ABSTRACT
The present study was to investigate the effects of administrating bromelain, inulin, or a mixture of these ingredients on different fecal parameters in a rat model. Our results showed that taking bromelain (120 CDU/kg body weight) apparently increased fecal moisture by 18% and declined fecal mucinase activity (−36.3%). The incorporation of inulin (260 mg/kg body weight) could also result in some desirable changes including increased fecal moisture by 19%, declined fecal mucinase activity (−43.9%). The feeding of same amounts of bromelain and inulin could lead to significant increases in the presumptive counts of Bifidobacterium and Lactobacilli as well as the concentrations of various fecal short chain fatty acids (by 54–95%). This study suggested that the consumption of bromelain and inulin together might exert favorable effects in improving certain fecal parameters, and provide more hints for the development of functional food formulations.
RESUMEN
El presente estudio se propuso investigar los efectos que la administración de bromelina, inulina o una mezcla de estos ingredientes tiene sobre distintos parámetros fecales en un modelo en ratas. Los resultados dan cuenta de que la administración de bromelina (120 CDU/kg de peso corporal) aparentemente aumentó la humedad fecal en 18% y disminuyó la actividad de la mucinasa fecal (−36.3%). Por otra parte, se constató que la administración de inulina (260 mg/kg de peso corporal) puede conllevar algunos cambios deseables, entre los que se incluye el incremento de humedad fecal en 19% y la reducción de la actividad de la mucinasa fecal (−43.9%). Alimentar a las ratas con cantidades iguales de bromelina e inulina puede producir aumentos significativos en los recuentos presuntivos de Bifidobacterium y Lactobacilli y en la concentración de varios ácidos grasos fecales de cadena corta (54-95%). Los resultados del estudio sugieren que el consumo de bromelina e inulina juntas puede provocar efectos favorables, mejorando ciertos parámetros fecales y proporcionando elementos para desarrollar ciertas formulaciones de alimentos funcionales.
Introduction
Human gut does not merely retain the nutrients from digested foods (Cencic & Chingwaru, Citation2010), but also provides a place for an enormous microbial growth. Various studies have revealed a relationship between the intestinal health and some intestinal lumen and feces-related parameters. These parameters included intestinal transit time, defecation frequency, intestinal lining integrity, fecal short chain fatty acids (SCFAs), fecal pH, and fecal moisture (Meyer & Stasse-Wolthuis, Citation2009; Olivares et al., Citation2006). The measurement of these parameters could be a useful clue to reflect the changes in the intestinal health and integrity.
Pineapple (Ananas comosus) is one of the most known nutritional fruits around the world. In addition to its flavorful taste, it has been used as both the traditional medicinal and food purposes (Chau & Wu, Citation2006). Pineapple possesses a proteolytic enzyme, namely, bromelain. It is categorized as one of the GRAS (Generally Recognized As Safe) enzymes. It could be found in both pineapple fruits and stems, but it exists in a larger quantity in stems than in fruits. Bromelain could be absorbed into blood circulation system (Bhattacharyya, Citation2008), and the typical daily dosage for different therapeutic applications (e.g. digestive disorders) ranged roughly from 4 to 40 CDU/kg body weight for a 60 kg adult (Roxas, Citation2008). It might benefit patients with postoperative ileus and prevent the enterotoxigenic Escherichia coli induced diarrhea by proteolytically reducing the binding ability of enteral mucosa (Mynott, Luke, & Chandler, Citation1996; Wen et al., Citation2006).
Inulin with a degree of polymerization (DP) > 3 is regarded as a non-digestible soluble fiber (Dai & Chau, Citation2017). It stays undigested in the small intestine and is gradually fermented in the large intestine (Schaafsma & Slavin, Citation2015). According to Meyer and Stasse-Wolthuis (Citation2009), adults, unlike infants who only require 2 g of inulin per day, require at least 4–5 g of inulin daily to improve intestinal health and to trigger a bifidogenic effect. It contributed to multiple intestinal health functions such as promoted growth of intestinal microflora, micronutrient absorption, fermentation byproducts (i.e. SCFA) production, and relief of constipation (Meyer & Stasse-Wolthuis, Citation2009; Roberfroid, Citation2005).
