 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study investigated the effects of cooking treatments (boiling and steaming) and season of the year (spring and fall) on vitamin C content, total phenolics content (TPC), total flavonoids content (TFC), antioxidant activity and scavenging of nitric oxide (NO•), and superoxide radical (O2–). After cooking, a significant decrease in vitamin C was observed (50 and 48.8% for boiling and 21 and 27.9% for steaming in spring and fall, respectively). TPC and TFC increased during steaming from 145–1480 mg GAE/kg and 348.6–797.2 mg QE/kg, respectively. DPPH values ranged from 2806–3801 μmol TE/kg and was affected by season whereas ORAC ranged from 1231.3–36,167.2 μmol TE/kg and was affected by cooking. The ability to scavenge NO• and O2–, were not affected by neither cooking nor season. However, the inhibition of O2– was higher for quintonil steamed in spring.
RESUMEN
Este estudio evaluó los efectos de la cocción (hervido y al vapor) y época del año (primavera y otoño) sobre vitamina C, compuestos fenólicos totales (TPC), flavonoides totales (TFC), actividad antioxidante e inhibición de óxido nítrico (NO•) y superóxido (O2–). La cocción afectó significativamente el contenido de vitamina C observándose reducciones de 50 y 48.8% en el hervido y para vapor 21 y 27.9%. Los valores de TPC y TFC incrementaron durante la cocción al vapor de 145 a 1480 mg GAE/kg y 348.6 a 797.2 mg QE/kg, respectivamente. Los valores de DPPH variaron de 2806 a 3801 μmol TE/kg mientras que los de ORAC de 1231.3 a 36,167.2 μmol TE/kg y se vieron afectados por la época del año y cocción, respectivamente. La capacidad de inhibir NO• y O2– no se afectaron por la cocción ni por la época del año, sin embargo, la inhibición de O2– fue mayor en el quintonil sometido a vapor colectado en primavera.
Introduction
Quintonil (Amaranthus hybridus) belongs to the quelites family, which is a wild and arable group of plants with edible foliage. Previous reports found in the technical literature have pointed out the remarkable nutritional value of quintonil leaves by the presence of polysaccharides, vitamins, minerals and amino acids (Akubugwo, Obasi, Chinyere, & Ugbogu, Citation2007; Mepba, Eboh, & Banigo, Citation2007). Quintonil is able to contribute with ca. 30% of the daily fibre requirements for humans. It also contributes with almost 25% of the recommended daily protein intake when 100 g of dried leaves are consumed (FND, Citation2002). Typically, quintonil is consumed crude (as salad) or after cooking (frying, boiling or steaming). The latter has been mentioned to affect the content of phytochemicals such as vitamin C (Olayinka et al., Citation2012; Lin et al., Citation2016), phenolic compounds (Xie et al., Citation2015) and antioxidant activity (Routray & Orsat, Citation2014; Jiménez-Aguilar & Grusak, Citation2017).
Consumption of a diet of vegetables has been underlined for its contribution towards decreasing the risks of several diseases such as heart disease, stroke, diabetes and different types of cancer (Akdaş & Bakkalbaşı, Citation2017; Tomas et al., Citation2017). Such benefits are related to the content of polyphenols, flavonoids, carotenoids and vitamins. Among the aforementioned phytochemicals, polyphenols are largely recognized as anti-inflammatory and antioxidant agents (Leyva-López, Nair, Bang, Cisneros-Zevallos, & Heredia, Citation2016). The concentrations of phenolic and other secondary metabolites in fruits and vegetables are influenced by many factors, including soil, irrigation and climatic conditions, as well as the time of the year (Routray & Orsat, Citation2014).
For the previous aspect, quintonil offers a natural source of micronutrients and phytochemicals; however, it is considered a neglected, underutilized crop. In this study we evaluated the effects of cooking treatments (boiling and steaming), and season of the year (spring and fall) on vitamin C concentration, total phenolics content (TPC), total flavonoids content (TFC) and the antioxidant activity of the extracts prepared from quintonil leaves in order to highlight its health benefits and promote its consumption.
Materials and methods
Plant material
Quintonil was identified and authenticated at the National Herbarium of Mexico, by Dra. Hilda Flores Olvera and was registered with the number 1,434,640. It was harvested in San Lorenzo Tlacotepec, Atlacomulco, State of México (19°49´10.84¨N and 99°55´2.19¨W) during spring and fall of 2015–2016. The quintoniles were planted and harvested according to the agronomic practices of the place. Leaves, as the edible portion, were employed to carry out this study and were harvested at 5 weeks after planting from three plants (15 g of fresh leaves were collected per plant).
Chemical reagents
The reagents Folin–Ciocalteu, DPPH (1,1-diphenyl-2-picrylhydrazyl), AAPH (2,2-azobis(2-amidinopropane) dihydrochloride) and Trolox (6-hydroxy-2,3,7,8-tetramethylchroman-2-carboxylic acid), as well as gallic acid, ferulic acid, sodium nitroprusside, PMS (Phenazine methosulfate), NADH (Nicotinamide adenine dinucleotide), NBT (Nitrotetrazolium blue), TCA (Trichloroacetic acid) and L ascorbic acid were all purchased from Sigma–Aldrich (Mexico City). Solvents used in methods were of analytical grade.
