 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This research was conducted to identify the optimum harvest maturity stage/s that yielded the highest nutritional quality and longest marketable shelf-life. “TY Megaton” and “Yureka” cultivars were harvested at the breaker, pink, and red stages and stored up to 20 days at 12°C with 85 ± 5% relative humidity (RH). Quality attributes, the contents of important secondary metabolites, and antioxidant activity were assessed during the storage period. Storing and marketing of tomato fruits up to 3 weeks could be possible. However, a gradually decreasing trend of ascorbic acid and total phenolic content was observed as the maturity stage and storage period proceeded, irrespective of the cultivar. Results of lycopene content and antioxidant activity suggest that better nutritional benefit from the breaker and pink stages could be obtained after 16 and 12 days of storage, respectively, and from the beginning to eighth day of storage for the red stage.
RESUMEN
La presente investigación se propuso identificar las etapas de maduración y cosecha de cultivares de tomate óptimas para obtener un producto de mayor calidad nutricional y mayor vida útil comercial. Con este objetivo se cosecharon los cultivares “TY Megaton” y “Yureka” en sus etapas de verde maduro, rosado y rojo, almacenándose posteriormente hasta 20 días a 12°C y a una humedad relativa (RH) de 85±5%. Durante el periodo de almacenamiento se valoraron los atributos de calidad, los contenidos de metabolitos secundarios importantes y la actividad antioxidante. Tras este proceso se determinó que los tomates pueden ser almacenados y comercializados durante un periodo máximo de tres semanas. Sin embargo, a medida que transcurrían las etapas de maduración y continuaba el periodo de almacenamiento se observó que, sin importar el cultivar, el ácido ascórbico y el contenido fenólico total tendieron a disminuir. Los registros tomados del contenido de licopeno y la actividad antioxidante sugieren que puede obtenerse mayor beneficio nutricional de las etapas verde maduro y rosado después de 16 y 12 días de almacenamiento, respectivamente, y desde el primero hasta el octavo día de almacenamiento para la etapa rojo.
1. Introduction
The tomato (Solanum lycopersicum L.) is a Solanaceae vegetable crop that believed to have originated from the Andean region of South America; its popularity during the last half-century causes an expanded cultivation to large scale (Preedy & Watson, Citation2008). Tomato is an economically important crop, with a worldwide production of 177.04 million tons from 4.78 million ha valued at $95.62 billion. China leads the global tomato cultivation, producing about 56.42 million tons annually, followed by India, which produces 18.40 million tons (FAOSTAT, Citation2016). According to FAOSTAT (Citation2016), tomato production in the Republic of Korea was 466,992 tons from 7,132 ha in 2016, which was valued at $682.43 million.
Tomatoes are widely consumed in different forms and can provide a significant amount of secondary metabolites which are responsible for providing free radical scavenging effects (Borguini & Torres, Citation2009; Lenucci, Cadinu, Taurino, Piro, & Dalessandro, Citation2006). The development of variety of cancers (Giovannucci, Rimm, Liu, Stampfer, & Willett, Citation2002; Martí, Roselló, & Cebolla-Cornejo, Citation2016) and cardiovascular diseases (Rao & Rao, Citation2007; Sesso, Liu, Gaziano, & Buring, Citation2003) have been shown to be prevented through the regular and sufficient intake of fresh tomatoes or tomato products.
Evaluation of different varieties and cultivars for selection and identification of the superior genotype for high antioxidant capacity (Brovelli, Citation2006) and storage at proper storage conditions to avoid chilling injury (Maul et al., Citation2000; Roberts, Sargent, & Fox, Citation2002) have been subjects of active research in the last decades.
Tomatoes can be harvested at stages of maturity ranging from mature green stage to full ripe. Harvesting before the climacteric rise is considered as the best strategy to prolong shelf-life and reduce spoilage rate (Saltveit, Citation2005). Since fully ripened tomatoes are prone to damage, mature green or breaker stage tomatoes can be harvested and ripened during transit or at destination (Kader, Stevens, Albright-Holton, Morris, & Algazi, Citation1977). Meanwhile, tomato fruits could be harvested at full-ripe red stage for immediate consumption but its shelf-life is too short due to loss of texture and sensitivity to the attack of phytopathogenic fungi. So, it is necessary to explore the appropriate maturity stages during picking for optimum benefit of the consumers in both storability and nutritional qualities. Therefore, the aim of this work was to identify the optimum harvest maturity stage/s for better nutritional quality and longer marketability of the common locally grown “TY Megaton” and “Yureka” tomato cultivars stored at 12°C and 85 ± 5% relative humidity (RH).