In accordance to the fact that bromelain could be beneficial to gut health, our preliminary study has shown that the benefits from bromelain and inulin were different, for instance, with no apparent growth of lactic acid bacteria being observed with bromelain. This study tended to fill the literature gap to see if more beneficial effect could be achieved by feeding bromelain and inulin together.
Therefore, the aim of this study was to evaluate the effects of the bromelain and inulin consumption on different fecal parameters including fecal bacterial growth, fecal bacterial enzyme, and SCFA profiles.
Materials and methods
Sample
In this study, bromelain (cat. no. Well3LE) was obtained from Top One Biotech Co. Ltd., Taiwan. The enzyme activity expressed in casein digestion unit (CDU) was about 1400 CDU/g. Inulin, soluble dietary fiber from chicory root (cat. no. AF-01), was obtained from CNI Venture (M) Sdn Bhd, Malaysia.
Diets and experimental design
The study protocol was approved by the Animal Care and Use Committee of National Chung Hsing University. Forty male Sprague Dawley (SD) rats aged seven weeks were purchased from BioLASCO Company, Taiwan. The SD rats weighing from 217.1 to 326.5 g were placed in animal room at 22 ± 1°C, 60 ± 5% humidity, and with 12-h light/dark cycle lightning. All animals were caged individually in a stainless steel cage and were fed chow diet (Laboratory Rodent Diet 5001, PMI Nutrition International/Purina Mills LLC, St. Louis, MO).
After an acclimation for 1 week, animals were divided into eight weight classes of five each. The rats in each weight class were randomly assigned to one of the five diet groups, including one control (feeding chow diet only) and four experimental groups, namely ‘2B’, ‘2I’, ‘B+ I’, and ‘2B+ 2I’ groups. Specifically, the animals in group ‘2B’ were given bromelain at a single dose of 120 CDU/kg bw. Group ‘2I’ was given inulin (260 mg/kg bw). Group ‘B+ I’ was given a mix of bromelain (60 CDU/kg bw) and inulin (130 mg/kg bw) while the group ‘2B+ 2I’ was provided with a mix of bromelain (120 CDU/kg bw) and inulin (260 mg/kg bw).
Throughout the experiment, water and feed were provided ad libitum. The feeding experiment was carried out for 28 days. Food intakes and body weights were recorded daily. Feces were collected, weighed, and analyzed for routine measurements. Some of the fecal samples left unused were stored at – 20°C for further use.
Determination of fecal pH and moisture
According to the methods as described by Chau, Huang, and Chang (Citation2005), fecal samples without urine and feed waste contamination were collected and analyzed for pH and moisture content. Fecal moisture content was determined by drying the fecal sample on aluminum foil trays to a constant weight in a 105°C air-oven. Fecal pH values were measured by homogenizing the fresh feces with deionized H2O in a 1:4 (w/v) ratio, followed by centrifugation at 1,006g for 10 min.
Determination of fecal mucinase activities
Fecal mucinase activity was determined by using the method of Shiau and Chang (Citation1983). Fresh fecal samples were homogenized in 0.01 M phosphate buffer (pH 7.2, 1:50 w/v) for 30 min. After a centrifugation at 1,006g for 10 min, the supernatant was analyzed for mucinase activity. Protein in the supernatant was also determined by a protein assay kit (Cat No 500–0006, Bio-Rad). Mucinase activity, which was expressed as μmol, of reducing sugar released per min per mg of fecal protein was estimated by measuring the amount of reducing sugar released from porcine gastric mucin (M1778, Sigma).
