 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Opuntia genus belongs to the Cactaceae family, Mexico is the major producer in the world. Due to Opuntia’s phytochemical content and their health-related benefits, was proposed the elaboration of liquor using Opuntia fruit. Phenolic content, volatile aromatic profile, antioxidant activity, and sensory evaluation were carried out to characterize the liquor. Sensorial evaluation results showed that L-2D (2 day-macerated liquor) has the higher consumers‘ acceptance. Moreover, L-2D had the highest content of volatile compounds (348 mg/L), and the highest antioxidant activity (1.29, 1.63 and 2.05 mmol Trolox/L, according to ABTS, DPPH and FRAP methods, respectively). We could identify and quantify 29 volatile compounds, being ethyl acetate and D-limonene those with the highest content. Quercitrin was the most abundant phenolic compound in the liquor. The findings of this study demonstrate that Opuntia liquor is a rich source of phenolics and aromatic compounds that would be a drink with the probability of being commercialize.
RESUMEN
El género Opuntia pertenece a la familia Cactaceae, México es el mayor productor del mundo. Debido al contenido fitoquímico de Opuntia y sus beneficios relacionados con la salud, se propuso la elaboración de un licor utilizando la fruta de Opuntia. El contenido fenólico, el perfil fenólico, el perfil aromático volátil, la actividad antioxidante y la evaluación sensorial se llevaron a cabo para caracterizar el licor. Los resultados de la evaluación sensorial mostraron que L-2D (licor de 2 días macerado) tuvo la mayor aceptación de los consumidores. Además, L-2D tuvo el mayor contenido de compuestos volátiles (348 mg/L) y la mayor actividad antioxidante (1.29, 1.63 y 2.05 mmol de Trolox/L, según los métodos ABTS, DPPH y FRAP, respectivamente). Con respecto a los compuestos volátiles aromáticos, pudimos identificar y cuantificar 29 de ellos, siendo el acetato de etilo y el D-limoneno los que tuvieron el mayor contenido. La quercitrina fue el compuesto fenólico más abundante en el licor. Los hallazgos de este estudio demuestran que el licor de Opuntia es una fuente rica de compuestos fenólicos y aromáticos, siendo una bebida con probabilidad de ser comercializada.
Introduction
Opuntia fruits, belonging to the Opuntia genus and Cactaceae family, is a tropical/subtropical plant cultivated mainly in drought regions (Cejudo-Bastante, Chaalal, Louaileche, Parrado, & Heredia, Citation2014; Melgar et al., Citation2017; Özcan & Al Juhaimi, Citation2011; Ventura‐Aguilar, Bosquez‐Molina, Bautista‐Baños, & Rivera‐Cabrera, Citation2017). Opuntia is native to the Americas and currently grown in the Mediterranean basin, the Middle East and India (Farag, Maamoun, Ehrlich, Fahmy, & Wesjohann, Citation2017; Msaddak et al., Citation2017). Mexico is the major producer of the Opuntia genus, with 44% of the world´s production coming from Puebla, which is the largest producer state (Barba et al., Citation2017). The Opuntia genus is highly diverse, and the species includes O. ficus-indica, O. stricta, O. dellenii, and O. robusta Wendl var. robusta, which are the most common variants grown commercially for both food and feed consumption (Ventura‐Aguilar et al., Citation2017).
Opuntia fruits are nutritional and functional valuable plant material due to its antioxidant properties and health-related benefits. Thus, increasing the interest to use it into new products developing (Barba et al., Citation2017; Cejudo-Bastante et al., Citation2014). The current industrial use of Opuntia fruits is as food additive to obtain natural pigments (betacyanin, betaxanthin, and betalain). The current industrial use for Opuntia fruits is as a food additive to obtain natural pigments (betacyanin, betaxanthin, and betalain) (Figueroa-Cares et al., Citation2010), dietary fiber and galacturonic acid obtention as thickening agent (Barba et al., Citation2017). Opuntia fruits are commonly consumed fresh manner; nonetheless, products such as juices, marmalades, jams, sweets, ice cream, candies, sauces, and beverages are elaborated (Barba et al., Citation2017; Melgar et al., Citation2017; Ramírez-Moreno, Hervert-Hernández, Sánchez-Mata, Díez-Marqués, & Goñi, Citation2011).
