ABSTRACT
The mucilage obtained from cladodes of Opuntia spinulifera has been used in industries and traditional applications. In the present work, the chemical, morphological, thermal and structural features of cladode mucilage from Opuntia spinulifera Salm-Dyck were studied. The 2D 1H-1H COSY NMR confirmed ten different residues within the repeating saccharide unit, six with α and four with β configurations. The FTIR showed the presence of galactose and pectins. The XRD detected minerals as calcium salts. The SEM exhibited aggregations and irregular morphology of the particles. The TGA registered the highest mass loss between 246–378°C and the DSC showed the transition state at 55.7°C and at 113.2°C was observed the typical endothermic peak, where it corresponds to the organic compound materials.
RESUMEN
El mucilago obtenido de cladodios de Opuntia spinulifera ha sido utilizado en varias industrias y en aplicaciones tradicionales. En el presente estudio, se estudiaron las características químicas, morfológicas, térmicas y estructurales del mucilago proveniente de cladodios de Opuntia spinulifera Salm-Dyck. La espectroscopía de correlación-resonancia magnética nuclear (EC-RMN) de 1H-1H en 2D confirmó la existencia de 10 residuos diferentes al interior del grupo sacárido que se repite, seis con configuraciones α y cuatro con configuraciones β. El espectrofotómetro de transformada de Fourier (FTIR) constató la presencia de galactosa y pectinas. La difracción de rayos X (DRX) detectó la existencia de minerales en forma de sales de calcio. La microscopía electrónica de barrido (MEB) reveló la presencia de agregaciones, así como la morfología irregular de las partículas. El análisis termogravimétrico (ATG) registró la mayor pérdida de masa entre 246–378°C, mientras que la calorimetría diferencial de barrido (DSC) precisó que se produjo un estado de transición a los 55.7°C, observándose el pico endotérmico típico de los materiales orgánicos compuestos a los 113.2°C.
1. Introduction
Opuntia is a complex genus that includes species used for their edible young cladodes called “nopalitos” obtained mainly from Opuntia ficus–indica, or for their fruits (from many species) known as cactus pears (tunas in Spanish) and xoconostles. Xonocostle fruit differs from cactus pear in having very thick edible pericarp, almost absent and highly acidic pulp, and long shelf life. The word “nopal” refers to each plant of most of the Opuntia species disregarding if they are used for fresh fruit, vegetable, or as animal feed (Gallegos-Vázquez, Barrientos-Priego, Reyes-Agüero, Núñez-Colín, & Mondragón-Jacobo, Citation2011).
Cactus pear utilization for human consumption started in Central Mexico with the collection of plant organs susceptible of use with minimal effort, ripe fruits, tender and mature cladodes (Mondragón-Jacobo, Gallegos-Vázquez, & Reyes-Agüero, Citation2009). According to the official statistics of the Mexican government 2014, the production of cactus pear fruit production was to 568,404.90 tonnes in 51,598.15 ha of harvest surface with a production value of US $91,748,556.57, and the young cladodes production was to 824,602.36 tonnes in 10,996.16 ha of harvest surface with a production value of US $91,244,953.92 (SIAP, Citation2015).
The chemical composition of cladode mucilage from Opuntia spp has been studied in several researches, however, the physicochemical characterization of the mucilage has not been fully determined. Majdoub et al. (Citation2001) mentioned that the mucilage is a heteropolysaccharide formed by arabinose, galactose, rhamnose, xylose, and neutral sugars. Sáenz, Sepúlveda, and Matsuhiro (Citation2004) pointed out that the mucilage of Opuntia ficus-indica is composed of 24.6–42% of arabinose, 21–40% of galactose, 8–12.7% of galacturonic acid, 7–13.1% of rhamnose and 22–22.2% of xylose, the complexity of the mucilage analysis is due to the variability of composition with respect to the species.
Besides, Saenz and Cuevas (Citation2013) reported several non-food products from this plant; among them, the use of mucilage for diverse industrial process is currently important. The application of mucilage from cactus pear is promising in agroindustry, as a component or matrix for coating and films in vegetables postharvest technology (Espino-Díaz et al., Citation2010; Zegbe, Mena-Covarrubias, & Dominguez-Canales, Citation2015). Also, it has potential medical application in the treatment of gastric mucosa and in other gastric ailments (Saenz & Cuevas, Citation2013). Petera et al. (Citation2015) mentioned that the mucilage from Cereus triangularis cladodes is rich in an arabinogalactans fraction, functioning as a good source of fiber and antioxidant compound and Jaramillo-Flores et al. (Citation2003) reported that Opuntia ficus-indica cladodes are rich in mucilage and pectin which form complex with bioactive compounds (phenolic acids and flavonoids) providing its antioxidant activity, also, it has been reported its potential use as coagulation-flocculation agent for bioremediation of wastewaters (Nharingo & Moyo, Citation2016).
However, from our knowledge the structural and thermal features of Opuntia spinulifera have not been completely reported. It belongs to Opuntia spp and identified by Salm-Dyck (family Cactaceae) and verified by L. Scheinvar (Soberon, Golubov, & Sarukhán, Citation2001). The aims of this study are to report the extraction, proximate analysis, chemical, thermal, and morphological characterization of the cladode mucilage of Opuntia spinulifera as an insight of the chemical composition of mucilage from this specie.