Presumptive enumerations of Bifidobacterium, Lactobacilli and Escherichia coli
Fresh fecal samples were analyzed within 20 min immediately after the collection. The samples were immersed and mixed well in a sterile and anaerobic diluting solution (1:10 w/v). Serial ten-fold dilutions were prepared to acquire desired concentrations for analysis (Shieh, Shang, Liao, Zhu, & Chien, Citation2011). Presumptive counts of Bifidobacterium and Lactobacilli in the solutions were analyzed using Bifidobacterium iodoacetate medium 25 (BIM-25) and Rogosa agar, respectively, in an anaerobic incubator at 37°C for 72 h (Muñoa & Pares, Citation1988). For the enumeration of presumptive E. coli count, LEMB agar (Merck KGaA, Darmstadt, Germany) was used as a selective and differential medium, and cultured in an anaerobic chamber at 37°C for 48 h.
Determination of fecal short-chain fatty acids (SCFAs)
According to the methods as described by Huang, Chu, Dai, Yu, and Chau (Citation2012) with slight modifications, the SCFA concentrations in the fecal samples were determined. Fresh fecal sample was homogenized with cold saline (0.9% w/v) at a ratio of 1:10 (w/v), followed by centrifugation at 1,006g for 10 min. Two millilitres of the supernatant was then mixed with 10 μL of isocaporic acid (internal standard) and 20 μL of 50% (w/v) sulfuric acid. After the SCFA extraction using diethyl ether, 1 μL of the ether layer was analyzed by a column (Agilent J & W HP-INNO Wax GC Column, 30 m, 0.25 mm. 0.25 µm) using a gas chromatograph (Agilent Technologies 7890A, California, USA) fitted with a flame ionization detector. The conditions were as follows: oven temperature, initially held at 80°C for 1 min and raised to 140°C at a rate of 20°C/min, then held at 140°C for another 1 min and raised again to 220°C at a rate of 20°C/min, followed by holding at 220°C for 2 more min; injector temperature, 140°C; detector temperature, 250°C; gas flow rate, 7 mL/min (carrier gas, helium).
Statistical analysis
All determinations expressed in mean ± standard deviation (SD) were analyzed by one-way ANOVA using the software of Statistical Product and Service Solutions (SPSS) (IBM Corp, version 20.0, Armonk, NY, USA). Values of P < 0.05 were considered statistically significant.
Results and discussion
All animals remained healthy and active over the whole experimental period. summarizes the body weight gain, daily food intake, and daily water intake of rats among the five dietary groups. After 28 days of feeding, no apparent differences in the body weight gain (5.8–6.2 g), daily food intake (28.2–29.1 g/day), and daily water intake (42.7–43.9 g/day) were noted among the five groups.
Table 1. Effect of different diets on body weight gain, daily food intake, daily water intake, fecal pH, fecal moisture, and fecal weight of rats.
Tabla 1. Efecto de distintas dietas en el aumento de peso corporal, ingesta alimentaria diaria, ingesta de agua diaria, pH fecal, humedad fecal y peso fecal en ratas.
displays the comparison of fecal pH, fecal moisture and fecal weight in animals fed different diet groups. A significant reduction in fecal pH was observed in the 2B+ 2I group over the control while no apparent differences in fecal pH were noted among the control, 2B, 2I, and B + I groups. As compared with the control, fecal moisture contents were significantly (P < 0.05) increased by the inclusion of bromelain in 2B and 2B+ 2I groups (118–119%). A similar trend was also observed in fecal weight in the 2B and 2B+ 2I groups (123–128%). Since some studies have demonstrated that inulin could increase fecal water content and weight (Drabińska, Zieliński, & Krupa-Kozak, Citation2016; Slavin, Citation2013), our results revealed that the inclusion of inulin in diets at a dose of 260 mg/kg bw were not high enough to trigger an apparent change in these fecal indexes. However, indicates that the feeding of bromelain at a relatively higher dosage (i.e. 2B and 2B+ 2I) was able to increase the moisture content and weight in fecal output. Wen et al. (Citation2006) have also reported that bromelain was capable of increasing fecal moisture content for postoperative disorders and ameliorating constipation problem. More specifically, bromelain might improve defecation by increasing fecal moisture, fecal wet weight, and number of fecal pellets in postoperative rats, at least in part, by inhibiting colonic iNOS overexpression via NF-kappaB pathway.
