miles, C. A., & Ghelashvili, M. (1999). Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophysical Journal, 76(6), 3243–3252.
- Naveena, B., Muthukumar, M., Sen, A., Babji, Y., & Murthy, T. (2006). Improvement of shelf-life of buffalo meat using lactic acid, clove oil and vitamin C during retail display. Meat Science, 74(2), 409–415.
- Nishimura, T. (2010). The role of intramuscular connective tissue in meat texture. Animal Science Journal, 81(1), 21–27.
- Nishimura, T., Hattori, A., & Takahashi, K. (1996). Relationship between degradation of proteoglycans and weakening of the intramuscular connective tissue during post-mortem ageing of beef. Meat Science, 42(3), 251–260.
- Nishimura, T., Hattori, A., & Takahashi, K. (1999). Structural changes in intramuscular connective tissue during the fattening of Japanese black cattle: Effect of marbling on beef tenderization. Journal of Animal Science, 77(1), 93–104.
- Pelton, J. T., & McLean, L. R. (2000). Spectroscopic methods for analysis of protein secondary structure. Analytical Biochemistry, 277(2), 167–176.
- Sawyer, J., Apple, J., & Johnson, Z. (2008). The impact of lactic acid concentration and sodium chloride on pH, water-holding capacity, and cooked color of injection-enhanced dark-cutting beef. Meat Science, 79(2), 317–325.
- Voutila, L., Ruusunen, M., & Puolanne, E. (2008). Comparison of the thermal characteristics of connective tissue in loose structured and normal structured porcine M. Semimembranosus. Meat Science, 80(4), 1024–1030.
- Wang, F., Zhang, Y., Li, J., Guo, X., Cui, B., & Peng, Z. (2016). Contribution of cross-links and proteoglycans in intramuscular connective tissue to shear force in bovine muscle with different marbling levels and maturities. LWT - Food Science and Technology, 66, 413–419.
- Wang, R., Peng, Z., Hui, T., Wang, F., Yao, Y., Zhang, Y., & Zhou, G. (2013). Potential use of crude extracts from Alaska pollock muscle as meat tenderizer. Cyta - Journal Of Food, 11(1), 50–59.
- Weber, I. T., Harrison, R. W., & Iozzo, R. V. (1996). Model structure of decorin and implications for collagen fibrillogenesis. Journal of Biological Chemistry, 271(50), 31767–31770.
- Xu, S. Q., Zhou, G. H., Peng, Z. Q. I., Zhao, L. Y., & Yao, R. (2009). The influence of polyphosphate marination on Simmental beef shear value and ultrastructure. Journal of Muscle Foods, 20(1), 101–116.
