?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Vanilla is currently sold as an ethyl alcoholic extract, typically at a concentration of 35% (v/v). Alcoholic extracts have some disadvantages, such as high concentrations of organic residues, long extraction times and the requirement of large volumes when used as a flavoring. In contrast, supercritical fluid extraction (SFE) allows isolation of high-purity oleoresins without co-extraction of undesired organic residues, thereby preserving the desired volatile compounds in vanilla. In this study, we identified and quantified volatile compounds and fatty acids in four oleoresins and two ethanolic extracts of cured Vanilla planifolia Andrews beans following 10 or 20 sun-drying and sweating cycles (SS). SFE was carried out at a pressure of 20 MPa at 40°C, 50°C, or 60°C, and the resulting vanilla oleoresins were analyzed with GC-MS and GC-FID. The major compounds identified in the extracts were o-guaiacol, p-creosol, p-vinylguaiacol, p-hydroxybenzaldehyde, vanillic acid and vanillin.
RESUMEN
La vainilla es actualmente vendida en forma de extracto alcohólico a una concentración 35% (v/v). Sin embargo, los extractos alcohólicos presentan algunas desventajas como la alta concentración de residuos orgánicos, largos tiempos de extracción y altos volúmenes para transportar la vainilla como saborizante. En contraste, la extracción con fluidos supercríticos (SFE) permite separar los compuestos volátiles de la vainilla con alta pureza sin la presencia de residuos orgánicos. En este estudio, se identificaron y cuantificaron compuestos volátiles y ácidos grasos en cuatro oleorresinas y dos extractos etanólicos de Vanilla Planifolia Andrews utilizando 10 o 20 ciclos de secado al sol-sudoración (SS). SFE se efectuó a presión de 20 MPa a 40, 50 o 60°C, las oleorresinas de vainilla resultantes fueron analizadas por GC-MS and GC-FID. Los compuestos mayoritarios identificados fueron o-guayacol, p-creosol, p-vinylguayacol, p-hidroxibenzaldehído, ácido vainíllico y vanillina. Otra contribución importante de este estudio es la detección y cuantificación de ácidos grasos en las oleorresinas. La composición de ácidos grasos principalmente consistió en ácido palmítico y ácido linoleico.
1. Introduction
After Saffron, vanilla is the most expensive spice in the world due to its prolific uses as a flavoring, its volatility, its scent for the manufacture of fragrances and perfumes, and its functionality as a critical intermediate for pharmaceutical products. Vanilla is obtained from the beans of Vanilla Pompona, Vanilla Tahitiensis and V. planifolia Andrews (de Guzman & Zara, Citation2012).
For vanilla production, the flowers of the plant must be pollinated manually. The fruit ripens in the form of bean and is harvested nine months later. Because green vanilla lacks flavor, green beans are cured for six months to develop the compounds responsible for vanilla’s flavor (Korthou & Verpoorte, Citation2007). This process is divided into three stages, the first of which is heat treatment to prevent dehiscence, or splitting, of the fruit. The beans are then dried to stabilize vanillin and to prevent the development of microorganisms, particularly mold. The beans then undergo conditioning, during which several enzymatic and biochemical reactions occur that produce considerable changes in color, texture, and flavor. The green beans are subsequently cured for six months to obtain the final product, which contains all of the vanilla flavor compounds. In Mexico, the drying process consists of placing the beans in sweating boxes (rectangular boxes 1 x 1 × 1 m made of wood handcrafted) for 24 to 40 h. The beans are then removed from the sweating boxes and placed on mats of woven palm, called petates, and exposed to the sun for between three and four hours. Finally, the beans are returned to their sweating boxes for additional cycles of sun drying and sweating. The cycle is repeated until the moisture content of the beans reaches 18 to 20%, corresponding to 0.75–0.80 water activity (aw). The beans are fully ripe after 20 − 24 sun drying-sweating cycles (Hernández-Hernández, Citation2011; Odoux, Citation2006).
More than 300 compounds have been identified in cured vanilla beans. After vanillin, the predominant compounds are vanillic acid, p-hydroxybenzaldehyde and p-hydroxybenzoic acid (Pérez-Silva, Odoux, & Brat et al., Citation2006). The most important volatile compound in cured vanilla beans is vanillin, which confers sweet and creamy notes that markedly enhance the vanilla flavor (Ranadive, Citation2006). Vanillin content ranges from 1.2% to 2.5% depending on the species, plant maturity, and the curing process used (Toth, Lee, Havkin-Frenkel, Belanger, & Hartman, Citation2011). Only 1% of the vanillin consumed worldwide is obtained from vanilla beans. The rest is artificially synthesized from lignin and eugenol raw materials (Gallage et al., Citation2015; Harshvardhan, Suri, & Goswami, Citation2017).
Natural vanilla is currently sold in four forms, which are an ethanolic vanilla extract, oleoresin, absolute vanilla, and vanilla sugar. The flavor compounds in ethanolic vanilla extract (35% v/v) are obtained through solvent extraction with ethanol following reduction of the bean volume by maceration, the vanillin concentration varies between 0.2% and 1.0% in commercial products. Oleoresin is a crystalline yellow, semi-fluid resin obtained through supercritical CO2 extraction, the range of vanillin concentration is very wide depending on supercritical extraction conditions, and it ranges from 1% to 6%. Absolute vanilla is a dark brown and solid perfumery product isolated by selective solvent extraction. The vanillin concentration in commercial products is >1.4%. In this process, an initial extraction is performed with a non-polar solvent such as benzene and this product is then isolated by extraction into a polar solvent such as ethanol. Vanilla sugar is prepared by mixing one or two ground cured beans with sugar (final sugar content is ≤30%), the vanillin concentration is <0.05% in commercial products (de Guzman & Zara, Citation2012). Of the four forms, the most heavily traded product worldwide is ethanolic extract. However, extraction is time-intensive and extracts contain high concentrations of undesired organic residues. The employed solvents must be removed under vacuum, so the desired volatile compounds could be lost if the extracts are not handled carefully (Rodríguez-Jimenes et al., Citation2013). Furthermore, alcohol consumption is forbidden in some faith-based cultures, such as the Church of Latter Day Saints or Islam. Consumption of vanilla in the form of an alcoholic extract is thus restricted in these communities.