Opuntia fruits beverages artisanal liquors are found. Liquors are flavored hydroalcoholic beverages obtained by maceration, infusion or distillation, with an alcohol content ranging between 15º and 50º (Herstein & Gregory, Citation2013). However, to our knowledge, Opuntia fruits liquor made by maceration process has not been standardized nor characterized. (Da Silva Alves, Berríos, Pan, & Ramírez-Ascheri, Citation2018). Therefore, the objectives of this work were to develop aromatic and functional Opuntia fruits liquor that would be accepted by consumers and to investigate the volatile compound profile. To achieve these goals Opuntia robusta Wendl. var. robusta was chosen to develop the liquor by the maceration process, and headspace solid-phase microextraction and gas chromatography-mass spectrometry (HS-SPME/GC-MS) was the technique used to analyze the volatile compounds in the liquor.
Materials and methods
Plant material
Fresh Opuntia robusta Wendl. var. robusta fruits, weighing approximately 120–150 g and with uniform shape and maturity, were harvested from Santiago Tolman, Otumba village in Estado de Mexico, Mexico. The fruits were washed and rinsed with distilled water and then they were divided into two batches: 1) whole fruit (WF) and 2) peeled fruit (PF), and they were chopped into 1 cm3 pieces to make the Opuntia fruits liquor.
Chemical composition
Ash, moisture, protein, and fat content were determined by AOAC methods (AOAC, Citation2016). Moisture (g water/100 g sample) was determined by drying a 3 g sample at 105°C to a constant weight. Ash (g ash/100 g) was performed at 550°C for 2 h. Protein (g protein/100 g) was analyzed according to the Kjeldahl method. Fat (g fat/100 g) was calculated by weight loss after a six-cycle extraction with petroleum ether in a Soxhlet apparatus (Soxtec ® System HT). Crude fiber was determined following the acid and alkaline digestion with subsequent calcination. Carbohydrates were determined using the difference from the crude fiber, lipid, protein and ash contents. Each assay was carried out in triplicate using fresh fruit.
Opuntia fruits liquor elaboration
Different liquors were prepared from the Opuntia fruits. It was used peeled fruit and it was left for one week of maceration in closed plastic trays at 20°C, time based on preliminary results (data not shown). Liquor preparation was carried out by mixing 0.707 kg of PF or UF with 0.394 kg of deodorized potable ethanol (96 ºGL), which helps extract the Opuntia fruits pigments and volatile compounds. Fruits were macerated at room temperature (20ºC) for 7 days. Thereafter, the samples were filtered through filter paper in order to remove the remaining pulp and seed particles. The extracts obtained in the previous step (0.19 L) were mixed with inverted sucrose syrup (0.58 L, 35 ºBrix) and packaged in amber glass bottles. The daily liquors were labeled as liquor-one day (L-1D), liquor-two days (L-2D), liquor-three days (L-3D), liquor-four days (L-4D), liquor-five days (L-5D), liquor-six days (L-6D) and liquor-seven days (L-7D).
Color measurement
Color measurement parameters (Lightness L*, redness a* and yellowness b*) were carried out using a color reader (CR-10, Konica–Minolta Sensing Inc., Osaka, Japan). The L* value indicated the lightness, 0–100 representing dark to light. The a* value gave the degree of the green–red color, with a higher positive a* value indicating more red. The b* value indicated the degree of the blue–yellow color, with a higher positive b* value indicating more yellow. The color index was estimated using equation (Solórzano, Martín, Salazar, Sandoval, & Kirschbaum, Citation2015):
Sensory evaluation of the liquor
The liquors produced were subjected to sensory evaluation. This test was designed to evaluate consumer acceptance of the liquor. Liquors were given to the participants in a randomized order, in a glass container with randomly assigned three-digit codes. Water was provided to rinse the mouth between each sample. Liquors were evaluated for color, odor, appearance, flavor and overall quality. A five-point hedonic scale was used (5 = like very much, 4 = like moderately, 3 = like slightly, 2 = dislike moderately, 1 = dislike very much) to assess the acceptance of the liquors by the consumers (Lesschaeve, Citation2007).
Extraction and identification of aromatic compounds in the liquor by headspace solid-phase microextraction (HS-SPME)
Extraction of aromatic compounds
Aromatic compounds were extracted from the L-1D, L-2D, L-3D, L-4D, L-5D, L-6D and L-7D liquors by HS-SPME. An SPME device (Supelco, Bellefonte, PA, USA) with a 10 mm fiber coated with 100 µm of polydimethylsiloxane was used for the extraction. As an internal standard, 2-nonanone 10 ppm (Sigma) was added to 5 mL of liquor and the mix was placed in a 10 mL vial (Supelco, Bellefonte, PA, USA). The extraction was performed using the headspace mode (20 mm from the liquid surface) at 50 ºC for 15 min and at 50ºC for 30 min with shaking (250 rpm). All the analyses were conducted in triplicate.
