ABSTRACT
This study analysed the effect of calcium ions from gluconate and lactate salts on the rheological properties of wheat flour dough. The empirical rheological properties of wheat flour dough were evaluated on Farinograph, Amylograph, Falling Number and Rheofermentometer devices. In the Farinograph test, both forms of calcium ions increased water absorption and dough stability, whereas the dough development time increased when calcium gluconate was added and decreased when calcium lactate was incorporated. The addition of calcium ions to wheat flour dough increased Amylograph gelatinization temperature and decreased peak viscosity. The Falling Number values decreased and the Rheofermentometer parameter retention coefficient increased with the increased level of calcium ions addition. Dynamic oscillatory rheology in the linear viscoelastic range (frequency sweep and oscillatory temperature sweep test) was studied. Each of the calcium ion forms influenced the rheological behaviour of wheat flour dough; thus, the results obtained are similar to those reported by the Farinograph and Amylograph devices.
RESUMEN
El presente estudio analizó el efecto que provoca la adición de iones de calcio obtenidos de sales glucónicas y lácticas en las propiedades reológicas de la masa de harina de trigo. Las propiedades reológicas empíricas de la masa de harina de trigo fueron evaluadas con los siguientes aparatos: farinógrafo, amilógrafo, reofermentómetro y de número de caída. La prueba con el farinógrafo mostró que ambas variedades de iones de calcio causaron el aumento de la absorción de agua y la estabilidad de la masa, mientras que el tiempo de desarrollo de la masa se elevó con la adición de gluconato cálcico y bajó cuando se incorporó lactato de calcio. La adición de iones de calcio a la masa de harina de trigo incrementó la temperatura de gelatinización registrada por el amilógrafo y disminuyó la viscosidad pico. Asimismo, con la adición de un nivel mayor de iones de calcio se redujeron los valores de número de caída y el coeficiente de retención del parámetro en el reofermentómetro aumentó. Además, se estudió la reología oscilatoria dinámica en el rango viscoelástico lineal (prueba de barrido de frecuencia y prueba de barrido de temperatura oscilatoria). Cada una de las forma de los iones de calcio incidió en el comportamiento reológico mostrado por la masa de harina de trigo, por lo que los resultados obtenidos presentan similitud con los datos reportados por el farinógrafo y el amilógrafo.
Introduction
Calcium is an essential nutrient whose deficiency has a negative effect on human body and may lead to improper brain function, muscular symptoms like pains, aches, twitching, spasms, cramps and low bone mineral density. Supplementation of foods with calcium may enhance bone density and reduce the possibility of its fracture (Chung, Tang, Fu, Wang, & Newberry, Citation2016). According to the Institute of Medicine (IOM) of the National Academy of Science, people should consume at least 1000–1200 mg/day of calcium in order to prevent calcium deficiency (Ross et al., Citation2011). In the Western countries, the average calcium intake is 700–900 mg/day, whereas in Asia and Africa it is lower. In the United States, according to the National Health and Nutrition Examination Survey (NHANES), up to 90% of the population did not meet the calcium needs from diet and supplements (Sharma, Kolahdooz, Vik, Sheehy, & Kolonel, Citation2016). Therefore, in many countries, many people take calcium supplements (Bolland et al., Citation2015). According to NHANES data, about 43% of the US population use dietary supplements containing calcium (Bailey et al., Citation2010) and in the Western countries more than 30–50% of older women take calcium supplements (Bolland et al., Citation2015) and even so, they were not sufficient in obtaining the recommended intakes (Mangano, Walsh, Insogna, Kenny, & Kerstetter, Citation2011). Therefore, nowadays it is recommended worldwide to increase the level of calcium supplement used in order to reduce the hypocalcemia risk among people. It is well known that cereals are the most important food sources for human consumption, out of which wheat and rice are the most important calories-providers. Wheat, the main ingredient of bread, is one of the basic food products in human nutrition. The most commercial bread products are those made from white wheat flour which has a high extraction rate (Dewettinck et al., Citation2008). Although wheat flour contains calcium of about 0.45% by wheat weight, this element is present in a lower amount as compared to people’s needs. Also, it must be taken into account that through the refining process, wheat flour loses almost all its calcium content because a high amount of calcium is localized in the bran fraction. Moreover, calcium bioavailability from wheat bran is very low due to its high phytic acid content which has a strong ability to chelate calcium in calcium, which reduces the calcium absorption (Frontela, Ros, & Martinez, Citation2011). Therefore, the bread made from white wheat flour represents a poor source of calcium from the point of view of the recommended daily calcium intake. The addition of calcium in wheat flour may be a solution in order to provide the adequate nutrition and to prevent calcium deficiency. The calcium addition may change the bread’s technological properties. Some factors that must be taken into consideration are the calcium source and the level at which it is incorporated. A high number of inorganic or organic salts may be used in order to improve the nutritional value of bread products. The calcium carbonate and calcium citrate are the most common calcium salts used (Abrams et al., Citation1991) but also other forms like calcium lactate, calcium chloride, calcium phosphate, calcium propionate, calcium gluconate are very used due to their preservation effect on food products (Martín-Diana et al., Citation2007). The effect of calcium ions from different sources on dough rheological properties was investigated by different researchers (Kaur, Bala, Singh, & Rehal, Citation2011; Morita, Nakamura, Hamauzu, & Toyosawa, Citation1994, Citation1996; Salovara, Citation1982; Sehn, Nogueira, Almeida, Chang, & Steel, Citation2015; Tuhumury, Small, & Day, Citation2016). According to those authors, it seems that, in general, the calcium ions addition in wheat flour presented a weakening effect on dough physical properties. This study aims to bring further contribution to the studies previously made by analysing the effect of calcium ions from lactate and gluconate salt at the level of 100, 150 and 200 mg/100g on dough fundamental and empirical rheological properties. In our report, lactate and gluconate salt were chosen as calcium ions sources due to the fact that this type of salt is a very good source of calcium when is incorporated in food and provides the food products with high bioavailability, solubility, textural and sensory properties during storage and extends the shelf life (Anino, Salvatori, & Alzamora, Citation2006; Yang & Lawsless, Citation2003). To our knowledge, few previous studies have been made regarding the effect of calcium ions from gluconate and lactate salts on dough rheological properties (Morita et al., Citation1994). In addition to these studies, we intend to carry out a full analysis on the effect of calcium ions from lactate and gluconate salts on dough rheological properties during mixing, pasting and fermentation process in order to anticipate its effect on bread technological process.
Materials and methods
Materials
Wheat flour was provided by S.C. Dizing S.R.L. Brusturi, Neamț, Romania. The calcium salts, calcium lactate ((CH3CHOHCOO)2Ca) and calcium gluconate (C12H22CaO14-H2O) were provided by Corbion, Netherlands. Wheat flour used in this study had 0.55 g/100g ash content (ICC 104/1), 14.0 g/100g moisture content (ICC method 110/1), 13.2 g/100g protein content (ICC 105/2) and 31 g/100g wet gluten content (ICC 106/1). Dough samples with four levels (0, 100, 150 and 200 mg/100g) of calcium ions from gluconate (CGF) and lactate salt (CLF) were prepared separately in order to evaluate their effect on dough rheological properties. In order to achieve the 100, 150 and 200 mg/100g of calcium ions, calcium gluconate was added in wheat flour in the amount of 1.11 g/100 g, 1.66 g/100 g, 2.22 g/100 g and calcium lactate in the amount of 0.75 g/100 g, 1.1 g/100 g, 1.45 g/100g.
Empirical rheological measurement
Dough empirical rheological measurements were analysed using a Farinograph (ICC 115/1), Amylograph (ICC 126/1), Falling Number device (ICC 107/1) and Rheofermentometer (AACC 89–01.01). The mixing properties of blends were determined to Farinograph (Brabender, Germany) using 300 g of flour were water absorption (WA), dough development time (DT), dough stability (DS) and degree of softening at 10 min (DS) were determined. Pasting properties of the blends were analysed to Amylograph (Brabender, Germany) where the gelatinization temperature (Tg, °C), temperature at peak viscosity (Tmax, °C) and peak viscosity (PVmax, BU) were investigated. The Falling Number (Perten Instruments AB, Sweden) method was used to evaluate the alpha-amylase activity of blends. The Rheofermentometer (Chopin Rheo, type F3, Villeneuve-La-Garenne Cedex, France) was used to obtain information on dough development time and gas production during the fermentation process. The measurements were conducted according to Chopin protocol by testing a dough sample under 2000 g cylindrical weight constraint at 30°C for 3 h. The dough for Rheofermentometer measurements was prepared by mixing 250 g blend flour, 7 g compressed yeast, 5 g salt and water according to Farinograph water absorption value for 8 min in a Brabender Farinograph. The parameters obtained were evaluated as follows: maximum height of gaseous production (H’m, mm), total CO2 volume production (VT, mL), volume of the gas retained in the dough at the end of the test (VR, mL) and retention coefficient (CR, %).