2. Materials and methods
2.1. Tomato samples and storage conditions
“TY Megaton” and “Yureka” tomato cultivars were grown at Gangwon province of South Korea, in the greenhouse during spring 2016. Biological color chart of USDA (Citation1991) was used to select fruit maturity level in the field and harvested at breaker, pink, and red stages. In order to secure uniform maturity stage of sample fruits, a careful selection was made again in the laboratory. After washing and wiping to dryness, fruits were placed in a commercial packaging box of tomatoes and stored up to 20 days at 12°C and 85 ± 5 % RH. Data for all the parameters were taken at 4 days' interval.
2.2. Physicochemical changes
2.2.1. Fresh weight loss and firmness
The methods described by Tilahun et al. (Citation2018) were used to determine fresh weight loss and firmness of tomato during the storage period. Weight loss was measured by subtracting the sample weight at the end of the storage period from its previous recorded weight at the beginning of storage; the result was presented as the percentage (%) of weight loss compared with its initial weight. Measurements of the firmness of the fruit were taken at the equator of the fruit by a Rheo meter (Sun Scientific Co. Ltd., Tokyo, Japan) fitted with a 3 mm diameter round stainless steel probe having a flat end, and results were expressed in N.
2.3. Respiration and ethylene production rate
Respiration rate of tomato was measured as a function of CO2 concentration by using the closed system method and expressed as mg CO2 kg−1.h−1. Tomato fruit samples were placed in airtight 4 L volume container, and CO2 concentration was analyzed at the beginning and after 3 h (Belew, Park, Tilahun, & Jeong, Citation2016; Neelam, Mehar, Puneet, Manoj, & Pravendra, Citation2003) of incubation using PBI Dan-sensor (Check mate 9900, Ringsted, Denmark) gas analyzer. To measure ethylene production rate, a gastight syringe was used to take 1 mL gas sample from a headspace of each airtight 4 L volume containers and to inject into a gas chromatography (Shimadzu Corporation, Kyoto, Japan). BP 20 Wax column (30 m 0.25 mm
0.25 µm, SGE Analytical Science, Ringwood, Australia) and a flame ionization detector (FID) were equipped with the gas chromatography. The operating temperature for detector and injector was 127°C, and the oven was set at 50°C; flow rate of the carrier gas (N2) was 0.67 mL.s−1. The result was expressed as μL C2H4 kg−1.h−1.
2.3.1. Color changes
Hunter a* (redness), b* (yellowness), and L* (brightness) values (McGuire, Citation1992) were determined using a CR-400 Chroma meter (Minolta, Tokyo, Japan) from the sides (near to the equatorial section) of three tomatoes, and three readings from each and the average was determined.
2.3.2. Soluble solid content (SSC), titratable acidity (TA), and pH
SSC, TA, and pH were measured according to Tilahun, Park, et al. (Citation2017a). Atago DR-A1 digital refractometer (Atago Co. Ltd., Tokyo, Japan) was used to measure soluble solid content (SSC) from five sample fruits at 20ºC and expressed in %.
Titration of the diluted tomato juice (1 mL juice: 19 mL distilled water) was done with 0.1 N NaOH up to pH 8.1 using a DL22 Food and Beverage Analyzer (Mettler Toledo Ltd., Zurich, Switzerland) to obtain TA, and the result was expressed as mg.100 g−1 of fresh tomato weight. pH was measured with a Mettler Toldo InLab 413 pH meter.
2.4. Antioxidant properties
2.4.1. Lycopene content
Lycopene content was determined from triplicate tomato samples according to the method of Fish, Perkins-Veazie, and Collins (Citation2002), with some modifications as stated by Tilahun et al. (Citation2018).
2.4.2. Ascorbic acid
Vitamin C was analyzed using reversed-phase liquid chromatography with UV detection, according to the method described by Kim et al. (Citation2011).
2.4.3. Total phenolics
Total phenolic content was quantified using a method described by Tilahun, Park, et al. (Citation2017a).
2.4.4. Antioxidant activity
Using duplicates of the same extracts used to quantify total phenolic content analyses, antioxidant activity was measured by spectrophotometric assay (Thermofisher Scientific, Madison, WI, USA) and the DPPH method was reported by Pataro, Sinik, Capitoli, Donsì, and Ferrari (Citation2015).
2.5. Statistical analysis
The experiment was conducted in a completely randomized design with nine replications for color; five replications (weight loss, ethylene production rate, respiration rate, firmness, SSC, TA, and pH); and in triplicate for lycopene, ascorbic acid, total phenolics, and antioxidant activity. Analysis of variance (ANOVA) at p < 0.05 was done by using SAS (SAS/STAT® 9.1, SAS Institute Inc., Cary, NC, USA) statistical software. When significant differences were detected, the Duncan’s multiple range test was performed to determine which particular means were significantly different (p < 0.05).