Cooking treatments
Boiling and steaming were selected to perform cooking and were conducted according to our previous study (López-García et al., Citation2017). Briefly, boiling was performed with water at 100°C and atmospheric pressure during 10 min. In the case of steaming, quintonil leaves were cooked in a stainless-steel steamer by direct incidence of saturated steam for 10 min. After the treatments, the samples were placed in a water bath at 4°C during 30 s to stop cooking. Crude and cooked samples were cut in pieces of 0.5 cm × 0.5 cm.
Preparation of the extracts
Aqueous extracts (AE) were prepared for crude and cooked samples according to our previous report (López-Martínez & García-Galindo, Citation2009), with slight modifications. For aqueous extraction, 5 g of crude and cooked quintonil leaves were placed in tubes (40 mL). 20 mL of distilled water were added and then the tubes were placed in an orbital shaker (Lab-Line Orbit Environ, Model 3527, Melrose Plaza, IL) at 200 rpm during 4 h at room temperature in the absence of light. After shaking, all the extracts were centrifuged at 11 000 rpm during 15 min. The supernatant was recovered and stored at −20°C and kept in the dark until used.
Vitamin C content
Vitamin C was extracted and quantified according to the method adapted by Jiménez-Aguilar and Grusak (Citation2015). Vitamin C extraction was performed on 40 mg of sample with 6% TCA, followed by centrifugation (13,000 g and 4°C) for 5 min. The supernatant (50 mL) was recovered and mixed with 75 mM potassium phosphate buffer (25 mL). Total vitamin C was evaluated by mixing 75 mL of buffered sample and 25 mL 10 mM DL-dithiothreitol. After 10 min, 25 mL of 0.5% N-ethylmaleimide were added. Then, 375 mL of a mixture consisting of 3% FeCl3 (50 mL), 4% α-α’ bipyridyl (100 mL), 10% TCA (125 mL) and 43% H3PO4 (100 mL) was added. The samples were incubated for 1 h at 37°C, and then 200 mL were transferred to a 96-well Costar® flat-bottom microplate. The absorbance was measured at 525 nm in a Synergy HT Absorbance Microplate Reader (BioTek Co., USA). Reagent-grade L-Ascorbic acid was used as standard. Results are expressed in mg L-ascorbic acid per kg of sample (mg AA/kg).
Analysis of the TPC
TPC of the extracts was analysed according to the method described by Singleton, Orthofer, and Lamuela-Raventos (Citation1999) with some modifications. Briefly, 15 µL of the extract were mixed with 240 µL of distilled water and 15 µL of 2 N Folin-Ciocalteu reagent in a 96-well Costar® flat-bottom microplate. After incubation for 3 min, 30 µL of 4 N Na2CO3 were added to neutralize the reaction mixture and the plates were then allowed to stand in the dark for 2 h. The absorbance was measured at 725 nm using a Synergy HT Absorbance Microplate Reader (BioTek Co., USA). A standard calibration curve was prepared using gallic acid, and the amount of total phenolic compounds in each extract was calculated and expressed as mg of Gallic Acid Equivalent per kg of sample (mg GAE/k g).
Analysis of TFC
TFC was determined by a colorimetric method reported by Ghasemi, Ghasemi, and Ebrahimzadeh (Citation2009). 200 μL of samples were used, and 112 μL of deionized water was added, followed by 60 μL of methanol and 40 μL of 10% AlCl3; finally, 40 μL of 1 M C2H3KO2 was added and incubated for 30 min. Absorbance was measured at 415 nm using a Synergy HT Absorbance Microplate Reader (BioTek Co., USA). A quercetin curve (from 0 to 0.4 mg/mL) was used to calculate the concentration of TFC and the results were expressed as mg of Quercetin Equivalents per kg of sample (mg QE/kg).
Antioxidant capacity assays
Radical scavenging of DPPH (2,2-diphenyl-1-picryl-hydrazyl)
The free radical scavenging activity of the extracts was measured using the stable DPPH· radical as described by Huang, Ou, and Prior (Citation2005) with some modifications. Briefly, a 20-µL aliquot of each sample was transferred to a 96-well Costar® flat bottom microplate and allowed to react with 200 µM DPPH solution (280 µL). The mixture was incubated in the dark for 30 min at room temperature, and the decrease in absorbance at 540 nm was determined with a microplate reader. The Trolox curve from 0.05–1 mmol TE/g was employed to calculate the results, which are expressed as μmol of Trolox Equivalents per kg (μmol TE/kg).
Oxygen radical absorbance capacity (ORAC)
The ORAC assay was conducted using fluorescein as the fluorescent probe, AAPH (2,2-azobis (2-amidino-propane) dihydrochloride) was used as a peroxyl radical generator, and Trolox was used as a standard, as previously described by Huang, Ou, Hampsch-Woodill, Flanagan, and Prior (Citation2002). The reaction mixture contained 25 μL of extract, 25 μL of 75 mM phosphate buffer (pH 7.4), 75 μL of 0.8 M AAPH and 200 μL of 0.106-μM fluorescein. The 75 mM phosphate buffer was used as a blank. The extracts, phosphate buffer and fluorescein were pre-incubated at 37°C for 15 min. AAPH was added to start the reaction, and fluorescence was measured every 70 s during 70 min with a 485 nm excitation filter and a 580 nm emission filter using a Synergy HT spectrophotometer. The values were calculated using a regression equation describing the relationship between the Trolox concentration and the net area under the fluorescein decay curve. The Trolox curve from 6.25–125 (μmol TE/g) was used to estimate the results, which are expressed as μmol of Trolox Equivalent per kg (µmol TE/kg).