Fundamental rheological measurement
Oscillation measurements were performed by using a HAAKE MARS 40 rheometer. The dough samples were prepared in the Farinograph mixer by using the amount of distilled water in which the calcium salt was dissolved and the mixing time (DT) to reach 500 UB consistency determined by Farinograph studies. After mixing, the dough samples were allowed to rest for 30 min before being analysed to the rheometer. The plate and plate system was used with a diameter of 40 mm and a gap between plates of 2 mm. A vaseline oil was used to prevent drying of the sample exposed edge during testing. In order to determine the linear viscoelastic region of the wheat flour dough samples, stress sweep tests were performed (1 Hz at 25°C). A stress value of 15 Pa was chosen for all the frequency tests. Frequency sweep tests from 0.01 to 100 Hz were performed at 25°C for all dough samples. For the temperature sweep test, the samples were heating from 25°C to 100°C at a heating rate of 4°C per min at a fixed strain of 0.001 and a frequency of 1 Hz. During the frequency sweep tests, were carried out and during heating, the storage modulus (G’) and loss modulus (G”) were determined.
Statistical analysis
The statistical analysis was done using XLSTAT (free trial version 2016, Addinsoft, Inc., Brooklyn, NY, USA) at a significance level of p < 0.05. All data were analysed using variance analysis (ANOVA) and the Tukey test for mean comparison. The measurements were performed in triplicate for each sample, and results were expressed as mean value ± standard deviation.
Results and discussion
The effect of calcium ions addition on empirical dough rheological properties
Dough empirical rheological properties during mixing were analysed with the Farinograph device. As can be seen from , the calcium ions addition in wheat flour dough significantly increased the water absorption values (p < 0.001), probably due to its interaction with the major wheat flour components starch and proteins. According to Sehn et al. (Citation2015), calcium ions may favour an increase in wheat flour proteins solubility and hydration capacity. On the other hand, the wheat flour starch component may present a destabilization effect leading to higher spaces between the amylopectin chains from the damaged starch of wheat flour. These facts may lead to an increase in Farinograph water absorption values for dough samples in which calcium ions were incorporated. Similar results were also obtained by Morita et al. (Citation1994); Morita et al. (Citation1996), who concluded that calcium ions addition from lactate and gluconate salts in wheat flour dough slightly increased the water absorption values. Regarding dough development time, this increased when calcium ions from gluconate salt were added and decreased when calcium ions from lactate salt were incorporated. This may be due to the anion salt type, which has a much greater effect than the cation on wheat flour dough. According to Miller and Hoseney (Citation2008), the anion decreased the electrostatic repulsion between proteins allowing them to associate, thus forming a more compact dough. Due to the fact that gluconate anion is in a higher amount than the lactate one at the same level of calcium ions addition, the effect of the increase in the dough strength is more evident when gluconate salt was incorporated in wheat flour dough. Probably when calcium lactate salt was added, gluten proteins interact less in a shorter mixing time having a positive charge. Also, as regards dough stability, the calcium ions from gluconate salt led to an increase in these values showing that it had a strengthening effect on wheat flour dough. Contrary, the calcium ions addition from lactate salt led to a decrease in these values when low levels were incorporated in wheat flour and to an increase in these values when high levels were added in dough samples. This may be due to the fact that calcium ions decreased the structure of water, which destabilizes the hydrophobic interactions between gluten proteins (Sehn et al., Citation2015), leading to low stability values. However, when salt is added in wheat flour dough, both ions interact with chemical groups of the gluten proteins. According to Tuhumury et al. (Citation2016), the effect of anion salt compared to the cation one on gluten structure is higher, probably due to the anion ability to bind more effectively with proteins and screening of the electrostatic repulsion on specific binding sites. This explains the high amount of anion from lactate salts present in wheat flour dough, which increased its stability. Taking into account the Farinograph values, it may be concluded that at high levels both types of calcium salts presented a strengthening effect on wheat flour dough, more pronounced in the case of calcium ions addition from gluconate form (p < 0.001) than in the case of calcium ions addition from lactate form (p < 0.05). However, at low levels, calcium ions addition from lactate form presented a weakening effect on dough rheological properties, highlighting thus the importance of the anion nature and level of the calcium salt used. The degree of softening values confirms the results previously obtained showing that the degree of softening decreases with the addition of calcium lactate salt, which correlates with the strengthening effect on the wheat flour dough, whereas the softening degree increases with the addition of low levels of lactate salt, which correlates with the weakening effect. However, when added at high levels, the lactate salt slightly decreases the softening degree, thus increasing wheat dough strength. These results are in disagreement with those obtained by Sehn et al. (Citation2015); Kaur et al. (Citation2011), who found a decrease in dough stability, dough development time and an increase in the degree of softening values by addition of calcium ions derived from calcium sulphate and calcium chloride salts. According to Salinas and Puppo (Citation2015); Salinas, Zuleta, Ronayne, and Puppo (Citation2012), these results are probably different due to the salt anion level addition which is interacting electrostatically with gluten proteins in a different negative charge proportion, generating structures with different consistency and stability during mixing and affecting dough rheological properties. It seems that the anions of calcium salts favour the aggregation of gluten, probably by the formation of cross-links through ionic interactions (Morita et al., Citation1996). The presence of the calcium salts exhibited a more pronounced effect on dough strength for gluconate salt than for the lactate one due to the fact there are more gluconate ions than lactate ones for the levels used in this study of calcium ions concentration.