2. Materials and methods
2.1. Mucilage extraction
The cladodes from Opuntia spinulifera with two years old were recollected and cleaned for the mucilage extraction. The medullar parenchyma was separated manually with a knife, and then crushed. For 100 g of parenchyma, 250 mL of water was added. Subsequently, the material was stirred at 100 rpm for 12 h at room temperature, and then was filtered in a domestic sieve (first filtrate); then the process is repeated, adding equal volume of water, resting and filtering to obtain, 2nd, 3rd and 4th. Then, the mucilage was recuperated of the filtrate obtained by precipitating with ethanol (96%). Specifically, 100 mL of the filtrate was mixed with 300 mL of ethanol. Finally, the powder mucilage was obtained by drying at 50°C for 3 h (Vargas-Rodríguez et al., Citation2016).
2.2. Proximate composition analysis
Total nitrogen content was determined by the micro-Kjeldahl method 12.960.52 (AOAC, Citation2007) and the crude protein content was calculated using a 6.25 factor. Also, the Bradford method was used to determine total protein according to Bradford (Citation1976) with some modifications. Fat content was determined by the Soxhlet method 996.01 (AOAC, Citation2007). Ash content was obtained according to method 900.02 (AOAC, Citation2007). These determinations were analyzed at least in triplicates. The carbohydrates were estimated as the percentage remaining to reach 100% of the proximal composition according to FAO (Citation2003).
2.3. Structural analysis
2.3.1. FTIR spectroscopy
The polysaccharide (exopolysaccharide) fraction was analyzed using Fourier transform infrared spectrometer. The mucilage was dried into a vacuum oven 60°C for an overnight, and then was ground with potassium bromide (KBr) spectroscopic grade (1 mg of dried sample and 100 mg of dried KBr). The accessory diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) for analysis of powdered sample was used to determine spectra in absorbance mode at room temperature (mid-infrared region, 4000–500 cm−1) using a Nicolet spectrometer model Nexus 670 coupled with personal computer loaded with OMNIC software. A total of 100 scans were measured with a resolution of 4 cm−1 (Rodríguez-Núñez, Madera-Santana, Sánchez-Machado, López-Cervantes, & Soto Valdez, Citation2014).
2.3.2. X-ray diffraction (XRD)
To determine the crystalline structure of the dried mucilage from Opuntia spinulifera, a sample powder was placed in a sample holder for X-ray diffractometry. The XRD patterns were recorded in the reflexion mode in an angular range of 10–90° (2θ) at room temperature using a diffractometer Siemens model D5000 (Karlsruhe, Germany), with a Bragg Brentano geometry and monochromatic CuKα radiation (λ = 1.5418 Å), at 30 kV, 1 s/0.005°, 30 rpm, and 40 mA (Rodríguez-Núñez et al., Citation2014).
2.3.3. NRM spectra
The 1H and 2D 1H-1H COSY NMR spectra of mucilage extracted from O. spinulifera was obtained in Bruker Avance III spectrometer (EU, Massachusetts) operating at 500 MHz using 1.0 s of pulse delayed at 25ºC and D2O as solvent. The NMR spectra were referenced by using the residual HOD signal at 4.79 ppm (chemical shifts). The sample (10 mg) was dissolved in 0.6 mL of D2O then freeze-dried two times before NMR analysis (Habibi, Heyraud, Mahrouz, & Vignon, Citation2004).
2.4. Morphological analysis
2.4.1. Scanning electron micrographs (SEM)
Morphological analysis of powder mucilage surface was performed at magnifications of 100X and 1000X using a SEM from FEI Corp. model Phillips XL 30 ESEM-FEG (Hillsboro, OR). The particles of mucilage sample were coated with a thin layer of Au-Pd using a sputter coater (Quorum model QI5OR-ES, Sussex, UK) before scanning. The morphological analysis of the particles of mucilage powder was performed to investigate the particle size, the shape and form of the particles (Habibi et al., Citation2004).
2.5. Thermal analyses
2.5.1. Thermogravimetric analysis (TGA)
TGA of the polysaccharide (exopolysaccharide) fraction was performed on equipment TGA from TA Instruments Inc. model Discovery (New Castle, DE). A sample of approximately 20 mg was heated from 50 to 600°C at a heating rate of 10°C/min, under inert atmosphere of N2 with a flow rate of 60 mL/min (Rodríguez-Núñez et al., Citation2014).
2.5.2. Differential scanning calorimetry (DSC)
A DSC analysis was performed on a TA Instruments Inc. model Discovery (New Castle, DE). The sample weight was from 4 to 10 mg and it was introduced into cells of aluminum and sealed under pressure. An empty cell of aluminum was used as reference. Both cells under inert atmosphere were heated up from 20 to 180°C at a heating rate of 10°C/min, and then cooled down rapidly to 20°C. The samples were subjected to a second heating in the same temperature interval, and the thermal parameters determined were: glass transition temperature (Tg), crystallization temperature (Tc), melting temperature (Tm), and the enthalpy of crystallization (ΔHc) and of melting (ΔHm) (Rodríguez-Núñez et al., Citation2014).
