ABSTRACT
The antioxidant and antimicrobial effect of Damsissa leaf powder extract (DLPE) for application to cold-stored minced beef was investigated. DLPE exhibited inhibition zones of 14.0 and 12.7 mm against Escherichia coli and Staphylococcus aureus, respectively. Total polyphenol and flavonoid contents of DLPE were 188.06 mg gallic acid equivalent (GAE)/g and 2.56 mg catechin equivalent (CE)/g, respectively. DLPE exhibited a concentration-dependent increase (P ≤ 0.05) in total reducing power ability (TRPA), scavenging of H2O2, and chelation of Fe2+ ions. Incorporation of different concentrations of DLPE in minced beef significantly (P ≤ 0.05) enhanced the physicochemical characteristics, sensory features, and microbial quality of the minced beef during cold storage (4°C). DLPE (2.5%) was effective (P ≤ 0.05) in delaying lipid oxidation and preventing microbial growth in stored minced beef. Therefore, DLPE at 2.5% concentration is recommended as a useful preservative to prolong the shelf-life of minced beef during cold storage.
RESUMEN
En el presente estudio se investigaron los efectos antioxidantes y antimicrobianos del extracto en polvo de la hoja de Damsissa (DLPE) al ser aplicado a carne de res picada almacenada en frío. Se comprobó que el DLPE exhibe zonas de inhibición de 14.0 mm y 12.7 mm contra Escherichia coli y Staphylococcus aureus, respectivamente. El contenido total de polifenoles y flavonoides de DLPE fue registrado en 188.06 mg equivalentes a ácido gálico (GAE)/g y el de flavonoides en 2.56 mg equivalentes a catequina (CE)/g. El DLPE mostró un incremento en su capacidad de poder reductor total (TRPA), eliminación de H2O2 y quelación de iones Fe2+, vinculado a la concentración (P ≤ 0.05). Así, la incorporación de distintas concentraciones del DLPE a la carne de res picada aumentó significativamente (P ≤ 0.05) las características fisicoquímicas, los aspectos sensoriales y la calidad microbiana de la misma cuando fue almacenada en frío (4°C). Asimismo, se constató que el DLPE (2.5%) fue efectivo (P ≤ 0.05) para retrasar la oxidación de lípidos y evitar el crecimiento microbiano en la carne de res picada almacenada, por lo que se recomienda su uso a una concentración de 2.5% como un conservante útil que permite extender la vida útil de la carne de res picada durante su almacenamiento en frío.
1. Introduction
The recent decades witnessed a substantial increase in the worldwide production and consumption of minced beef. This has been owing to the population explosion, quick growth in the fast food market, and great nutritional value and palatability of beef meat products (Eltilib, Elgasim, & Mohamed Ahmed, Citation2016; Hawashin, Al-Juhaimi, Mohamed Ahmed, Ghafoor, & Babiker, Citation2016). However, minced beef is susceptible to lipid oxidation and microbial spoilage because the mincing process disturbs muscles integrity, and with the increased surface area generated by mincing, facilitates the uptake of oxygen and microorganisms that leads to reduced shelf life (Honikel, Citation2014). Microbial activity and lipid oxidation adversely affect the safety, nutritional and organoleptic qualities, and economic value of meat products during storage (Hawashin et al., Citation2016; Jayawardana, Liyanage, Lalantha, Iddamalgoda, & Weththasinghe, Citation2015). Thus, retarding the microbial growth and lipid oxidation in minced meat by using natural or synthetic antioxidant and antimicrobial agents would be a useful strategy to reduce such harmful effects of increased lipid oxidation and microbial activity (Elhadi, Elgasim, & Mohamed Ahmed, Citation2017; Eltilib et al., Citation2016). To date, several synthetic antioxidants and antimicrobials have been used to retard oxidative reactions, inhibit microbial growth, and consequently extend the shelf life of minced meat products (Fernandes, Trindade, Lorenzo, Munekata, & de Melo, Citation2016; Honikel, Citation2014). However, most of these synthetic preservatives are considered as unsafe ingredients by both consumers and health experts (Tang, Kerry, Sheehan, Buckley, & Morrissey, Citation2001) and thus their use in food preparations is regulated by several rules in recent years. Accordingly, interest in the use of natural antimicrobials and antioxidants to prolong the shelf life of stored meat has markedly increased in last decades because of the expected health benefits and safety of these natural materials (Das, Rajkumar, Verma, & Swarup, Citation2012; Jayawardana et al., Citation2015). The use of natural antioxidants and antimicrobials in minced meat products is of high interest for both customers and meat producers to promote confidence in the safety of meat products and extend the marketing time of the products (Eltilib et al., Citation2016).