3. Results and discussion
3.1. Physicochemical changes
3.1.1. Weight loss and firmness
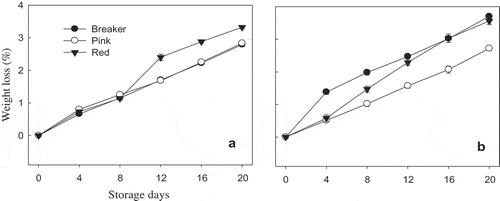
The percentage weight loss increased significantly (p < 0.05) as the storage period proceeded in all the three maturity stages of both cultivars (). “TY Megaton” cultivar that harvested at breaker and pink stages were ripened into well-colored fruits with a similar rate of water loss during storage at 12°C. The weight loss after 3 weeks of storage period was 2.79% and 2.83% for breaker and pink maturity stages, respectively. In the case of red maturity stage, the highest (3.32%) weight loss of “TY Megaton” was recorded at the end of 20 days' storage period (). On the other hand, “Yureka” cultivar exhibited lowest weight loss (2.72%) at the end of 20 days storage period for pink maturity stage. Unlike “TY Megaton” cultivar, “Yureka” cultivar has shown relatively high water loss of 3.70% for breaker stage and 3.56% for red stage. The maximum acceptable weight loss before a tomato becomes unsaleable has been reported to range from 6% to 7% (Nunes, Citation2008). Getinet, Seyoum, and Woldetsadik (Citation2008) also suggested 10% physiological loss in weight to be considered as an index of termination of the shelf-life (threshold level) of commodities. In this study, the weight loss values of all the three maturity stages of both cultivars were found lower than those suggested by Nunes (Citation2008) and Getinet et al. (Citation2008) at the end of storage period; it could be due to the storage at the recommended temperature (12°C) with proper management of RH at 85 ± 5%. Considering the significant difference between cultivars on weight loss in the present study and as reported by Tilahun, Park, et al. (Citation2017a) for the same cultivars, it could be explained that tomato cultivars markedly vary in response to weight loss at different maturity stages.
Figure 1. Fresh weight loss percentage of “TY Megaton” (A) and “Yureka” (B) tomato cultivars as affected by maturity stages during the 20 days of storage at 12°C and 85 ± 5% RH. Each data point is the mean of five sample replicatesstandard error.
Figura 1. Porcentaje de pérdida de peso en seco de los cultivares de tomate “TY Megaton” (A) y “Yureka” (B) en relación con las etapas de maduración durante 20 días de almacenamiento a 12°C y a una RH de 85 ± 5%. Cada dato representa la media de cinco réplicas de la muestra ± error estándar.
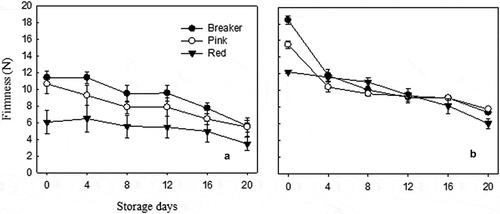
Firmness of both cultivars was reduced significantly (p < 0.05) as the storage period proceeded in all the three maturity stages (). The highest firmness of 18.45 N was recorded from “Yureka” at breaker stage immediately after harvest and the lowest firmness of 3.45 N was recorded from “TY Megaton” at red stage after 20 days of storage. The results revealed that tomato cultivars differ in response to firmness at different maturity stages; “Yureka” was firmer than “TY Megaton” at all stages throughout the storage period. The firmness of both cultivars at three maturity stages was above the minimum limit of marketing after 3 weeks of storage at 12°C and 85 ± 5% RH. Two possible minimum firmness limits suggested by Batu (Citation2004) for tomato fruits at the point of retail marketing and at home were 1.45 N and 1.28 N, respectively.
Figure 2. Firmness of “TY Megaton” (A) and “Yureka” (B) tomato cultivars as affected by maturity stages during the 20 days of storage at 12°C and 85 ± 5% RH. Each data point is the mean of five sample replicatesstandard error.
Figura 2. Firmeza de los cultivares de tomate “TY Megaton” (A) y “Yureka” (B) en relación con las etapas de maduración durante 20 días de almacenamiento a 12°C y a una RH de 85 ± 5%. Cada dato representa la media de cinco réplicas de la muestra ± error estándar.
3.1.2. Ethylene production and respiration rate
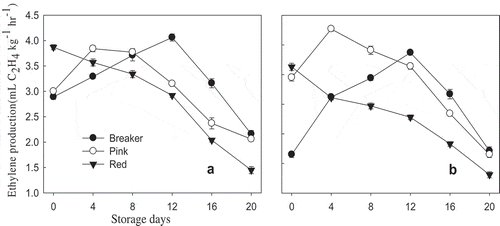
Significant (p < 0.05) differences were observed between maturity stages on ethylene production rate of both varieties. Ethylene production rate of both cultivars followed the same trend of increment up to day 12 and was reduced significantly (p < 0.05) after the twelfth day of storage for breaker stage. In the case of pink stage, ethylene production rate of both cultivars increased up to the fourth day of storage and decreased afterward. Red stage showed a continuous decreasing trend from day 0 up to the final storage day (). Nonsignificant (p > 0.05) difference was observed between breaker and pink stages on day 0 for “TY Megaton,” while Yureka exhibited a significant (p < 0.05) difference between breaker and pink stages and the lowest (2.15 μL.kg−1.h−1) was observed from breaker stage at the beginning of the storage period. de Jesús Dávila-Aviña et al. (Citation2011) reported 3.61 and 2.6 μL.kg−1.h−1 for breaker and pink stage “Grandela” tomato cultivar, respectively; they also reported the highest ethylene production of 5.7 and 8.0 μL.kg−1.h−1 for breaker and pink tomatoes, respectively, on the second day of storage at 20°C.