Inhibitory effect of nitric oxide (NO•) formation
The determination was performed using sodium nitroprusside and the Griess reagent (Giraldo, Hernández, Angulo, & Fuertes, Citation2003). The absorbance was measured at 546 nm. A control sample containing no extract and a Blank sample contained all reagents except Griess reagent to correct for background absorbance conferred by the quintonil leaves extract or reference. Ferulic acid was used as reference. The percentage of inhibition of NO• formation was calculated according to the following equation:
where Acontrol, Asample and Ablank are the absorbances of the control, sample and blank, respectively.
Inhibitory effect of superoxide radical formation (O2–)
It was determined according to a standardized methodology (Rojano, Zapata, & Cortes, Citation2012). O2– was generated by means of a PMS-NADH-NBT system in a 75 mM phosphate buffer pH 7.4. Blanks were prepared for each extract and contained the entire mixture except NADH and the control contained no extracts. Ferulic acid was used as reference. The percentage of inhibition of O2– formation was calculated using the latter equation.
Statistical analysis
All the trials were performed by triplicate (r), using a completely randomized design to evaluate the effects of thermal processing and season of the year on Vitamin C, TPC, TFC, DPPH, ORAC, and the inhibitory effect of NO• and O2–. The treatments (t) were crude, boiled and steamed quintonil leaves and the seasons (s) were spring and fall. Data were analysed by GLM – ANOVA using SAS 9.0; when needed mean treatments were compared using Tukey’s multiple range procedure. A p < 0.05 was regarded as a significantly different.
Results and discussion
Mexico owns a very vast biodiversity. However, ancient crops, quintonil included, are unexploited and undervalued because they are disregarded and underutilized. In consequence, such species are not fully exploited and the research conducted has been devoted on cultivation, adaptation and some aspects of its nutritional value. In an effort to promote its consumption and to improve access to more nutritious food, the present study evaluated the effect of traditional cooking on bioactive compounds from quintonil harvested on spring and fall.
Vitamin C content
From the data we can observe that crude quintonil contained 902 and 361 mg/kg sample in spring and fall, respectively, which are higher compared to other vegetables such as cabbage (322 mg/kg), lettuce (153 mg/kg) and cauliflower (63.5 mg/kg), but lower than spinach (1180 mg/kg) and broccoli (1174 mg/kg) (Singh, Upadhyay, Prasad, Bahadur, & Rai, Citation2007; Vallejo, Tomás-Barberán, & Garcia-Viguera, Citation2002).
Once cooking treatments were applied to quintonil leaves, significant changes in content of vitamin C were noted (). Quintoniles submitted to cooking underwent a significant reduction (p < 0.05; ), and the largest degree of degradation was observed for boiled quintoniles harvested on fall (79%). Mathooko and Imungi (Citation1994) studied vitamin C contents in fresh green leaves of Amaranthus hybridus, and they found that vitamin C losses between 57 and 83%, depending on boiling time (5 and 20 min, respectively). Many studies indicated that the loss of vitamin C content during cooking could be attributed to the fact that vitamin C is unstable at high temperatures. Because heat is known to enhance the oxidation process of ascorbic acid, thermal processing results in loss of vitamin C content in fruits and vegetables, loss of vitamin C occurs primarily by chemical degradation that involves oxidation of ascorbic acid to dehydroascorbic acid, followed by hydrolysis to 2,3-diketogulonic acid and further polymerization to form other nutritionally inactive products (Gregory, Citation1996). Thus, temperature could have inactivated most of the vitamin C in the vegetables by oxidative degradation, while water could have also dissolved vitamin C during cooking treatments (Oboh, Citation2005; Somsub, Kongkachuichai, Sungpuag, & Charoensiri, Citation2008). The loss of vitamin C during cooking of vegetables also depends on the method of cooking, the difference in Vitamin C content between boiling and steaming could be related with the volume of water used. In this study, quintonil leaves were immersed in hot water during boiling, which resulted in vitamin C leach into the cooking water, while during steaming Vitamin C loss was likely produced by steam condensing on the surface of quintonil leaves.
Table 1. Effect of cooking treatments and harvesting season on vitamin C concentration, total phenolics content (TPC) and total flavonoids content (TFC) for quintonil leaves (Amaranthus hybridus). The results are expressed in fresh weight as the main value ± standard deviation.
Tabla 1. Efecto del tratamiento térmico y época de cosecha sobre la concentración de vitamina C, contenido de fenólicos totales (TPC) y contenido de flavonoides totales (TFC) en hojas de quintonil (Amaranthus hybridus). Los resultados están expresados como la media del peso fresco ± desviación estándar.