Ambrosia maritima L. (Asteraceae) is a widely available weed in the Mediterranean region and African countries, particularly Egypt and Sudan, where it locally known as Demsissa and it grows abundantly near water catchments and on the banks of the Nile River (Saeed, Abdelgadir, Sugimoto, Khalid, & Efferth, Citation2015). This weed plant is widely used in Sudanese traditional medicine for the treatment of urinary tract infections, gastrointestinal disturbance, kidney stones, diabetes, hypertension, asthma, rheumatic pain, and cancer (Dirar et al., Citation2014). It contains important sesquiterpene lactones, such as neoambrosin, ambrosin, and damsin, which have molluscidal and cytotoxic activities (Saeed et al., Citation2015). In addition, this plant contains several phytochemicals, such as coumarins, flavonoids, sterols and tannins, and exhibits considerable antioxidant activity (Dirar et al., Citation2014). Despite the common use of Damsissa in traditional medicine, to date, there are no reports on its use as a natural preservative of food products. Therefore, the primary aim of the current study was to investigate the antioxidant and antimicrobial potential of Damsissa leaf powder extract (DLPE) and to assess its influence on the physicochemical, microbial, and sensory qualities of minced beef.
2. Materials and methods
2.1. Preparation of Damsissa leaf powder extracts
Fresh Damsissa leaves were obtained from Toti Island (Khartoum, Sudan). The leaves were hand-cleaned to remove dust and any foreign material thoroughly washed with double-distilled water (ddH2O), dried at ambient temperature, ground to a fine powder using an electrical mill, and stored in polyethylene bags at 4°C. Water extract was prepared by mixing 30 g of Damsissa leaf powder with 300 mL of distilled water and placed on an orbital shaker (Model OM 11, Ratek Instrument Ltd., Australia) at 150 rpm for 5 h at ambient temperature (25°C). After that, the extract was filtered using Whatman No. 1 filter paper and then centrifuged (HettichZentrifugen, Tuttlingen, Germany) at 4000 × g for 10 min. The resultant supernatant was concentrated under reduced pressure at 40°C for 3 h using a rotary evaporator (IKA-WERKE-RV06ML, Stanfer, Germany). The concentrated extract was freeze-dried and stored in a freezer at −20°C until utilized. Prior to analysis of antimicrobial and antioxidant activities and the contents of total polyphenols and flavonoids, dried extract was reconstituted in ddH2O at a final concentration of 30 mg/mL stock solution.
2.2. Preparation of minced meat
Fresh beef was obtained from a local meat market (Bahri, Khartoum North, Sudan). The meat was sliced, minced through a 0.75-inch plate using a meat mincer, and stored at 4 ± 1°C. Thereafter, DLPE at final concentrations (w/w) of 0%, 2.5%, and 5% were added to minced beef and then the content was thoroughly mixed. Instantly at the first day of preparation, minced beef samples from different treatments (0%, 2.5%, and 5% DLPE) were used for the analysis of the physicochemical, microbiological, and sensory attributes. The remaining samples were kept in a refrigerator (4 ± 1°C) for up to 10 days. At intervals of 5 days, minced beef samples were taken from each treatment and analyzed for physicochemical and microbiological properties.
2.3. Determination of chemical composition of Damsissa leaf powder and minced meat
The amounts of moisture, protein, fat, ash, and fiber in Damsissa leaf powder and minced beef were assessed as described in the standard method (AOAC, Citation2003). Carbohydrate was calculated by differences.
2.4. Determination of polyphenols and flavonoids of DLPE
Total polyphenols of DLPE was estimated as described by Price and Butler (Citation1977) using Prussian blue spectrophotometric method. Gallic acid was used as external standard. Total polyphenol content was expressed as mg gallic acid equivalent (GAE) g−1 sample. The flavonoids content was determined using a colorimetric assay as described by Zhishen, Mengcheng, and Jianming (Citation1999). Briefly, 1 mL of DLPE was added to 0.3 mL sodium nitrite (5%, w/v) in a test tube, followed by 0.3 mL of 10% (w/v) aluminum chloride solution. The test tubes were incubated for 5 min at room temperature (25°C), and thereafter 2 mL of 1 M sodium hydroxide were added to the mixture. Instantly, the volume of the mixture was raised to 10 mL with ddH2O, the mixture was vortexed, and the absorbance was determined at 510 nm. A calibration curve was prepared with catechin, and the results were presented as mg catechin equivalents (CE)/g.
2.5. Determination of antibacterial and antioxidant activities of DLPE
In the assay of antibacterial activity of DLPE, pure cultures of indicator microorganisms (Escherichia coli ATCC 29522 and Staphylococcus aureus ATCC 2818) were cultivated on agar plates and antibacterial activity was assessed using disc diffusion method as described by Kilani, Abdelwahed, Ammar, and Hyder (Citation2005). In brief, DLPE saturated filter paper discs (10 µL extract/6 mm disc) were appropriately positioned on the surface of the cultured plates and the plates were incubated at 37°C for 24 h. After that, the plates were inspected for the occurrence of inhibition zones of bacterial growth around discs. Ampicillin (10 µg/disk) and chloramphenicol (30 µg/disk) were used as control standard antibiotics.