Figure 3. Ethylene production rate of “TY Megaton” (A) and “Yureka” (B) tomato cultivars as affected by maturity stages during the 20 days of storage at 12°C and 85 ± 5% RH. Each data point is the mean of five sample replicatesstandard error.
Figura 3. Tasa de producción de etileno de los cultivares de tomate “TY Megaton” (A) y “Yureka” (B) en relación con las etapas de maduración durante 20 días de almacenamiento a 12°C y a una RH de 85 ± 5%. Cada dato representa la media de cinco réplicas de la muestra ± error estándar.
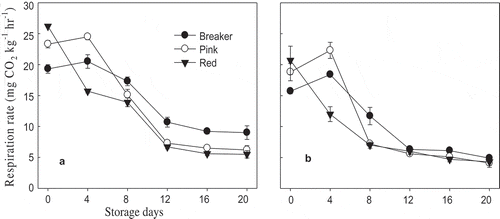
Respiration rate of both varieties was significantly (p < 0.05) affected by maturity stages. The highest respiration rate was observed on day 0 for red (26.2 and 20.7 mL.kg−1.h−1) and on day 4 for pink (24.5 and 22.3 mL.kg−1.h−1) “TY Megaton” and “Yureka”, respectively (). The lowest respiration rates up to day 4 for both cultivars were observed on breaker stage, but it was higher than both pink and red stages after day 4. Fast decline of respiration rate was observed in red stage and followed by pink and breaker, respectively, on both varieties. Lee, Sargent, and Huber (Citation2007) reported a similar trend of respiration rate; they reported the highest respiration rate (33 mL.kg−1.h−1) for Roma type tomatoes. de Jesús Dávila-Aviña et al. (Citation2011) measured initial CO2 production rate of “Grandela” tomato cultivar grown in greenhouse and reported 33.4 and 31.5 mL.kg−1.h−1 for the breaker and pink stages, respectively, at 20. In our study, the delay to reach the peaks in ethylene production rate for both breaker and pink stages and lower respiration rates for all the three stages than previously reported by other authors could be due to the effect of storage temperature.
Figure 4. Respiration rate of “TY Megaton” (A) and “Yureka” (B) tomato cultivars as affected by maturity stages during the 20 days of storage at 12°C and 85 ± 5% RH. Each data point is the mean of five sample replicatesstandard error.
Figura 4. Tasa de respiración de los cultivares de tomate “TY Megaton” (A) y “Yureka” (B) en relación con las etapas de maduración durante 20 días de almacenamiento a 12°C y a una RH de 85 ± 5%. Cada dato representa la media de cinco réplicas de la muestra ± error estándar.
3.1.3. SSC, TA, and pH
The present study revealed that there was a significant (p < 0.05) interaction between cultivars and maturity stages on the biochemical characteristics of tomatoes (). SSC of “TY Megaton” remained to be higher than “Yureka” under all maturity stages. On the breaker stage, the maximum SSC for “TY Megaton” (5.86%) was recorded on day 8, and for “Yureka” (5.72%) the same was recorded on day 12; on the pink stage, maximum SSC was revealed on day 4 for both “TY Megaton” (6.00%) and “Yureka” (5.64%). For the red stage, “TY Megaton” (6.32%) and “Yureka” (5.68%) were recorded on the eighth day of storage. It has been reported that SSC increases as the maturity proceeds (Getinet et al., Citation2008; Renquist & Reid, Citation1998), and the changes are dependent on ripeness at harvest, storage temperature, and storage period (Tilahun, Park, et al., Citation2017a, Citation2017b; Žnidarčič & Požrl, Citation2006). The TA decreased as the maturity stage proceeded in both cultivars (). The minimum (0.65 mg.100g−1) for “TY Megaton” was observed on red stage, while the maximum acidity (1.00 mg.100g−1) was observed on the breaker stage. Similarly, for “Yureka,” the minimum (0.51 mg.100g−1) and maximum (1.27 mg.100g−1) were recorded on the red and breaker stages, respectively. Getinet et al. (Citation2008) also reported a decrease in TA as maturity and storage period proceed. The reduction in acidity as the maturity proceeded might be associated with the conversion of organic acid into sugar and their derivatives or their utilization in respiration (Rai et al., Citation2012). The range of pH in the present study varied from 4.23 to 5.05 in “TY Megaton” and 4.19 to 5.90 in “Yureka” (). As the storage days proceeded, there was an increasing trend of pH on both cultivars. Rai et al. (Citation2012) studied four cultivars of tomato and found that the pH varied from 3.43 to 4.63.