Analysis by gas chromatography with mass spectrometry detection (GC-MS)
After the extraction, the SPME device was introduced in the splitless injector of a 7890A gas chromatograph (Agilent Technologies Inc., USA) at 230ºC for 5 min. The initial oven temperature was 40ºC and increased to 230ºC at 5ºC/min for 5 min. It was then increased to 300ºC at a rate of 20ºC/min for 3 min. Helium flow (99.99% purity, 1 mL/min) was the carrier, using a DB-5MS capillary column (60 m long, 250 µm diameter, 0.25 µm wide phase; Agilent Technologies Inc., USA). The aromatic compounds were identified with a 5975C quadrupole mass analyzer (Agilent Technologies Inc., USA), using the following specifications: EI mode at 70 eV, gain factor 1, transference line temperature (250ºC), ionization source (230ºC) and quadrupole (150ºC). The mass range was 33–800 u. The spectra were compared with NIST/EPA/NIH Mass Spectra Library database v1.7 (USA), and 80% of the identity was assumed positive. The volatile compounds were positively identified by comparing Kovats retention indices (Kováts, Citation2004) and retention time with those obtained for authentic standards or with mass spectra in the Wiley7n.l Database (Hewlett-Packard, Palo Alto, CA).
Antioxidant activity
ABTS radical scavenging assay
The 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay was carried out in the liquors (1–7 days) as stated by Re et al. (Citation1999). Afterward, 7 mM of ABTS (Sigma) was mixed (1:1) with 2.45 mM of potassium persulfate (Sigma). The mix was kept for 12–16 h at room temperature in the dark to generate free radicals (ABTS*+). The ABTS*+ solution was then diluted in ethanol (1:100) to obtain 0.7 ± 0.02 absorbance units (734 nm), measured by a spectrophotometer (S-22 UV/Vis BOECO, Germany). Next, 10 µL of liquor was mixed with 990 µL of ABTS*+ solution. Then, the absorbance was measured at 734 nm for 7 min. A standard curve (500–3000 µM) was constructed using 6-hydroxy-2, 5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, Sigma), and the ABTS*+ scavenge value was obtained by interpolation from a linear regression analysis. The results were expressed in µM Trolox equivalent antioxidant capacity/L (TE). All the samples were analyzed in triplicate. When the value exceeded the linear range of the standard curve, additional dilutions were carried out.
DPPH radical scavenging assay
The 2,2-diphenyl-1-picrylhydrazyl-hydrate (DPPH) assay of the liquors (1–7 days) was carried out following the method described by Brand-Williams, Cuvelier, and Berset (Citation1995). A solution of 0.2 mM of DPPH (Sigma) in methanol (80%, Meyer) was prepared and 100 µL of the liquor was added to 2.9 mL of the DPPH+ solution. The mix was shaken (5 s) using a vortex and left at rest (120 min) at room temperature. After, the absorbance was measured at 515 nm using a spectrophotometer (S-22 UV/Vis BOECO, Germany), and a standard curve (Trolox, 200–1000 µM) was constructed. The DPPH+ scavenge value was obtained by interpolation from a linear regression analysis, and the results were expressed in TEAC. All the samples were analyzed in triplicate. When the value exceeded the linear range of the standard curve, additional dilutions were conducted.
Ferric reducing power assay
The ferric reducing power assay was conducted on the liquors (1–7 days) as stated by Benzie and Strain (Citation1996) method. The liquor (0.5 mL) was mixed with a sodium phosphate buffer (0.5 mL, 200 mM, pH 6.6, JTBaker) and potassium ferricyanide (0.5 mL, 1% w/v, Sigma). Then, the mix was incubated (50ºC, 20 min) and trichloroacetic acid (10% v/v, 0.5 mL, Sigma) was added. The mix (0.8 mL) was placed in microplate wells with deionized water (0.8 mL) and ferric chloride (0.16 mL, 0.1% w/v, Sigma). Finally, the absorbance was measured at 700 nm in a microplate reader. The results were expressed in Trolox equivalent using a standard curve (Trolox, 200–1000 µM). All the samples were analyzed in triplicate.