Table 1. Analysis of variance of the influence of calcium ions from the gluconate salt (CGF) and lactate salt (CLF) addition on wheat flour dough farinograph parameters.
Tabla 1. Análisis de la varianza respecto al efecto provocado por la adición de iones de calcio obtenidos de sales glucónicas (CGF) y sales lácticas (CLF) a la masa de harina de trigo, con parámetros del farinógrafo.
The Amylograph pasting properties and Falling Number values of wheat flour dough with different levels of calcium ions from the gluconate salt (CGF) and lactate salt (CLF) addition are shown in . The falling number value of wheat flour dough slightly decreased with the increased level of calcium ions addition from CGF and CLF, indicating a decrease in dough viscosity of the mixes, more obvious in the case of the calcium ions addition from the lactate form than in the case of the calcium ions addition from the gluconate form. This decrease may be due to the fact that the alpha-amylase enzyme activity depends on the presence of calcium ions from its structure (Suandarram, Pandurangappa, & Murthly, Citation2014) and also due to the interactions between wheat flour components with the calcium salts used. It is well known that dough viscosity decreased in the presence of salt (Samustri & Suphantharika, Citation2012) due to the amount of amylose which leached out the starch granules (Venkatesh & Boldizar, Citation2017). Therefore, the increase in the calcium level from wheat flour dough stabilizes the alpha-amylase enzyme activity unable to function in calcium absence (Mironeasa & Codină, Citation2017) and destabilizes the wheat flour starch component. According to the Amylograph data, the gelatinization temperature increased (p < 0.05) with the increased level of calcium ions addition indicating a delay of starch gelatinization, these data being in agreement with those obtained by Kaur et al. (Citation2011) regarding calcium ions addition derived from chloride salt. Regarding the peak viscosity, these values were significantly decreased (p < 0.001) when calcium ions from lactate salt were incorporated and increased (p < 0.05) when low levels of calcium ions from gluconate salt were added. This may be explainable due to the fact that the amylolitic activity in wheat flour dough increased by calcium ions addition and therefore the peak viscosity decreased. In the case of calcium ions addition from the gluconate source, the peak viscosity presented higher values than the control sample, even if the falling number values decreased, probably due to the stretching effect that this form of calcium salt has on the wheat flour dough, according to the Farinograph data. However, when high levels of calcium ions from gluconate salt were added the Amylograph peak viscosity begins to decrease as a consequence of the increase in alpha-amylase activity and to destabilize the effect that calcium salts have on wheat flour starch component (Samustri & Suphantharika, Citation2012). The temperature at peak viscosity had a similar trend with peak viscosity values which decreased when calcium ions from lactate salt were incorporated and increased up to an addition level of 100 mg/100g, followed by a slight decrease when calcium ions from gluconate form were added in the wheat flour dough. This behaviour may be due to the well-known effects of salts on starch, which depends on the type of anions and cations from the salt used, as well as on its concentrations. According to Oosten (Citation1990), the starch granule has a negative potential, which repels the anion and attracts the cation. He suggested that the anion promotes the starch gelatinization by breaking the hydrogen bonds between starch molecules. The absorption of calcium cation is more limited, due to the fact that in the dough system there is no agent to bind the released H+ and in that way, the increase in temperature at peak viscosity to Amylograph device was more limited (Samustri & Suphantharika, Citation2012) in the case of the lactate salt than in the case of the gluconate one.