3. Results and discussion
3.1. Proximate composition analysis
The proximate composition of powder mucilage from O. spinulifera is shown in . Gebresamuel and Gebre-Mariam (Citation2012) reported the proximate composition of mucilage from cactus pear (Opuntia spp.) obtained from the Northern Ethiopia. They reported 6.82% of proteins, 0.42% of fat content; these values are similar with the reported in the present work. However, the value of ashes (33.96%) differ greatly; these authors did not mention the carbohydrates percentage. In a similar work made in nopal (Opuntia ficus-indica) by Espino-Díaz et al. (Citation2010) pointed out values of 1.04, 20.08, and 64.15% of protein, ash, and carbohydrates, respectively. These values are different with the reported in the present work. Meanwhile, Sepúlveda, Sáenz, Aliaga, and Aceituno (Citation2007) mentioned a range of proteins of 6.1–7.9%, 34.9–39.1% for ash content, and 0.98–1.28% for total nitrogen. The results of proteins and total nitrogen are similar to the values obtained in the present work and it differs in the percentage of ashes. De Souza et al. (Citation2016) reported an ash value of 0.35% in mucilage from Cereus peruvianus cactus and Kalegowda, Chauhan, and Nanjaraj (Citation2017) pointed out a value of 4.70% of ash in mucilage from Opuntia dillenii cladode, agreeing with the low percentage obtained in the present work. According to Villareal et al. (Citation1963) the reason that may explain these differences are the influence of several factors such as the soil, species, as well as the complex phenomena related to plant nutrition. In addition, Granados and Castañeda (Citation1991) mentioned that the high values of ashes in cladode reported for some authors can be attributed to the high salinity of the soil and to the mineral bioavailability.
Table 1. Analysis of proximal composition of O. spinulifera powder mucilage.
Tabla 1. Análisis de composición proximal de mucilago en polvo obtenido de O. spinulifera.
3.2. Structural analysis
3.2.1. FTIR spectroscopy
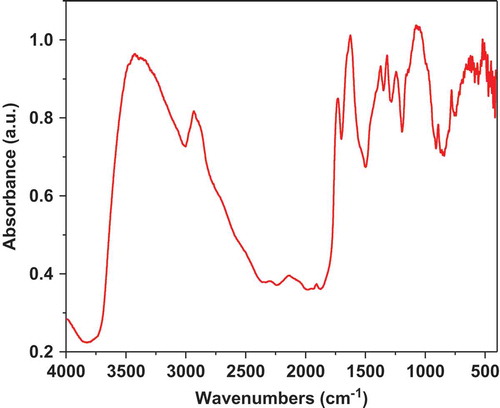
The FTIR spectrum of polysaccharide fraction is shown in . The results were analyzed in three characteristic regions: -OH stretching band in the region of 3600–3200 cm−1; C-H (methyl group) stretching bands (3000–2800 cm−1) and the common fingerprint region of polysaccharides or carbohydrate units (1300–1050 cm−1) (Kamnev, Colina, Rodriguez, Ptitchkina, and Ignatov (Citation1998); Mohan, Singh, & Singh, Citation2005). A typical broad absorption band at 3454 cm−1 is due to stretching frequency of – OH group and the bands between 2920 and 2830 cm−1 are attributed to the C-H stretching vibration (Sawut, Yimit, Sun, & Nurulla, Citation2014). Both bands are attributed to functional groups of neutral polysaccharide components of the mucilage, such as arabinose, rhamnose, galactose and xylose; as it was reported in the literature (Matsuhiro, Lillo, Sáenz, Urzua, & Zárate, Citation2006). The absorption band between 1185 and 1045 cm−1 is related to the stretching vibrations of the glycosidic bonds (C-O, ether linkage) and pyranoid rings (C-C) attributed to polysaccharides that are part of pectins and mucilage (Pereira et al., Citation2016). According to Lefsih et al. (Citation2016) and Jouini et al. (Citation2018) the pectins from cladodes of Opuntia ficus indica and Opuntia microdasys can be precipitated with ethanol, explaining the presence of pectins in the samples of mucilage. The absorption bands between 1750 and 1735 cm−1 correspond to C = O stretching vibration of methyl esterified groups (Kpodo et al., Citation2017) and the regions of 1650–1600 cm−1 are related with the carboxyl group (COOH) (Kpodo et al., Citation2017; Nejatzadeh-Barandozi & Enferadi, Citation2012) meanwhile, Synytsya, Čopíková, Matějka., and Machovic (Citation2003) reported the stretching vibrations of carboxylate anion (COO−) at 1633 cm−1. The band at 1320 cm−1 is assigned to C-H methyl vibration of the rhamnose ring commonly present in the mucilage obtained from Opuntia spp (Vargas-Rodríguez et al., Citation2016). The bands at 1300 and 1050 cm−1 indicate the presence of carbohydrate units, corroborated by the galactose absorption band at 778 cm−1, it is in conformity with the monosaccharide composition of this fraction. In a similar work made in mucilage from Dioscorea opposite, Ma et al. (Citation2017) reported that the pyranose stretching vibration showed the peaks at 1069 cm−1 and the peak at 923 cm−1 is concerned as β-pyranose bending vibration. These results agree with the obtained in the present work corroborating the presence of carbohydrates in the mucilage.