Table 1. Interaction effect of cultivars and maturity stage on soluble solid content (SSC), titratable acid (TA), and pH of tomato fruit during 20 days of storage at 12°C and 85 ± 5% RH.
Tabla 1. Efecto de interacción de los cultivares y la etapa de maduración en el contenido de sólidos solubles (SSC), el ácido titulable (TA) y el pH del tomate durante 20 días de almacenamiento a 12°C y a una RH de 85 ± 5%.
3.1.4. Color changes
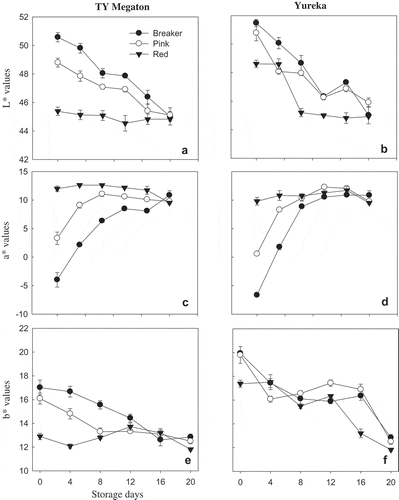
Color is determined by skin and flesh pigmentation, and it is one of the most important quality factors that affect tomato appearance and consumers' purchase decision (Brandt, Pék, Barna, Lugasi, & Helyes, Citation2006; Fraser, Truesdale, Bird, Schuch, & Bramley, Citation1994). In the present study, there was a significant (p < 0.05) interaction between cultivars and maturity stages on color values. Hunter a* values of both cultivars showed an increasing trend as the maturity stage progresses from breaker to red stage. Hunter L* values showed a reducing trend as the maturity proceeded irrespective of variety. The lowest (−4.62) and the highest (+12.64) Hunter a* values were recorded at the breaker and red stages, respectively, for “TY Megaton,” whereas −6.65 and +12.31 were recorded as the lowest and highest a* values for “Yureka” (). The trend observed for Hunter a* in the present study was similar to the trend observed for lycopene content. Arias, Lee, Logendra, and Janes (Citation2000) and Tilahun, Park, et al. (Citation2017a) also reported a direct correlation of lycopene content with measurements of color a* values. Among Hunter L*, a*, and b* values, Hunter a* values are best for evaluating the maturation process of tomatoes based on color. Color development in tomato as measured by a Chroma meter is characterized by lower L* value (lightness) readings and a change from negative to positive a* values (redness) (Shewfelt, Thai, & Davis, Citation1988). Red color is the result of chlorophyll degradation as well as synthesis of lycopene and other carotenoids, as chloroplasts are converted into chromoplasts (Fraser et al., Citation1994).
Figure 5. Color variables of “TY Megaton” (A, C, E) and “Yureka” (B, D, F) tomato cultivars as affected by maturity stages during the 20 days of storage at 12°C and 85 ± 5% RH. Each data point is the mean of nine sample replicatesstandard error.
Figura 5. Variación de color de los cultivares de tomate “TY Megaton” (A, C, E) y “Yureka” (B, D, F) en relación con las etapas de maduración durante 20 días de almacenamiento a 12°C y a una RH de 85 ± 5%. Cada dato representa la media de cinco réplicas de la muestra ± error estándar.
3.2. Antioxidant properties
3.2.1. Lycopene
Results of the present study revealed a significant (p < 0.05) interaction between cultivars and maturity stages on lycopene content (). Although there exists variation due to the nature of the cultivar as stated by George, Kaur, Khurdiya, and Kapoor (Citation2004), lycopene content increased as the maturity stage and storage period proceeded. Lycopene content of “TY Megaton” and “Yureka” at breaker stage was 2.67 and 2.56 mg.kg−1, respectively; increased to 9.30 and 7.41 mg.kg−1, respectively at the pink stage; and reached 20.40 and 20.41 mg.kg−1, respectively, at red maturity stage. Lycopene content of breaker stage “TY Megaton” reached its peak (20.32 mg.kg−1) and of “Yureka” reached its peak (20.22 mg.kg−1) on day 20. Peak of pink stage “TY Megaton” (21.05 mg.kg−1) and “Yureka” (21.71 mg.kg−1) was attained on the 16th and 12th days, respectively; red stage attained its peak earlier on the fourth day for “TY Megaton” (21.64 mg.kg−1) and on the eighth day for “Yureka” (23.15 mg.kg−1). It has been reported by different authors that as the fruit develops from the mature green stage to the red stage, lycopene concentration increases significantly (Brandt et al., Citation2006; Dumas, Dadomo, Di Lucca, & Grolier, Citation2003; Helyes, Pék, & Lugasi, Citation2006). Lycopene begins to accumulate after the breaker stage, and at the ripe-red stage, lycopene comprises 95% of all colored carotenoids and 73% of the total carotenoids (Dumas et al., Citation2003).
