ABSTRACT
Total antioxidant capacity (TAC) and total polyphenol content (TPC) of 52 fruits sold in Spain were evaluated by FRAP, ABTS, DPPH and Folin-Ciocalteu. For berries, TAC was 9, 17 and 31 times greater than for oranges, bananas and apples, respectively. Consumption of unpeeled fruit increases TAC and TPC by 2.4 times. Oranges contribute most to TAC and TPC (33% and 32% respectively) in the Spanish diet. Oranges, strawberries, tangerines, grapes and bananas account for 76% of the TAC of fruits consumed in Spain. The consumption of fruit in Spain has remained stable for the last eight years, but falls below WHO recommendations for daily intake. Greater consumption of fruits is recommended, to increase TAC and TPC and to reduce the risk of developing chronic disease.
RESUMEN
La capacidad antioxidante total (CAT) y el contenido total de polifenoles (PT) de 52 frutas adquiridas en España se evaluaron por FRAP, ABTS, DPPH y Folin-Ciocalteu. Las bayas poseían una CAT de 9, 17 y 31 veces mayor que las naranjas, plátanos y manzanas, respectivamente. El consumo de fruta sin pelar aumenta la CAT y PT en 2,4 veces. Las naranjas son las que más contribuyen a CAT y PT (33% y 32% respectivamente) en la dieta española, si añadimos fresas, mandarinas, uvas y plátanos esta sube al 76%. El consumo de fruta en España se ha mantenido estable durante los últimos ocho años, pero está por debajo de las recomendaciones de la OMS para la ingesta diaria. Se recomienda un mayor consumo de frutas, para aumentar CAT y PT y así reducir el riesgo de desarrollar enfermedades crónicas.
1. Introduction
Fruits and vegetables are important components of a healthy diet and it is widely accepted that their consumption helps prevent chronic diseases. In 2003, the World Health Organization (World Health Organization-WHO, Citation2003) recommended consuming at least 400 g of fruit and vegetables per day (excluding potatoes and other starchy tubers) to prevent chronic disorders such as heart disease, cancer, diabetes and obesity. According to epidemiological studies there is an inverse association between the intake of fruits and vegetables and the risk of cardiovascular disease (Williams & Hord, Citation2005) and cancer (Riboli & Norat, Citation2003). It is not known which dietary constituents are responsible for this association, but it is assumed that antioxidants play a significant role in this respect. Fruits contain a variety of compounds with antioxidant activity, including ascorbic acid, carotenoids and polyphenols such as flavonoids and phenolic acid.
Total antioxidant capacity (TAC) describes the cumulative ability of all antioxidants present in food to scavenge free radicals. It is considered a valid measure of the antioxidant quality of a diet and a means of monitoring the protective effects of plant foods in epidemiological studies (Kamiloglu et al., Citation2016). Different TAC assays have been reported, using various radicals and a variety of measurement methods. The precise evaluation of antioxidant activity requires the use of several assay methods to ensure that different mechanisms of inhibition are included (Frankel & Meyer, Citation2000).
There are not many bibliographical references that analyze a high quantity of fruits of the Spanish market with the same extraction procedure and determination methods, so it is very difficult to use them in epidemiological analyzes.
Spain is the main exporter of fresh fruits and vegetables to the other EU countries, supplying 12.3 million tonnes (7.1 of fruits and 5.2 of vegetables), followed by Germany and France. Of this total, citrus fruits account for 50.35%. Sales of berries (currants, blackberries, strawberries, cranberries, etc.), mainly grown in and around the province of Huelva (SW Spain), constitute 5.42% of all fruit exported from Spain to the EU. In 2016, export volumes of these fruits rose, especially cranberries and raspberries, which increased by 26% (FEPEX (Federación Española de Asociaciones de Productores Exportadores de Frutas, Hortalizas, Flores y Plantas vivas), Citation2016).
The aim of this study is primarily to determine the TAC and TPC of fruits produced and/or sold in Spain, and thus to report a database of this information useful to epidemiological studies. In addition, the intake of TAC and TPC of the most consumed fruits in Spain were estimated, using a panel of fruit consumption data for Spanish households.