3.2.2. X-ray diffraction
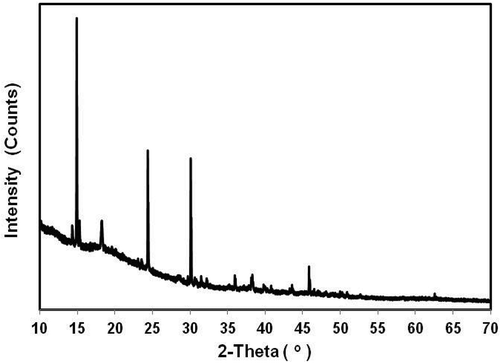
XRD of the mucilage from nopal cladodes was performed in order to examine its amorphous, semi-crystalline, or crystalline structure. The X-ray patterns identified only crystalline structures characteristic of calcium salts (calcium oxalates) that are typical in nopal mucilage and in other cactus (Sepúlveda et al., Citation2007), . This result agrees with that reported by Contreras-Padilla et al. (Citation2011) who pointed out the presence of oxalates in nopal powders (form of druses) and identified minerals as whewellite (C0CaO4.H2O), calcite (CaCO3), fairchildite (K2Ca(CO3)2), potassium peroxydiphosphate (K4P2O8), and sylvine (KCl). This result is quite interesting because, the mucilage from Opuntia spinulifera shows an excellent source of minerals as Ca+2, and these compounds would have bioavailability for the human body (Tucker et al., Citation1999).
3.2.3. NMR spectra of mucilage
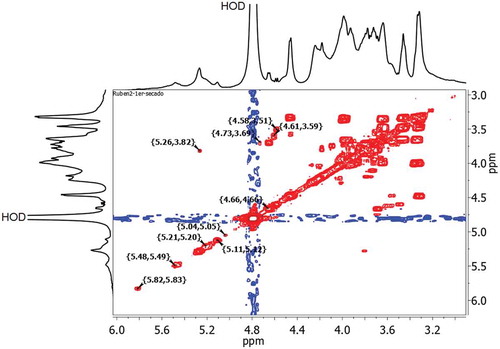
shows the 1H NMR spectrum of mucilage in the range of 0.0–8.0 ppm. The NMR spectrum confirms the presence of polysaccharides, N-acetyl group at 1.90 ppm, aliphatic alkyl groups at 0.95 ppm, uronic acid at 2.4 ppm and aromatic compounds in the region of 6.83–7.21 ppm. The signals in the regions of 5.81–5.11 and 4.66–4.58 were attributed to proton of α- and β-anomeric carbon of hexose or pentose, respectively. The signals in the region of 4.18–3.32 ppm were assigned to hydrogen next to the functional –OH group. The regions corresponding to α- and β-anomeric carbon could be attributed to the presence of approximately 55 sugar residues, composed of arabinose, galactose, rhamanose, and xylose as neutral sugars (Sáenz et al., Citation2004). Also, other studies mentioned the presence of acid sugars as D-galacturonic acid (Trachtenberg & Mayer, Citation1981) agreeing with the uronic acid found in this study.
Figure 3. 1H-1H COSY NMR spectra of mucilage extracted from O. spinulifera.
Figura 3. Espectros de EC-RMN 1H-1H de mucilago extraído de O. spinulifera.
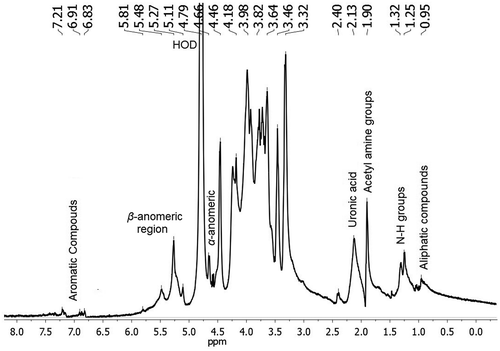
shows the 2D 1H-1H COSY NMR spectrum of mucilage. It confirmed that there were ten different residues within the repeating saccharide unit; six of them with α- configuration at 5.82, 5.48, 5.26, 5.21, 5.11, and 5.04 ppm and four with β- configuration at 4.73, 4.66, 4.61, and 4.58 ppm and according to Bubb (Citation2003), the signals of the anomeric protons in carbohydrates are typical at 4.4–5.5 ppm.