Table 2. Interaction effect of cultivars and maturity stage on lycopene and ascorbic acid content of tomato fruit during 20 days of storage at 12°C and 85 ± 5% RH.
Tabla 2. Efecto de interacción de los cultivares y la etapa de maduración en el licopeno y el contenido de ácido ascórbico del tomate durante 20 días de almacenamiento a 12°C y a una RH de 85 ± 5%.
Based on the result of lycopene content, it could be possible to get an optimum benefit of “TY Megaton” tomato fruits stored at 12°C after storing breaker stage for 16 to 20 days, pink stage for 12 to 16 days and red stage for 0 to 12 days. On the other hand, an optimum benefit of “Yureka” could be found after storing breaker stage for 12 to 20 days, pink stage for 8 to 20 days and red stage for 0 to 12 days.
3.2.2. Ascorbic acid
In this study, significant (p < 0.05) interaction was observed between cultivars and maturity stages on ascorbic acid content (). The content ranged from 200.1 to 311.4 mg.kg−1 and 209.7 to 301.6 mg.kg−1 for “TY Megaton” and “Yureka,” respectively. Maximum content was recorded at the breaker stage irrespective of cultivars, and the ascorbic acid content decreased gradually as the maturity stage and storage period proceeded and the minimum values of (200.1 mg.kg−1) and (209.7 mg.kg−1) were recorded for red stage “TY Megaton” and “Yureka”, respectively, on 20th day of storage. The results revealed that not all antioxidant compounds necessarily increase in tomatoes picked at the red stage. Previously, Rai et al. (Citation2012) reported similar decreasing trends in tomatoes stored under ambient condition. Sharma, Mahajan, and Bajaj (Citation1996) also reported a wider range of ascorbic acid (112.1 to 532.9 mg.kg−1) in different genotypes.
3.2.3. Total phenolics
Significant (p < 0.05) interaction was observed between cultivars and maturity stages on total phenolic contents from 0 to eighth storage days and nonsignificant (p > 0.05) difference was observed afterward (). Helyes et al. (Citation2006) also reported that polyphenol content did not change significantly during the ripening process. In the present study, the maximum values (239.51 mg GAE.kg−1) and (256.08 mg GAE.kg−1) were recorded at the breaker stage for “TY Megaton” and “Yureka”, respectively, on day 0, whereas the lowest values were found from the fruits at red stage irrespective of cultivars. The range of total phenolic contents found in the present study is in agreement with the result reported by Park, Kim, and Shin (Citation2016) and Tilahun, Park, et al. (Citation2017a, Citation2017b). Higher polyphenol content ranging from 330 to 480 mg from ‘Lamance F1ʹ tomato fruits was also reported by Helyes et al. (Citation2006).
Table 3. Interaction effect of cultivars and maturity stage on total phenolics and antioxidant activity of tomato fruit during 20 days of storage at 12°C and 85 ± 5% RH.
Tabla 3. Efecto de interacción de los cultivares y la etapa de maduración en los fenólicos totales y la actividad antioxidante del tomate durante 20 días de almacenamiento a 12°C y a una RH de 85 ± 5%.
3.2.4. Antioxidant activity
Significant (p < 0.05) interaction between cultivars and maturity stages was observed on DPPH radical scavenging of tomato fruit (). Higher DPPH radical scavenging percentage for both cultivars was recorded after 16 days of storage for breaker stage, after 12 days of storage for pink stage, and from the beginning to eighth day of storage for the red stage. In this study, the trends of DPPH radical scavenging follow the same trends as lycopene content. In agreement with the present results, lycopene was reported to be a highly effective antioxidant owing to its ability to act as a free radical scavenger and has the highest singlet oxygen quenching rate of all the carotenoids tested from biological systems (Rao & Agarwal, Citation1999). An increased consumption of tomatoes and tomato products has been reported to be associated with a decreased risk of prostate cancer, and their assumptions were related to the antioxidant properties of lycopene (Etminan, Takkouche, & Caamano-Isorna, Citation2004; Giovannucci et al., Citation2002).
4. Conclusions
Although storing and marketing of tomato fruits up to 3 weeks could be possible, better nutritional benefit from breaker and pink stages could be obtained after 16 and 12 days of storage, respectively, and from the beginning to eighth day of storage for red stage, irrespective of cultivars. With an assumption of packing 20 fruits per box for 20 days, packing and storing red, pink, and breaker stages in 2:2:1 (8:8:4) ratio will help consumers to obtain optimum benefit in both storability and nutritional qualities up to 20 days at 12°C and 85 ± 5% RH.