The antioxidant activities of DLPE were measured using three different antioxidant approaches, namely: total reducing power ability (TRPA), chelation of Fe+2 ions, and scavenging of hydrogen peroxide (H2O2) as described by Mohamed, Fageer, Eltayeb, and Mohamed Ahmed (Citation2014). For TRPA assay, 1 mL of DLPE (125–1000 µg/mL) was mixed with sodium phosphate buffer (2.5 mL, 200 mM, pH 6.6) and potassium ferricyanide (2.5 mL, 1%). After 20 min incubation at 50°C, 2.5 mL of 10% trichloroacetic acid (TCA) was added, and then the reaction mixture was centrifuged at 2000 × g for 10 min. Thereafter, 2.5 mL of the supernatant was mixed with 2.5 mL of ddH2O and 0.5 mL of freshly prepared FeCl3 solution (0.1%), and then the absorbance was recorded at 700 nm against a blank. A higher absorbance indicates a higher reducing power. For the chelating activity of Fe2+ ions, 1 mL of DLPE (125–1000 µg/mL) was mixed with 1 mL of a solution containing 50 mM FeSO4 and 50 mM NaCl (pH 7.0), and the mixture was incubated at room temperature for 20 min. Thereafter, 2 mL of 1 mM 2,2-dipyridyl was added, the absorbance of the complex of ferrous–dipyridyl was examined at 525 nm, and the results were expressed as a percentage of inhibition of 2,2-dipyridyl–Fe2+ complex formation (Harris & Livingstone, Citation1964). For H2O2 scavenging ability assay, 1 mL of DLPE (125–1000 µg/mL) in phosphate buffer (pH 7.4, 40 mM) was added to 1 mL of H2O2 solution (40 mM) in phosphate buffer (pH 7.4, 40 mM) and the reaction mixture was incubated for 10 min at room temperature. Then, the absorbance of H2O2 was determined at 230 nm against a blank solution containing phosphate buffer without H2O2, and the results were expressed as percent scavenging of H2O2 by the sample (Jayaprakasha, Rao, & Sakariah, Citation2004). In all assays, the spectrophotometric readings were carried out using a 1061-Shimadzu UV–VIS spectrophotometer (Shimadzu, Tokyo, Japan).
2.6. Determination of peroxide and pH values of minced beef treated with DLPE
The estimation of peroxide value was carried out following a standard official method (AOAC, Citation2003). Briefly, about 50 g of minced beef sample was soaked in 200 mL of chloroform for 8 h and then filtered through Whatman No. 4 filter paper. Thereafter, a saturated potassium iodide solution was added to a mixture of filtrate and chloroform/acetic acid (2:1.5, v/v) and kept in the dark. Then, released iodine was titrated with a sodium thiosulfate solution, and the peroxide value was then expressed as milli-equivalents of active oxygen per kilogram of the sample (meq/kg).
For pH measurement, 10 g of minced beef sample was suspended in distilled water, homogenized for 1 min in a homogenizer, and then filtered through Whatman No 1 filter paper. The pH of the filtrate was measured using a digital pH meter (model 210, HANNA instruments microprocessor pH meter).
2.7. Microbiological analysis
The microbiological analysis methods described by Harrigan (Citation1998) were used to determine the microbial quality of minced beef samples from different treatments and storage time. In brief, about 10 g of minced beef sample was homogenized with 90 mL of 0.1% sterile peptone water under aseptic conditions. Then, appropriate serial dilutions from each sample were prepared. Thereafter, 1 mL of each dilution was inoculated onto total plate count agar (PCA), MacConkey broth and Brilliant green bile lactose broth, MacConkey agar, and Baired-Parker broth (Oxoid, UK). After incubating the plates at 37°C for 24–48 h, they were respectively examined for total plate, coliform, E. coli, and S. aureus counts.
2.8. Sensory analysis
Sensory analysis of prepared minced beef samples was carried out instantly after processing following standard guidelines for sensory evaluation (AMSA, Citation2015). A panel consisting of 20 members (10 male, age 26–40, and 10 female, age 24–33) of the staff and post-graduate students at the Department of Food Science and Technology, Faculty of Agriculture, University of Khartoum provided sensory evaluation of cooked samples. Prior to the sensory evaluation, the panel received preliminary training sessions (n = 3) to focus attention on the sensory attributes so that each panelist was able to carefully discuss and identify each attribute. The samples with three-digit code numbers were served randomly to the panelists. Tap water was provided to the panel members between the samples to flush their palate from the previous sample taste. The panelists were requested to record their preference on a 7-point hedonic scale (7 = very acceptable, 1 = extremely unacceptable) for color, flavor, tenderness, juiciness, and overall acceptability of cooked minced meat products. Three independent sessions of sensory evaluations were performed, and the mean values of the scores of 20 panellists for each sample and session were calculated and used in data analysis. The cut-off score was set as 4 and the mean scores higher than 4 were considered acceptable.
2.9. Statistical analysis
Three minced beef batches were produced and all measurements were repeated three times. In each batch, three treatments (0.0% DLPE, 2.5% DLPE, and 5% DLPE) were performed and measurements (n = 3) were carried out at three storage days (0, 5, and 10). SAS program V. 8.1 (SAS Institute Inc., Cary, NC) was used for the statistical analysis of the results. The obtained data of physicochemical properties, microbiological, and sensory qualities from different treatments and storage times was subjected to analysis of variance (one-way ANOVA) using the general linear model (GLM) procedure. In the analysis models, the treatments, storage times, and assessors were considered as fixed factors, while replications of the experiments as a random factor. LSD was used to separate means and results were expressed as mean ± SD, and significances of the data were accepted at probability of P ≤ 0.05.