shows the fecal presumptive counts of Bifidobacterium and Lactobacilli. When comparing with the control group (6.68 log CFU/g), the presumptive Bifidobacterium counts of the 2B (6.71 log CFU/g) and 2I (6.93 log CFU/g) groups did not demonstrate an apparent difference. A slight increase in the bacterial count of the B + I group (7.23 log CFU/g) over the control group was observed, but not as significant (P < 0.05) as the one shown in the 2B+ 2I group (7.95 log CFU/g). As for the presumptive Lactobacilli count, the trend was somehow similar as the one seen in presumptive Bifidobacterium count. There was a major rise (P < 0.05) of the presumptive Lactobacillus count from 7.72 log CFU/g (control group) to 8.64 log CFU/g (2B+ 2I group). It could be induced that consumption of the mixture of bromelain and inulin at a higher dose (2B+ 2I) effectively (P < 0.05) enhanced the growth of Bifidobacterium and Lactobacilli.
Table 2. Changes in the presumptive Bifidobacterium, Lactobacilli, and Escherichia coli counts in fecal samples of rats fed different diet groups.
Tabla 2. Cambios en los presuntivos recuentos de Bifidobacterium, Lactobacilli y Escherichia coli en las muestras fecales de ratas alimentadas con distintas dietas.
As shown in , a dosage of 260 mg/kg bw of inulin (2I group) did not result in any apparent growth in both the Bifidobacterium and Lactobacilli only until bromelain was added in the 2B+ 2I group. Consistent with these findings, animals fed with 260 mg/kg bw of inulin alone in this experiment did not demonstrate any growth promoting effect. Intriguingly, a supplement of bromelain (120 CDU/kg bw) together with inulin (260 mg/kg bw) was found to enhance the growth of these two bacteria to a further extent. With a stimulation of the presumptive counts of Bifidobacterium and Lactobacilli, proteolytic bacteria growth in the colon would decrease and result in a reduction of protein fermentation that produced carcinogenic end products, such as phenols and ammonia (Pattananandecha et al., Citation2016).
Among the five diet groups, there were no significant differences in the presumptive E. coli count. However, merely a slight decline in the presumptive E. coli count between the control (6.11 log CFU/g) and 2B+ 2I (5.65 log CFU/g) groups was noted (). According to Hale (Citation2004), bromelain administered orally might induce proteolytic activity in the intestinal tract, leading to an elimination of cell surface receptors for E. coli. It was inferred that the slight decrease in the presumptive E. coli count between the 2I and 2B+ 2I groups might be partly due to the declined adhesion of E. coli on the intestinal lining by bromelain.
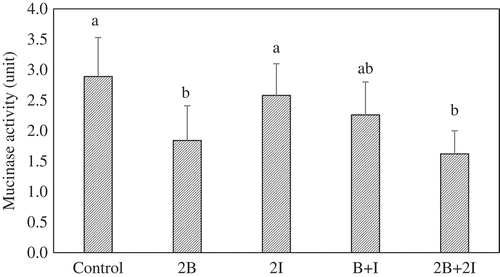
On the intestinal mucosa, mucin serves as a defense barrier layer to protect against bacterial invasion, enzymatic degradation, and toxic substances (Satchithanandam, Klurfeld, Calvert, & Cassidy, Citation1996). Mucinase in the hindgut and feces might catalyze a broad of metabolic transformations and the formation of toxic and carcinogenic substances. It also further hydrolyzes the protective mucin layer to exposes the intestinal cells to harmful substances (Shiau & Chang, Citation1983). illustrates that the basal mucinase activity in the control group was 2.9 units. No significant changes in the fecal mucinase activity were observed in the groups 2I and B + I (2.3–2.8 units) as compared with that of the control. Feeding the rats with bromelain at a higher dosage in both the 2B and 2B+ 2I groups could significantly (P < 0.05) decrease the mucinase activity by −36.3% and −43.9%, respectively, against the control.