2. Materials and methods
2.1. Chemical
2,2´-Azino-bis (3-ethylbenzo-thiazoline-6-sulphonic acid) (ABTS), 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), gallic acid, iron (II) sulphate-7-hydrate, iron (III) chloride hexahydrate, sodium carbonate, sodium acetate, acetone and hydrochloric acid 37% were purchased from Sigma-Aldrich (Madrid, Spain). Potassium persulphate, sodium di-hydrogen phosphate anhydrous and methanol were purchased from Panreac (Barcelona, Spain). Folin-Ciocalteu´s phenol reagent was purchased from Merck. All chemicals used were analytical grade, and the water used was Milli-Q.
2.2. Fruit samples
Fifty-two fruits were obtained from various local markets in Granada (Spain) in 2016. The origin, fruit varieties, scientific name, moisture content, edible portion and weight of each portion are listed in . For some fruits – apples, pears, oranges, plums, melon, grapes and berries – several varieties were chosen for analysis. All samples were obtained at eating ripeness. All assays were performed using the edible portion of the fruit (). Five of the fruits (two varieties of pear and three of apple) were studied both with and without peel (Figure S1). The selection of the fruits was based on those sold in Spain at different times of the year and taking into account the diverse varieties available. Sixteen of these fruits represent 91% of all the fruit consumed in Spain (Table S1) (Ministerio de Agricultura, Alimentación y Medio Ambiente, & MAGRAMA, Citation2017).
Table 1. Fruits analysed, percentage of moisture and edible portion.
Tabla 1. Frutas analizadas, porcentaje de humedad y porción comestible.
Table 2. Total antioxidant capacity (FRAP, TEAC and DPPH) and total polyphenol content of fresh fruits marketed in Spain.
Tabla 2. Capacidad antioxidante total (FRAP, TEAC y DPPH) y polifenoles totales de frutas frescas comercializadas en España.
2.3. Sample preparation
The fresh fruits were cleaned with tap water and separated into edible and non-edible portions, after which the percentage of edible portion was calculated (). The edible portion was chopped and triturated for 10 seconds using an Oster blender, and then homogenised using an IKA T25 digital Ultraturrax homogeniser immersed in an ice bath. The amount of sample homogenised depended on the size of the fruit. The moisture content was determined by gravimetric method according to the AOAC method 934.06 (Association of Official Analytical Chemists (AOAC), Citation1990) and the samples were then stored at −80°C until analysis.
Fruit extracts were obtained following the method described by Contreras-Calderón, Calderón-Jaimes, Guerra-Hernández, and García-Villanova (Citation2011) with slight modifications. Two grams of sample were placed in a capped centrifuge tube and 8 ml of acidic methanol-water (60:40, v/v pH 2) were added, after which the tube was vortexed for 1 min at normal atmosphere in a vortex mixer (Cleaver Scientific Ltd) and shaken at room temperature in a shaker (P-Selecta Rotaterm) for 1 h. The tube was then centrifuged at 9000 rpm/10 min (centrifuge Sigma 2–16 Pk Sartorius) at 4°C and the supernatant was recovered. 8 mL of acetone-water (70:30) were added to the residue, followed by stirring and sonication (P-Selecta Ultrasons) for 1 and 15 min respectively and centrifuged at 4°C and 9000 rpm/10 min. The last extraction was repeated without sonication. The supernatants were combined and transferred to a 25 mL volumetric flask, and acetone-water (70:30) was added to reach a final volume of 25 mL. Finally, the extract was stored at −80°C until analysis.
Two antioxidant extraction methods were assayed prior to the present study. In these assays, of tangerine and kiwi, the FRAP and Folin-Ciocalteu methods were applied to determine the antioxidant capacity and total phenolic content, as follows. Method 1: two extraction solvents: acidic methanol/water (60/40 v/v, pH = 2) and acetone/water (70/30, v/v) and three extraction steps (Contreras-Calderón et al., Citation2011) with slight modifications. Method 2: two extraction solvents: acidic methanol/water (60/40 v/v, pH = 2) and methanol/water (50/50, v/v) and four extraction steps (Rodríguez-Pérez, Quirantes-Piné, Fernéndez-Gutiérrez, & Segura-Carretero, Citation2015). The extraction varied according to the fruit considered and the compounds measured. Thus, with method 1 higher values of FRAP and lower ones of TPC were obtained for tangerine, and vice versa for kiwi; nevertheless, the differences were not very large. Finally, method 1 was chosen because it is a faster procedure, with less reagent expense, and because it facilitates comparison with other Colombian exotic fruits previously analysed by our group (Contreras-Calderón et al., Citation2011).