Table 2. Statistical analysis to evaluate the effects of thermal processing and season of the year on vitamin C concentration, total phenolics content (TPC) and total flavonoids content (TFC), DPPH and ORAC, NO. y O2–.
Tabla 2. Análisis estadístico para evaluar los efectos del tratamiento térmico y época de cosecha sobre la concentración de vitamina C, compuestos fenólicos totales (TPC) and flavonoides totales (TFC), DPPH, ORAC, NO. and O2–.
On the other hand, climate conditions during the harvesting periods may have also influenced vitamin C contents. A reduction was observed in crude quintonil harvested in fall (361 mg/kg) compared to those harvested in spring (902 mg/kg) (). The amount and intensity of light during the growing season exerts a remarkable influence on vitamin C formation, which is synthesized from sugars supplied through photosynthesis in plants; thus, plants exposed to maximum sunlight contain higher amounts of vitamin C than those receiving less sunlight, In general, the lower light intensity during growth, the lower vitamin C content in plant tissues (Harris, Citation1975). Lee and Kader (Citation2000), who studied the variation in vitamin C content at different times of the year on horticultural crops, refer lower vitamin C contents during fall; they suggest that high illumination intensity may be the main reason promoting the accumulation of vitamin C in leaves. The behaviour of vitamin C present in quintonil was similar to that previously reported by Lee and Kader (Citation2000).
In both cooking treatments (steaming and boiling) at the same season of the year there was a difference in vitamin C content (). This can be explained by the different initial contents of vitamin C at each time of year (902 and 361 mg/kg for spring and fall, respectively) and by the principles of cooking treatments applied, due to boiling requires quintonil leaves to be immersed in hot water, whereas steaming only requires condensing steam.
TPC and TFC
depicts both TPC and TFC values for quintonil leaves determined at different seasons of year and under different cooking treatments. For crude samples, TPC ranged from 145 to 583 mg GAE/kg (spring and fall, respectively) and TFC varied from 789 to 348 mg QE/kg (spring and fall, respectively). These results show that season of the year clearly influenced the content of both phenolic and flavonoid compounds. On the same hand, those contents are affected by stress conditions: temperature variations during day and night, irregular rainfall, drought and length of daytime. The latter could explain the elevated phenolic compounds content during spring compared to fall, which serves as protection and defence to the plant (Oke, Citation1983). In a previous study with quintonil, López-García et al. (Citation2017) also reported that after cooking treatments the content of fatty acids was higher in fall, which represents a defence mechanism of the plant to strengthen the stability of the cell membrane. On the other hand, Wu et al. (Citation2017) observed that leaves of Vaccinium ashei from spring exhibited higher TPC and TFC values (2221 mg GAE/kg and 391 mg RE/kg, respectively) in comparison to those on winter (1100 mg GAE/kg and 195 mg RE/kg, respectively). They suggest that strong illumination intensity and low humidity of soil moisture in spring may be the main reasons promoting the accumulation of phenolic compounds in the leaves. It is difficult to determine which environmental factor is mainly responsible for the variations in TPC and TFC observed in the aforementioned studies. As a result, further studies are required in order to elucidate the induction of biosynthesis of total phenols by environmental parameters.
With respect to cooking treatments (steaming and boiling), they exhibited significant effects on both TPC and TFC (). Steaming had a significant effect compare to crude and boiling treatments. As it can be seen in , steamed quintonil (1480 and 1706 mg GAE/kg) showed an increase over crude quintonil (145 and 583 mg GAE/kg), regardless the season. When quintonil was boiled, samples presented a significant decrease in comparison to crude quintonil (135 and 292 mg GAE/kg), regardless the season of the year. TFC of crude samples were 789 and 348 mg CE/kg in spring and fall, respectively; after boiling, TFC value for sample harvested in fall was reduced to the half. However, a significant raise (p < 0.05) was observed when samples where submitted to steaming: 6.2 and 2.2-fold in spring and fall, respectively. Losses or gains of phenolic compounds are the consequence of cooking or processing methods, length of exposure to a processing technique and the sensitivity of the compounds to modification or degradation (Bernaert, De Loose, Van Bockstaele, & van Droogenbroeck, Citation2013; Lee et al., Citation2008); boiling seemed to be somewhat detrimental on the contents of phenolic compounds ().
Degradation of phenolic compounds depends, besides the cooking treatment, on the chemical structure of the compound present in the fruit or vegetable. The loss in phenolic compounds during quintonil cooking is not only related to the chemical breakdown of flavonoid conjugates or by formation of new compounds, but also by leaching of phenolic compounds into the cooking water (Xu & Chang, Citation2008; Bernaert et al., Citation2013).
Cooking treatments may release more bound phenolic acids from the breakdown of cellular constituents; this disruption also releases the oxidative enzymes that can destroy the phenolic compounds in fruits and vegetables (Chism & Haard, Citation1996). Steaming seems to deactivate those enzymes in order to avoid the loss of phenolic acids.