Research highlights
Packing and storing red, pink, and breaker stages in 2:2:1 (8:8:4) ratio will help consumers to obtain optimum benefit from tomato fruit up to 20 days.
Lycopene content and antioxidant activity suggest that better nutritional benefit from tomato fruit could be obtained after 16 and 12 days of storage for breaker and pink stages, respectively, and from the beginning to eighth day of storage for the red stage.
Not all biologically active compounds necessarily increase as the maturity stage proceeds.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arias, R., Lee, T. C., Logendra, L., & Janes, H. (2000). Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. Journal of Agricultural and Food Chemistry, 48, 1697–1702.
- Batu, A. (2004). Determination of acceptable firmness and color values of tomatoes. Journal of Food Engineering, 61(3), 471–475.
- Belew, D., Park, D. S., Tilahun, S., & Jeong, C. S. (2016). The effects of treatment with ethylene-producing tablets on the quality and storability of banana (Musa sp.). The Journal of Horticultural Science and Biotechnology, 34(5), 746–754.
- Borguini, R., & Torres, E. (2009). Tomatoes and tomato products as dietary sources of antioxidants. Food Reviews International, 25, 313–325.
- Brandt, S., Pék, Z., Barna, E., Lugasi, A., & Helyes, L. (2006). Lycopene content and color of ripening tomatoes as affected by environmental conditions. Journal of the Science of Food and Agriculture, 86, 568–572.
- Brovelli, E. A. (2006). Pre-and postharvest factors affecting nutraceutical properties of horticultural products. Stewart Postharvest Review, 2(2), 1–6.
- de Jesús Dávila-Aviña, J. E., Villa-Rodríguez, J., Cruz-Valenzuela, R., Rodríguez-Armenta, M., Espino-Díaz, M., Ayala-Zavala, J. F., & González-Aguilar, G. (2011). Effect of edible coatings, storage time and maturity stage on overall quality of tomato fruits. American Journal of Agricultural and Biological Sciences, 6, 162–171.
- Dumas, Y., Dadomo, M., Di Lucca, G., & Grolier, P. (2003). Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. Journal of the Science of Food and Agriculture, 83, 369–382.
- Etminan, M., Takkouche, B., & Caamano-Isorna, F. (2004). The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiology and Prevention Biomarkers, 13(3), 340–345.
- FAOSTAT. (2016). Food and agriculture organization of the united nations cropping database Retrieved from http://faostat3.fao.org/home/index.html
- Fish, W. W., Perkins-Veazie, P., & Collins, J. K. (2002). A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. Journal of Food Composition and Analysis, 15(3), 309–317.
- Fraser, P. D., Truesdale, M. R., Bird, C. R., Schuch, W., & Bramley, P. M. (1994). Carotenoid biosynthesis during tomato fruit development (evidence for tissue-specific gene expression). Plant Physiology, 105(1), 405–413.
- George, B., Kaur, C., Khurdiya, D. S., & Kapoor, H. C. (2004). Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chemistry, 84(1), 45–51.
- Getinet, H., Seyoum, T., & Woldetsadik, K. (2008). The effect of cultivar, maturity stage and storage environment on quality of tomatoes. Journal of Food Engineering, 87(4), 467–478.
- Giovannucci, E., Rimm, E. B., Liu, Y., Stampfer, M. J., & Willett, W. C. (2002). A prospective study of tomato products, lycopene, and prostate cancer risk. CancerSpectrum Knowledge Environment, 94, 391–398.
- Helyes, L., Pék, Z., & Lugasi, A. (2006). Tomato fruit quality and content depend on stage of maturity. HortScience, 41, 1400–1401.
- Kader, A. A., Stevens, M. A., Albright-Holton, M., Morris, L. L., & Algazi, M. (1977). Effect of fruit ripeness when picked on flavor and composition in fresh market tomatoes. Journal of the American Society for Horticultural Science, 102(6), 724–731.
- Kim, H. S., Jung, J. Y., Kim, H. K., Ku, K. M., Suh, J. K., Park, Y. M., & Kang, Y. H. (2011). Influences of meteorological conditions of harvest time on water-soluble vitamin contents and quality attributes of oriental melon. Journal of Bio-Environment Control, 20(4), 290–296.
- Lee, E., Sargent, S. A., & Huber, D. J. (2007). Physiological changes in Romatype tomato induced by mechanical stress at several ripeness stages. HortScience, 42, 1237–1242.
- Lenucci, M. S., Cadinu, D., Taurino, M., Piro, G., & Dalessandro, G. (2006). Antioxidant composition in cherry and high-pigment tomato cultivars. Journal of Agricultural and Food Chemistry, 54, 2606–2613.