2.4. Antioxidant assays
2.4.1. Ferric ion reducing antioxidant power (FRAP) assay
The FRAP assay was performed as previously described by Benzie and Strain (Citation1996), 20 μL of test sample, diluted appropriately with water, or Trolox standard, or ferrous sulphate standard or blank (distilled water) were placed in each well of a 96-well polystyrene microplate, after which 280 μL of the FRAP reagent (containing TPTZ, FeCl3 and acetate buffer) freshly prepared and warmed at 37°C were added. The absorbance values at 595 nm after 30 min were measured using a Fluostart omega microplate reader (BMG Labtech, GmbH, Ortenberg, Germany) thermostatted at 37°C. The standard curves were constructed using FeSO4 (115–1150 μM) and Trolox solutions (20–400 μM) and the results are expressed as micromoles of Trolox equivalent (TE) per 100 gram of fresh weight (µmol of TE/100 g of FW) and as µmol of Fe2+ per 100 g of FW.
2.4.2. Trolox equivalent antioxidant capacity (TEAC) assay
The TEAC (ABTS) assay was carried out according to Re et al. (Citation1999). ABTS stock solution was prepared from 7 mM ABTS and 2.45 mM potassium persulphate in a volume ratio 1:1, and then incubated in the dark for 16 h at room temperature. The radical ABTS•+ solution was obtained by diluting ABTS stock solution with phosphate buffer 5 mM at pH = 7.4 to obtain an absorbance of 0.7 ± 0.02 at 730 nm. 30 µL of test sample, diluted appropriately with water, or Trolox standard or blank (distilled water) were placed in each well of a 96-well polystyrene microplate, after which 270 µL of radical ABTS•+ were added. After 30 min at 30°C, the absorbance was measured at 730 nm using a Fluostart omega microplate reader (BMG Labtech). Aqueous solutions of Trolox concentrations (20–200 μM) were used for calibration and the results are expressed as micromoles of Trolox equivalent (TE) per 100 gram of fresh weight (µmol of TE/100 g of FW).
2.4.3. DPPH antioxidant assay
The DPPH (1,1-Diphenyl-2-picryl-hydrazyl) assay was carried out according to Brand-Williams, Cuvelier, and Berset (Citation1995). The working DPPH solution was obtained by dissolving DPPH powder in methanol to obtain an absorbance of 0.7 ± 0.02 at 517 nm. 20 µL of test sample, diluted appropriately with water, or Trolox standard or blank (distilled water) were placed in each well of a 96-well polystyrene microplate, after which 280 µL of working DPPH solution were added. After 30 min at 30°C, the absorbance was measured at 517 nm using a Fluostart omega microplate reader (BMG Labtech). Aqueous solutions of Trolox concentrations (50–500 μM) were used for calibration and the results are expressed as micromoles of Trolox equivalent (TE) per 100 gram of fresh weight (µmol of TE/100 g of FW).
2.4.4. Total phenolic content (TPC)
The total phenolic content was determined using the Folin-Ciocalteu assay (Singleton, Orthofer, & Lamuela-Raventos, Citation1999). 190 µL distilled water were placed in each well of a 96-well polystyrene microplate, and then 30 µL of test sample diluted appropriately with water, or gallic acid standard or blank (distilled water) were added. Finally, 15 µL of Folin-Ciocalteu reagent and 60 µL of 10% sodium carbonate solution were added quickly. The absorbance was measured at 725 nm after 60 min by using a Fluostart omega microplate reader (BMG Labtech) at 30°C. Aqueous solutions of gallic acid (10–100 mg/L) were used for calibration and the results are expressed as mg of gallic acid equivalent (GAE) per 100 grams of fresh weight (mg of GAE/100 g of FW).
2.5. Estimated consumption
Fruit intake in the Spanish diet was estimated from national food consumption data, (Ministerio de Agricultura, Alimentación y Medio Ambiente. (MAGRAMA), Citation2017) which are obtained annually from daily budget questionnaires. The data analysed were obtained from 12,000 households, and from 12,500 in mid-March, with daily purchases being recorded using an optical reader.
2.6. Statistical analysis
The extraction assays were carried out in duplicate, and a triplicate of each extract was analysed. Results are expressed as means ±standard deviation (SD). The normality of the variables analysed, the Pearson correlation coefficients (p < 0.05) and Principal Component Analysis (PCA) were evaluated using Statistica 8.0 software (2007, StatSoft, Tulsa, OK)
3. Results and discussion
3.1. Total antioxidant capacity and total phenolic content
Three antioxidant assays (FRAP, TEAC and DPPH) were applied to obtain accurate evaluations of antioxidant activities because no universal assay can accurately reflect all of the antioxidants in a complex system (Wang et al., Citation2015).