3. Results and discussion
3.1. Chemical composition of Damsissa leaf powder and extract
shows the chemical composition of Damsissa leaf powder and extract. The results indicated that Damsissa leaf powder contains high amounts of carbohydrate (29.40 g/100 g), protein (26.20 g/100 g), and ash (20.67 g/100 g) and an appreciable amount of crude fiber (10.07 g/100 g), moisture (8.37 g/100 g), fat (5.30 g/100 g), whereas, DLPE contains substantial amounts of polyphenol (188.06 mg GAE/g) and flavonoids (2.56 mg CE/g). Strikingly, these results demonstrate that Damsissa leaves are a potentially excellent source of protein, carbohydrate, fiber, ash, polyphenols, and flavonoids. Thus, DLPE could be used as a source of these essential nutrients in the diet to contribute to protein and energy requirements and provide the human body with bioactives that protect them from diseases arising from malnutrition and carcinogens (Salah & Yagi, Citation2011). The composition results show that Damssisa leaves contain nutritional and health potential that could find application as a food ingredient, in infant formula, food supplements, and food formulations. Several studies demonstrated wide range of bioactivities for Damssisa plant and its extracts, such as anthelmintic, antispasmodic, antidiabetic anti-inflammation, and antihypertension (Barakat, Al-Hizab, & Bakhiet, Citation2012; Makkawi, Keshk, ElShamy, & Abdel-Mogib, Citation2015) that are very relevant to many regions, such as Africa, South America, and Asia where the genus Ambrosia can be found. Dietary intake of up to 10% were found to be safe in animal models and exhibited significantly higher weight gain and significantly lower serum cholesterol and glucose levels in rats fed A. maritima compared to control treatment group (Barakat et al., Citation2012). A related plant, Ambrosia artemisiifolia L. was reported to have wide range of bioactive compounds that demonstrated a wide range of biological activities (Ding, Huang, Zhou, & Li, Citation2015). Collectively, these studies highlight the potential use of Ambrosia to provide functional role in food and pharmaceutical applications.
Table 1. Chemical composition of Damsissa leaf powder and extract.
Tabla 1. Composición química de polvo de la hoja de Damsissa y extracto.
3.2. Antibacterial activity of DLPE
The in vitro antibacterial activity of DLPE against a Gram-negative (E. coli) and a Gram-positive (S. aureus) bacteria displayed inhibition zones of 14.0 mm (E. coli) and 12.7 mm (S. aureus), signifying high to moderate antimicrobial activities of DLPE against the two organisms (). Similarly, EL-Kamali and EL-amir (Citation2010) reported that a methanol extract of A. maritima leaves had antibacterial activity against E. coli (15 mm, inhibition zone) and S. aureus (12 mm, inhibition zone). In addition, Wang, Kong, and Zhang (Citation2006) reported similar observations on the antibacterial activity of leaf extracts of a closely related plant (Ambrosia trifida), grown in China, against E. coli and S. aureus. The inhibition potential of leaf extracts of A. maritima could be attributed to its high content of polyphenols and flavonoid compounds observed in the present study. In addition, leave extracts of A. maritima are reported to contains several bioactive compunds such as ambrosin, anhydrofranserin, apigenin, s-amyrin, carvone, caryophyllene, chloroambrosin, cineole, comphor, coumarins, damsin, damsinic acid, farnserin, hymendin, hymenin, neoambrosin, stamonin-b, sterols, β-sitosterol, tannins, and triterpenes with high antimicrobial and antioxidant activities (Badawy, Abdelgaleil, Suganuma, & Fuji, Citation2014; El-Sawy et al., Citation2017). These findings collectively demonstrate the protective potentials of leaf extracts of these plants, and thus, may pave the way for their utilization as a natural preservative in food products.
Table 2. Antibacterial activity of DLPE.
Tabla 2. Actividad antimicrobiana del DLPE.
3.3. Antioxidant activity
DLPE exhibited substantial antioxidant activity as determined by three commonly used in vitro methods, such as TRPA, chelation of Fe+2 ions, and scavenging of H2O2 (). Previous reports have indicated that leaf extracts of A. maritima exhibited high antioxidant activity in the DPPH free radical scavenging assay (Dirar et al., Citation2014; Hilmi, Abushama, Abdalgadir, Khalid, & Khalid, Citation2014). Furthermore, Maksimovic (Citation2008) found that leaf extracts of A. artemisiifolia L. expressed considerable DPPH radical scavenging and ferric-reducing antioxidant power activities. Our results show significant (P ≤ 0.05) increases in TRPA, Fe+2 ions chelation, and H2O2 scavenging activities with the increase in the concentration of DLPE from 125 to 1000 µg/mL. Previous reports have shown similar observations where TRPA, Fe2+ ions chelation, and H2O2 scavenging activities were increased with an increase in the concentration of leaf extracts of Moringa (Muthukumar et al., Citation2014; Shah, Bosco, & Mir, Citation2015) and Cyperus rotundus rhizome extract (Eltilib et al., Citation2016). Our findings suggest that DLPE may act as an iron chelator, free radical and H2O2 scavenger and thus, it could prevent the formation of free radicals and the initiation of free radical-mediated chain reactions by stabilizing reactive species before they can participate in harmful reactions (Maksimovic, Citation2008). Due to its antioxidant potential, DLPE could be used in functional food formulations and therapeutic agents designed to prevent or slow the progress of aging and age-associated oxidative stress-related degenerative diseases.