From our data, a maximum TPC value of 1706 mg GAE/kg was estimated for steamed quintonil harvested in fall. Studies performed previously on Amaranthus hybridus (Gutiérrez Avella, Ortíz García, & Cisneros Mendoza, Citation2008; Muñiz-Márquez et al., Citation2014; Jiménez-Aguilar & Grusak, Citation2017) reported 4943 mg GAE/kg, 508 mg GAE/kg and 370 mg GAE/kg, which are larger TPC values than the values reported in this study. However, TPC level measured for quintonil was higher than the TPC reported for spinach (1200 mg GAE/kg), the most consumed quelite in Mexico (Isabelle et al., Citation2010). The difference observed among the studies could be attributed to the plant species and cultivar, different isolation procedures, applied solvents mixtures and agrotechnological practices and growing location.
Antioxidant activity
Antioxidant activity determination is a reaction mechanism dependent, the specificity and sensitivity of a single method do not lead to the complete examination of all phytochemicals in the extract; a combination of several tests could provide a more reliable assessment of the antioxidant activity profiles of the samples. In this study the inhibition of DPPH, ORAC and the ability to scavenge NO• and O2– were selected for that purpose.
The ability of quintonil leaves extract to act as a free radical scavenger or electron donor was determinate by the DPPH radical scavenging activity assay. Total DPPH radical scavenging of crude quintonil were 2839 and 3776 µmol TE/kg in spring and fall (), respectively, which may be related to the content of phenolic compounds present in quintonil leaves. Those values decreased 2 and 7% during boiling and increased 7 and 1.3% with steaming in spring and fall, respectively. However, the differences were found to be nonsignificant. There were significant differences in the values of inhibition of the DPPH radical in both cooking treatments () performed at the same season of the year, which may be related to the content of phenolic compounds present in quintonil leaves () and that the nature of cooking treatments that were previously discussed. In this study the values of antioxidant activity were not significantly influenced by different cooking treatments, but it was affected by the season of the year ().
Table 3. Effects of cooking treatments and harvesting season on DPPH and ORAC assays for quintonil leaves (Amaranthus hybridus). The results are expressed in fresh weight as the main value ± standard deviation.
Tabla 3. Efectos del tratamiento térmico y época de cosecha sobre los ensayos DPPH y ORAC en hojas de quintonil (Amaranthus hybridus). Los resultados están expresados como la media del peso fresco ± desviación estándar.
The capacity of quintonil leaves extract to transfer hydrogen atoms (protons) was quantified by the ORAC method. Cooking treatments increased ORAC values significantly. For instance, the value measured for crude quintonil in spring was 1231.3 µmol TE/kg, whereas for the boiled and steamed product were 3186.2 and 36,167.2 µmol TE/kg, respectively. A similar behaviour was also observed on fall: 3161.0, 6190.4 and 32,228.8 µmol TE/kg for crude, boiled and steamed samples, respectively (). The increase in ORAC value agrees with the increase in the total phenol contents, and this could be attributed to the presence of more proton donors and thermal treatments breaking the glucosides of flavonoids to form aglycones, which possess higher antioxidant properties (Rohn, Buchner, Driemel, Rauser & Kroh, Citation2007).
Although variations in ORAC values with respect to the harvest season were observed, they were not significant (); in general, antioxidant activity was lowest in spring than in fall, which may be related to the content of phenolic compounds present in quintonil leaves ().
Crude quintonil samples displayed a range of NO• inhibition from 66.7 to 79.2%. By comparison, NO• scavenging activities of the reference compound ferulic acid in the assay was, 93%, whilst for boiled quintonil ranged from 66.5–78.9%, and the steamed product ranged from 78.3 to 83.3%. Scavenging activity did not show definite seasonal trends or a clear effect of cooking (). The scavenging activities of each extract against NO• are attributable to phenolic compounds, which are recognized as free radical scavenger, effectives in suppressing NO• and peroxinitrite (ONOO) (Lopez-Martinez, Parkin, & Garcia, Citation2012). NO• is a molecule with a free radical character, and it was reported that are directly scavenged by phenolic compounds attributed to their hydroxyl groups.
Figure 1. Effect of cooking treatments and harvesting season on inhibition of nitric oxide and superoxide for quintonil (Amaranthus hybridus). The results are expressed in FW as the main value ± standard deviation. The different letters in fresh and thermal treatment indicate the significant difference of the heat treatments.
Figura 1. Efecto del tratamiento térmico y época de cosecha sobre la inhibición de los radicales óxido nítrico y super óxido en hojas de quintonil (Amaranthus hybridus). Los resultados están expresados como la media del peso fresco ± desviación estándar.
