?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The best enzymolysis conditions that fit actual production were determined via multiple-enzyme hydrolysis of waste shrimp heads through orthogonal experiment, taking frozen Litopenaeus vannamei as the starting material. The parameters including a material-water ratio of 1:4, initial pH of 8.5 and enzyme dosage of 0.4% contributed to average DH (degree of hydrolysis) value of 18.38% when the reaction was carried out at 55°C for 5 h. Microwave Maillard reaction of enzymatic hydrolysate was then performed to develop shrimp flavor juice, the optimal preparation conditions were determined based on reaction extent and sensory evaluation, including a power of 200W, temperature of 90°C and duration time of 30 min, with the addition of 3% reducing sugar and 3% amino acid.
RESUMEN
A través de la hidrólisis de las cabezas de camarón de desecho con múltiples enzimas se determinaron las mejores condiciones de enzimólisis, que se ajustan a la producción real, empleando para ello un experimento ortogonal. Como material de partida se utilizó Litopenaeus vannamei congelado. Los parámetros, que incluyen una relación material-agua de 1:4, un pH inicial de 8.5 y una dosis de enzima de 0.4%, contribuyeron a la obtención de un valor promedio de DH (grado de hidrólisis) de 18.38% cuando la reacción se llevó a cabo con una temperatura de 55°C durante 5 horas. Posteriormente se realizó la reacción de Maillard mediante microondas del hidrolizado enzimático para elaborar jugo con sabor a camarón. Así, según el grado de reacción y la evaluación sensorial, incluida una potencia de 200 W, una temperatura de 90°C y un tiempo de duración de 30 minutos, con la adición de 3% de azúcar reductora y 3% de aminoácidos, se determinaron las condiciones de preparación óptimas.
PALABRAS CLAVE:
1. Introduction
Litopenaeus vannamei, which belongs to crustacean species, is one of the most important economic penaeid shrimps (Lightner et al., Citation2006). It is also known as Penaeus vannamei and constitutes the world’s three major cultured penaeid together with Penaeus monodon and Fenneropenaeus chinensis (Lightner et al., Citation2006). Since the introduction of L. vannamei in 1988, coupled with further promotion and expanded aquaculture, it has now become the main species in shrimp aquaculture (Chan, Susan, & Keeley, Citation1988; Muñoz et al., Citation2000). It is typically featured with high-protein containing food on account of the crude protein content of 22.76%, in addition to 17 kinds of amino acids with a total amount of 80.67%, among which the content of flavor amino acid reaches up to 31.52%. Litopenaeus vanname is popular because of its delicious meat and high-nutrition-value character, the by-products of which also have considerable use value. For example, free amino acid content of shrimp head and shell is up to 2.36%, in which the essential amino acid (EAA) accounts for 41.52%, in addition to the rich protein, ash and chitin contents. The contents of oleic acid, linoleic acid, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are also rich (Deng, Yang, Pang, Shi, & Li, Citation2002).
At present, the processing of L. vanname in China mainly focuses on shrimp meat, fried shrimp and other products. Large amount of products including shrimp head and shell can be involved during the processing, their additional value may decrease evidently due to the absence of reasonable use, thereby giving rise to both resource waste and environmental pollution. In this regard, extensive efforts have been devoted to well address this issue. Some researchers processed them into shrimp meal to serve as additive for fish feed (Gao, Qian, & Hu, Citation1996). Others used the raw materials to produce shrimp head sauce (Deng et al., Citation2002; Zhou, Shunzhu, Xiaomei, Huang, & Li, Citation2008). Shrimp head and soybean were used as raw materials to carry out starter propagation by Aspergillus oryzae, followed by mixed fermentation to finally make shrimp head soy sauce (Duan et al., Citation2013). Monomeric amino acid and reducing sugar were added after concentrating the enzymatic hydrolysate of shrimp head, which induced Maillard reaction and further produced seasoner with full-bodied shrimp flavor high simulation degree (Ren & Zhang, Citation2005). Therefore, scientific and reasonable use of shrimp by-products is a hot topic in resource optimization.