A relatively new method of extracting essential oils, aromas, and fragrances is supercritical fluid extraction (SFE). The solvating power of supercritical fluids can be manipulated through adjustments of pressure and temperature, thus allowing highly selective extraction. The low viscosity and relatively high diffusivity of supercritical fluids enable them to penetrate porous solid materials more effectively than liquid solvents, resulting in higher yields than can be obtained through extraction with organic solvents. SFE is sustainable, environmentally friendly and efficient (Naik et al., Citation2014). In addition, SFE isolates and dries products of high purity without requiring high temperatures, which is a great advantage in the extraction of volatile vanilla compounds (Knez et al., Citation2014; Sinha, Sharma, & Sharma, Citation2008). In this study, oleoresins and ethanolic extracts were isolated from V. planifolia Andrews after curing with 10 and 20 sun-drying and sweating cycles (SS), the concentration of the most important compounds was also compared.
Despite the information made available through studies of supercritical vanilla extraction (Castillo-Ruiz, Guillermo-Alcocer, Bojórquez-Gamboa, & Rocha-Uribe, Citation2011; Fang, Shi, & Zhang, Citation2002; Nguyen, Barton, & Spencer, Citation1991; Romero-de la Vega, Salgado-Cervantes, García-Alvarado, Romero-Martínez, & Hegel, Citation2016), little is known about volatile compounds other than vanillin in vanilla oleoresins. The concentrations of volatile compounds present after a time of half the one used during traditional have not been evaluated, and the composition of fatty acids in vanilla oleoresins has not been determined. The aims of this study were to obtain the highest concentrations of vanillin and other major compounds in supercritical extracts without the use of organic solvents and to decrease the traditional curing time. Another important contribution of this study is the determination of the fatty acid composition in the oleoresins. These compounds not only improve the quality of vanilla flavor; they also confer advantages for facile transport and storage, greater uniformity and more flexible application in food products that is provided by ethanolic extracts.
2. Materials and methods
2.1. Materials and reagents
All standards (grade HPLC) were purchased from Sigma-Aldrich® (St Louis, MO, USA). Dichloromethane, hexane, ethanol and methanol were HPLC grade Merck® (Darmstadt, Germany).
2.2. V. planifolia Andrews samples
Cured vanilla beans from V. planifolia Andrews cured with 10SS and 20SS were collected from the Totonacapan region near Papantla de Olarte, Veracruz, México (20°27’N, 97°19’W). Cured vanilla beans were stored in hermetically sealed packages at 4°C. Alcoholic extracts from V. planifolia Andrews were prepared by macerating and percolating 100 g vanilla beans (10SS and 20SS) per liter in ethanolic solution (35% w/v) in triplicate. For S-CO2 extraction, the beans (10SS and 20SS) were first cut using a sharp new stainless-steel knife into pieces 1 − 25 mm thick, 1 − 9 mm wide and 5 − 68 mm long. Extraction was then performed with an SFE-500 extraction system (Thar Technologies, Pittsburgh, USA). The experimental conditions to obtain alcoholic extracts and oleoresins are presented in .
Table 1. Experimental conditions to obtain alcoholic extracts and oleoresins from V. planifolia Andrews.
Tabla 1. Condiciones experimentales para la obtención de extractos alcohólicos y oleorresina de V. planifolia Andrews.
The extraction system was equipped with two solvent pumps and two heat exchangers, one for solvent cooling and the other for heating. A recirculator (Polyscience 9506) containing an ethanol/water mixture was used to cool the solvent exchanger and pump. The system was also equipped with an extraction cylinder and a separation vessel. The system was controlled via computer using the Process SuiteTM software package. For each supercritical extraction, 100 g cured vanilla beans were placed in the extraction cylinder. Carbon dioxide at 3°C was fed to the SFE-500 system at 5.7 MPa at a flow rate of 3 g/min. The S-CO2 carrying the desired compounds was transferred from the extraction vessel to the separator, during which the pressure and temperature decreased to 0.1 MPa and 20°C, respectively. The dissolved solutes precipitated as an oleoresin, which impregnated the walls and bottom of the separator. Finally, the oleoresin was collected manually for analysis.
Four oleoresins were extracted at 20 MPa and temperatures of 40°C, 50°C, and 60°C in triplicate according to previous research of Rojas-Ávila, Pimentel-Rodas, Rosales-García, Dávila-Ortiz, and Galicia-Luna (Citation2016). The yield of each oleoresin was calculated by:
2.3. Identification and quantification of volatile compounds in V. planifolia Andrews oleoresins and ethanolic extracts by GC-MS
Analysis of volatile compounds was performed with an Agilent 7890A GC-MS (Santa Clara, CA) equipped with an MPS2 automatic sampler (GERSTEL Inc., Switzerland). Separation was performed on an Agilent DB-5 column (60 m × 0.250 mm × 0.25 μm). A 1:100 (v/v) dilution of each extract was prepared in dichloromethane, and 1 µL was injected in split mode with a split ratio of 5:1. Helium was used as the carrier gas at a constant flow rate of 1.0 mL∙min−1. The oven temperature was held at 40°C for 5 min, raised to 200°C at a rate of 3°C/min, then ramped from 200°C to 280°C at 20°C/min and held at 280°C for 5 min. The detector and injector were maintained at 280°C.
The mass spectrum of each sample was compared to a library of authentic pure compounds (NIST, Citation2008). A match of >80% was required for positive identification. Peak retention times were compared to those of reference standards, and a linear retention index (RI) was calculated using n-alkanes (C6− C27). Six external calibration standards were prepared by diluting the stock solution in dichloromethane to concentrations between 1 and 10 000 mg/kg. Each standard was injected with six replicates.
2.4. Identification and quantification of fatty acids in oleoresins from V. planifolia Andrews by GC-MS and GC-FID
Prior to fatty acid analysis, a derivatization step was performed to convert to their methyl esters (FAMEs). For each sample, 8 mL NaOH solution (0.5 N in methanol) was added to 500 mg oleoresin in a 100 mL Florence flask. The flask was placed in a heating mantle and connected to a water-cooled condenser (4°C). The sample solution was brought to a boil and maintained at that temperature for 15 min, then 9 mL of 20% boron trifluoride solution in methanol was added through the condenser. The mixture was boiled for 1 min, followed by addition of 2 mL hexane through the condenser. The mixture was allowed to boil for an additional minute, then the flask was removed from the condenser. Saturated sodium chloride solution (15 mL) was added to the mixture and stirred for 15 s. Saturated sodium chloride solution was added until the flask was full, and the organic phase was transferred by pipette to a vial containing 0.1 g sodium sulfate. Finally, the dried organic phase was transferred to a 1 mL amber vial for GC-FID analysis. Samples were diluted to 1:100 (v/v) in dichloromethane prior to injection.