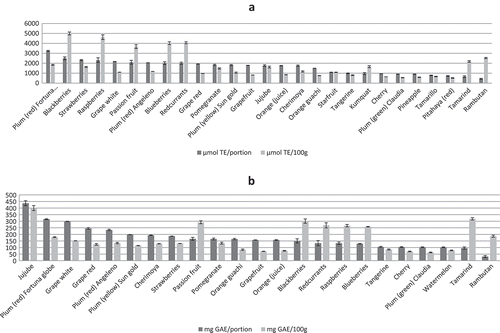
The results obtained by FRAP assay were expressed in two ways, as μmol TE or as μmol Fe2+. The antioxidant capacities of the fruit extracts tested varied from 8.1 to 8634 μmol of Fe2+/100 g (). 30% of the fruits presented values >1000 μmol of Fe2+/100 g and 21% of them were <100 μmol of Fe2+/100 g. The fruits with the highest FRAP values were raspberries, blackberries, redcurrants and blueberries. The fruits with the lower FRAP values were pear var. conferencia, apple var. granny (peeled), and chayote.
Pellegrini et al. (Citation2003) studied 104 foods, including 30 fruits, acquired in markets in Italy. Twenty of these fruits coincided with those analysed in the present study. The antioxidant content in these fruits, according to the FRAP method, ranged from 1.13 mmol Fe2+/kg for watermelon to 51.53 mmol Fe2+/kg for blackberry. Our values are statistically different to those obtained in the Italian study, probably due to differences in the extraction method employed and the varieties of fruit analysed. In the Italian study, as in our own, berries presented the highest antioxidant activity and Cucurbitaceae family presented the lowest values. However, in contrast to our results, TAC values were higher in oranges than in white grapes.
Carlsen et al. (Citation2010) studied the antioxidant capacity of 3100 foods, thus generating a large database. These foods included 278 fruits and fruit juices, purchased in local stores and markets, from Norway and other countries. The antioxidant content in fruits varied from 0.02 mmol Fe2+/100 g FW for watermelon to 55.5 mmol Fe2+/100 g FW in the yellow pith of Spanish pomegranate.
Morales-Soto et al. (Citation2014) analysed 15 kinds of fruits grown in Andalusia (Spain), harvested in different seasons. Nine of these fruits were similar types to those analysed in the present study. The fruits presented variation of 17.7% a value that rose to 40% for quince and cantaloupe.
Although the values obtained by other authors were statistically different from ours, nevertheless, there was a very high correlation between the two sets of results between r = 0.5685–0,857; (p < 0.01).
The antioxidant activity values determined by TEAC method ranged from 70.3 to 4998 μmol of TE/100 g (). 30% of the fruits considered presented values >1000 μmol of TE/100 g and 7% of them had <100 μmol of TE/100 g. Blackberries, raspberries, redcurrants and blueberries had the highest TEAC values. The fruits with the lowest TEAC values were pear var. blanquilla, apple var. red delicious and apple var. granny (peeled, in every case).
Pellegrini et al. (Citation2003) analysed the antioxidant content in fruits, by TEAC, and reported values ranging from 0.64 mmol TE/kg for banana to 20.24 mmol TE/kg for blackberry. The values obtained were statistically different from ours.
The TEAC values determined by Morales-Soto et al. (Citation2014) ranged from 0.0539 mmol TE/100 g for watermelon to 0.8571 mmol TE/100 g for red grape, which are similar to the values we obtained for medlar, melon (galia and cantaloup), quince, mango and red grape among the nine fruits that are coincident in the two studies. These authors obtained a medium CV of 29% for the same samples harvested in different seasons.
Although the values obtained by other authors were statistically different from ours, nevertheless, there was a very high correlation between the two sets of results between r = 0.840–0,8805; (p < 0.01).
The antioxidant capacity of the fruit extracts analysed by DPPH assay ranging from 16.5 to 2210 μmol of TE/100 g (). 12% of the fruits analysed presented values >1000 μmol of TE/100 g and 37% of them had <100 μmol of TE/100 g. The fruits with the highest DPPH values were blackberries, raspberries, redcurrants and blueberries. The fruits with the lowest DPPH values were pear var. ercolini (peeled), pear var. blanquilla (peeled) and pear var. conference.