In this study, frozen L. vannamei was used as raw material for orthogonal experiment to acquire the optimal conditions for hydrolyzing shrimp head with enzyme complex. The enzymatic hydrolysate was then employed as staring material and subjected to Maillard reaction, full-bodied flavor of shrimp sauce was obtained by taking fuzzy sensory evaluation result as indicator. The combined results are expected to provide technical support for both comprehensive development and utilization of shrimp by-products.
2. Materials and methods
2.1. Preparation of shrimp head enzymatic hydrolysate
The chemical composition of shrimp head includes protein and EAA, among which glycine, proline, alanine and threonine constitute the main free amino acids. Various kinds of free amino acids contribute to the flavor of shrimp head, in which the content of inosine monophosphate (IMP) was the highest, followed by adenosine monophosphate (AMP). The taste intensity value of both IMP and AMP are higher than 1, thus showing greater contribution to shrimp flavor.
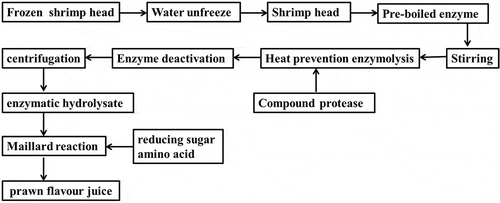
Flow water was utilized to thaw the complete shrimp head, which was then mixed with distilled water with a mass ratio of 1:1. The mixture was pre-cooked for 4 min in boiling water to well cook the shrimp head and weighted again, additional distilled water was added to ascertain that the total mass was the same with that of pre-cooked one. According to the designed solid-liquid ratio of shrimp paste, corresponding distilled water was added to complete the dilution process, the pH value of which was then adjusted with 1 mol/L HCl to match well with that of initially designed one. Then they were put into the corresponding water bath kettle to preheat for 5 mins. Subsequently, the paste was pre-heated in water bath for 5 min with certain temperature. Timing was then started after adding activated protease. The shrimp paste was subjected to stirring with glass rod to make uniform distribution of protease. The opening part of container was sealed with plastic wrap to reduce the evaporation effect induced by the water in shrimp paste, which exerts impact on solid-liquid ratio, and simultaneously, decreases the volatilization of flavor staff during enzymolysis process. After a certain duration time for enzymolysis, the enzyme deactivation lasted for 20 min by transferring the container into boiling water bath. The cooled shrimp paste after enzyme deactivation was then centrifuged at 4000 rpm for 10 min to obtain the supernatant, which was denoted as the expected enzymatic hydrolysate of shrimp head. Raw materials including amino acids, reducing sugar, thiamine and ascorbic acids, as well as seasoners including salts, monosodium glutamate and I + G were added into the above mentioned enzymatic hydrolysate of shrimp head to induce Millard reaction, which was initiated under different conditions in microwave rapid reactor, shrimp flavor sauce functioned as the reaction liquid. Note that the free amino acids including glycine and alanine involved in Maillard reaction would react with sugar, the as-produced substance with meaty flavor would enrich the flavor of product. The overall procedures are briefly summarized and shown in .
2.2. Shrimp head enzymatic hydrolysis
Five different proteases were used to hydrolyze the shrimp head. The properties of each protease are presented in . The ratio of material to liquid was controlled to be 1: 1, and 0.3% enzyme was added. The initial pH value and the reaction temperature were identical with the optimum conditions of various enzymes, the reaction was carried out for 5 h. The DH value acted as an indicator to evaluate the enzymatic hydrolysis effect. The aim was to screen out three enzymes with favorable enzymolysis effect and suitable combination with each other. Formaldehyde titration method was used in this study to detect the nitrogen attached to amino acid (Bao et al., Citation2017). The DH of the protein represents the degree or percentage of peptide bond cleavage during the hydrolysis process of protein (Bao et al., Citation2017), which is represented as
Table 1. The characteristics of various proteases.
Tabla 1. Características de varias proteasas.
where A and B represent the nitrogen contents of amino acid in the enzymatic hydrolysate and starting material, respectively. C represents the total nitrogen content in raw material. The as-screened complex enzyme was then subjected to orthogonal enzymolysis test of shrimp head protein. The reaction level and parameters that affect the reaction are listed in , upon which the optimal enzymolysis conditions can be obtained, and subsequently, enzymatic hydrolysate can be prepared under this condition.