The esterified fatty acids were analyzed on an Agilent 7890A GC (Agilent technologies, France) equipped with an FID and a multifunctional automatic sampler (GERSTEL Inc., Switzerland). Fatty acids were separated on an Agilent J&W HP-88 column (100 m × 0.250 mm × 0.20 μm). Nitrogen was used as carrier gas at a constant flow rate of 1.0 mL min−1, and 2 μl of each sample were injected with a split ratio of 100:1. The oven temperature was held at 70°C for 4 min, then increased to 110°C at a rate of 8°C/min. The temperature was then increased to 170°C at a rate of 5°C/min and held for 10 min, followed by an increase to 215°C at a rate of 4°C/min. The temperature was held at 215°C for 23 min, then finally increased to 250°C at a rate of 10°C/min and held there for 5 min. The detector and injector temperature were maintained at 260°C. The percentage of fatty acid methyl esters in the oleoresins was determined by comparison with a FAMEs standard calibration curve (C4-C24) constructed with standards from 1 to 10 000 mg/kg, which were injected with six replicates.
2.5. Method validation
Solutions of individual compounds were prepared at a concentration of 10 000 mg/kg and used to make calibration standards by serial dilution. Standard solutions were made at concentrations of 1, 10, 100, 1000, 5000, and 10 000 mg/kg. Peak areas of each compound were recorded, and calibration curves were constructed by plotting peak area vs. concentration. Limit of detection (LOD) and limit of quantification (LOQ) were defined as 3σ and 10σ, respectively. Precision at the LOD and LOQ was calculated for each compound based on six injections of the standard at each concentration. To evaluate the precision of the method, six standard replicates at two concentrations were analyzed. Precision was determined by the %RSD of the measured standards at each concentration. Extraction recoveries (%) were determined by comparing the peak areas of analytes spiked into blank vanilla samples (matrix standards) with those of compounds in mixed standard solutions at two concentrations. Analysis was performed with six replicates at two concentrations (Liang et al., Citation2018; Magnusson & Örnemark, Citation2014).
3. Results
3.1. Effect of SFE extraction conditions on vanilla oleoresin yield
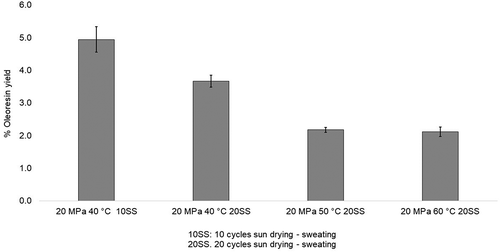
The oleoresin yields ranged from 2.12% to 4.95% (w/w) (), and the maximum yield was obtained from SFE performed at 20 MPa and 40°C. These values were consistent with those reported in previous studies (Castillo-Ruiz et al., Citation2011; Nguyen et al., Citation1991). Generally, oleoresin yield increased as the pressure and temperature were increased. Nguyen et al. (Citation1991) reported yields ranging from 0.3% to 8.0% (w/w) with extraction temperatures from 33°C to 36°C at a pressure of 11.0 MPa. Castillo-Ruiz et al. (Citation2011) obtained yields between 4.0% and 7.77% (w/w), and their maximum yield was obtained from extraction at 476.19 MPa and 50°C. On the other hand, Naik et al. (Citation2014) reported a yield of 3.2% (w/w) from extraction at 30 MPa and 50°C. The differences between the oleoresin yields obtained in this study and others reported in the literature are a consequence of several factors. These include extraction conditions such as pressure and temperature; use of a co-solvent; the origin of the vanilla beans; the type and duration of curing; and the dimensions of the vanilla beans placed in the extraction cylinder.
3.2. Identification of volatile compounds in V. planifolia Andrews oleoresins and ethanolic extracts
Four oleoresins from V. planifolia Andrews were analyzed by GC-MS. Twenty-two volatile compounds were identified, which included nine phenols, three aliphatic acids, two aldehydes, five esters, one hydrocarbon, and two ketones (). Six volatile compounds were identified in the ethanolic extracts. These were guaiacol, p-vinylguaiacol, vanillin, p-hydroxybenzaldehyde, vanillyl alcohol, and vanillic acid (). The ethanolic extracts tended to contain the highest vanillin concentrations, whereas SFE employs a single supercritical solvent, such as S-CO2, to selectively separate only the most important volatile compounds in vanilla. These are vanillin, vanillic acid, p-hydroxybenzyl alcohol, p-hydroxybenzaldehyde, p-hydroxybenzoic, guaiacol, p-creosol, p-vinylguaiacol, anisyl alcohol, and anisaldehyde (Cicchetti & Chaintreau, Citation2009; Da Costa & Pantini, Citation2006; Havkin-Frenkel, French, & Frenkel, Citation2005; Pérez-Silva et al., Citation2006; Sinha et al., Citation2008; Zhang & Mueller, Citation2012).
Table 2. Volatile compounds identified in oleoresins from V. planifolia Andrews obtained at different pressure and temperature conditions.
Tabla 2. Compuestos volátiles identificados en oleorresinas de V. planifolia Andrews obtenidos a diferentes condiciones de presión y temperatura.
Table 3. Volatile compounds identified in alcoholic extracts from V. planifolia Andrews obtained at different sun drying-sweating cycles.
Table 3. Compuestos volátiles identificados en extractos alcohólicos de V. planifolia Andrews obtenidos a diferentes ciclos de secado al sol-sudoración.
In this study, the largest number of volatile compounds from V. planifolia Andrews was extracted at 20 MPa and 40°C. As the temperature is increased, the solubility of volatile compounds in S-CO2 decreases due to competition with non-volatile compounds. The important volatile compounds may also degrade at elevated temperatures (Romero de la Vega et al., Citation2016). In a report by Rojas-Ávila et al. (Citation2016), the best conditions for solubilizing vanillin and vanillic acid were observed at pressures from 16 to 30 MPa and temperatures from 35°C to 65°C. A decrease in solubility was observed in ternary systems at higher pressures and temperatures. On the other hand, Nguyen et al. (Citation1991) obtained higher concentrations of vanillin at pressures from 10 to 13 MPa and temperatures from 33°C to 36°C. Romero-de la Vega et al. (Citation2016) also reported that the best conditions for vanillin extraction with S-CO2 were 14 MPa and 37°C. Castillo-Ruiz et al. (Citation2011) obtained their highest vanillin concentration at 40.8 MPa and 40°C.