Table 2. Analysis of variance of the influence of calcium ions from the gluconate salt (CGF) and lactate salt (CLF) addition on wheat flour dough amylograph and falling number values.
Tabla 2. Análisis de la varianza respecto al efecto provocado por la adición de iones de calcio obtenidos de sales glucónicas (CGF) y sales lácticas (CLF) a la masa de harina de trigo, con valores del amilógrafo y del número de caída.
During the fermentation process, the dough behaviour with different levels of calcium ions addition is shown in . According to the Rheofermentometer data, the total CO2 volume production significantly decreased (p < 0.001) with the increased level of calcium salt addition in wheat flour dough. According to Codină, Zaharia, Ropciuc, and Dabija (Citation2017), this may be due to the increase in the osmotic pressure on the yeast cells, which may lead to an inhibitory impact on the yeast metabolism. In the case of calcium gluconate salt addition, the volume of the gas retained in the dough at the end of the test (VR) had higher values than the control sample, whereas in the case of calcium ions from lactate salt these values were lower when levels of 150 and 200 mg/100g were incorporated. Higher VR values are probably due to the fact that at these levels of calcium ions addition, the gluten network has more strength, as can be seen from the Farinograph data. Therefore, the wheat flour dough was more capable of retaining CO2 (Codină, Mironeasa, Voica, & Mironeasa, Citation2013; Lynch, Dal Bello, Sheehan, Cashman, & Arendt, Citation2009).
Table 3. Analysis of variance of the influence of calcium ions from the gluconate salt (CGF) and lactate salt (CLF) addition on wheat flour dough Rheofermentometer values.
Tabla 3. Análisis de la varianza del efecto provocado por la adición de iones de calcio obtenidos de sales glucónicas (CGF) y sales lácticas (CLF) a la masa de harina de trigo, con valores del reofermentómetro.
The effect of calcium ions addition on empirical dough rheological properties
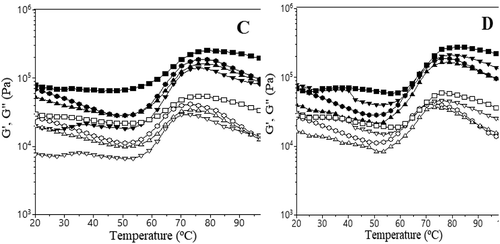
The fundamental rheological properties of wheat flour dough with different levels of calcium ions addition from the gluconate and lactate form are shown in and . ) shows the frequency sweep results for wheat flour with different levels of calcium ions addition from gluconate and lactate salts. ) presents the evolution with temperature of G’ and G” of dough samples in which the CLF and CGF were incorporated.
Figure 1. Evaluation with frequency at 20°C of G’ values (represented by solid symbols) and G” (open symbols) for samples with different levels of calcium ions addition.
Figura 1. Evaluación con frecuencia a 20°C de los valores G’ (representados por los símbolos sólidos) y G” (símbolos abiertos) para las muestras a las que se adicionaron diferentes niveles de iones de calcio: 0 mg/100g (●),100 mg/100g (▼), 150 mg/100g (▲) y 200 mg/100g (■) de sales lácticas (CLF) (A) y sales glucónicas (CGF) (B).
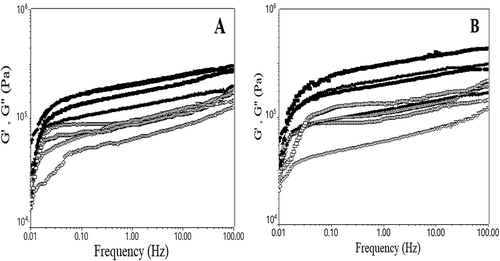
Figure 2. Evaluation with temperature of G’ values (represented by solid symbols) and G” (open symbols) for samples with different levels of calcium ions addition.
Figura 2. Evaluación con temperatura de los valores G’ (representados por los símbolos sólidos) y G” (símbolos abiertos) para las muestras a las que se adicionaron diferentes niveles de iones de calcio: 0 mg/100g (●),100 mg/100g (▼), 150 mg/100g (▲) y 200 mg/100g (■) de sales lácticas (CLF) (A) y sales glucónicas (CGF) (B).