HPLC phenolic compound analysis
The liquor with the best antioxidant activity was analyzed by HPLC-UV-DAD (Agilent 1100; Agilent Technologies Inc., USA) to quantify the phenolic acids and flavonoids using a C-18 Silica Gel column (150 x 4.6 mm, 5 µm particle size, Zorbax), according to Ramamurthy, Maiti, Thomas, and Nair (Citation1992). The eluents were water/acetic acid (98/2, v/v, phase A) and water/acetonitrile/acetic acid (68/30/2, v/v, phase B), using a linear gradient flow (1.5 mL/min) from B to A (10–100%) for 30 min. The detector was set at 280 nm. The liquor and standards were injected using an automatic system. Each standard was injected by triplicate at different concentrations. Then, the compounds were identified by association to the retention times of the standards, while the quantification of the polyphenols was estimated using linear regression equations of the ratio of concentration/absorbance for each standard. The content of each compound was expressed as µg/mL of liquor.
Statistical analysis
The statistical analyses were performed using Sigma Plot® Software v12 (2015, USA), and the significant differences (p < 0.05) between the mean responses were evaluated using a one-way ANOVA with Tukey’s test. The values are expressed as the means of the triplicates ± standard deviation (SD).
Results and discussion
Chemical composition
shows the results of the chemical characterization of WF and PF. It can be observed that Opuntia fruit is a product with high moisture content (>80%). Protein and fat content did not show significant differences among the samples. Crude fiber was higher in WF, which might be due to the presence of the Opuntia peel, which is richer than pulp in fiber and minerals (El Kossori, Villaume, El Boustani, Sauvaire, & Méjean, Citation1998).
Table 1. Chemical composition of Opuntia robusta Wendl. var. robusta.
Tabla 1. Composición química de Opuntia robusta Wendl var. robusta.
In general, the results obtained in this study of Opuntia robusta Wendl. var. robusta fruits are comparable to those reported by Aquino Bolaños et al. (Citation2012) and El Kossori et al. (Citation1998), on different varieties of Opuntia fruits.
Color measurement
The color was calculated by tristimulus colorimetry, and the values of L*, a*, b*, ΔE, and the color index are presented in . The lightness (L) of the liquors obtained at different days of maceration was similar to that of the Opuntia fruit fresh pulp. However, the a* value of the liquor showed a decrease compared to the a* value of the fresh fruit pulp, suggesting that liquors presented red tones; this might be related to the fruit betalain content and stability (Herbach, Stintzing, & Carle, Citation2006). The b* values for the liquor were positive and close to the b* value for those presented by the fresh fruit pulp, indicating that Opuntia liquors are lighter (yellow color) than the fresh fruit. The ΔE is an indicator of the difference between liquor and fruit; the liquor obtained after two days of maceration presented a negative ΔE compared with the fresh fruit. On the other hand, the highest CI values were presented by L-1D and L-2D, possibly due to a higher extraction of pigments, such as the betalain family, which includes betacyanin (red-purple color) and betaxanthin (yellow color). Thus, the liquor’s color depends on the betalain concentration and the type that is extracted during maceration (Herbach et al., Citation2006; Stintzing et al., Citation2005). Hence, L-2D is the liquor that had the minus ΔE and the highest CI, indicating that it is the closest one to the color of fresh pulp.
Table 2. Fruit Opuntia liquor color parameters at different days of maceration.
Tabla 2. Parámetros del color del licor del fruto de Opuntia a diferentes días de maceración.
Sensory evaluation of the liquor
One focus group was used as a brief sensory characterization of the product. The test was conducted according to Gomes Da Silva et al. (Citation2017), with 9 participants, three men, and six women, ranging from 35 to 63 years old, recruited from their involvement with correlated areas, such as food science or food technology.
The panelists assessed the following descriptors: color, aroma, flavor, and appearance. The frequencies of each suggested application and the descriptive terms were calculated (). All attributes varied significantly with the maceration time (1–7 days), and L-2D was perceived as the most accepted Opuntia liquor among the consumers; moreover, L-2D had the highest values of flavor, odor, and appearance, followed by L-3D and L-7D. On the other hand, L-5D had the overall lowest acceptance rate, according to the panelists; it presented an unpleasant odor and flavor. Considering the sensorial characteristics, L-2D was optimal. Therefore, two days were selected as the maceration time to prepare the liquors.
Table 3. Influence of maceration days on the sensory acceptability of Opuntia fruit liquors.
Tabla 3. Influencia del tiempo de maceración en la aceptabilidad sensorial de licores de fruto de Opuntia.