Historically, the extraction of volatile compounds from vanilla has most often been performed with organic solvents. Dignum, Kerler, and Verpoorte (Citation2002) studied extraction at different stages of the curing process and detected only 10 volatile compounds in extracts obtained with 60:40 pentane:dichloromethane. Pérez-Silva et al. (Citation2006) compared different extraction solvents to determine which yielded the largest number of vanilla volatile compounds. The best results were obtained with 1:1 pentane:diethyl ether (v/v), in which 65 compounds were identified. Extraction of over 240 volatile compounds from vanilla with other solvents, such as dichloromethane, used in conjunction with solvent-assisted flavor evaporation (SAFE) distillation has been reported (Zhang & Mueller, Citation2012). Considering the solvents employed in these methods, SFE presents itself as an alternative green technology by which maximize yields for a large number of vanillin compounds without the use of organic solvents.
3.3. Validation of the quantitative method
Linear range, LOD, LOQ, precision, and accuracy were determined for each compound during chromatographic method validation. The results are summarized in and . The linear relationship between concentration and the peak area can be expressed as y = mx + c, where y is the instrumental response (peak area), x is the analyte concentration in the sample, m is the slope of the line in a plot of y vs. standard concentration, and c is the y-intercept of the plot. Good linearity was achieved (R2 > 0.98) with all of the compounds. LODS for the volatile compounds and fatty acids fell within the range of concentrations used to construct the calibration curves. These values ranged from 0.02 to 1689.43 mg/kg, while LOQs fell in the range of 0.07 − 3096.30 mg/kg. Accuracy of the GC-MS method was considered as acceptable between 83.16% and 122.69%. Good precision was achieved with the developed method, as %RSD ranged from 2.54% to 7.89%. Analyte recoveries fell between 74.19% and 89.34%, which indicated the method was sufficiently accurate for quantification of the vanilla oleoresin compounds.
Table 4. Coefficient of correlation, % Accuracy, LOD, and LOQ values of quantified compounds in vanilla oleoresins.
Tabla 4. Coeficiente de correlación, % Exactitud, LOD y LOQ de compuestos cuantificados en oleorresinas de vainilla.
Table 5. Precision (%RSD) and % Extraction recovery for the analysis of vanilla compounds.
Tabla 5. Precisión (%RSD) y % de recuperación de extracción para el análisis de compuestos de vainilla.
3.4. Quantification of volatile compounds in V. planifolia Andrews oleoresins
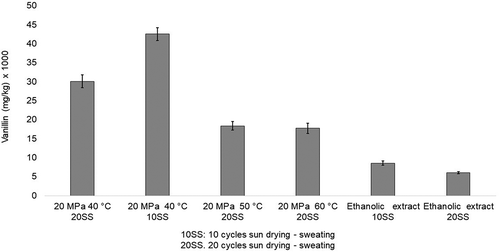
Vanillin is considered the most important volatile compound in vanilla extracts (Adedeji, Hartman, & Ho, Citation1993; Odoux, Escoute, & Verdeil, Citation2006; Sinha et al., Citation2008; Zhang & Mueller, Citation2012). The concentrations of the major components in V. planifolia Andrews beans obtained after traditional curing in Mexico with 20SS are given in . The concentrations shown are the mass of analyte (mg) per mass of vanilla bean (kg). Vanillin concentrations of 17 707 mg/kg and 30 648 mg/kg were determined from oleoresins extracted at 20 MPa at 60°C and 40°C, respectively. Increasing the SFE temperature from 40°C to 50°C while maintaining a pressure of 20 MPa reduced the apparent vanillin concentration by 56%. Increasing the SFE temperature to 60°C reduced the apparent vanillin concentration by 73% (). On the other hand, the oleoresin obtained from V. planifolia Andrews beans cured with 10SS by SFE at 20 MPa and 40°C yielded an apparent vanillin concentration of 41 046.70 mg/kg, which was 34% higher than that of beans cured with 20SS. This was consistent with previously reported findings, in which the solubility of vanillin in S-CO2 decreased as temperature increased along with possible degradation of the important volatile compounds. The vanillin concentrations determined in the present study were similar to those of Fang et al. (Citation2002), who reported a concentration of 20 050 mg/kg following SFE at 35 MPa and 45°C. Nguyen et al. (Citation1991) reported vanillin concentrations of 10 000 to 16 800 mg/kg following extraction at 11 MPa and 33°C, while Naik et al. (Citation2014) indicated a vanillin concentration of 14 208 mg/kg after extracting at 30 MPa and 50°C. Nevertheless, Castillo-Ruiz et al. (Citation2011) obtained a higher vanillin concentration (~57 000 mg/kg) following extraction at 40.8 MPa and 40°C.
Table 6. Concentration of major compounds in oleoresins from V. planifolia Andrews obtained at different pressure and temperature conditions.
Tabla 6. Concentración de los compuestos mayoritarios en oleorresinas de V. planifolia Andrews obtenidos a diferentes condiciones de presión y temperatura.
Results from the present study are shown in and and in . Compared to the vanillin concentrations based on ethanolic extracts (35% w/v), the vanillin concentrations determined with oleoresins extracted from vanilla beans with 10SS and 20SS by SFE were 4.45 and 4.88 times higher, respectively. The range of vanillin concentrations determined with the ethanolic extracts in this study was similar to reported values obtained with organic extractions, which range from 9296 to 19 400 mg/kg in (Adedeji et al., Citation1993; Cicchetti & Chaintreau, Citation2009; Gassenmeier, Riesen, & Magyar, Citation2008; Hartman et al., Citation1992; Pérez-Silva et al., Citation2006).
Table 7. Concentration of major compounds in alcoholic extracts from V. planifolia Andrews obtained at different pressure and temperature conditions.
Tabla 7. Concentración de los compuestos mayoritarios en extractos alcohólicos de V. planifolia Andrews obtenidos a diferentes condiciones de presión y temperatura.
Table 8. Comparison of volatile compounds in vanilla beans from diverse extraction methods.
Tabla 8. Comparación de los compuestos volátiles en vainas de vainilla utilizando diferentes métodos de extracción.
Figure 2. Effect of extraction and curing time on vanillin content.
Figura 2. Efecto de la extracción y tiempo de beneficio en el contenido de vainillina.
According to the Official Mexican Standard (NMX-FF-074-SCFI-Citation2009), the vanillin content of a gourmet bean must exceed 2% (w/w). An alcoholic extract of cured V. planifolia beans must contain 0.11 g of vanillin per 100 mL, and the ethyl alcohol content must be below 35%. Based on our results, oleoresin obtained through extraction with S-CO2 meets the specifications of the Mexican Official Standard.