As can be seen from , the addition of CLF conducted to a decrease in G’ and G” for wheat flour dough with 100 mg/100g calcium ions addition followed by an increase in the moduli values when high levels of calcium ions from lactate salt were incorporated. Taking into account these results, we may presume that these decreases may be attributed to the reduction of hydrophobic intermolecular interactions with the increased amount of free water in the dough system. When high levels of calcium lactate salt were added in wheat flour, it may be possible for the ions to exert a dehydration action on gluten network and decrease the amount of water osmotically bounded, which led to dough structure with more strength and compactness.
For dough in which CGF was incorporated, the sample with 150 mg/100g calcium ions addition presented the lowest values of G’ and G” moduli. The samples with 100 mg/100 g and 200 mg/100g calcium ions addition presented smaller changes in dough rheological properties compared to the control sample, suggesting that at these level calcium ions did not induce fundamental changes in the structure of dough samples.
For the dough samples with 200 mg/100g calcium ions addition, the trend was an increase in G’ and G” showing that at this level the dough presented a higher elasticity and solid-like behaviour than the control sample. It is evident from that dough samples with calcium ions addition from gluconate salt presented higher values for G’ and G” than dough samples with calcium ions addition from lactate salt, indicating the fact that these samples presented stronger and more rigid characteristics.
During heating, the G’ and G” moduli decreased to a minimum up to a certain temperature due to the protein weakening, followed by an increase due to the fact that starch gelatinization takes place. Finally, at high temperatures, dough viscosity and elasticity decreased due to the physical breakdown of starch granules. At 200 mg/100g calcium ions addition, the calcium salt decreased the protein weakening phase and increased dough elasticity and viscosity during the gelatinization phase. This may be due to the strengthening effect that calcium salt at this level has on wheat flour dough, which is more evident in the case of gluconate salt than in the case of lactate salt. However, when 150 mg/100 g calcium ions were added in wheat flour dough, the G’ and G” moduli during the protein weakening and starch gelatinization phase decreased probably due to the increased level of the α-amylase activity caused by the high level of calcium content from calcium salt addition, which stabilizes this calcium metalloenzyme unable to function in the absence of calcium (Mironeasa, Codină, & Mironeasa, Citation2012). Compared to the control sample, the dough samples with 100 mg/100g of calcium ions addition presented lower values of G’ and G” moduli in the case of calcium lactate addition and higher ones when calcium gluconate was incorporated. It may be possible that the gluconate anion absorbs a higher amount of water during dough mixing than the lactate one. When dough temperature increases, the water entrapped by anion salts is released during heating, causing an increase of water availability for the starch granules. This fact will lead to a better gelatinization of the starch granules in the presence of gluconate salt than in the presence of the lactate one, which will increase dough consistency.
The relationship between the empirical and fundamental rheological characteristics
PCA loadings of empirical and fundamental rheological characteristics of dough samples with different levels of calcium ions addition are shown in . The two plots represent 84.90% of the total variance. The plot of PC1 vs. PC2 loadings shows a close association between the G’ and G” values with frequency at 20°C, as well as and between these moduli during heating. Also, there may be noticed a close relationship between Farinograph parameters stability (ST) and dough development time (DT) and Rheofermentometer value the retention coefficient (CR). This correlation is in agreement with those obtained by Sroan, Bean, and MacRitchie (Citation2009), who found that the stability of gas cells during their expansion depends on dough strength.
Figure 3. Principal component analysis between the dough samples with different levels of calcium ions addition to G’ and G” moduli, the farinograph, amylograph, falling number and rheofermentometer characteristics.
Figura 3. Análisis de los componentes principales de las muestras de masa a las que se adicionaron diferentes niveles de iones de calcio para los módulos G’ y G”, según las características indicadas por el farinógrafo, el amilógrafo, el número de caída y el reofermentómetro.
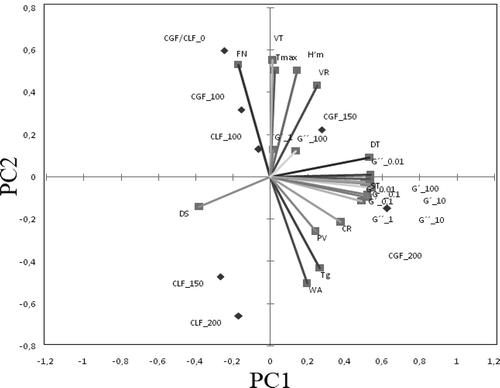
The first main component opposes the dough samples with 150 mg/100g calcium ions addition and the dough sample with 100 mg/100g calcium ions from lactate salt and the G’ and G” moduli. It seems that at this level of calcium ions addition, the changes on dough fundamental rheological properties are smaller compared to the control sample and presented a negative effect. A close positive association may be noticed between the dough samples with 100 mg/100g calcium ions addition and VT dough Rheofermentometer values and a maximum height of gaseous production (H’m) and a negative one between the dough samples when calcium ions from the gluconate salt were added at the levels of 150 mg/100g and 200 mg/100g.