Table 2. Enzymatic hydrolysis of shrimp head protein orthogonal test level factors.
Tabla 2. Hidrólisis enzimática de los factores de nivel de prueba ortogonales de la proteína de la cabeza de camarón.
Table 3. Microwave Maillard reaction orthogonal test level factor.
Tabla 3. Factor de nivel de prueba ortogonal de la reacción de Maillard obtenida por microondas.
2.3. Microwave Maillard reaction orthogonal experiment and degree experiment
The enzymatic hydrolysate of shrimp head prepared under the optimum reaction conditions in orthogonal test was used as raw material, followed by adding with 1% thiamine, 0.9% ascorbic acid, 10% salt, 5% MS, I + G 0.2% and reducing sugar (Glucose: xylose = 1: 1) and amino acids (glycine: alanine = 1: 1). The orthogonal tests with five levels and four factors were conducted at respective reaction conditions, the reaction levels are shown in . A total of 2 ml microwave reaction solution of shrimp flavor was accurately extracted and 2 ml 25% trichloroacetic acid was added, the mixture was then centrifuged at 4000 rpm for 10 min to collect the supernatant. The mixed solution that did not experience Maillard reaction (hydrolyzate + additive) was selected as reference, the absorbance of which under 420 nm and 550 nm were determined. Maillard reaction degree was represented by the difference between the two absorbance values, and can be expressed using the following formula:
2.4. Fuzzy mathematical evaluation
Sixteen groups of shrimp flavor derived from microwave Maillard reaction were subjected to sensory evaluation by 10 professional assessment staff, the results are summarized in .
Table 4. Sensory evaluation of microwave Maillard reaction of shrimp flavor.
Tabla 4. Evaluación sensorial de la reacción de Maillard obtenida por microondas del sabor a camarón.
3. Results and discussion
3.1. Screening test for optimum protease
The DH value of enzymatic hydrolysate was selected for the evaluation of enzymolysis effect. As shown in , the variation of enzymolysis effect goes identical with varying time for the other four proteases, with the exception of acidic protease. The enzymolysis effect got evidently enhanced with prolonging enzymolysis time within 4 h. The DH values of enzymolysis hydrolysate of trypsin (11.12%), pepsin (12.42%), neutral protease (16.20%) and flavor protease (17.52%) reached the highest at 4 h. However, the DH values of hydrolysate of the fours proteases decreased in the subsequent enzymatic process. Evidently, the enzymatic hydrolysis effect and the enzymolysis time are not always positively correlated. The following reasons are believed to be responsible for the above results. First, pH of shrimp head paste changed with proceeding of enzymatic hydrolysis, which inhibited protease activity. Second, the concentration of zymolyte changed, which was evident in the enzymatic hydrolysis process of acid protease. The enzymatic hydrolysate of acidic protease got very viscous after enzymatic hydrolysis for 3 h, thus giving rise to the failure in determining the content of free amino acid. Third, the amino acids and peptides of the enzymatic hydrolysate accumulated over time, thus resulting in gradually increased concentration, and eventually, inhibiting the enzymatic reaction to a certain extent.
Figure 2. Effects of different proteases on the hydrolysis degree of shrimp head.
Figura 2. Efectos de diferentes proteasas sobre el grado de hidrólisis de la cabeza de camarón.
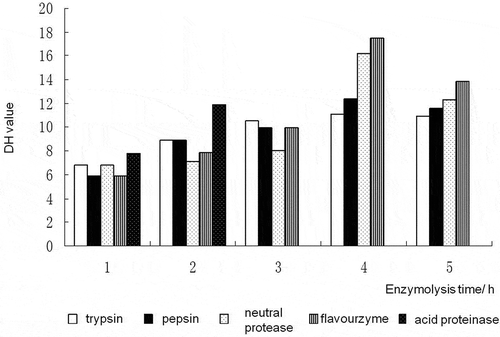
The highest hydrolysis degree of protease can be realized when enzymolysis time reached 4 h, followed by neutral protease. The enzymolysis conditions for the two proteases were similar and therefore suitable for combination. As an endonuclease with the highest specificity, trypsin is of diverse sources, low cost and suitable for combination (Ágústa, Hilmar, & Stefansson, Citation2013; Alloy et al., Citation2015; Haerteis et al., Citation2014; Wicht et al., Citation2014). Taking all factors into consideration, the combination of trypsin, flavor protease and neutral protease with a mass ratio of 1:2:2 was selected for enzymolysis of shrimp head.