Similar results were obtained by Stratil, Klejdus, and Kubán (Citation2007) for redcurrants, with approximately 20 mmol TE/kg, and for pear, with approximately 0.1 mmol TE/kg. The DPPH method obtained values that were several times lower than those provided by TEAC, and also somewhat lower than those provided by the FRAP method. The high stability of the DPPH reagent could account for these differences.
The TPC values obtained in the present study ranged from 14.9 to 400 mg of GAE/100 g (). The fruits with the highest content were jujube, tamarind and blackberries. Maracuya, pear var. ercolini (peeled) and paraguayan contained the lowest values.
In some countries, TAC and TPC studies of foods have been performed to create a national database that could be useful in epidemiological studies. Thus, Brat et al. (Citation2006) used the Folin-Ciocalteu method to analyse the polyphenol content of 24 fruits of different origins (including France, Spain, Italy and Morocco) bought in French markets. The values obtained ranged from 7.8 to 264 mg GAE/100 g for melon and strawberries, respectively. Although this range is similar to the one we obtained, and although strawberries and melon occupied the first and last positions, respectively, in both studies, the correlation obtained was low (r = 0.293) and not significant. This could be explained by the different origin and cultivars of the fruits analysed, and also because Brat et al. (Citation2006) considered polyphenols in particular because other reducing substances had been eliminated by solid phase extraction.
The US Department of Agriculture Food Composition Database (USDA, Citation2017) has determined the total polyphenols of 326 foods, including 59 fruits. However, because the fruits analysed were from different origins, the TPC values obtained differ considerably from ours. The correlation between our results and those of the USDA database was r = 0.365 (p < 0.05). The fruits with the highest antioxidant activity according to USDA coincide with ours, i.e., berries.
Correlations higher than r = 0.95 (p < 0.01) were obtained between different TAC methods and the lowest correlations (r = 0.857–0.874; p < 0.001) were obtained between TAC methods and total polyphenols. According to Tabart, Kevers, Pincemail, Defraigne, and Dommes (Citation2009) the weighted average of the results obtained by different assays was calculated to get an overall impression of the antioxidant potential of the samples (). Therefore, the results of antioxidant capacity obtained by the specific assay (DPPH, ABTS and FRAP) was divided by the average activity of the all samples by the same assay, and the calculated values in each assay were added and divided by the number of assays used. TEAC (ABTS) was the method that had the highest correlation with the weighted average antioxidant capacity.
Principal component analysis (PCA) was applied to samples and methods to know their distribution. The two first principal components accounted for 98% of total system variability (Figure S2). According to principal component analysis, PC1 discriminate between the samples with high TPC content (TPC values >250 mg GAEs/100 g FW) from the others. Morever, considering the high values of TPC group, PC2 discriminate between berry fruits from tamarind, jujube and passion fruit.
Apples and pears were analysed with and without peel, to reflect varying consumption habits.
The antioxidant capacity of these fruits was found to be greater with than without peel. The differences ranged from 1.11 times for apple var. red delicious measured by DPPH to 12.43 times for apple var. granny measured by FRAP and were statistically significant (p < 0.05) except for apple var. red delicious measured by DPPH.
The difference in TPC, in favour of fruits with peel, was higher for apples than pears, ranging from 1.12 times for apple var. golden to 2.6 times for var. red delicious. However, a mean difference of 1.15 times was obtained for pears. Figure S1 shows the results obtained by TEAC and TPC assay.
Wang et al. (Citation2015) studied TAC by FRAP, ABTS and DPPH and TPC in the peel and flesh of apples from China and the USA, extracted with 70% methanol. These authors reported higher values in peel than in flesh: ranging from 2.3 times for ABTS to 38 times for the FRAP assay.The smaller differences found in our study of whole fruits in comparison with others in which the peel was separated from the flesh may be due to the small proportion of the peel in the fruits analysed in the varieties grown in Europe.