Pérez-Silva, Gunata, Lepoutre, and Odoux (Citation2011) compared the total glycosylated and non-glycosylated vanillin concentrations in green beans and beans obtained after the traditional Mexican curing process. The concentrations found in green and cured beans were 51 018 and 22 361 mg/kg, respectively, demonstrating that the total vanillin concentration in green beans was higher than in cured beans. This is because the second stage of the Mexican curing process, which involves “killing” at 60°C for 48 h and sweating for 48 h, causes decompartmentalization of cells within tissues. The endogenous enzyme β-D-glucosidase thus comes into contact with its substrate, glucovanillin, which is then hydrolyzed to yield vanillin. This process takes from two to three months and liberates aromatic vanillin (Brillouet & Odoux, Citation2010; Dignum et al., Citation2002; Odoux, Citation2011; Odoux et al., Citation2006; Pérez-Silva et al., Citation2006).
The loss of vanillin during the curing process has also been attributed to the formation of dimers by peroxidase activity, sublimation or co-evaporation with water, oxidation of vanillin to vanillic acid (Frenkel & Havkin-Frenkel, Citation2006; Pérez-Silva et al., Citation2006), and possible formation of a covalent bond between lignin and vanillin released by β-D-glucosidase (Gatfield et al., Citation2007). When these findings are considered, it can be argued that beans cured with 10SS contain higher vanillin concentrations than beans cured with 20SS. The pressures and temperatures employed during SFE with S-CO2 cause compaction and rupture of tissues, which allows the release of vanillin.
After vanillin, vanillic acid and p-hydroxybenzaldehyde were the second and third most abundant compounds, respectively, in oleoresins obtained from V. planifolia Andrews. The concentration of vanillic acid ranged from 23.89 to 78.96 mg/kg, while that of p-hydroxybenzaldehyde ranged from 18.69 to 48.18 mg/kg. Following SFE, Nguyen et al. (Citation1991) reported p-hydroxybenzaldehyde concentrations of 270 to 2014 mg/kg and vanillic acid concentrations of 30 to 1378 mg/kg. In contrast, the concentrations of p-hydroxybenzaldehyde in our ethanolic extracts were higher than those of vanillic acid. Concentrations of p-hydroxybenzaldehyde ranged from 618.07 to 941.26 mg/kg, while vanillic acid was found at concentrations between 17.61 and 44.87 mg/kg. After using organic solvents for extraction of Bourbon beans (V. planifolia), Hartman et al. (Citation1992) reported p-hydroxybenzaldehyde and vanillic acid concentrations of 1040 mg/kg and 440 mg/kg, respectively. In contrast, Adedeji et al. (Citation1993) reported V. planifolia p-hydroxybenzaldehyde and vanillic acid concentrations of 635 mg/kg and 994 mg/kg, respectively. Pérez-Silva et al. (Citation2006) reported p-hydroxybenzaldehyde and vanillic acid concentrations of 873.3 mg/kg and 1315 mg/kg, respectively, in V. planifolia G. Jackson. Using extracts of Bourbon and Ugandan V. planifolia beans from Madagascar, Zhang and Mueller (Citation2012) reported p-hydroxybenzaldehyde concentrations of 27.06 and 8.01 mg/kg and vanillic acid concentrations of 0.57 and 0.48 mg/kg. Our comparatively low p-hydroxybenzaldehyde and vanillic acid concentrations might have been a consequence of incomplete solvation of these compounds during SFE. SFE may also extract vanillin more selectively than other volatile compounds present in vanilla beans. The curing method and the origin of the beans are also factors that affect oleoresin p-hydroxybenzaldehyde and vanillic acid concentrations (Odoux, Citation2011).
Guaiacol was the fourth most abundant volatile compound based on analysis of the oleoresins, from which cured bean concentrations range from 5.56 to 68.50 mg/kg were determined. In contrast, the ethanolic extracts indicated guaiacol concentrations between 162.95 and 275.77 mg/kg. Using organic extraction, Pérez-Silva et al. (Citation2006) reported a guaiacol concentration of 9.3 mg/kg in V. planifolia Jackson of Mexican origin, while Zhang and Mueller (Citation2012) reported concentrations of 105 and 169.5 mg/kg in Bourbon and Ugandan V. planifolia beans, respectively. Although one study reported a concentration of 19 mg/kg in Bourbon beans (Hartman et al., Citation1992), this compound has not been detected in vanilla beans from Madagascar, Bali, Tahiti or Java (Adedeji et al., Citation1993). These differences were attributed primarily to differences in bean origin and the extraction methods used (Odoux, Citation2011; Zhang & Mueller, Citation2012).
Other compounds identified in the oleoresins at concentrations below 2.5 mg/kg included p-creosol, p-vinylguaiacol, and acetovanillone. Pérez-Silva et al. (Citation2006) reported concentrations of 3.8 mg/kg (p-creosol), 1.2 mg/kg (p-vinylguaiacol) and 13.7 mg/kg (acetovanillone) in V. planifolia Jackson of Mexican origin. Following organic extraction of Bourbon beans, Zhang and Mueller (Citation2012) reported p-cresol, p-vinylguaiacol and acetovanillone concentrations of 5.55, 0.06, and 1.49 mg/kg, respectively. They also reported p-cresol, p-vinylguaiacol, and acetovanillone concentrations of 77.71, 0.09, and 0.82 mg/kg, respectively, in Ugandan beans. It is worth mentioning that in spite of the relatively low concentrations of these compounds relative to vanillin, they are responsible for phenolic, sweet, balsamic, spicy, woody, vanilla, and roasted notes. Variable concentrations of these compounds conferred a characteristic aroma to each vanilla oleoresin obtained in this study. Likewise, the oleoresins contained higher vanillin concentrations and more compounds overall than the ethanolic extracts. This may be the origin of the sensory and physical differences observed among vanilla extracts obtained with the two extraction methods. Oleoresins were bright yellow with a creamy consistency, while ethanolic extracts were dark brown and smelled strongly of alcohol. These differences can be attributed to the solvent selected for extraction (S-CO2 vs. ethanol) and its polarity (Odoux, Citation2011; Pérez-Silva et al., Citation2006).