Table 3. Total reducing power ability (TRPA), Fe+2 ion chelating, and H2O2 scavenging activities of different concentrations of DLPE.
Tabla 3. Capacidad de poder reductor total (TRPA), quelación de iones Fe+2 y eliminación de H2O2 con distintas concentraciones de DLPE.
3.4. Chemical composition of minced beef treated with DLPE
The chemical composition of minced beef was significantly (P ≤ 0.05) affected by the addition of DLPE () in a concentration-dependent manner. Increasing the concentration of DLPE in minced beef concurrently (P ≤ 0.05) improved protein, fat, and ash contents, while the moisture content exhibited a concomitant (P ≤ 0.05) decrease. A reverse association of moisture content and DLPE level may be correlated with the increase in the total solids, increased moisture binding ability or modification of the pH of the minced beef – DLPE system (Eltilib et al., Citation2016). Similarly, a concurrent reduction of moisture content following addition of increased concentrations of bambara groundnut seed flour and moringa seed and leaf powder to meat patties has been reported (Alakali, Irtwange, & Mzer, Citation2010; Al-Juhaimi, Ghafoor, Hawashin, Alsawmahi, & Babiker, Citation2016; Elhadi et al., Citation2017). In addition, integration of bambara groundnut seed flour or moringa seed or leaf powder in meat patties simultaneously increased the protein, fat, and ash contents of the products (Alakali et al., Citation2010; Al-Juhaimi et al., Citation2016; Elhadi et al., Citation2017). The increase in the crude protein, fat, and ash contents of minced beef supplemented with DLPE might be due to the high contents of these constituents in DLPE (). These findings established that integration of DLPE in minced meat could improve the nutritional value of the product.
Table 4. Chemical composition (g/100 g) of minced beef treated with different concentrations of DLPE.
Tabla 4. Composición química (g/100 g) de carne de res picada y tratada con distintas concentraciones de DLPE.
3.5. pH and peroxide values of minced beef treated with DLPE during cold storage
Minced beef incorporated with increasing amounts of DLPE showed a significantly (P ≤ 0.05) high pH values as the storage period progressed ()). At all storage days, the pH of the control (0% DLPE) minced beef samples was lower (P ≤ 0.05) than those incorporated with 2.5% and 5% DLPE indicating that DLPE enhances the pH of minced beef that could be due to the nature of bioactive compounds of DLPE that might not contain much acidic compounds. Generally, the reduction rate of pH was higher in control samples compared to DLPE-containing ones suggesting that DLPE enhances the pH stability of minced beef and could thus regulate the growth of spoilage microorganisms (Hawashin et al., Citation2016). Similarly, previous reports have shown that meat products formulated with moringa leaf and seed powder extracts exhibited higher pH values compared to unformulated controls (Al-Juhaimi et al., Citation2016; Muthukumar, Naveena, Vaithiyanathan, Sen, & Sureshkumar, Citation2014; Shah, Bosco, & Mir, Citation2015). Contrasting reports have indicated that incorporation of moringa leaf powder or extract in chicken sausages significantly reduced the pH of the product (Elhadi et al., Citation2017; Jayawardana et al., Citation2015). The divergent results obtained from these studies might be due to differences in materials and extracts used. Our findings also showed that over the storage period the pH of minced beef reduced significantly (P ≤ 0.05). Similar observations on pH reduction during storage of sausages formulated with moringa leaf powder or extract (Elhadi et al., Citation2017; Jayawardana et al., Citation2015) and microencapsulated Jabuticaba (Myrciaria cauliflora) extract (Baldin et al., Citation2016) have been reported.
Figure 1. pH (a) and peroxide (b) values of minced beef treated with different concentrations (0.0%, ◊; 2.5%, O; and 5%, ∆) of DLPE during cold storage (4°C). Error bars specify the standard error of triplicate samples.
Figura 1. Valores de pH (a) y peróxido (b) en carne de res picada tratada con distintas concentraciones (0.0%, ◊; 2.5%, O; y 5%, ∆) de DLPE durante su almacenamiento en frío (4°C). Las barras de error indican el error estándar de tres muestras.