3.2. TAC and TPC per 100 g or per portion
From the nutritional standpoint, it is very interesting to establish the antioxidant capacity and total polyphenol content per portion, since nutritional recommendations (dietary guidelines) are usually expressed in terms of portions. Thus, in Spain adults are advised to consume at least five portions per day of fruits and vegetables (Aranceta et al., Citation2016). compares the TAC measured by TEAC and TPC per 100 g and per portion of fruits (see also ). It can be seen that the contribution of antioxidants varies according to the fruit. One hundred grams of berries contributed more to TAC and TPC than the same quantity of other fruits. Although the portion of berries contained high values of TAC and TPC, other fruits such as plums, grapes and oranges also presented high values. However, other fruits such as rambutan and tamarind contained low TAC and TPC values per portion.
3.3. Antioxidant capacity provided by fruit consumption in the Spanish diet
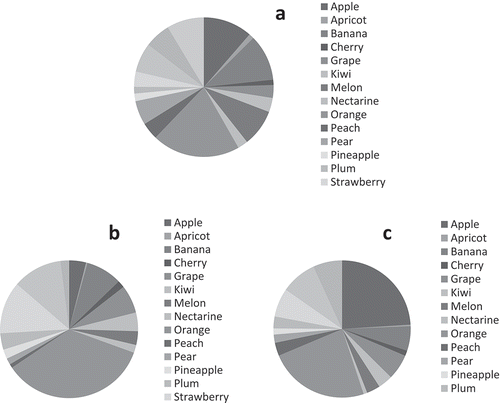
According to the MAGRAMA (Citation2017) study, the panel of food consumption for 2016 recorded values for fresh fruit consumption of 272.7 grams/person/day (207 g/p/d edible portion) (Table S1) corresponding to eighteen fruits. However, only the consumption of sixteen fruits was specified; avocado and lemon were included in ‘other fruit’, which accounted for 9% (). Oranges were most commonly consumed, followed by apples and bananas. The fruits that were least consumed were apricots and cherries.
Figure 2. Contribution to the daily intake of total antioxidant capacity and total polyphenol content according to fruit consumption: a. Percentage of fruits consumed in Spain; b. Antioxidant capacity (TEAC); c. Total polyphenols.
Figura 2. Contribución a la ingesta diaria de capacidad antioxidante total y polifenoles totales según el consumo de fruta: a. Porcentaje de frutas consumidas en España; b. Capacidad antioxidante (TEAC); c. Polifenoles totales
Analysis shows that the consumption of fruit decreased between 2008 and 2016, from 285.7 to 272.7 g/p/d, a reduction of 4.54%. The fruits that underwent the greatest falls in consumption were plums (31%), pears and peaches (27%) and citrus fruits (17%). Those for which consumption increased were watermelon (16%) and grapes, apricots, strawberries and bananas (8%).
The fresh fruit consumption in Spain in 2017 was 207 g/p/d edible portion (Table S1). Other authors compared (Pérez-Jiménez & Saura-Calixto, Citation2015) fruit consumption levels in four European countries: France, Germany, Netherlands and Spain, and reported values of 176, 160, 144, 219 g/p/d (edible portion) respectively, concluding that Spain is the country where most fruit is consumed.
The WHO recommends the regular consumption of 400 g/day of fruits and vegetables (Aranceta et al., Citation2016). In Spain, fruits contribute 4.5% to daily energy consumption, but this varies according to age; from a little over 2% in children to more than 8% in adults aged over 65 years. At present, the average consumption is around 270 g/day, although in those aged over 65 years it is 400 g.
According to consumer panel data, the fruits that provided the greatest antioxidant capacity were oranges (35%), followed by strawberries, tangerine and bananas (). The consumption of oranges, strawberries, tangerines, bananas and grapes contributed 75% of antioxidant capacity intake. The fruits found to have least antioxidant capacity were apricots, peaches, nectarines, cherries, pineapples, pears and watermelon.
Similar percentages were obtained for total polyphenols, except for apples, banana, oranges, pear, tangerine, watermelon and strawberries. The consumption of oranges, apples, tangerines, watermelon and strawberries contributes 70% of the total polyphenol intake.
According to the Phenol Explorer Database (Neveu et al., Citation2010), the individual polyphenols obtained from fruits consumed in Spain are distributed as follows (Ministerio de Agricultura, Alimentación y Medio Ambiente, (MAGRAMA), Citation2017) (Table S2): 26.7% of total polyphenols comes from flavanones, 25.1% from flavanols, approximately 22% from anthocyanins, 18.3% from hydroxycinamic acids and 5.5% from lignans and, to a lesser extent, other phenolic compounds. The fruit that contribute most flavanones are oranges, flavanols mainly come from apples and peaches, anthocyanins mainly come from strawberries, grapes and cherries. Apple, plums and cherries provide hydroxycinamics acids. Lignans are derived from oranges, melons and tangerines.