Figure 1. Effects of different diets on fecal bacterial mucinase activity.
a-b Bars (mean ± SD, n = 8) among different groups with different letters are significantly different (P < 0.05).c Control: given chow diet only; 2B: given bromelain at a dose of 120 CDU/kg bw; 2I: given inulin at a dose of 260 mg/kg bw; B + I: given a mix of bromelain (60 CDU/kg bw) and inulin (130 mg/kg bw); 2B+ 2I: given a mix of bromelain (120 CDU/kg bw) and inulin (260 mg/kg bw).
Figura 1. Efectos de distintas dietas en la actividad bacteriana en la mucinasa fecal.
a-b Las barras (media ± DE, n = 8) con diferentes letras entre los distintos grupos son significativamente diferentes (P < 0.05).c Control: recibieron solo una dieta de alimentos; 2B: recibieron bromelina a una dosis de 120 CDU/kg bw; 2I: recibieron inulina a una dosis de 260 mg/kg bw; B + I: recibieron una mezcla de bromelina (60 CDU/kg bw) e inulina (130 mg/kg bw); 2B+ 2I: recibieron una mezcla de bromelina de (120 CDU/kg bw) e inulina de (260 mg/kg bw).bw = peso corporal
Based on the above findings, major differences were observed among the control, 2B, and 2B+ 2I groups, while the results of the other groups (including 2I, and B + I) did not differ significantly from the control. Accordingly, the SCFA profiles analyses were solely conducted among the control, 2B, and 2B+ 2I groups (). The changes in fecal SCFA profiles among these three groups were similar to those observed with the elevation in the presumptive counts of Bifidobacterium and Lactobacilli (). In general, SCFA concentrations in hindgut were associated with the consumption level of fermentable carbohydrate and the extent of microbial fermentation (Högberg & Lindberg, Citation2004). The SCFAs (i.e. acetate, propionate, and butyrate) produced in the colon could play an indispensable role in maintaining intestinal lining integrity and suppressing the pathogen growth (Meyer & Stasse-Wolthuis, Citation2009; Slavin, Citation2013).
Figure 2. Changes in the fecal short-chain fatty acids among the control, 2B, and 2I+ 2B groups.
a Bars (mean ± SD, n = 8) of each fatty acid denoted with * differ from its corresponding control significantly (P < 0.05).b Control: given chow diet only; 2B: given bromelain at a dose of 120 CDU/kg bw; 2B+ 2I: given a mix of bromelain (120 CDU/kg bw) and inulin (260 mg/kg bw).
Figura 2. Cambios en los ácidos grasos fecales de cadena corta entre los grupos de control, 2B y 2I+ 2B.