- Martí, R., Roselló, S., & Cebolla-Cornejo, J. (2016). Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers, 8(6), 58.
- Maul, F., Sargent, S. A., Sims, C. A., Baldwin, E. A., Balaban, M. O., & Huber, D. J. (2000). Tomato flavor and aroma quality as affected by storage temperature. Journal of Food Science, 69(8), S310–S318.
- McGuire, R. G. (1992). Reporting of objective color measurements. HortScience, 27, 1254–1255.
- Mcn, N. (2008). Color atlas of postharvest quality of fruits and vegetables (pp.239–243). John Wiley & Sons, Inc.. doi:10.1002/9780813802947
- Neelam, P., Mehar, A. H., Puneet, D., Manoj, S. K., & Pravendra, N. (2003). Expression and activities of ethylene biosynthesis enzymes during ripening of banana fruits and effect of 1-MCP treatment. Plant Growth Regulation, 40, 11–19.
- Park, C. Y., Kim, Y. J., & Shin, Y. (2016). Effects of an ethylene absorbent and 1-methylcyclopropene on tomato quality and antioxidant contents during storage. Horticulture, Environment, and Biotechnology, 57(1), 38–45.
- Pataro, G., Sinik, M., Capitoli, M. M., Donsì, G., & Ferrari, G. (2015). The influence of post-harvest UV-C and pulsed light treatments on quality and antioxidant properties of tomato fruits during storage. Innovative Food Science & Emerging Technologies, 30, 103–111.
- Preedy, V. R., & Watson, R. R. (2008). Tomatoes and tomato products: Nutritional, medicinal and therapeutic properties. NH, USA: Science Publishers, Enfield.
- Rai, G. K., Kumar, R., Singh, A. K., Rai, P. K., Rai, M., Chaturvedi, A. K., & Rai, A. B. (2012). Changes in antioxidant and phytochemical properties of tomato (Lycopersicon esculentum mill.) under ambient condition. Pakistan Journal of Botany, 44(2), 667–670.
- Rao, A. V., & Agarwal, S. (1999). Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutrition Research, 19(2), 305–323.
- Rao, A. V., & Rao, L. G. (2007). Carotenoids and human health. Pharmacological Research : the Official Journal of the Italian Pharmacological Society, 55(3), 207–216.
- Renquist, A. R., & Reid, J. B. (1998). Quality of processing tomato (Lycoperscion esculentum) fruit from four bloom dates in relation to optimal harvest timing. New Zealand Journal of Crop and Horticultural Science, 26(2), 161–168.
- Roberts, P. K., Sargent, S. A., & Fox, A. J. (2002). Effect of storage temperature on ripening and postharvest quality of grape and mini-pear tomatoes. Proceedings of Florida State Horticulture Society, 115, 80–84.
- Saltveit, M. E. (2005). Aminoethoxyvinylglycine (AVG) reduces ethylene and protein biosynthesis in excised discs of mature-green tomato pericarp tissue. Postharvest Biology and Technology, 35(2), 183–190.
- Sesso, H. D., Liu, S., Gaziano, J. M., & Buring, J. E. (2003). Dietary lycopene, tomato-based food products and cardiovascular disease in women. The Journal of Nutrition, 133(7), 2336–2341.
- Sharma, S., Mahajan, R., & Bajaj, K. L. (1996). Biochemical evaluation of some tomato varieties. Journal of Vegetation Science, 23(1), 42–47.
- Shewfelt, R. L., Thai, C. N., & Davis, J. W. (1988). Prediction of changes in color of tomatoes during ripening at different constant temperatures. Journal of Food Science, 53, 1433–1437.
- Tilahun, S., Park, D. S., Seo, M. H., Hwang, I. G., Kim, S. H., Choi, H. R., & Jeong, C. S. (2018). Prediction of lycopene and β-carotene in tomatoes by portable chroma-meter and VIS/NIR spectra. Postharvest Biology and Technology, 136, 50–56.
- Tilahun, S., Park, D. S., Taye, A. M., & Jeong, C. S. (2017a). Effects of storage duration on physicochemical and antioxidant properties of tomato (Lycopersicon esculentum Mill.). The Journal of Horticultural Science and Biotechnology, 35, 88–97.
- Tilahun, S., Park, D. S., Taye, A. M., & Jeong, C. S. (2017b). Effect of ripening conditions on the physicochemical and antioxidant properties of tomato (Lycopersicon esculentum Mill.). Food Science and Biotechnology, 26, 473–479.
- USDA. (1991). United States standards for grades of fresh tomatoes. Washington, DC, USA: Agricultural Marketing Service.
- Žnidarčič, D., & Požrl, T. (2006). Comparative study of quality changes in tomato cv. ‘Malike’ (Lycopersicon esculentum Mill.) whilst stored at different temperatures. Acta Agriculturae Slovenica, 87(2), 235–243.