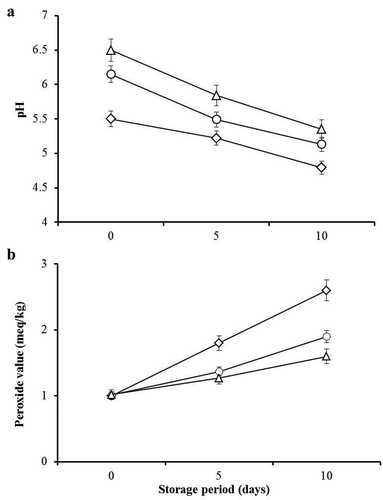
DLPE and storage period significantly influenced (P ≤ 0.05) the peroxide value of minced beef ()). Progress in storage period increased (P ≤ 0.05) peroxide value of minced beef, whereas, increase of DLPE concentration significantly (P ≤ 0.05) decreased the peroxide value of the product. At the beginning of the storage period (0 time), minced beef samples with or without DLPE had similar (P ≥ 0.05) peroxide values. The incorporation of DLPE in minced beef delayed (P ≤ 0.05) lipid oxidation development because the peroxide values of DLPE containing samples were lower during storage than those of the control samples. This is in agreement with several reports that showed the incorporation of moringa leaf and seed extracts (Al-Juhaimi et al., Citation2016; Das et al., Citation2012; Shah et al., 2015) and microencapsulated Jabuticaba extract (Baldin et al., Citation2016) in meat products significantly reduced lipid peroxidation during cold storage. Reduction of peroxide values in the current study might be attributed to polyphenols and flavonoids of DLPE that possess antioxidant activity. Therefore, the incorporation of DLPE in minced beef inhibits the lipid oxidation and lower formation of H2O2, and it will thus contribute to extending the shelf life and the quality of the minced beef.
3.6. Microbiological characteristics of minced beef treated with DLPE
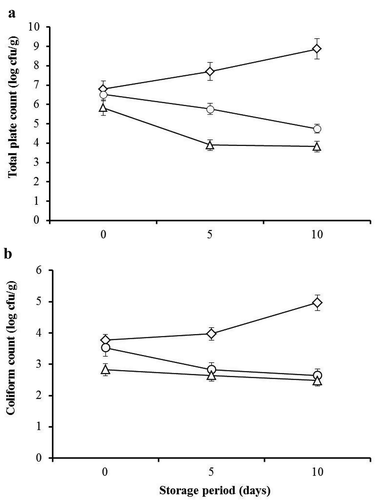
The microbiological analysis indicated that the counts of E.coli and S. aureus were less than 102 cfu/g (data not shown), indicating that good hygienic conditions were followed during the minced beef processing. The microbiological characteristics of minced beef were different (P ≤ 0.05) among all treatments and storage periods (). The number of total plate and coliform counts of control samples were increased (P ≤ 0.05) with the progress of the storage time. DLPE-containing samples exhibited significantly (P ≤ 0.05) lower total plate and coliform counts throughout the storage period compared to untreated control. This showed that the DLPE treatment had significant (P ≤ 0.05) antimicrobial activity and supported the aforementioned observations of the in vitro antimicrobial activity of the DLPE against E.coli and S. aureus (). Interestingly, the total plate counts of minced beef formulated with 2.5% and 5.0% DLPE were significantly reduced to 4.75 and 3.82 cfu/g, respectively after 10 days of storage. These values are considerably below the recommended detection limit (6.7 cfu/g) of the microbiological shelf life of minced beef (AFNOR, Citation2004). In contrast, the untreated control was significantly increased to 7.5 and 8.8 cfu/g after 5 and 10 days of storage, respectively. Our results show that the growth of bacteria in minced beef declined concurrently (P ≤ 0.05) with the increase of DLPE level. Similarly, a reduction of total plate count in meat products incorporated with extracts of leaves and seeds of moringa have previously been reported (Al-Juhaimi et al., Citation2016; Jayawardana et al., Citation2015). By contrast, other reports indicated that inclusion of moringa leaf extracts, at relatively low doses, in meat products did not affect the microbial load of the products (Muthukumar et al., Citation2014; Shah et al., 2015). Overall, our findings demonstrate that incorporation of DLPE in minced beef substantially reduced the microbial load and thus extended the storability of the product for up to 10 days at 4°C.
Figure 2. Total plate count (a) and total coliform count (b) of minced beef treated with different concentrations (0.0%, ◊; 2.5%, O; and 5%, ∆) of DLPE during cold storage (4°C). Error bars specify the standard error of triplicate samples.
Figura 2. Recuento total de gérmenes (a) y recuento total de coliformes (b) de carne de res picada tratada con distintas concentraciones (0.0%, ◊; 2.5%, O; y 5%, ∆) de DLPE durante su almacenamiento en frío (4°C). Las barras de error indican el error estándar de tres muestras.