The PC2 axis shows a close association between the dough samples with 150 mg/100g and 200 mg/100g from lactate salt which are inversely correlated with Rheofermentometer values VR, VT, H’m and Farinograph data DT and ST.
Conclusions
The addition of different levels (0, 100, 150 and 200 mg/100g) of calcium ions from gluconate and lactate salts significantly influence dough empirical and rheological characteristics. At high levels, both forms of calcium ions had a strengthening effect on dough rheological properties, more in the case of gluconate form than in the case of lactate one, due to the increase in stability and decrease in degree of softening according to the Farinograph data and to the increased values of G’ and G” moduli according to the dynamic rheometer data compared to the control sample. The alpha-amylase activity increased with the increased level of calcium ions addition from CGF and CLF and during heating at high levels of calcium ions addition, dough elasticity and viscosity during the gelatinization phase increased. During the fermentation process, the gas retention coefficient had higher levels for all the dough samples in which calcium salts were incorporated as compared with the control sample. The correlation analysis of data set shows that during heating, the dough samples with 200 mg/100g calcium ions presented the highest positive influence on dough fundamental rheological properties. During mixing and fermentation, the dough samples with 100 mg/100g calcium ions addition had a positive correlation with Farinograph parameters stability (ST), dough development time (DT) and Rheofermentometer values the gas retained in the dough at the end of the test (VR), the total CO2 volume production (VT).
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS/CCCDI – UEFISCDI, project number PN-III-P2-2.1-BG-2016-0079, within PNCDI III.
Additional information
Funding
References
- Abrams, S. A., Sidbury, J. B., Muenzer, J., Esteban, N. V., Vieira, N. E., & Yergey, A. L. (1991). Stable isotopic measurement of endogenous fecal calcium excretion in children. Journal of Pediatric Gastroenterology and Nutrition, 12, 469–473.
- Anino, S. V., Salvatori, D. M., & Alzamora, S. M. (2006). Changes in calcium level and mechanical properties of apple tissue due to impregnation with calcium salts. Food Research International, 39, 154–164.
- Bailey, R. L., Dodd, K. W., Goldman, J. A., Gahche, J. J., Dwyer, J. T., Moshfegh, A. J., … Picciano, M. F. (2010). Estimation of total usual calcium and vitamin D intakes in the United States. Journal of Nutrition, 140, 817–822.
- Bolland, M. J., Leung, W., Tai, V., Bastin, S., Gamble, G. D., Grey, A., & Reid, I. R. (2015). Calcium intake and risk of fracture: Systematic review. British Medical Journal, 351(4580), h4580.
- Chung, M., Tang, A. M., Fu, Z., Wang, D. D., & Newberry, S. J. (2016). Calcium intake and cardiovascular disease risk: An updated systematic review and meta-analysis. Annals of Internal Medicine, 165, 856–866.
- Codină, G. G., Mironeasa, S., Voica, D. V., & Mironeasa, C. (2013). Multivariate analysis of wheat flour dough sugars, gas production, and dough development at different fermentation times. Czech Journal of Food Science 31, 222–229. doi:10.17221/216/2012-CJFS
- Codină, G. G., Zaharia, D., Ropciuc, S., & Dabija, A. (2017). Influence of magnesium gluconate salt addition on mixing, pasting and fermentation properties of dough. The EuroBiotech Journal, 3, 222–225.
- Dewettinck, K., Van Bockstaele, F., Kuhne, B., Van de Walle, D., Courtens, T. M., & Gellynck, X. (2008). Nutritional value of bread: Influence of processing, food interaction and consumer perception. Journal of Cereal Science, 48, 243–257.
- Frontela, C., Ros, G., & Martinez, C. (2011). Phytic acid content and “in vitro” iron, calcium and zinc bioavailability in bakery products: The effect of processing. Journal of Cereal Science, 54, 173–179.
- Kaur, A., Bala, R., Singh, B., & Rehal, J. (2011). Effect of replacement of sodium chloride with mineral salts on rheological characteristics of wheat flour. American Journal of Food Technology, 6, 674–684.