3.3. Morphological analysis
3.3.1. Scanning electron micrographs
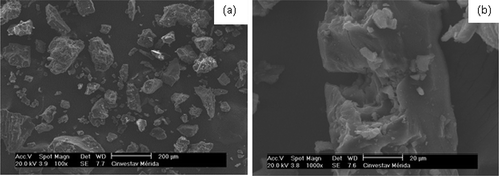
The mucilage sample showed a wide distribution of particle size as well as irregular morphology (). It is possible to observe that bigger particles are able to show aggregations with smaller particles, it could be due to electrostatic charge between the particles (). Moreover, the morphology of the mucilage sample varied from submicron particles to complex structures, and the small singles particles were not spherical. Kalegowda et al. (Citation2017) reported aggregates of irregular shape and rough surface in the mucilage extracted from Opuntia dillenii. Likewise, De Souza et al. (Citation2016) observed agglomerated crystalline particles in samples of mucilage extracted from Cereus peruvianus. These results agree with the reported in the present work. However, Otálara, Carriazo, Iturriga, Nazareno, and Osorio (Citation2015) obtained spherical and non-agglomerated particles with uniform appearance in mucilage from Opuntia ficus-indica cladodes and they attributed these characteristic to the drying process (air convection oven). García-Cruz, Rodríguez-Ramírez, Mendez, and Medina-Torres (Citation2013) pointed out that the agglomeration of the particles depends on the properties of the material and the drying conditions, whilst Boudries, Nadjemi, Bekhaneche-Bensemra, and Sindic (Citation2014) mentioned that the type of polysaccharides in the mucilage affects the shape of the particle, in this sense, the morphology and superficial characteristics of the particles obtained in the present work can be related with the extraction method, its chemical composition and drying process used.
3.4. Thermal analyses
3.4.1. Thermogravimetric analysis
According to the thermogravimetric curve of the dried extract (), the weight loss occurred in several stages, probably due to the complexity of the sample, which was a mucilage extract from O. spinulifera and it contained many different chemical compounds. The details of thermal behavior and thermal stability data according to the primary thermogram and derivative thermogram for the polysaccharide extract showed that heating at a rate of 10°C/min from 50°C to 600°C results in four mass loss events.
Figure 6. TGA thermogram curve of mucilage extracted from O. spinulifera. Insert: derivative thermogram curve.
Figura 6. Curva termográfica ATG de mucilago extraído de O. spinulifera. Recuadro: curva termográfica derivada
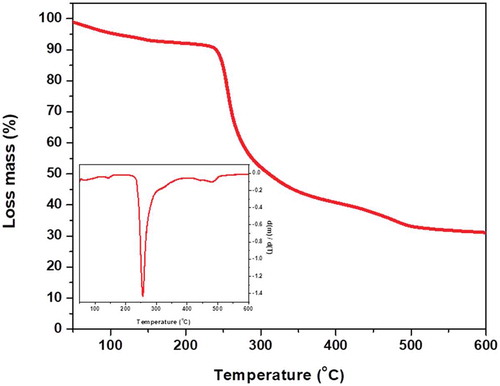
According with the at 100.50°C, it was observed a weight loss associated with loss of water. This result was consistent with the value reported of polysaccharides from several biopolymers (Bothara & Singh, Citation2012). Some polysaccharides release occluded humidity of the material at temperatures above 100°C, due to the loss of water bound to the saccharide molecules (Martin, Alves De Freitas, Lanzi, Labiak, & Sierakowski, Citation2017). Alpizar-Reyes et al. (Citation2017) pointed out the first mass change of 4.94% at temperatures higher than 75°C in tamarind seed mucilage and it was attributed to the loss of moisture corresponded to free water in mucilage particles, besides Martin et al. (Citation2017) mentioned a 9% of lost water at 90°C in the mucilage from leaves of Pereskia aculeta. This result was similar to the observed in the present work at 138°C where the sample showed a mass loss close to 5.05%. This transition can be variable due to hydrophilic nature of the functional groups of each polysaccharide in the mucilage.
The step with the highest mass loss (53.5%) was seen between the temperatures 246°C and 387ºC, agree with the findings of Silva Júnior, Vieira, Barbosa, and Pereira (Citation2012) on the dry extract of Symphytum officinale L. In addition, Munir, Shahidb, Anjumc, and Mudgil (Citation2016) reported a weight loss in crude Dalbergia sissoo gum at 238.85°C. This event can be attributed to the thermal degradation onset of the material and branch ruptures (ej. polysaccharides and pectins decomposition) due to the considerable mass loss (Rodríguez-Núñez et al., Citation2017), as it was described by the thermogram. The mean cumulative mass loss until the end of the test (at 600°C) was 59.7%. Residual mass at 600°C could mean that the content of inorganic compounds presents in the mucilage.