3.5. Identification and quantification of fatty acids in oleoresins from V. planifolia Andrews
In addition to the volatile acids, lactones, sulfur compounds, ethers, furans, alcohols, aldehydes, ketones, esters, acetals, phenols, pyrans and coumarins in pure vanilla extracts (Toth et al., Citation2011; Zhang & Mueller, Citation2012), the oleoresins obtained in this study contained significant quantities of fatty acids. However, fatty acids were not detected in the ethanolic extracts. Previous studies have detected the presence of fatty acids in cured vanilla beans by non-polar solvent extractions, palmitic and linoleic acids have been the most abundant among these (Adedeji et al., Citation1993; Brunschwig et al., Citation2012; Lechat-Vahirua & Bessiere, Citation1998; Pérez-Silva et al., Citation2006). In this study, the following nine fatty acids were identified: myristic acid, pentadecanoic acid, palmitic acid, palmitoleic acid, heptadecanoic acid, stearic acid, oleic acid, linoleic acid, and γ-linoleic acid. These fatty acids confer a yellow and glossy appearance to the oleoresins. In addition, the butter and oil notes in vanilla extracts have been accredited to these compounds (Brunschwig et al., Citation2012; Dunphy & Bala, Citation2014).
The most abundant fatty acids in the oleoresins were palmitic acid (35.11 − 42.29 mg/kg) and linoleic acid (59.42 − 98.76 mg/kg). However, considerable concentrations of stearic acid (10.53 − 16.88 mg/kg), oleic acid (13.39 − 22.54 mg/kg), and γ-linolenic acid (5.92 − 16.68 mg/kg) were also found (). Adedeji et al. (Citation1993) reported palmitic acid, stearic acid, and linoleic acid concentrations of 127, 67, and 70 mg/kg, respectively, in V. planifolia Andrews beans. Pérez-Silva et al. (Citation2006) identified a larger number of fatty acids in V. planifolia G. Jackson, the most important being palmitic acid and linoleic acid at 126.6 and 225 mg/kg, respectively.
Table 9. Concentration of fatty acids in oleoresins from V. planifolia Andrews obtained at different pressure and temperature conditions.
Tabla 9. Concentración de ácidos grasos en oleorresinas de V. planifolia Andrews obtenidos a diferentes condiciones de presión y temperatura.
Larger quantities of fatty acids (198.18 mg/kg) were extracted from vanilla beans cured with 20SS at 20 MPa and 50°C. As for most of the compounds in V. planifolia Andrews, increasing extraction temperature to 60°C at 20 MPa reduced the solubility of the fatty acids in S-CO2. This behavior has also been observed in extractions performed at 18 MPa and 55°C (Romero-de la Vega et al., Citation2016).
For extraction of vanilla beans cured with 20SS, increasing the extraction temperature by 10 degrees to 50°C at 20 MPa increased the concentrations of palmitic acid and linoleic acid in the extracts by 14% and 66%, respectively. Increasing the temperature by 20 degrees to 60°C increased the palmitic acid and linoleic acid concentrations by 20% and 45%, respectively. A comparison of vanilla oleoresins obtained from beans cured with 10SS or 20SS extracted under the same conditions (20 MPa and 40°C) showed that the palmitic acid and linoleic acid concentrations in the 10SS oleoresins were higher by 10% and 44%, respectively.
It is important to note that during the curing process, lipolytic activity increases, which promotes the activation of enzymes such as lipases and phospholipases. The most complex lipids are thus hydrolyzed, and free fatty acids are released. The fatty acids are then oxidized by lipoxygenases to yield hydroxyperoxides. Finally, hydroxyperoxide lyase catalyzes the cleavage of the hydroxyperoxides, which produces aldehydes associated with vanilla flavor. These include hexanal, (Z)-3-hexanal, (Z)-3-nonenal, and (Z,Z)-3,6-nonadienal. In addition, the presence of four other aldehydes produced through autoxidation of the linoleic and oleic fatty acids esters after 25 days of curing has been reported. These are 2-heptenal, (E)-2-decenal, (E,Z)-2,4, decadienal, and (E,E)-2,4-decadienal (Dunphy & Bala, Citation2014; Ho & Chen, Citation1994; Pérez-Silva et al., Citation2011). However, none of these compounds were detected in the oleoresins obtained by SFE in this study, which was probably because long-chain oleic, linoleic, and γ-linolenic fatty acids were not completely degraded through enzymatic or auto-oxidative pathways.
3.6. Application of vanilla compounds identified
The large variety of compounds found in vanilla extract is a consequence of the diverse factors to which the vanilla beans are exposed. Generally, the prevailing vanilla flavor in extracts is due to the presence of volatile compounds such as acids, esters, alcohols, acetals, phenols, and non-volatile compounds like polyphenols, amino acids, and fatty acids. It is important to note that although the quality of an extract is directly related to its vanillin concentration, the presence of non-volatile compounds confers its characteristic aromatic notes. This improves its quality and helps to retain the characteristic aromas of vanilla for long periods (Sinha et al., Citation2008).
In addition, each of the compounds quantified in this study has one or more applications in the food, perfume, and pharmaceutical industries (Burdock & Fenaroli, Citation2005; Fladby et al., Citation2004; Jacobson, Citation2009). These include the following:
Vanillin is the main flavoring ingredient in vanilla, and it is added to chocolate, confectionery and alcoholic beverages such as whiskey, wine, and beer in concentrations of 1 to 50 mg/kg. The two most important food-related uses for vanillin are for the production of ice cream and soft drinks. In the beauty care and perfumery industries, natural vanillin is much more valued than synthetic vanillin for the manufacture of moisturizers, aromatic soaps, colognes, and perfumes. In addition, vanillin is proposed as a treatment to improve memory in people suffering from Alzheimer’s disease.
After vanillin, p-Hydroxybenzaldehyde has the highest commercial value and is used to enhance fruit and chocolate flavors in desserts, confections, and other baked goods. It is usually found in concentrations of 1 to 50 mg/kg. Although its flavoring power is two to four times greater than that of vanillin, it is not very stable.
Vanillic acid in oxidized form is an intermediate in the bioconversion of ferulic acid to vanillin. Like vanillin, it is generally added in low concentrations (1–50 mg/kg) to confectionery, chocolate, coffee, and tea due to its pleasant and creamy flavor.
Vanillyl alcohol is most frequently used to replace vanillin in coffee, cola, baked goods, confectionery and products containing coconut. At concentrations from 1 to 50 mg/kg, it imparts a mild, sweet, and balsamic flavor.
Because they have strong odors, guaiacol, p-creosol, and p-vinylguaiacol are added to foods in lower concentrations, about 1 mg/kg. The flavors attributed to these compounds are smoky, phenolic, medicinal and woody. They are generally used in evaporated milk, parmesan cheese, butter, cognac, whiskey, tobacco, wine, and floral perfumes.