3.2. Combined enzymatic hydrolysis of shrimp head orthogonal test
The results of the orthogonal test are clearly displayed in , which indicate that the effect of each reaction factor on the enzymatic hydrolysis of shrimp head was: time> temperature> ratio of material to liquid> enzyme> initial pH. The optimum reaction conditions were as follows: A4B1C4D4E4, that is, the ratio of material to liquid was 1: 4, the initial pH was 7.0, the amount of enzyme was 0.4%, the enzymolysis temperature was 55 ºC and the enzymolysis time was 5 h.
Table 5. Combined enzymatic hydrolysis of shrimp head orthogonal test.
Tabla 5. Hidrólisis enzimática combinada de la prueba ortogonal realizada en cabezas de camarón.
The variation of material to liquid ratio would lead to concentration changes of both zymolyte and enzyme in the enzyme reaction system, thus affecting the overall enzymatic reaction effect (Yang & Tong, Citation2008). As shown in , DH value increased with the ratio of material to liquid, and the highest value could be obtained when the ratio was 1: 4. The change of pH mainly exerted impact on enzyme activity and further affected the enzymatic reaction effect. In addition, the variation of DH value with changing pH level was not obvious, a gradual decrement tendency can be observed.
The initial pH value of shrimp head raw material was around 8.5, which was more convenient and economical for practical production. The enzymatic hydrolysis effect of complex enzyme over shrimp head paste and DH value gradually increased when the enzyme amount increased from 0.1% to 0.4%.
3.3. Microwave Maillard reaction
In the Maillard reaction process, the color change of the reaction solution would affect its absorbance value, which can be utilized to determine the degree of Maillard reaction. As shown in , No. 4 exhibited the highest microwave Maillard reaction degree, followed by No. 3 and No. 8. Meanwhile, No. 1, No. 9, No. 10 and No. 16 showed the lowest degree of reaction. One can carefully draw the conclusion that No. 4 and No.10 exhibited the highest and lowest Millard reaction degree, respectively.
Table 6. Maillard reaction degree determination results.
Tabla 6. Resultados de la determinación del grado de reacción de Maillard.
3.4. Fuzzy sensory evaluation results
The overall scores of the 16 groups of sensory evaluation are listed in . It is obvious that No. 3 had the highest overall score; however, the reaction degree of No. 3 only ranked second among all reactions. Also, No. 4 with highest reaction degree only possessed a lower score in the sensory assessment, indicating that the Maillard reaction degree was not an efficient criterion for the evaluation of optimal processing condition.
Table 7. Sensory evaluation score.
Tabla 7. Puntuación de la evaluación sensorial.
Repeated experiments were performed using the reaction condition of No.3, harmonious, pure and salty flavor of shrimp sauce can be obviously detected. Taking all these factors into consideration, the microwave Millard reaction condition of No. 3 was selected as the optimal processing condition for preparation of shrimp flavor, that is, a power of 200W, a temperature of 90 ºC reaction and duration time of 30 min, the percent of reducing sugar and amino acid were both 3%.
4. Conclusions
The orthogonal experiments of enzymolysis over shrimp head using enzyme complex showed that the effect of each reaction factor on the enzymatic hydrolysis of shrimp head was as follows: time> temperature> ratio> the amount of enzyme> the initial pH value.
The optimal conditions of enzymolysis were determined as follows: the ratio of material to water was 1:4, the initial pH was 7.0, the amount of enzyme was 0.4%, the temperature was 55 ºC, the time was 5 h. The above parameters were then taken as the reaction conditions on the account of the factors involved in practical processing, upon which the average DH value of enzymatic hydrolysate can reach up to 18.38%.