Aromatic compound profile
Aroma is one of the most important sensory attributes that contribute to a liquor’s quality and acceptance by the consumer (Lasekan & Peng Yap, Citation2018; Sha, Chen, Qian, Wang, & Xu, Citation2017). HS-SPME/GC-MS was used to characterize the volatile compounds present in the Opuntia liquors. As summarized in , 29 volatile compounds were identified in Opuntia liquors macerated at different days, with esters, aldehydes, hydrocarbons, terpene, benzene, and sulfide-compounds being the most important compounds. The compounds that were quantitatively present in the liquors were ethyl acetate (pineapple odor), benzene, 1,4-dichlorobenzene, D-limonene (lemon) and toluene. They were all between 65% and 75% of the total peak area. The content varied depending on the day of maceration.
Table 4. Odor-active volatiles identified in Opuntia fruit liquors by HS-SPME GC-MS.
Tabla 4. Productos volátiles con olor activo identificados en licores de fruto de Opuntia por HS-SPME GC-MS.
These results are in accordance with the literature, since compounds such as octanal, nonanal, ethyl ester octanoic acid, decanal, hexadecane, tetradecane were also found in the pulp and peel of some Opuntia ficus-indica varieties (red “Rose”, yellow-orange “Gialla” and greenish-white “Bianca”) and pomegranate juices (Farag et al., Citation2017; Yi et al., Citation2016). Esters might minimize the acidity and bitter flavors in food (Curioni & Bosset, Citation2002). Aldehydes such as hexanal and nonanal provide an herbaceous aroma (Garde, Avila, Fernández-García, Medina, & Nunez, Citation2007); moreover, nonanal contributes to the aroma by exhibiting a strong odor of orange peel, and a fatty, rose-like odor (Farag et al., Citation2017). Alcohols were also reported as predominant cactus fruit volatile compounds, though identified herein at lower levels (Farag et al., Citation2017). S chain acids such as nonanoic acid and octanoic acid were also found in this work. L-2D showed a higher concentration of volatile compounds compared to the other liquor samples, relating to sensory acceptance. Nonetheless, differences in the volatiles profile and quantification in this work and in the existing literature could be attributed to the addition to the liquor preparation, also the geographical origin or agricultural practices the volatile extraction method.
Antioxidant activity
The main antioxidants in food are phytochemicals such as phenolic acids, flavonoids, carotenoids, and anthocyanins, among other compounds. During antioxidation, different mechanisms are involved making it difficult to assess the antioxidant activity using a single method; therefore, it is necessary to combine more than one method to determine the in vitro antioxidant activity of different foods (López-Vargas, Fernández-López, Pérez-Álvarez, & Viuda-Martos, Citation2013; Martínez et al., Citation2012).
It is well known that the Opuntia genus has antioxidant properties, specifically the purple-skinned fruits (Kuti, Citation2004), or purple fruit juices (Stintzing et al., Citation2005). Chavez-Santoscoy, Gutierrez-Uribe, and Serna-Saldívar (Citation2009), and Madrigal-Santillán et al. (Citation2013) attributed this to its phenolic content, as phenols act as reducing agents, donating hydrogen quenching single oxygen molecules and chelating metals (Leopoldini, Russo, & Toscano, Citation2011; Rice-Evans, Miller, & Paganga, Citation1996).
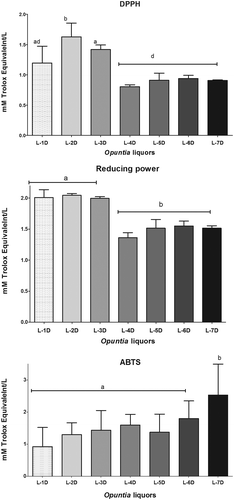
shows the results of the assessment of the antioxidant capacity of Opuntia liquors. The antioxidant activity, measured by DPPH and FRAP, showed that the maceration time had a positive effect on the extraction of compounds with antioxidant properties, L-2D was the liquor that presented the highest activity, except for ABTS•+ method. Despite these differences, these assays indicate that the liquors obtained have good antioxidant properties (Schlesier, Harwat, Böhm, & Bitsch, Citation2002). The antioxidant activity results are within the range reported by Di Majo, La Guardia, Giammanco, La Neve, and Giammanco (Citation2008) for different varieties of red wines. Nevertheless, our results were lower than those found by Stintzing et al. (Citation2005), who evaluated the antioxidant activity of different O. ficus-indica fruit samples using the ORAC method, resulting in green (5.4 mmol/L), orange (5.8 mmol/L) and red (6.8 mmol/L).