3.7. Sensory attributes of cooked minced beef treated with DLPE
Inclusion of DLPE in minced beef reduced (P ≤ 0.05) the panelist scores of the color, flavor, juiciness, tenderness, and overall acceptability compared to the untreated control (). The reduction trend in the scores of all sensory attributes was concomitant with increasing concentration of DLPE. Interestingly, all sensory attributes of minced beef incorporated with 2.5% DLPE had scores higher than 4.0 (cut-off score) indicating that minced beef treated with 2.5% DLPE was satisfactory and acceptable to the panelists. However, the flavor of 5% DLPE minced beef was acceptable and not different (P ≥ 0.05) from the other treated samples. Jayawardana et al. (Citation2015) stated that increasing the concentration of moringa leaf extract above 0.5% in chicken sausage negatively affected the sensory attributes compared to the control and those samples treated with 0.25% and 0.50% moringa leaf extract. In addition, other report has shown that increasing the concentrations of moringa seed powder in beef patties concurrently reduced the organoleptic attributes of the product (Al-Juhaimi et al., Citation2016). Nevertheless, inclusion of moringa leaf extract in meat burgers did not have any negative effect on the organoleptic characteristics of the product (Das et al., Citation2012; Muthukumar et al., Citation2014). The dissimilarities found in these reports could be due to variations in the parts of the moringa tree (seeds or leaves) that were used, the age of the trees, the percentage of the added plant materials, the meat type used, and the approaches used for addition of the extracts to the meat products. In the present study, the organoleptic attributes of cooked minced beef demonstrated that inclusion of up to 2.5% DLPE has resulted in very satisfactory sensory scores, albeit somewhat lower than those for non-treated controls, and thus could potentially be used in minced meat preservation.
Table 5. Sensory evaluation of minced beef treated with different concentrations of DLPE.
Tabla 5. Evaluación sensorial de carne de res picada y tratada con distintas concentraciones de DLPE.
4. Conclusion
This study provides, for the first time, evidence for the potential utilization of Damsissa leaf powder extract as a natural preservative for minced beef. Our study concludes that DLPE is an excellent source of flavonoids and polyphenols, and it contributes considerable antioxidant and antibacterial activity to minced beef during refrigerated storage. The incorporation of DLPE in minced beef significantly enhanced its physicochemical characteristics, oxidative and storage stability, and safety. While the majority of the sensory attribute scoring of the minced beef product were lower with addition of DLPE, 2.5% DLPE appeared to be well within the range of acceptability for consumers. Therefore, utilization of DLPE to prolong the shelf life and the safety of minced beef products could both satisfy the expectation of consumers for natural, healthy and safe food ingredients and add value to this underutilized Damsissa weed plant.
Acknowledgments
The authors extend their appreciation to the International Scientific Partnership Program ISPP at King Saud University for funding this research work through ISPP# 0073.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AFNOR. (2004) Hygiène des aliments - Lignes directrices pour la réalisation de tests de vieillissement microbiologique - Aliments périssables et très périssables réfrigérés (NF V01-003). Saint-Denis, France: Association française de Normalisation, p. 7.
- Alakali, J. S., Irtwange, S. V., & Mzer, M. T. (2010). Quality evaluation of beef patties formulated with bambara groundnut (Vigna subterranean L.) seed flour. Meat Science, 85, 215–223.
- Al-Juhaimi, F., Ghafoor, K., Hawashin, M. D., Alsawmahi, O. N., & Babiker, E. E. (2016). Effects of different levels of moringa (Moringa oleifera) seed flour on quality attributes of beef burgers. CyTA – Journal of Food, 14(1), 1–9.
- AMSA. (2015). Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of fresh meat (2nd ed., version 1.0). Chicago, IL: American Meat Science Association.
- AOAC. (2003). Official methods of analysis of AOAC international (17th ed.). Gaithersburg, MD: Association of the Official Analytical Chemists.
- Badawy, M. E. I., Abdelgaleil, S. A. M., Suganuma, T., & Fuji, M. (2014). Antibacterial and biochemical activity of pseudoguaianolide sesquiterpenes isolated from Ambrosia maritima against plant pathogenic bacteria. Plant Protection Science, 50, 64–69.
- Baldin, J. C., Michelin, E. C., Polizer, Y. J., Rodrigues, I., de Godoy, S. H. S., Fregonesi, R. P., … Trindade, M. A. (2016). Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Science, 118, 15–21.
- Barakat, S. E. M., Al-Hizab, F. A., & Bakhiet, A. O. (2012). Clinicophathological effects of various levels of dietary Ambrosia maritima on Wistar rats. Journal of Animal and Veterinary Advances, 11, 2672–2676.
- Das, A. K., Rajkumar, V., Verma, A. K., & Swarup, D. (2012). Moringa oleifera leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. International Journal of Food Science and Technology, 47, 585–591.
- Ding, W., Huang, R., Zhou, Z., & Li, Y. (2015). New sesquiterpenoids from Ambrosia artemisiifolia L. Molecules, 20, 4450–4459.
- Dirar, A. I., Mohamed, M. A., Khalid, H. S., Osman, B., Fadul, E., & Khalid, A. (2014). In vitro antioxidant activity and phytochemical properties of three antitumor medicinal plants grown in Sudan. World Journal of Pharmaceutical Research, 3(9), 136–142.
- Elhadi, D. A. E., Elgasim, E. A., & Mohamed Ahmed, I. A. (2017). Microbial and oxidation characteristics of refrigerated chicken patty incorporated with moringa (Moringa oleifera) leaf powder. CyTA - Journal of Food, 15(2), 234–240.
- EL-Kamali, H. H., & EL-amir, M. Y. (2010). Antibacterial activity and phytochemical screening of ethanolic extracts obtained from selected Sudanese medicinal plants. Current Research Journal of Biological Sciences, 2(2), 143–146.