In a study conducted in a neighbouring country, France, Brat et al. (Citation2006) found that the largest polyphenol contribution achieved through the consumption of fruit was obtained from apples, strawberries and grapes, which accounted for 70–80% of the polyphenol intake from fruit. In Portugal (Pinto et al., Citation2013), the consumption of fruit during 2010 was 365.6 g/day which was higher than in Spain during the same year (280 g/p/d). However, the data collection method used in the latter study was different from ours, being based on an online questionnaire of consumption frequency, which was answered by 1220 people. The fruits most consumed in Portugal were apples (23%) followed by citrus fruits (15%) and berries, cantaloup and bananas (10% each). The total polyphenols ingested in Portugal through fruit consumption is 783.9 mg GAE/day, almost five times higher than in Spain. This difference may be due to the greater consumption of fruits and/or to the fact that Pinto et al. (Citation2013) used the Phenol Explorer Database to determine the polyphenol content of the fruit (Neveu et al., Citation2010).
The intake of antioxidants from fruit in the UK population (Haleem, Barton, Borges, Crozier, & Anderson, Citation2008) is less than in Spain, although it varies by region, being higher in London and SE England. The main five fruits contributing to high intakes of antioxidant were apples, strawberries, oranges, pears and bananas, which varies from the results for Spain, where apples and pears do not appear, being replaced by grapes and plums.
If 400 g of fruit were consumed daily, as recommended in the Nutritional Goals for the Spanish Population (Sociedad Española de Nutrición Comunitaria, Citation2011) the intake of antioxidants (measured by TEAC) would be approximately 1400 μmol/day.
For people who find it difficult to meet the recommendation of 3–4 portions of fruit a day, a good strategy to obtain the benefits indicated by the WHO would be to modify the type of fruit consumed. In Spain, this could mean increasing the consumption of strawberries, plums and grapes and also including berries, which are grown in Spain.
4. Conclusions
The berries analysed (blackberry, blueberry and redcurrant) are the fruits presenting the highest levels of TAC and TPC, although their level of consumption in Spain is very low. Oranges contribute most dietary TAC and TPC. The Spanish population does not meet the recommended levels of fruit intake to obtain the health benefits indicated by the WHO, and therefore the consumption of fruits should be increased or the pattern of intake modified.
Supplemental Material
Download Zip (189.3 KB)Acknowledgments
This paper and results presented constitute part of Arancha Ruiz-Torralba’s Doctoral Thesis performed in the Nutrition and Food Science Doctorate Program of the University of Granada. She was supported by a Research Fellowship from the Government of Spain (FPU16/02536). We wish to thank Glenn Harding for assisting with the translation into English.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
References
- Aranceta, J. B., Arija, V. V., Maíz, E. A., Martínez, E. D. V. M., Ortega, R. A., Pérez-Rodrigo, C., … Salvador, G. C. (2016). Dietary guidelines for the spanish population (SENC, diciembre 2016); the new graphic icon of healthy food. Nutrición Hospitalaria, 33(suppl 8), 1–48.
- Association of Official Analytical Chemists (A.O.A.C.). (1990). Method 934.06. In Association of Official Analytical Chemists (A.O.A.C.) (Eds.), Official methods of analysis of the AOAC (15th ed.). (p. 912). Arlington, VA: EEUU.
- Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76.
- Brand-Williams, W., Cuvelier, M., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30.
- Brat, P., George, S., Bellamy, A., Chaffaut, L. D., Scalbert, A., Mennen, L., … Amiot, M. J. (2006). Daily polyphenol intake in france from fruit and vegetables. The Journal of Nutrition, 136(9), 2368–2373.
- Carlsen, M. H., Halvorsen, B. L., Holte, K., Bohn, S. K., Dragland, S., Sampson, L., … Sanada, C. (2010). The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutrition Journal, 9(1), 3.
- Contreras-Calderón, J., Calderón-Jaimes, L., Guerra-Hernández, E., & García-Villanova, B. (2011). Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from colombia. Food Research International, 44(7), 2047–2053.