Fatty acids lend waxy and creamy characteristics to oleoresins, making their application easier and protecting the volatile compounds present. For these reasons, fatty acids are used to produce emollients, hair conditioners, skin perfumes, lubricants, creams, lotions, lipsticks, powders, skin ointments, face cleaners, body shampoos, and soaps. Another important contribution of this work is the identification of linoleic and α-linoleic acids, also known as omega-6 and omega-3 fatty acids. These essential fatty acids are metabolized to yield arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid, which are involved in the regulation of blood pressure and inflammatory response (Rueda, Domingo, & Mach, Citation2011).
Vanillin may be the most important compound in vanilla. Nevertheless, vanilla contains other compounds for which uses have yet to be identified. These can also be extracted without consumption of large amounts of energy or solvents via environmentally friendly SFE. New methodologies for SFE extraction of non-volatile compounds with beneficial health impacts could soon be developed.
4. Conclusions
Extraction with supercritical fluids provides an important alternative for isolating oleoresins that contain higher vanillin concentrations than are found in alcoholic extracts. Shorter curing times may also yield higher quantities of vanillin. The product obtained through SFE exhibits high purity and retains the desired V. planifolia Andrews volatile compounds and therefore, it is recommended the reduction of curing time from 20 to 10 SS cycles. The maximum concentration of vanillin was obtained through extraction at 20 MPa and 40°C. Extraction at temperatures over 50°C decreased apparent vanillin concentrations, possibly because solubility in S-CO2 was reduced and degradation was accelerated. Despite the relatively high moisture content (>20%) of vanilla beans cured with 10SS, the highest concentration of vanillin (41 046 mg/kg) was found in beans cured in this manner. Fatty acids, which are the compounds responsible for the oily and glossy appearance of supercritical vanilla extracts, were detected in the oleoresins. The predominant fatty acids in the oleoresins were palmitic and linoleic acid, which were obtained in their highest concentrations at 20 MPa and 50°C. They are present in the semi-solid state and have a yellow hue in the absence of alcohol, which increases the consumption of this flavoring. The uniformity and facile transport of oleoresins can improve the quality of natural vanilla products in pharmaceutical, cosmetological, perfumery, food, and flavoring (drinks and pastries). Finally, there are other value compounds as omega-6 and omega-3 that can be extracted from vanilla beans to add them in different types of products in order to have a beneficial impact on health.
Acknowledgments
This research was supported by Consejo Nacional de Ciencia y Tecnología de México (CONACyT) and Instituto Politécnico Nacional (IPN) through scholarships awarded to Hernández Fernández Miguel Ángel (registry number 275957).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adedeji, J., Hartman, T. G., & Ho, C. (1993). Flavor characterization of different varieties of vanilla beans. Perfumer & Flavorist, 18, 115–133.
- Brillouet, J.-M., & Odoux, É. (2010). In vivo kinetics of β-glucosidase towards glucovanillin and related phenolic glucosides in heat-treated vanilla pod (Vanilla planifolia, Orchidaceae). Fruits, 65(2), 85–95.
- Brunschwig, C., Senger-Emonnot, P., Aubanel, M.-L., Pierrat, A., George, G., Rochard, S., & Raharivelomanana, P. (2012). Odor-active compounds of Tahitian vanilla flavor. Food Research International, 46, 148–157.
- Burdock, G. A., & Fenaroli, G. (2005). Fenaroli‘s handbook of flavor ingredients. Boca Raton, Fla: CRC Press.
- Castillo-Ruiz, M. C., Guillermo-Alcocer, G., Bojórquez-Gamboa, R. R., & Rocha-Uribe, J. A. (2011). Extraction of vanilla oleoresin (Vanilla planifolia Andrews) with supercritical CO2. Tecnología, Ciencia, Educación. (IMIQ), 26(2), 80–84.
- Cicchetti, E., & Chaintreau, A. (2009). Comparison of extraction techniques and modeling of accelerated solvent extraction for the authentication of natural vanilla flavors. Journal of Separation Science, 32, 1957–1964.
- Da Costa, N. C., & Pantini, M. (2006). The analysis of volatiles in Tahitian vanilla (Vanilla tahitensis) including novel compounds. Developments in Food Science, 43, 161–164.
- de Guzman, C. C., & Zara, R. R. (2012). Vanilla. In K. V. Peter (Ed.), Handbook of Herbs and Spices (2nd ed., pp. 547–589). Cambridge, UK: Woodhead Publishing in Food Science, Technology and Nutrition.
- Dignum, M. J. W., Kerler, J., & Verpoorte, R. (2002). Vanilla curing under laboratory conditions. Food Chemistry, 79, 165–171.
- Dunphy, P., & Bala, K. (2014). The Role of Lipids in Vanilla Beans and their Transformation during Curing. A review of the effects, incluiding flavor formation. Perfumer & Flavorist, 39, 20–28.
- Fang, Y., Shi, Z., & Zhang, K. (2002). Study on extraction of vanillin from Vanilla planifolia Andr. with supercritical CO2 fluid. Xianglio Xiangjing Huazhuangpin, 4, 22–24.
- Fladby, T., Bryhn, G., Halvorsen, O., Rosé, I., Wahlund, M., Wiig, P., & Wetterberg, L. (2004). Olfactory Response in the Temporal Cortex of the Elderly Measured with Near-Infrared Spectroscopy: A Preliminary Feasibility Study. Journal of Cerebral Blood Flow & Metabolism, 24, 677–680.
- Frenkel, C., & Havkin-Frenkel, D. (2006). The physics and chemistry of vanillin. Perfumer & Flavorist, 31, 28–36.
- Gallage, N. J., & Møller, B. L. (2015). Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Molecular Plant, 8, 40–57.
- Gassenmeier, K., Riesen, B., & Magyar, B. (2008). Commercial quality and analytical parameters of cured vanilla beans (Vanilla planifolia) from different origins from the 2006–2007 crop. Flavour and Fragrance Journal, 23(3), 194–201.
- Gatfield, I., Hilmer, J. M., Weber, B., Hammerschmidt, F., Reiss, I., Poutot, G., … Meier, D. (2007). Chemical and biochemical changes occurring during the traditional Madagascan vanilla curing process. Perfumer & Flavorist, 32, 20–28.
- Harshvardhan, K., Suri, M., & Goswami, A. (2017). T. Biological approach for the production of vanillin from lignocellulosic biomass (Bambusa tulda). Journal of Cleaner Production, 149, 485–490.