3.4.2. Differential scanning calorimetry
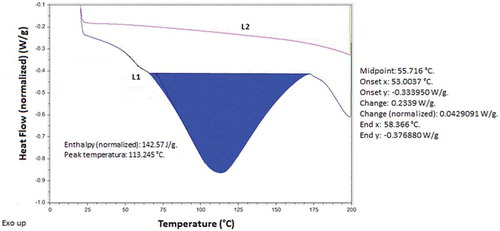
The DSC thermograms were a powerful physical tool to observe physical and chemical changes that occur in polysaccharides. showed the DSC with two heating curves of mucilage from nopal (Opuntia spinulifera), the thermal events registered were due to presence of polysaccharides in the mucilage. The first heating curve (L1) suggests that the glass transition temperature (Tg) was in the interval between 53 and 58.4°C (midpoint at 55.7°C), and it was attributed to the presence of compounds with lower molecular weights (short chains) that contain hydrophilic groups. The second thermal event was observed at 113.24°C (endothermic 142.57 J/g) and it was related with the melting point (Tm), indicating the semi-crystalline character of the mucilage. The Tg obtained was fairly different in comparison with the value reported by Bothara and Singh (Citation2012) for natural gums and polysaccharides, although it was near to 45°C resembling with the mentioned by León-Martínez, Méndez-Lagunas, and Rodríguez-Ramírez (Citation2010) for Opuntia ficus-indica specimens. However, these authors also observed that these biopolymers showed a thermal response (Tm) at temperatures around of 113.2°C, resulting similar to the Tm obtained in the present work. In this case, the thermogram started at 63°C and ended at 173°C, it was an evidence of water evaporation with a very broad endothermic peak on heating, typical of the organic compound materials Medina-Torres, Brito De-La Fuente, Gómez-Aldapa, Aragon-Piña, and Toro-Vazquez (Citation2006). The second heating process (L2) performed in the same sample did not showed these thermal events, because the thermal memory was erased, and the sample had not water to release. The structural and functional groups of the polysaccharides of the nopal mucilage have significant influence on the thermal behavior (transition and endothermic peak) which was observed in the thermogram.
4. Conclusions
The NMR and FTIR showed the presence of at least 10 residues of saccharides as galactose and uronic acid with α and β configurations in the mucilage powder of Opuntia spinulifera and pectins were also detected by these analyses. However, the presence of pectins could be due to the used extraction method. The SEM and XRD revealed particles with irregular shapes and forming particle aggregations, also these particles showed the presence of minerals into the polysaccharides matrix. The TGA thermogram indicated the presence of different chemical compounds by different stages of weight loss, and DSC thermogram in the first heating curve showed the release of occluded humidity and the presence of polysaccharides with low molecular weight in the samples of Opuntia spinulifera. The results found in the present work demonstrate the relationship between the chemical constituents of the mucilage from cladodes of Opuntia spinulifera and its thermal, structural, morphological behavior, which is important because of the great variety of applications that are attributed to this product.
Acknowledgments
SEM, FTIR, and XRD measurements were performed at LANNBIO Cinvestav Mérida, under support from projects FOMIX-Yucatán 2008-108160 and CONACYT LAB-2009-01 No. 123913. Technical help is acknowledging to MSC. D. Aguilar-Treviño and Mrs. A. Cristóbal for their assistance for XRD and SEM analyses, respectively. We also thank at Universidad de Guanajuato for the support with the project named Cátedra para Fortalecer la Excelencia Académica 2015 No. 012/2015.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alpizar-Reyes, E., Carrillo-Navas, H., Gallardo-Rivera, R., Varela-Guerrero, V., Alvarez-Ramirez, J., & Pérez-Alonso, C. (2017). Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. Journal of Food Engineering, 209, 68–75.
- AOAC. (2007). Official methods of analysis (18th ed.). Washington, DC: Association of Official Analytical Chemists.
- Bothara, S. B., & Singh, S. (2012). Thermal studies on natural polysaccharide. Asian Pacific Journal of Tropical Biomedicine, 2, S1031–S1035.
- Boudries, N., Nadjemi, B., Bekhaneche-Bensemra, N., & Sindic, M. (2014). Morphological and thermal properties of starches isolated from white and pigmented sorghum landraces grow in hyper arid regions. Journal of Agriculture Science Technology B, 4, 674–682.
- Bradford, M. M. (1976). Rapid and sensitive method for quantification of microgram quanties of protein utilizing the principle dye binding. Analytical Biochemistry, 72, 248–254.
- Bubb, W. A. (2003). NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts in Magnetic Resonance Part A, 19, 1–19.
- Contreras-Padilla, M., Pérez-Torrero, E., Hernández-Urbiola, M. I., Hernández-Quevedo, G., Del Real, A., Rivera-Muñoz, E. M., & Rodríguez-García, M. E. (2011). Evaluation of oxalates and calcium in nopal pads (Opuntia ficus-indica var. redonda) at different maturity stages. Journal of Food Composition Analysis, 24, 38–43.
- De Souza, F. M. T., De Almeida, A. C., Ambrosio, E., Brizola, S. L., De Souza Freitas, T. K. F., Domingos, M. D., … Carla, G. J. (2016). Extraction and use of Cereus peruvianus cactus mucilage in the treatment of textile effluents. Journal of the Taiwan Institute of Chemical Engineers, 67, 174–183.
- Espino-Díaz, M., Ornelas-Paz, J. J., Martínez-Téllez, M. A., Santillán, C., Barbosa-Cánovas, G. V., Zamudio-Flores, P. B., & Olivas, G. I. (2010). Development and characterization of edible films based of mucilage of Opuntia ficus-indica. Food Science, 75, E347–E352.
- Food and Agriculture Organization of United Nations (FAO). (2003). Food energy-methods of analysis and conversion factors. Food and Nutrition Paper, 77, 1–87.
- Gallegos-Vázquez, C., Barrientos-Priego, A. F., Reyes-Agüero, J. A., Núñez-Colín, C. A., & Mondragón-Jacobo, C. (2011). Clusters of commercial varieties of cactus pear and xoconostle using UPOV morphological traits. Journal of the Professional Association for Cactus Development, 13, 10–22.