Figure 1. Effect of maceration days on antioxidant activity of Opuntia liquors.
Liquor-one day (L-1D), liquor-two days (L-2D), liquor-three days (L-3D), liquor-four days (L-4D), liquor-five days (L-5D), liquor-six days (L-6D) and liquor-seven days (L-7D).Different letters within each bar indicate significant difference according to Tukey Test (p ≤ 0.05).
Figura 1. Efecto de los días de maceración en la actividad antioxidante de licores de Opuntia.
Licor de 1 día (L-1D), licor 2 días (L-2D), licor 3 días (L-3D), licor 4 días (L-4D), licor 5 días (L-5D), licor 6 días (L-6D) y licor 7 días (L-7D). Letras diferentes dentro de cada barra indican una diferencia significativa según la prueba de Tukey (p ≤ 0.05).
HPLC phenolic compounds analysis
The presence of polyphenol compounds in alcoholic beverages is important as their bitterness and astringency contribute to the sensorial profile, and some of them, such as betacyanins and flavonols, also contribute to the color. Moreover, Opuntia polyphenols have been associated with several health benefits.
The analysis of the phenolic compounds in Opuntia liquor resulted in the identification of 9 compounds, which are presented in . These results show that the phenolic compounds identified belong to the phenolic acid and flavonoid families. Kuti (Citation2004) analyzed different types of prickly pears and reported that the purple skinned fruits contained the highest amounts of flavonoids, which is in accordance with the literature reporting that Opuntia with red-purple skin and their juices are widely known to possess high concentration of polyphenols, mainly flavonoids (Leopoldini et al., Citation2011; Madrigal-Santillán et al., Citation2013).
Table 5. HPLC phenolic profile and quantification in fruit Opuntia liquor (Opuntia robusta Windl. var. robusta) at two days of maceration.
Tabla 5. Perfil de compuestos fenólicos y cuantificación de ellos en el licor de fruto de Opuntia (Opuntia robusta Windl. var. robusta) a dos días de maceración, obtenidos por HPLC.
As seen in , quercitrin is the most abundant compound identified in the liquor. Ginestra et al. (Citation2009) and Msaddak et al. (Citation2017) reported that the flavanol, quercetin, is one of the most abundant dietary flavonoids with diverse biological properties, such as antiproliferative and anticarcinogenic activities. Thus, the presence of quercitrin, which is a glucoside derived from quercetin, might give the liquor some of these health benefits. Moreover, Mata et al. (Citation2016) identified some derivatives of quercetin in Opuntia spp. juices.
Although the phenolic concentration in cactus pears varies depending on the genetic and environmental factors, Mata et al. (Citation2016) reported that fruit with purple and red pulp contains the highest concentrations of these compounds, suggesting a correlation between the color of the fruit and its phenolic content, which is in accordance with our results.
Conclusion
Opuntia robusta Wendl. var. robusta could be a good source to develop new aromatic liquors. The results indicate that the liquor obtained after two days of maceration resulted in the best sensory experience and had the highest content of polyphenolic and aromatic compounds and good antioxidant activity. Twenty-nine aromatic compounds and nine phenolic compounds were identified, with ethyl acetate and D-limonene being the most abundant aromatic compounds and quercitrin being the most abundant phenolic compound in the liquor. The findings of our work suggest that Opuntia liquor is rich in phenolics and aromatic compounds, so it would be a drink with antioxidant capacity and a good acceptance, with a high probability of being commercialized. Nevertheless, further studies are needed to assess the stability of the aromatic and phenolic compounds during storage, and to determine the parameters that might affect the processing of the liquor.
Acknowledgments
We thank the technical staff of the Fruit and Vegetable Pilot Plant and Spectroscopy Central of Escuela Nacional de Ciencias Biologicas of Instituto Politecnico Nacional and the Flavors and Fragrances Laboratory of CICATA-Queretaro for their support in the analytical assays.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AOAC. (2016). Official methods of analysis of AOAC international (20th ed.). Rockville, MD: AOAC.
- Aquino Bolaños, E. N., Chavarría Moctezuma, Y., Chávez Servia, J. L., Guzmán Gerónimo, R. I., Silva Hernández, E. R., & Verdalet Guzmán, I. (2012). Physicochemical characterization of seven red-purple prickly pear fruit varieties (Opuntia spp.) and pigment stability of two varieties with the highest concentration. Investigación y ciencia, 55, 3–10.