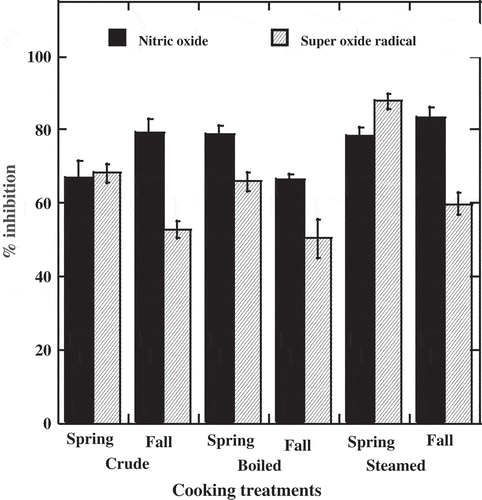
O2– radical scavenging capacity of extracts from fresh quintonil is indicated in . The activity of the reference ferulic acids was 63%. A significant increase in the percentage of radical inhibition can be noted, mainly in those that were steamed and harvested in spring (87.7%), compared to the crude and boiled samples (68.2 and 65.8%, respectively). Differences in O2– inhibition percentages were found among treatments and season of the year, O2–; however, they do not show statically differences (p < 0.05 (). Percent inhibition of boiled extracts ranged from 65.8 and 87.7%, and steamed from 50.4 and 59.8% in spring and fall, respectively. Extracts from quintonil showed strong O2– anion radical scavenging potential in spring; however, it decreased in the fall. Similar results were reported by Xie et al. (Citation2015) who examined the antioxidant activity of Dryopteris erythrosora at different seasons and mentioned that leaves extracts had strong O2– anion radical scavenging potential in spring; it was lower in summer and fall and it was further reduced in winter. It is concluded that phenolic compounds scavenged O2– caused by their redox activity, which allows them to act as reducing agents, hydrogen donors and free radical scavengers (Rice-Evans, Miller, Bolwell, Bramley, & Pridham, Citation1995). However, differences on the scavenging abilities are related to the concentration and specific composition on phenolic compounds. Hu et al. (Citation1995) illustrated that not only the number of the hydroxyl groups in ring B and the presence of a free hydroxyl group in position C3 are obvious for a reasonable activity, but also the presence of a saturated C2-C3 bond and the absence of a C4 carbonyl group remarkably increased the activity for scavenging NO• and O2–, and in the case of O2– the most potent scavenger should have ortho-dihydroxyl groups on B-ring of flavonoids (Zhang & Lu, Citation2006). In addition to other non-phenolic compounds extracted by water such as vitamin C can also scavenge NO• and O2– (Wang & Jiao, Citation2000). Flavonoids are 10 to 1000 times more efficient at NO• and O2– scavenging than glutathione, tocopherols, tocotrienols and vitamin C (Van Acker, Tromp, Haenen, Vandervijgh, & Bast, Citation1995).
As described above, cooking treatments applied on quintonil leaves exhibited positive effects on phytochemicals content and antioxidant activity. In some reports, it has been indicated that phenolic content and antioxidant activity increased after canning (Sablani et al., Citation2010); TPC was reduced after processing and heating, but unpredictable changes in antioxidant activity were noted during jam making (Kim & Padilla-Zakour, Citation2004). In contrast, other studies have indicated that TPC was reduced after dehydration, while antioxidant activity in dried plums increased when compared to that of fresh ones (Piga, Del Caro, & Corda, Citation2003), or that TPC was not altered when purple wheat bran was baked at 177°C for 20 min (Li, Pickard, & Beta, Citation2007). It was also reported that the total phenolics and total antioxidant activity of sweet corn increased by 54 and 44%, respectively, after thermal processing at 100–121°C for 10–50 min (Dewanto, Wu, & Liu, Citation2002). In other studies, antioxidant activity in processed tomatoes (Re, Bramley, & Rice-Evans, Citation2002) and coffee (Nicoli, Anese, Parpinel, Franceschi, & Lerici, Citation1997) was retained or found higher than their fresh equivalents
These findings allowed us to propose that antioxidant activity depends on phenolic compounds found on the sample, type and nature of the compounds present, polarity of the components and the radical tested for the inhibition assay.
Conclusions
In order to promote the consumption and revalorize a neglected and underutilized specie with preventive effects against malnutrition and some chronic diseases such as quintonil, we conducted this study. According to our findings, both cooking treatments as well as the time of the year exhibited significant effects on phenolic compounds and on vitamin C. In the case of flavonoids, only cooking treatments had a significant effect. With respect to treatments, steaming had a significant effect for both total phenolic compounds and total flavonoids, and for vitamin C both thermal treatments reduced its content. Regarding the season of the year, quintonil harvested in fall exhibited a higher content of phenolic compounds, whereas quintonil harvested in spring showed a higher content of flavonoids. For antioxidant activity, DPPH assay was affected by season of the year, being quintonil harvested in fall, which exhibited a higher activity. For ORAC assay, treatments had a significant effect: steaming > boiling > crude. The inhibition of both NO and O2– were not affected by neither treatments nor seasons; however, the inhibition of O2– was higher for crude quintonil (crude > steaming > boiling).
Acknowledgments
Author López-García gratefully acknowledges financial support for her MSc studies (scholarship no. 680332/581488) from the National Council of Science and Technology (CONACYT) of Mexico.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akdaş, Z. Z., & Bakkalbaşı, E. (2017). Influence of different cooking methods on color, bioactive compounds, and antioxidant activity of kale. International Journal of Food Properties, 20, 877–887.
- Akubugwo, I. E., Obasi, N. A., Chinyere, G. C., & Ugbogu, A. E. (2007). Nutritional and chemical value of Amaranthus hybridus L. leaves from Afikpo, Nigeria. African Journal of Biotechnology, 6, 2833.
- Bernaert, N., De Loose, M., Van Bockstaele, E., & van Droogenbroeck, B. (2014). Antioxidant changes during domestic food processing of the white shaft and green leaves of leek (Allium ampeloprasum var. porrum). Journal of the Science of Food and Agriculture, 94, 1168–1174.