a Las barras (medias ± DE, n = 8) de cada ácido graso indicado con * son significativamente diferentes de su control correspondiente (P < 0.05).b Control: recibieron solo una dieta de alimentos; 2B: recibieron bromelina a una dosis de 120 CDU/kg bw; 2B+ 2I: recibieron una mezcla de bromelina (120 CDU/kg bw) e inulina (260 mg/kg bw).bw = peso corporal
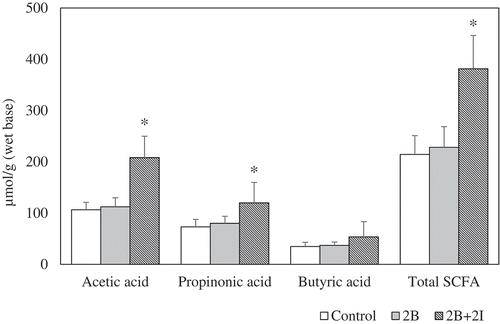
The results depicted that the concentrations of acetic acid, propionic acid, butyric acid, and total SCFA in the fecal samples between the control and 2B groups were comparable to each other, and were found to be 106.5–112.3, 73.1–80.0, 34.7–37.1, and 214.4–228.4 μmol/g, respectively. On the other hand, the fecal SCFA profiles of the 2B+ 2I group showed a consistently higher amounts of acetic acid, propionic acid, and butyric acid, which were up to 195%, 164%, 154%, and 178%, respectively, over the control. Some studies have showed that each of the SCFAs would perform a unique role. For instances, acetate could enhance the mucin secretion, propionate had the capability to decrease the de novo synthesis of fatty acid, and butyrate might suppress the neoplastic alterations in cancer cells (Barcelo et al., Citation2000; Nishina & Freedland, Citation1990; Pryde, Duncan, Hold, Stewart, & Flint, Citation2002).
An elevation in the concentration of SCFAs would stimulate mucosal cells to trigger a peristaltic reflex and hence to shorten gastrointestinal tract time (Grider & Piland, Citation2007). It is speculated that the increased gut motility might reduce the time for water reabsorption and therefore led to a significantly higher fecal moisture in 2B+ 2I group (). The elevated moisture retention in feces might, in turn, support a better growth of intestinal microflora, such as Bifidobacterium and Lactobacilli. A relative higher level of fermentation metabolites (i.e. lactic acid) was produced and led to a greater decline in fecal pH (Meyer & Stasse-Wolthuis, Citation2009).
Conclusion
Based on the above findings in this study, as compared to taking bromelain alone, the administration of inulin together with bromelain could result in some further desirable changes including increased fecal moisture (by 19%), declined fecal mucinase activity (−43.9%), promoted growth of Bifidobacterium and Lactobacilli, and elevated concentrations of various fecal SCFAs (by 54–95%). Our results revealed that the consumption of bromelain and inulin together might exert favorable effects in improving certain fecal parameters. Future investigations should be directed to understanding the possible interactions between bromelain with inulin or some other prebiotic dietary fiber on potentiating the improvement of different fecal parameters.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barcelo, A., Claustre, J., Moro, F., Chayvialle, J. A., Cuber, J. C., & Plaisancié, P. (2000). Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut, 46(2), 218–224.
- Bhattacharyya, B. K. (2008). Bromelain: An overview. Natural Product Radiance, 7(4), 359–363.
- Cencic, A., & Chingwaru, W. (2010). The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients, 2(6), 611–625.
- Chau, C. F., Huang, Y. L., & Chang, F. Y. (2005). Effects of fibre derived from passion fruit seed on the activities of ileum mucosal enzymes and colonic bacterial enzymes in hamsters. Journal of the Science of Food and Agriculture, 85(12), 2119–2124.
- Chau, C. F., & Wu, S. H. (2006). The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends in Food Science & Technology, 17(6), 313–323.
- Dai, F. J., & Chau, C. F. (2017). Classification and regulatory perspectives of dietary fiber. Journal of Food and Drug Analysis, 25(1), 37–42.
- Drabińska, N., Zieliński, H., & Krupa-Kozak, U. (2016). Technological benefits of inulin-type fructans application in gluten-free products–A review. Trends in Food Science & Technology, 56, 149–157.
- Grider, J. R., & Piland, B. E. (2007). The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. American Journal of Physiology-Gastrointestinal and Liver Physiology, 292(1), G429–G437.
- Hale, L. P. (2004). Proteolytic activity and immunogenicity of oral bromelain within the gastrointestinal tract of mice. International Immunopharmacology, 4(2), 255–264.