- FEPEX (Federación Española de Asociaciones de Productores Exportadores de Frutas, Hortalizas, Flores y Plantas vivas). (2016). FEPEX. exportación/importación española de frutas y hortalizas - datos del sector. Retrieved from http://www.fepex.es/datos-del-sector/exportacion-importacion-espa%C3%B1ola-frutas-hortalizas
- Frankel, E. N., & Meyer, A. S. (2000). The problems of using one‐dimensional methods to evaluate multifunctional food and biological antioxidants. Journal of the Science of Food and Agriculture, 80(13), 1925–1941.
- Haleem, M. A., Barton, K. L., Borges, G., Crozier, A., & Anderson, A. S. (2008). Increasing antioxidant intake from fruits and vegetables: Practical strategies for the scottish population. Journal of Human Nutrition and Dietetics, 21(6), 539–546.
- Kamiloglu, S., Toydemir, G., Boyacioglu, D., Beekwilder, J., Hall, R. D., & Capanoglu, E. (2016). A review on the effect of drying on antioxidant potential of fruits and vegetables. Critical Reviews in Food Science and Nutrition, 56(sup1), S129.
- Ministerio de Agricultura, Alimentación y Medio Ambiente, (MAGRAMA). (2017). Informe del consumo de alimentación en españa 2016.
- Morales-Soto, A., García-Salas, P., Rodríguez-Pérez, C., Jiménez-Sánchez, C., de la Luz Cádiz-Gurrea, M., Segura-Carretero, A., & Fernández-Gutiérrez, A. (2014). Antioxidant capacity of 44 cultivars of fruits and vegetables grown in andalusia (spain). Food Research International, 58, 35–46.
- Neveu, V., Perez-Jiménez, J., Vos, F., Crespy, V., Du Chaffaut, L., Mennen, L., … Wishart, D. (2010). Phenol-explorer: An online comprehensive database on polyphenol contents in foods. Database, 2010, bap024.
- Pellegrini, N., Serafini, M., Colombi, B., Del Rio, D., Salvatore, S., Bianchi, M., & Brighenti, F. (2003). Total antioxidant capacity of plant foods, beverages and oils consumed in italy assessed by three different in vitro assays. The Journal of Nutrition, 133(9), 2812–2819.
- Pérez-Jiménez, J., & Saura-Calixto, F. (2015). Macromolecular antioxidants or non-extractable polyphenols in fruit and vegetables: Intake in four european countries. Food Research International, 74, 315–323.
- Pinto, P., Cardoso, S., Pimpao, R. C., Tavares, L., Ferreira, R. B., & Santos, C. N. (2013). Daily polyphenol intake from fresh fruits in portugal: Contribution from berry fruits. International Journal of Food Sciences and Nutrition, 64(8), 1022–1029.
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237.
- Riboli, E., & Norat, T. (2003). Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. The American Journal of Clinical Nutrition, 78(3), 569S.
- Rodríguez-Pérez, C., Quirantes-Piné, R., Fernéndez-Gutiérrez, A., & Segura-Carretero, A. (2015). Optimization of extraction method to obtain a phenolic compounds-rich extract from moringa oleifera lam leaves. Industrial Crops and Products, 66, 246–254.
- Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in enzymology. 299, 152–178.
- Sociedad Española de Nutrición Comunitaria. (2011). Objetivos nutricionales para la población española. Rev.Esp.Nutr.Comunitaria, 4, 178–199.
- Stratil, P., Klejdus, B., & Kubán, V. (2007). Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta, 71(4), 1741–1751.
- Tabart, J., Kevers, C., Pincemail, J., Defraigne, J., & Dommes, J. (2009). Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chemistry, 113(4), 1226–1233.
- USDA (United States Department of Agriculture). (2017). Agricultural Research Service. Food composition databases. Retrieved from https://ndb.nal.usda.gov/ndb/
- Wang, X., Li, C., Liang, D., Zou, Y., Li, P., & Ma, F. (2015). Phenolic compounds and antioxidant activity in red-fleshed apples. Journal of Functional Foods, 18, 1086–1094.
- Williams, M. T., & Hord, N. G. (2005). The role of dietary factors in cancer prevention: Beyond fruits and vegetables. Nutrition in Clinical Practice, 20(4), 451–459.
- World Health Organization, & (WHO). (2003). Fruit and vegetable promotion initiative/a meeting report/25-27/08/03. Retrieved from http://apps.who.int/iris/bitstream/handle/10665/68395/WHO_NMH_NPH_NNP_0308.pdf;jsessionid=10DB1FBE9367CDFD9A06FE4D562F3C00?sequence=1