- Chism, G. W., & Haard, N. F. (1996). Characteristics of edible plant tissues. In O. R. Fennema (Ed.), Food Chemistry (3rd ed., pp. 943−1011). New York: Dekker.
- Dewanto, V., Wu, X. Z., & Liu, R. H. (2002). Processed sweet corn has higher antioxidant activity. Journal of Agricultural and Food Chemistry, 50, 4959–4964.
- FND. (2002). Dietary reference intake for energy, carbohydrate, fibre, fat, fatty acids, cholesterol, protein and amino acid (Micronutrients). FND. USA: National Academy of Sciences.
- Ghasemi, K., Ghasemi, Y., & Ebrahimzadeh, M. A. (2009). Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pakistan Journal of Pharmaceutical Sciences, 22, 277–281.
- Giraldo, B. L., Hernández, P. M., Angulo, H. P., & Fuertes, R. C. (2003). Actividad antinitrosativa y antiinflamatoria de los flavonoides de las hojas de Uncaria tomentosa Willd. D.C. (uña de gato). Revista De La Sociedad Química Del Perú, 69, 229–242.
- Gregory, J. F., III. (1996). Vitamins in food chemistry. In O. R. Fennema (ed.), Food chemistry (3rd ed., pp. 531−616). New York: Dekker.
- Gutiérrez Avella, D. M., Ortíz García, C. A., & Cisneros Mendoza, A. (2008). Medición de fenoles y actividad antioxidante en malezas usadas para alimentación animal. En Centro Nacional de Metrología. Simposio de metrología. Octubre, 2008, Santiago de Queretaro, Querétaro, Universidad Autonoma de Queretaro, pp 1–5.
- Harris, R. S. (1975). Effects of agricultural practices on the composition of foods. Nutritional Evaluation of Food Processing, 2, 33–57.
- Hu, J. P., Calomme, M., Lasure, A., De Bruyne, T., Pieters, L., Vlietinck, A., & Berghe, D. V. (1995). Structure-activity relationship of flavonoids with superoxide scavenging activity. Biological Trace Element Research, 47, 327–331.
- Huang, D., Ou, B., Hampsch-Woodill, M., Flanagan, J. A., & Prior, R. L. (2002). High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. Journal of Agricultural and Food Chemistry, 50, 4437–4444.
- Huang, D., Ou, B., & Prior, R. L. (2005). The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry, 53, 1841–1856.
- Isabelle, M., Lee, B. L., Lim, M. T., Koh, W. P., Huang, D., & Ong, C. N. (2010). Antioxidant activity and profiles of common fruits in Singapore. Food Chemistry, 123, 77–84.
- Jiménez-Aguilar, D. M., & Grusak, M. A. (2015). Evaluation of minerals, phytochemical compounds and antioxidant activity of Mexican, Central American and African green leafy vegetables. Plant Foods for Human Nutrition, 70, 357–364.
- Jiménez-Aguilar, D. M., & Grusak, M. A. (2017). Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. Journal of Food Composition and Analysis, 58, 33–39.
- Kim, D. O., & Padilla-Zakour, O. I. (2004). Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: Cherry, plum, and raspberry. Journal of Food Science, 69, SS395–S400.
- Lee, S. K., & Kader, A. A. (2000). Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology, 20, 207–220.
- Lee, S. U., Lee, J. H., Choi, S. H., Lee, J. S., Ohnisi-Kameyama, M., Kozukue, N., & Friedman, M. (2008). Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. Journal Of Agricultural and Food Chemistry, 56, 8541–8548.
- Leyva-López, N., Nair, V., Bang, W. Y., Cisneros-Zevallos, L., & Heredia, J. B. (2016). Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. Journal of Ethnopharmacology, 187, 302–312.
- Li, W. D., Pickard, M. D., & Beta, T. (2007). Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chemistry, 104, 1080–1086.
- Lin, Y. W., Liu, Y. H., Wang, L., Xie, L., Xie, Y. C., Zhang, Q., & Xiao, H. W. (2016). Vitamin C degradation and polyphenol oxidase inactivation of lotus root under boiling water blanching and steam blanching. International Agricultural Engineering Journal, 25, 257–266.
- López-García, G., López-Martínez, L. X., Dublán-García, O., & Baeza-Jiménez, R. (2017). Extraction and characterization of the fatty acid profile of quintonil (amaranthus hybridus). Revista Mexicana De Ingeniería Química, 16, 835–844.
- López-Martínez, L. X., & García-Galindo, H. S. (2009). Actividad antioxidante de extractos metanólicos y acuosos de distintas variedades de maíz mexicano. Nova Scientia, 2, 51–65.
- Lopez-Martinez, L. X., Parkin, K. L., & Garcia, H. S. (2012). Effect of processing of corn for production of masa, tortillas and tortilla chips on the scavenging capacity of reactive nitrogen species. International Journal of Food Science and Technology, 47, 1321–1327.
- Mathooko, F. M., & Imungi, J. K. (1994). Ascorbic acid changes in three indigenous Kenyan leafy vegetables during traditional cooking. Ecology of Food and Nutrition, 32, 239–245.