- Högberg, A., & Lindberg, J. E. (2004). Influence of cereal non-starch polysaccharides on digestion site and gut environment in growing pigs. Livestock Production Science, 87(2), 121–130.
- Huang, Y. L., Chu, H. F., Dai, F. J., Yu, T. Y., & Chau, C. F. (2012). Intestinal health benefits of the water-soluble carbohydrate concentrate of wild grape (Vitis thunbergii) in hamsters. Journal of Agricultural and Food Chemistry, 60(19), 4854–4858.
- Meyer, D., & Stasse-Wolthuis, M. (2009). The bifidogenic effect of inulin and oligofructose and its consequences for gut health. European Journal of Clinical Nutrition, 63(11), 1277.
- Muñoa, F. J., & Pares, R. (1988). Selective medium for isolation and enumeration of Bifidobacterium spp. Applied and Environmental Microbiology, 54(7), 1715–1718.
- Mynott, T. L., Luke, R. K., & Chandler, D. S. (1996). Oral administration of protease inhibits enterotoxigenic Escherichia coli receptor activity in piglet small intestine. Gut, 38(1), 28–32.
- Nishina, P. M., & Freedland, R. A. (1990). Effects of propionate on lipid biosynthesis in isolated rat hepatocytes. The Journal of Nutrition, 120(7), 668–673.
- Olivares, M., Díaz-Ropero, M. P., Gómez, N., Lara-Villoslada, F., Sierra, S., Maldonado, J. A., … Xaus, J. (2006). Oral administration of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. International Journal of Food Microbiology, 107(2), 104–111.
- Pattananandecha, T., Sirilun, S., Duangjitcharoen, Y., Sivamaruthi, B. S., Suwannalert, P., Peerajan, S., & Chaiyasut, C. (2016). Hydrolysed inulin alleviates the azoxymethane-induced preneoplastic aberrant crypt foci by altering selected intestinal microbiota in Sprague–Dawley rats. Pharmaceutical Biology, 54(9), 1596–1605.
- Pryde, S. E., Duncan, S. H., Hold, G. L., Stewart, C. S., & Flint, H. J. (2002). The microbiology of butyrate formation in the human colon. FEMS Microbiology Letters, 217(2), 133–139.
- Roberfroid, M. B. (2005). Introducing inulin-type fructans. British Journal of Nutrition, 93(S1), S13–S25.
- Roxas, M. (2008). The role of enzyme supplementation in digestive disorders. Alternative Medicine Review, 13(4), 307–314.
- Satchithanandam, S., Klurfeld, D. M., Calvert, R. J., & Cassidy, M. M. (1996). Effects of dietary fibers on gastrointestinal mucin in rats. Nutrition Research, 16(7), 1163–1177.
- Schaafsma, G., & Slavin, J. L. (2015). Significance of inulin fructans in the human diet. Comprehensive Reviews in Food Science and Food Safety, 14(1), 37–47.
- Shiau, S. Y., & Chang, G. W. (1983). Effects of dietary fiber on fecal mucinase and β-glucuronidase activity in rats. The Journal of Nutrition, 113(1), 138–144.
- Shieh, M. J., Shang, H. F., Liao, F. H., Zhu, J. S., & Chien, Y. W. (2011). Lactobacillus fermentum improved intestinal bacteria flora by reducing. Clostridium Perfringens. European e-Journal of Clinical Nutrition and Metabolism, 6(2), e59–e63.
- Slavin, J. (2013). Fiber and prebiotics: Mechanisms and health benefits. Nutrients, 5(4), 1417–1435.
- Wen, S., Huang, T. H., Li, G. Q., Yamahara, J., Roufogalis, B. D., & Li, Y. (2006). Bromelain improves decrease in defecation in postoperative rats: Modulation of colonic gene expression of inducible nitric oxide synthase. Life Sciences, 78(9), 995–1002.