- Barba, F. J., Putnik, P., Kovačević, D. B., Poojary, M. M., Roohinejad, S., Lorenzo, J. M., & Koubaa, M. (2017). Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends in Food Science & Technology, 67, 260–270.
- Benzie, F. F. I., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: The FRAP assay. Analytical Biochemistry, 239, 70–76.
- Brand-Williams, W., Cuvelier, M.-E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28, 25–30.
- Cejudo-Bastante, M. J. S., Chaalal, M., Louaileche, H., Parrado, J., & Heredia, F. J. (2014). Betalain profile, phenolic content, and color characterization of different parts and varieties of Opuntia ficus-indica. Journal of Agricultural and Food Chemistry, 62, 8491–8499.
- Chavez-Santoscoy, R., Gutierrez-Uribe, J., & Serna-Saldívar, S. (2009). Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods for Human Nutrition, 64, 146–152.
- Curioni, P., & Bosset, J. (2002). Key odorants in various cheese types as determined by gas chromatography-olfactometry. International Dairy Journal, 12, 959–984.
- Da Silva Alves, P. L., Berríos, J. D. J., Pan, J., & Ramírez-Ascheri, J. L. (2018). Passion fruit shell flour and rice blendes processed into fiber-rich expanded extrudates. CyTA-Journal of Food, 16, 901–908.
- Di Majo, D., La Guardia, M., Giammanco, S., La Neve, L., & Giammanco, M. (2008). The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chemistry, 111, 45–49.
- El Kossori, R. L., Villaume, C., El Boustani, E., Sauvaire, Y., & Méjean, L. (1998). Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica spp.). Plant Foods for Human Nutrition, 52, 263–270.
- Farag, M. A., Maamoun, A. A., Ehrlich, A., Fahmy, S., & Wesjohann, L. A. (2017). Assessment of sensory metabolites distribution in 3 cactus Opuntia ficus-indica fruit cultivars using UV fingerprinting and GC/MS profiling techniques. LWT-Food Science and Technology, 80, 145–154.
- Figueroa-Cares, I., Martinez-Damian, M. T., Rodríguez-Pérez, E., Colinas- Leon, M. T., Valle-Guadarrama, S., Ramirez-Ramirez, S., & Gallegos-Vazquez, C. (2010). Pigments content, other compounds and antioxidant capacity in 12 cactus pear cultivars (Opuntia spp.) from México. Agrociencia, 44, 763–771.
- Garde, S., Avila, M., Fernández-García, E., Medina, M., & Nunez, M. (2007). Volatile compounds and aroma of hispánico cheese manufactured using lacticin 481-producing Lactococcus lactis subsp. lactis INIA 639 as an adjunct culture. International Dairy Journal, 17, 717–726.
- Ginestra, G., Parker, M. L., Bennett, R. N., Robertson, J., Mandalari, G., Narbad, A., … Waldron, K. W. (2009). Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. Journal of Agricultural and Food Chemistry, 57, 10323–10330.
- Gomes Da Silva, M. D. F., Dionísio, A. P., Ferreira Carioca, A. A., Silveira Adriano, L., Pinto, C. O., Pinto de Abreu, F. A., & Ferreira Pontes, D. (2017). Yacon syrup: Food applications and impact on satiety in healthy volunteers. Food Research International, 100(May), 460–467.
- Herbach, K. M., Stintzing, F. C., & Carle, R. (2006). Betalain stability and degradation structural and chromatic aspects. Journal of Food Science, 71. doi:10.1111/j.1750-3841.2006.00022.x
- Herstein, K. M., & Gregory, T. C. (2013). Liqueurs and cordials Cap. XII (pp. 190). Broking, NY: In Chemistry and Technology of Wines and Liquors; Amazon.
- Kováts, E. S. (2004). Gas‐chromatographische charakterisierung organischer verbindungen. Teil 1: Retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helvetica Chimica Acta, 41, 1915–1932.
- Kuti, J. O. (2004). Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chemistry, 85, 527–533.
- Lasekan, O., & Peng Yap, S. (2018). Characterization of the aroma compounds in fresh and dried sapodilla (Manikara zapota, L.) by the application of aroma extract dilution analysis. CyTA - Journal of Food, 16, 801–806.
- Leopoldini, M., Russo, N., & Toscano, M. (2011). The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chemistry, 125, 288–306.
- Lesschaeve, I. (2007). Sensory evaluation of wine and commercial realities: Review of current practices and perspectives. American Journal of Enology and Viticulture, 58, 252–258.