- Hartman, T. G., Karmas, K., Chen, J., Shevade, A., Deagro, M., & Hwang, H. (1992). Determination of vanillin, other phenolic compounds, and flavours in vanilla beans. In C. T. Ho, C. Y. Lee, & M. T. Huang ACS Symp. Ser. No. 506 Eds., Phenolic compounds in food and their effects on Health I (pp. 60–76). Washington, DC: American Chemical Society.
- Havkin-Frenkel, D., French, J. C., & Frenkel, C. (2005). Inside vanilla. Perfumer & Flavorist, 30, 36–55.
- Hernández-Hernández, J. (2011). Mexican Vanilla Production. In D. Havkin-Frenkel & F. Belanger (Eds.), Handbook of vanilla of science and technology (pp. 3–24). West Sussex, UK: Blackwell Publishing Ltd.
- Ho, C. T., & Chen, Q. (1994). Lipids in food flavors (pp. 2–24). (C. T. Ho & T. G. Hartman, Eds.). Washington, DC: American Chemical Society.
- Jacobson, M. F. (2009). Liquid candy. In How soft drinks are harming American’sHealth (2nd, pp. 17). USA: Center for Science in the Public Interest.
- Knez, Z., Markocic, M., Leitgeb, M., Primozic, M., Hrncic, M., & Skerget, M. (2014). Industrial applications of supercritical fluids: A review. Energy, 77, 235–243.
- Korthou, H., & Verpoorte, R. V. (2007). Vanilla. In R. G. Berger (Ed.), Flavours and Fragrances (pp. 203–217)). Berlin: Springer.
- Lechat-Vahirua, I., & Bessiere, J. M. (1998). Plantes aromatiques de Polynésie Française: La vanille tahitienne. Rivista Italiana EPPOS, 9, 569–578.
- Liang, Y., Liu, J., Zhong, Q., Shen, L., Yao, J., Huang, T., & Zhou, T. (2018). Determination of major aromatic constituents in vanilla using an on-line supercritical fluid extraction coupled with supercritical fluid chromatography. Journal of Separation Science, 41(7), 1600–1609.
- Magnusson, B., & Örnemark, U. (2014). Eurachem guide: the fitness for purpose of analytical methods – a laboratory guide to method validation and related topics (2nd ed.). USA: CRC Press, Taylor & Francis Group. Retrieved from http://www.eurachem.org
- Naik, S. N., Jadeja, G. C., Pradhan, R. C., & Rout, P. K. (2014). Extraction of natural products using supercritical fluids and pressurized liquids. Pharmaceutical and Biological Evaluations, 1(1), 9–24.
- Nguyen, K., Barton, P., & Spencer, J. (1991). Supercritical carbon dioxide extraction of Vanilla. The Journal of Supercritical Fluids, 4, 40–46.
- NIST. 2008. Nist chemistry webbook. Retrieved from https://webbook.nist.gov/chemistry/
- NMX-FF-074-SCFI-2009. (2009). Non Industrialized Food Products for Human Consumption –Vanilla (Vanilla fragrans (Salisbury) Ames*). México: Economy Secretary. Specifications and test methods.
- Odoux, E. (2006). Glucosylated aroma precursors and glucosidase(s) in vanilla bean (Vanilla planifolia G. Jackson). Fruits, 61(3), 171–184.
- Odoux, E. (2011). Developing the aromatic quality of cured vanilla beans (Vanilla planifolia G. Jackson). In E. Odoux & M. Grisoni (Eds.), Medicinal and aromatic plants-industrial profiles: Vanilla (Vol. 47, pp. 189–204). Boca Raton, FL: CRC Press.
- Odoux, E., Escoute, J., & Verdeil, J. L. (2006). The relation between glucovanillin, β-glucosidase activity and cellular compartmentation during the senescence, freezing and traditional curing of vanilla beans. Annals of Applied Biology, 149, 43–52.
- Pérez-Silva, A., Gunata, Z., Lepoutre, J., & Odoux, E. (2011). New insight on the genesis and fate of volatile-active compounds in vanilla beans (V. planifolia G. Jackson) during traditional curing. Food Research International, 44, 2930–2937.
- Pérez-Silva, A., Odoux, E., Brat, P., Ribeyre F., Rodriguez-Jimenes, G., Robles-Olvera V., García-Alvarado, M. A., & Günata, Z. (2006). GC–MS and GC olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chemistry, 99, 728–735.
- Ranadive, A. S. (2006). Inside look: Chemistry and biochemistry of vanilla flavor. Perfumer & Flavorist, 31(3), 8–44.
- Rodríguez-Jimenes, G. C., Vargas-García, A., Espinoza-Pérez, D. J., Salgado-Cervantes, M. A., Robles-Olvera, V. J., & García-Alvarado, M. A. (2013). Mass transfer during vanilla beans solid liquid extraction: Effect of extraction method. Food Bioprocess Technology, 10, 2640–2650.
- Rojas-Ávila, A., Pimentel-Rodas, A., Rosales-García, T., Dávila-Ortiz, G., & Galicia-Luna, L. A. (2016). Solubility of binary and ternary systems containing vanillin and vanillic acid in supercritical carbon dioxide. Journal of Chemical & Engineering Data, 61(9), 3225–3232.
- Romero-de la Vega, G., Salgado-Cervantes, M. A., García-Alvarado, M. A., Romero-Martínez, A., & Hegel, P. E. (2016). Fractionation of vanilla oleoresin by supercritical CO2 technology. The Journal of Supercritical Fluids, 108, 79–88.
- Rueda, F., Domingo, J. C., & Mach, N. (2011). Efectos de los ácidos grasos omega 3 y otros suplementos alimenticios en procesos patológicos relacionados con la tercera edad. Revista Española de Nutrición Humana y Dietética, 15(1), 20–29.
- Sinha, A. K., Sharma, U. K., & Sharma, N. (2008). A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanilla and other constituents. International Journal of Food Sciences and Nutrition, 59(4), 299–326.
- Toth, S., Lee, K. J., Havkin-Frenkel, D., Belanger, F. C., & Hartman, G. (2011). Volatile compounds in vanilla. In D. Havkin-Frenkel & F. Belanger (Eds.), Handbook of vanilla science and technology (pp. 183–218). West Sussex, UK: Blackwell Publishing Ltd.
- Zhang, S., & Mueller, C. (2012). Comparative analysis of volatiles in traditionally cured Bourbon and Ugandan vanilla bean (Vanilla planifolia) extracts. International Journal of Food Sciences and Nutrition, 60, 10433–10444.