- García-Cruz, E. E., Rodríguez-Ramírez, J., Mendez, L. L., & Medina-Torres, L. (2013). Rheological and physical properties of spray-dried mucilage obtained from Hylocereus undatus cladodes. Carbohydrate Polymers, 91, 394–402.
- Gebresamuel, N., & Gebre-Mariam, T. (2012). Comparative physico-phemical characterization of the mucilages of two cactus pears (opuntia Spp.) obtained from Mekelle, Northern Ethiopia. Journal of Biomaterials Nanobiotechnology, 03, 79–86.
- Granados, S. D., & Castañeda, P. A. D. (1991). El nopal: Historia, fisiología, genética e importancia frutícola. (11–80). México: Ed. Trillas.
- Habibi, Y., Heyraud, A., Mahrouz, M., & Vignon, M. R. (2004). Structural features of pectic polysaccharides from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydrate Research, 339, 1119–1127.
- Jaramillo-Flores, M., González-Cruz, L., Cornejo-Mazón, M., Dorantes-Álvarez, L., Gutiérrez-López, G. F., & Hernández-Sánchez, H. (2003). Effect of thermal treatment on the antioxidant activity and content of carotenoids and phenolic compounds of cactus pear cladodes (Opuntia ficus-indica). Food Science and Technology International, 9(4), 271–278.
- Jouini, M., Abdelhamid, A., Chaouch, M. A., Le Cerf, D., Bouraoui, A., Majdoub, H., & Ben, J. H. (2018). Physico-chemical characterization and pharmacological activities of polysaccharides from Opuntia microdasys var. Rufida Cladodes. International Journal of Biological Macromolecules, 107, 1330–1338.
- Kalegowda, P., Chauhan, A. S., & Nanjaraj, U. S. M. (2017). Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydrate Polymers, 157, 1057–1064.
- Kamnev, A. A., Colina, M., Rodriguez, J., Ptitchkina, N. M., & Ignatov, V. V. (1998). Comparative spectroscopic characterization of different pectins and their sources. Food Hydrocolloids, 12, 263–271.
- Kpodo, F. M., Agbenorhevi, J. K., Alba, K., Bingham, R. J., Oduro, I. N., Morris, G. A., & Kontogiorgos, V. (2017). Pectin isolation and characterization from six okra genotypes. Food Hydrocolloids, 72, 323–330.
- Lefsih, K., Delattre, C., Pierre, G., Michaud, P., Aminabhavi, T. M., Dahmoune, F., & Madani, K. (2016). Extraction, characterization and gelling behavior enhancement ofpectins from the cladodes of Opuntia ficus indica. International Journal of Biological Macromolecules, 82, 645–652.
- León-Martínez, F. M., Méndez-Lagunas, L. L., & Rodríguez-Ramírez, J. (2010). Spray drying of nopal mucilage (Opuntia ficus-indica): Effects on powder properties and characterization. Carbohidrate Polymers, 81, 864–870.
- Ma, F., Zhang, Y., Yao, Y., Wen, Y., Hu., W., Zhang, J., … Tikkanen-Kaukanen, C. (2017). Chemical components and emulsification properties of mucilage from Dioscorea opposita Thunb. Food Chemistry, 228, 315–322.
- Majdoub, H., Roudesli, S., Picton, L., Cerf, D. L., Muller, G., & Grisel, M. (2001). Prickly pear nopals pectin’s from Opuntia ficus indica physico-chemical study in dilute and semi-dilute solutions. Carbohydrate Polymers, 46, 69–79.
- Martin, A. A., Alves De Freitas, R., Lanzi, S. G., Labiak, E. P. H., & Sierakowski, M. R. (2017). Chemical structure and physical-chemical properties of mucilage from the leaves of Pereskia aculeata. Food Hidrocolloid, 70, 20–28.
- Matsuhiro, B., Lillo, L. E., Sáenz, C., Urzua, C. C., & Zárate, O. (2006). Chemical characterization of the mucilage from fruits of Opuntia ficus indica. Carbohydrate Polymers, 63(263–267). doi:10.1016/j.carbpol.2005.08.062
- Medina-Torres, L., Brito De-La Fuente, E., Gómez-Aldapa, C. A., Aragon-Piña, J. F., & Toro-Vazquez, J. F. (2006). Structural characteristics of gels formed by mixtures of carrageenan and mucilage gum from Opuntia ficus indica. Carbohydrate Polymers, 63, 299–309.
- Mohan, D., Singh, K. P., & Singh, V. K. (2005). Removal of hexavalent chromium from aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth. Industrial & Engineering Chemistry Research, 44, 1027–1042.
- Mondragón-Jacobo, C., Gallegos-Vázquez, C., & Reyes-Agüero, J. A. (2009). Cactus Pear genetic resources the cornerstone of the Mexican Cactus pear industry. Acta Horticulture, 811, 39–46.
- Munir, H., Shahidb, M., Anjumc, F., & Mudgil, D. (2016). Structural, thermal and rheological characterization of modified Dalbergia sissoo gum-A medicinal gum. International Journal of Biological Macromolecules, 84, 236–245.