- López-Vargas, J. H., Fernández-López, J., Pérez-Álvarez, J. A., & Viuda-Martos, M. (2013). Chemical, physico-chemical, technological, antibacterial and antioxidant properties of dietary fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Food Research International, 51, 756–763.
- Madrigal-Santillán, E., García-Melo, F., Morales-González, J. A., Vázquez-Alvarado, P., Muñoz-Juárez, S., Zuñiga-Pérez, C., … Hernández-Ceruelos, A. (2013). Antioxidant and anticlastogenic capacity of prickly pear juice. Nutrients, 5, 4145–4158.
- Martínez, R., Torres, P., Meneses, M. A., Figueroa, J. G., Pérez-Álvarez, J. A., & Viuda-Martos, M. (2012). Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chemistry, 135, 1520–1526.
- Mata, A., Ferreira, J., Semedo, C., Serra, T., Duarte, C., & Bronze, M. (2016). Contribution to the characterization of Opuntia spp. juices by LC–DAD–ESI-MS/MS. Food Chemistry, 210, 558–565.
- Melgar, B., Dias, M. I., Ciric, A., Sokovic, M., Garcia-Castello, E. M., Rodriguez-Lopez, A. D., … Ferreira, I. (2017). By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Industrial Crops and Products, 107, 353–359.
- Msaddak, L., Abdelhedi, O., Kridene, A., Rateb, M., Belbahri, L., Ammar, E., … Zouari, N. (2017). Opuntia ficus-indica cladodes as a functional ingredient: Bioactive compounds profile and their effect on antioxidant quality of bread. Lipids in Health and Disease, 16, 32.
- Özcan, M. M., & Al Juhaimi, F. Y. (2011). Nutritive value and chemical composition of prickly pear seeds (Opuntia ficus indica L.) growing in Turkey. International Journal of Food Sciences and Nutrition, 62, 533–536.
- Ramamurthy, M. S., Maiti, B., Thomas, P., & Nair, P. M. (1992). High- performance liquid chromatography determination of phenolic acids in potato tubers (Solanum tuberosum) during wound healing. Journal of Agricultural and Food Chemistry, 40, 569–572.
- Ramírez-Moreno, E., Hervert-Hernández, D., Sánchez-Mata, M., Díez-Marqués, C., & Goñi, I. (2011). Intestinal bioaccessibility of polyphenols and antioxidant capacity of pulp and seeds of cactus pear. International Journal of Food Sciences and Nutrition, 62, 839–843.
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26, 1231–1237.
- Rice-Evans, C. A., Miller, N. J., & Paganga, G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine, 20, 933–956.
- Schlesier, K., Harwat, M., Böhm, V., & Bitsch, R. (2002). Assessment of antioxidant activity by using different in vitro methods. Free Radical Research, 36, 177–187.
- Sha, S., Chen, S., Qian, M., Wang, C., & Xu, Y. (2017). Characterization of the typical potent odorants in chinese roasted sesame-like flavor type liquor by Headspace Solid Phase Microextraction–Aroma extract dilution analysis, with special emphasis on sulfur-containing odorants. Journal of Agricultural and Food Chemistry, 65, 123–131.
- Solórzano, A. C., Martín, A., Salazar, S. M., Sandoval, J. S., & Kirschbaum, D. S. (2015). Correlation between fruit color measurement and total soluble solids concentration in strawberry (Fragaria ananassa Duch.). Revista agronomica del noroeste argentino, 35, 55–60.
- Stintzing, F. C., Herbach, K. M., Mosshammer, M. R., Carle, R., Yi, W., Sellappan, S., … Felker, P. (2005). Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. Journal of Agricultural and Food Chemistry, 53, 442–451.
- Ventura‐Aguilar, R. I., Bosquez‐Molina, E., Bautista‐Baños, S., & Rivera‐Cabrera, F. (2017). Cactus stem (Opuntia ficus‐indica Mill): Anatomy, physiology and chemical composition with emphasis on its biofunctional properties. Journal of the Science of Food and Agriculture, 97, 5065–5073.
- Woerdenbag, H. J., Quax, W. J., Bos, R., Riswan, S., & Windono, T. (2004). Composition of the essential oils ofKaempferia rotunda L. andKaempferia angustifolia Roscoe rhizomes from Indonesia. Flavour and Fragrance Journal, 19, 145–148.
- Yi, Z., Feng, T., Zhuang, H., Ye, R., Li, M., & Liu, T. (2016). Comparison of different extraction methods in the analysis of volatile compounds in pomegranate juice. Food Analytical Methods, 9, 2364–2373.