- Mepba, H. D., Eboh, L., & Banigo, D. E. B. (2007). Effects of processing treatments on the nutritive composition and consumer acceptance of some Nigerian edible leafy vegetables. African Journal of Food, Agriculture, Nutrition and Development, 7, 1.
- Muñiz-Márquez, D. B., Rodríguez, R., Balagurusamy, N., Carrillo, M. L., Belmares, R., Contreras, J. C., & Aguilar, C. N. (2014). Phenolic content and antioxidant capacity of extracts of Laurus nobilis L., Coriandrum sativum L. and Amaranthus hybridus L. CyTA-Journal of Food, 12, 271–276.
- Nicoli, M. C., Anese, M., Parpinel, M. T., Franceschi, S., & Lerici, C. R. (1997). Loss and/or formation of antioxidants during food processing and storage. Cancer Letters, 114, 71–74.
- Oboh, G. (2005). Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT-Food Science and Technology, 38, 513–517.
- Oke, O. L. (1983). Amaranth. In H. T. Chan (eds), Handbook of Tropical Foods (pp. 1–14). New York: Marcel-Dekker, Inc.
- Olayinka, O. O., Kareem, A. M., Ariyo, I. B., Omotugba, S. K., & Oyebanji, A. O. (2012). Antioxidant contents (Vitamin C) of raw and blanched different fresh vegetable samples. Food and Nutrition Sciences, 3, 18.
- Piga, A., Del Caro, A., & Corda, G. (2003). From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. Journal of Agricultural and Food Chemistry, 51, 3675–3681.
- Re, R., Bramley, P. M., & Rice-Evans, C. (2002). Effects of food processing on flavonoids and lycopene status in a Mediterranean tomato variety. Free Radical Research, 36, 803–810.
- Rice-Evans, C. A., Miller, N. J., Bolwell, P. G., Bramley, P. M., & Pridham, J. B. (1995). The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Research, 22, 375–383.
- Rohn, S., Buchner, N., Driemel, G., Rauser, M., & Kroh, L.W. (2007). Thermal degradation of onion quercetin glucosides under roasting conditions. Journal of Agricultural and Food Chemistry, 55, 1568–1573.
- Rojano, B. A., Zapata, A. K., & Cortes, C. F. (2012). Capacidad atrapadora de radicales libres de Passiflora mollissima (Kunth) L. H. Bailey (curuba). Revista Cubana De Plantas Medicinales, 17, 408–419.
- Routray, W., & Orsat, V. (2014). Variation of phenolic profile and antioxidant activity of North American highbush blueberry leaves with variation of time of harvest and cultivar. Industrial Crops and Products, 62, 147–155.
- Sablani, S. S., Andrews, P. K., Davies, N. M., Walters, T., Saez, H., Syamaladevi, R. M., & Mohekar, P. R. (2010). Effect of thermal treatments on phytochemicals in conventionally and organically grown berries. Journal of the Science of Food and Agriculture, 90, 769–779.
- Singh, J., Upadhyay, A. K., Prasad, K., Bahadur, A., & Rai, M. (2007). Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. Journal of Food Composition and Analysis, 20, 106–112.
- Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Oxidants and Antioxidants, Methods in Enzymology, 299, 152–178.
- Somsub, W., Kongkachuichai, R., Sungpuag, P., & Charoensiri, R. (2008). Effects of three conventional cooking methods on vitamin C, tannin, myo-inositol phosphates contents in selected Thai vegetables. Journal of Food Composition and Analysis, 21, 187–197.
- Tomas, M., Beekwilder, J., Hall, R. D., Sagdic, O., Boyacioglu, D., & Capanoglu, E. (2017). Industrial processing versus home processing of tomato sauce: Effects on phenolics, flavonoids and in vitro bioaccessibility of antioxidants. Food Chemistry, 220, 51–58.
- Vallejo, F., Tomás-Barberán, F., & Garcia-Viguera, C. (2002). Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. European Food Research and Technology, 215, 310–316.
- Van Acker, S. A., Tromp, M. N., Haenen, G. R., Vandervijgh, W. J. F., & Bast, A. (1995). Flavonoids as scavengers of nitric oxide radical. Biochemical and Biophysical Research Communications, 214, 755–759.
- Wang, S. Y., & Jiao, H. (2000). Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. Journal of Agricultural and Food Chemistry, 48, 5677–5684.
- Wu, S., Li, J., Wang, Q., Cao, H., Cao, J., & Xiao, J. (2017). Seasonal dynamics of the phytochemical constituents and bioactivities of extracts from Stenoloma chusanum (L.) Ching. Food and Chemical Toxicology, 108, 458–466.
- Xie, Y., Zheng, Y., Dai, X., Wang, Q., Cao, J., & Xiao, J. (2015). Seasonal dynamics of total flavonoid contents and antioxidant activity of Dryopteris erythrosora. Food Chemistry, 186, 113–118.
- Xu, B. J., & Chang, S. K. C. (2008). Total phenolic content and antioxidant properties of eclipse black beans (Phaseolus vulgaris L.) as affected by processing methods. Journal of Food Science, 73, 2.
- Zhang, L., & Lu, W. M. (2006). Study on the relationship of antioxidant activity with structure on flavonoids as scavengers of superoxide anion. Journal-Zhejiang University-Sciences Edition, 33, 187.