- Nejatzadeh-Barandozi, F., & Enferadi, S. T. (2012). FT-IR study of the polysaccharides isolated from the skin juice, gel juice, and flower of aloe vera tissues affected by fertilizer treatment. Organic and Medicinal Chemistry Letters, 2, 33.
- Nharingo, T., & Moyo, M. (2016). Application of Opuntia ficus-indica in bioremediation of wastewaters. A critical review. Journal of Environmental Management, 166, 55–72.
- Otálara, M. C., Carriazo, J. G., Iturriga, L., Nazareno, M. A., & Osorio, C. (2015). Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chemistry, 187, 174–181.
- Pereira, P. H. F., Oliveira, T. Í. S., Rosa, M. F., Cavalcante, F. L., Moates, G. K., Wellner, N., … Azeredo, H. M. C. (2016). Pectin extraction from pomegranate peels with citric acid. International Journal of Biological Macromolecules, 88, 373–379.
- Petera, B., Delattre, C., Pierre, G., Wadouachi, A., Elboutachfaiti, R., Engel, E., … Fenoradoso, T. A. (2015). Characterization of arabinogalactan-rich mucilage from Cereustriangularis cladodes. Carbohydrate Polymers, 127(372–380). doi:10.1016/j.carbpol.2015.04.001
- Rodríguez-Núñez, J. R., Domínguez-López, A., Domínguez-López, C., Quintana Owen, P., López-Cervantes, J., Sánchez-Machado, D. I., … Madera-Santana, T. J. (2017). Evaluation of physicochemical and antifungal properties of polylactic acid-thermoplastic starch-chitosan biocomposites. Polymer-Plastics Technology and Engineering, 56, 44–54.
- Rodríguez-Núñez, J. R., Madera-Santana, T. J., Sánchez-Machado, D. I., López-Cervantes, J., & Soto Valdez, H. (2014). Chitosan/hydrophilic plasticizer-based films: Preparation, physicochemical and antimicrobial properties. Journal of Polymers and the Environmental, 22, 41–51.
- Saenz, C., & Cuevas, G. R. (2013). Industrial production of non-food products. In Saenz, C. (Ed.), Agro-industrial utilization of cactus pear (pp. 89–102). Rome, Italy: FAO-CACTUSNET.
- Sáenz, C., Sepúlveda, E., & Matsuhiro, B. (2004). Opuntia spp. mucilage’s: A functional component with industrial perspectives. Journal of Arid Environments, 57, 275–290.
- Sawut, A., Yimit, M., Sun, W., & Nurulla, I. (2014). Photopolymerisation and characterization of maleylatedcellulose-g-poly(acrylic acid) superabsorbent polymer. Carbohydrate Polymers, 101, 231–239.
- Sepúlveda, E., Sáenz, C., Aliaga, E., & Aceituno, E. (2007). Extraction and characterization of mucilage in opuntia spp. Journal of Arid Environment, 68, 534–545.
- Servicio de Información y Estadística Agroalimentaria y Pesquera (SIAP). (2015). Cierre de la Producción Agrícola por Cultivo 2014. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, México. Retrieved April 7, 2015, from http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-cultivo/
- Silva Júnior, J. O. C., Vieira, J. L. F., Barbosa, W. L. R., & Pereira, N. L. (2012). Caracterização físico-química do extrato fluido e seco por nebulização de Symphytum officinale L. Revista Brasileira De Farmacognosia, 16, 671–677.
- Soberon, J., Golubov, J., & Sarukhán, J. (2001). The importance of opuntia in Mexico and routes of invasion and impact of Cactoblastis cactorum (Lepeidoptera: Pyralidae). The Florida Entomologist, 84, 486–492.
- Synytsya, A., Čopíková, J., Matějka., P., & Machovic, V. (2003). Fourier transform Raman and infrared spectroscopy of pectins. Carbohydrate Polymers, 54, 97–106.
- Trachtenberg, S., & Mayer, A. M. (1981). Composition and properties of Opuntia ficus indica mucilage. Phytochemistry, 20, 2665–2668.
- Tucker, K. L., Hannan, M. T., Chen, H., Adrienne, L. P., Cupples, W. F., & Kiel, D. P. (1999). Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. The American Journal of Clinical Nutrition, 69, 727–736.
- Vargas-Rodríguez, L., Arroyo-Figueroa, G., Herrera-Méndez, C. H., Pérez-Nieto, A., García-Vieyra, M. I., & Rodríguez-Núñez, J. R. (2016). Propiedades físicas del mucílago de nopal. Acta Universitaria, 26, 8–11.
- Villarreal, F., Rojas, P., Arellano, V., & Moreno, J. (1963). Estudio químico sobre seis especies de nopales (opuntia spp.). Ciencia Mexicana, 22, 59–65.
- Zegbe, J. A., Mena-Covarrubias, J., & Dominguez-Canales, V. S. I. (2015). Cactus mucilage as a coating film to enhance shelf life of unprocessed guavas (Psidium guajava L.). Acta Horticulture, 1067, 423–427.