- El-Sawy, M. F. E.-D., El-Sawy, L. M. F. E.-D., Helmy, S. H. A., Day, M. L., Rubin, J. R., & Lorenzatti, G. (2017). Medicinal Ambrosia maritima extracts. Houston, TX: United States Patents, US 2017007 1994A1, p. 1–46.
- Eltilib, H. H. A. B., Elgasim, A. E., & Mohamed Ahmed, A. (2016). Effect of incorporation of Cyperus rotundus L. rhizome powder on quality attributes of minced beef meat. Journal of Food Science and Technology, 53, 3446–3454.
- Fernandes, R. P. P., Trindade, M. A., Lorenzo, J. M., Munekata, P. E. S., & de Melo, M. P. (2016). Effects of oregano extract on oxidative, microbiological and sensory stability of sheep burgers packed in modified atmosphere. Food Control, 63, 65–75.
- Harrigan, W. F. (1998). Laboratory method in microbiology (3rd ed.). London: Elsevier Academic Press Ltd. ISBN: 0-12-326043-4.
- Harris, G. M., & Livingstone, S. E. (1964). Bidentate chelates. In F. P. Dwyer & D. P. Mellor (Eds.), Chelating agents and metal chelates (1st ed., pp. 95–141). New York, NY: Academic Press, Inc.
- Hawashin, M. D., Al-Juhaimi, F., Mohamed Ahmed, I. A., Ghafoor, K., & Babiker, E. E. (2016). Physicochemical, microbiological and sensory evaluation of beef patties incorporated with destoned olive cake powder. Meat Science, 122, 32–39.
- Hilmi, Y., Abushama, M. F., Abdalgadir, H., Khalid, A., & Khalid, H. (2014). A study of antioxidant activity, enzymatic inhibition and in vitro toxicity of selected traditional Sudanese plants with anti-diabetic potential. BMC Complementary and Alternative Medicine, 14, 149.
- Honikel, K. O. (2014). Minced meat. In C. Devine & M. Dikeman (Eds.), Encyclopedia of meat sciences (2nd ed., pp. 422–424). London: Elsevier Academic Press.
- Jayaprakasha, G. K., Rao, L. J., & Sakariah, K. K. (2004). Antioxidant activities of flavidin in different in vitro model systems. Bioorganic and Medicinal Chemistry, 12(19), 5141–5146.
- Jayawardana, B. C., Liyanage, R., Lalantha, N., Iddamalgoda, S., & Weththasinghe, P. (2015). Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT - Food Scicence and Technology, 64, 1204–1208.
- Kilani, S., Abdelwahed, A., Ammar, R., & Hyder, N. (2005). Chemical composition, antimicrobial and antimutagenic activities of essential oil from Tunisian. Cyperus Rotundus. Journal of Essential Oil Research, 19, 368–379.
- Makkawi, A. J. J., Keshk, E. M., ElShamy, M. M., & Abdel-Mogib, M. (2015). Phytochemical and biological evaluation of Ambrosia maritima. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 6, 1678–1688.
- Maksimovic, Z. (2008). In vitro antioxidant activity of ragweed (Ambrosia artemisiifolia L., Asteraceae) herb. Industrial Crops Production, 28, 356–360.
- Mohamed, R. M. A., Fageer, A. S. M., Eltayeb, M. M., & Mohamed Ahmed, I. A. (2014). Chemical composition, antioxidant capacity, and mineral extractability of Sudanese date palm (Phoenix dactylifera L.) fruits. Food Science and Nutrition, 2(5), 478–489.
- Muthukumar, M., Naveena, B. M., Vaithiyanathan, S., Sen, A. R., & Sureshkumar, K. (2014). Effect of incorporation of Moringa oleifera leaves extract on quality of ground pork patties. Journal of Food Science and Technology, 51(11), 3172–3180.
- Price, M. L., & Butler, L. G. (1977). Rapid visual estimation and spectrophotometric determination of tannin in sorghum grain. Journal of Agriculture and Food Chemistry, 25, 1268–1273.
- Saeed, M. E., Abdelgadir, H., Sugimoto, Y., Khalid, H. E., & Efferth, T. (2015). Cytotoxicity of 35 medicinal plants from Sudan towards sensitive and multidrug-resistant cancer cells. Journal of Ethnopharmacology, 174, 644–658.
- Salah, O., & Yagi, S. (2011). Nutritional composition of Prosopis chilensis (Molina) Stuntz leaves and pods from Sudan. African Journal of Food Science and Technology, 2(4), 79–82.
- Shah, M. A., Bosco, S. J. D., & Mir, S. A. (2015). Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere packaged raw beef. Food Packaging and Shelf Life, 3, 31–38.
- Tang, S., Kerry, J. P., Sheehan, D., Buckley, D. J., & Morrissey, P. A. (2001). Antioxidative effect of added tea catechins on susceptibility of cooked red meat poultry and fish patties to lipid oxidation. Food Research International, 34, 651–657.
- Wang, P., Kong, C. H., & Zhang, C. X. (2006). Chemical composition and antimicrobial activity of the essential oil from Ambrosia trifida L. Molecules, 11, 549–555.
- Zhishen, J., Mengcheng, T., & Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64, 555–559.