According to the trials based on microwave Maillard reaction, the optimum conditions for preparing shrimp sauces were as follows: a power of 200 W, a temperature of 90 ºC and duration time of 30 min, the percent of reducing sugar and amino acid were both 3%. Under this condition, the shrimp sauce flavor is featured with harmonious, rich flavor and excellent aftertaste.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ágústa, G., Hilmar, H., & Stefansson, B. (2013). Potential use of atlantic cod trypsin in biomedicine. BioMed Research International, Article ID: 749078, 11. doi:10.1155/2013/749078
- Alloy, A. P., Kayode, O., Wang, R., Hockla, A., Soares, A. S., & Radisky, E. S. (2015). Mesotrypsin has evolved four unique residues to cleave trypsin inhibitors as substrates. Journal of Biological and Chemical, 290(35), 21523–21535.
- Bao, Z., Zhao, Y., Wang, X., & Chi, Y. (2017). Effects of Degree of Hydrolysis (DH) on the functional properties of egg yolk hydrolysate with alcalase. Journal of Food Science and Technology, 54(3), 669–678.
- Chan, S. M., Susan, R., & Keeley, L. L. (1988). Characterization of the molt stages in penaeus vannamei: setogenesis and hemolymph levels of total protein ecdysteroids, and glucose. Biological Bulletin, 175(2), 185–192.
- Deng, S., Yang, P., Pang, Y., Shi, J., & Li, Y. (2002). Preparation of Shrimp Head Sauce. Food and Fermentation Industries, 28(10), 80–82.
- Duan, S., Hu, X., Li, M., Miao, J., Du, J., & Wu, R. (2013). Composition and metabolic activities of the bacterial community in shrimp sauce at the flavor-forming stage of fermentation as revealed by metatranscriptome and 16S rRNA gene sequencings. Journal of Agricultural and Food Chemistry, 64(12), 2591–2603.
- Gao, R., Qian, N., & Hu, Y. (1996). Extraction of shrimp flavor materials. Chinese Condiment, 1, 24–26.
- Haerteis, S., Krappitz, A., Krappitz, M., Murphy, J. E., Bertog, M., Krueger, B., … Korbmacher, C. (2014). Proteolytic activation of the human epithelial sodium channel by trypsin iv and trypsin i involves distinct cleavage sites. Journal of Biological and Chemical, 289(27), 19067–19078.
- Lightner, D. V., Poulos, B. T., Tang-Nelson, K. F., Pantoja, C. R., Nunan, L. M., Navarro, S. A., … Mohney, L. L., 2006. Application of molecular diagnostic methods to penaeid shrimp diseases: advances of the past 10 years for control of viral diseases in farmed shrimp. In: New Diagnostic Technology: Applications in Animal Health and Biologics Controls, International Conference, Saint-Malo, October 2005: Proceedings (Editors: Vannier P., Espeseth D.), 978-3-8055-8116-5, pages 117–122.
- Muñoz, M., Cedeño, R., Rodrı́guez, J., Knaap, W. P. W., Mialhe, E., & Bachère, E. (2000). Measurement of reactive oxygen intermediate production in haemocytes of the penaeid shrimp, penaeus vannamei. Aquaculture, 191(1–3), 89–107.
- Ren, Y., & Zhang, S. (2005). Study on shrimp head hydrolyzate and reaction type shrimp flavor. Chinese Food Additives, 6, 38–45.
- Wicht, O., Li, W., Willems, L., Meuleman, T. J., Wubbolts, R. W., Kuppeveld, F. J. M., … Bosch, B. J. (2014). Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsinin cell culture. Journal of Virology, 88(14), 7952–7961.
- Yang, E., & Tong, Q. (2008). Optimization of technological conditions for hydrolysis of chicken with compound enzyme. Journal of Anhui Agricultural Science, 14(6083–6084), 6088.
- Zhou, Z., Shunzhu, Z., Xiaomei, H., Huang, M., & Li, C. (2008). Study on extraction technology of shrimp head oil. Fat China: How Expanding Waistlines are Changing a Nation, 33(5), 77–79.