- Lynch, E. J., Dal Bello, F., Sheehan, E. M., Cashman, K. D., & Arendt, E. K. (2009). Fundamental studies on the reduction of salt on dough and bread characteristics. Food Research International, 42, 885–891.
- Mangano, K. M., Walsh, S. J., Insogna, K. L., Kenny, A. M., & Kerstetter, J. E. (2011). Calcium intake in the United States from dietary and supplemental sources across adult age groups: New estimates from the National Health and nutrition examination survey 2003-2006. Journal of the American Dietetic Association, 111, 687–695.
- Martín-Diana, A. B., Rico, D., Henehan, G. T. M., Frías, J., Barat, J. M., & Barry-Ryan, C. (2007). Calcium for extending the shelf-life of fresh whole and minimally processed fruits and vegetables: A review. Trends in Food Science & Technology, 18, 210–218.
- Miller, R. A., & Hoseney, R. C. (2008). Role of salt in baking. Cereal Foods World, 53, 4–6.
- Mironeasa, S., & Codină, G. G. (2017). The mixolab rheological properties and dough microstructure of deffated mustard seed-wheat composite flours. Journal of Food Processing and Preservation, 41, 13130.
- Mironeasa, S., Codină, G. G., & Mironeasa, C. (2012). The effects of wheat flour substitution with grape seed flour on the rheological parameters of the dough assessed by mixolab. Journal of Texture Studies, 43, 40–48.
- Morita, N., Nakamura, M., Hamauzu, Z., & Toyosawa, I. (1994). Effects of calcium gluconate on physical properties of wheat flour dough and breadmaking. Journal of Applied Glycocscience, 43, 407–412.
- Morita, N., Nakamura, M., Hamauzu, Z., & Toyosawa, I. (1996). Effects of calcium gluconate and lactate on properties of dough and breadmaking in home baker. Journal of Applied Glycocscience, 43, 87–93.
- Oosten, B. J. (1990). Interactions between starch and electrolytes. Starch/Stärke, 42, 327–330.
- Ross, C. A., Manson, J. E., Abrams, S. A., Aloa, J. E., Brannon, P. M., Clinton, S. K., … Shapses, S. A. (2011). The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: What clinicians need to know. The Journal of Clinical Endocrinology & Metabolism, 96, 53–58.
- Salinas, M. V., & Puppo, M. C. (2015). Optimization of the formulation of nutritional breads based on calcium carbonate and inulin. LWT- Food Science and Technology, 60, 95–101.
- Salinas, M. V., Zuleta, A., Ronayne, P., & Puppo, M. C. (2012). Wheat flour enriched with calcium and inulin: A study of hydration and rheological properties of dough. Food Bioprocess and Technology, 8, 3129–3141.
- Salovara, H. (1982). Effect of partial sodium chloride replacement by other salts on wheat dough rheology and bread making. Cereal Chemistry, 59, 422–426.
- Samustri, W., & Suphantharika, M. (2012). Effect of salts on pasting, thermal, and rheological properties of rice starch in the presence of non-ionic and ionic hydrocolloids. Carbohydrate Polymers, 87, 1559–1568.
- Sehn, G. A. R., Nogueira, A. C., Almeida, E. L., Chang, Y. K., & Steel, C. J. (2015). Fortification of wheat dough with calcium and magnesium ions affects empirical rheological properties. Cereal Chemistry, 92, 405–410.
- Sharma, S., Kolahdooz, F., Vik, S., Sheehy, T., & Kolonel, L. N. (2016). Dietary sources of calcium, vitamin D, and the pattern of dairy products consumption in five ethnic groups in the United States. Journal of Food Research, 5, 58–66.
- Sroan, B. S., Bean, S. R., & MacRitchie, F. (2009). Mechanism of gas cell stabilization in bread making. I. The primary gluten-starch matrix. Journal of Cereal Science, 49, 32–40.
- Suandarram, A., Pandurangappa, T., & Murthly, K. (2014). α amylase production and applications: A review. Journal of Applied and Environmental Microbiology, 2, 166–175.
- Tuhumury, H. C. D., Small, D. M., & Day, L. (2016). Effects of hofmeister salt series on gluten network formation: Part I. Cation series. Food Chemistry, 212, 789–797.
- Venkatesh, A., & Boldizar, A. (2017). Plasticizing starch by adding magnesium chloride or sodium chloride. Starch/Stärke, 69, 1600191.
- Yang, H. H., & Lawsless, H. T. (2003). Descriptive analysis of divalent salts. Journal of Sensory Studies, 20, 97–113.
