ABSTRACT
This study aimed to develop and characterize a fermented persimmon juice with potential anti-hangover and anti-hypertensive effects using gamma-aminobutyric acid (GABA)-producing lactic acid bacteria. Results showed the co-fermentation of Lactobacillus plantarum C17 and Lactobacillus pentosus Lp-B produced the highest yield of 2.102 mmol/L of GABA. After 48 h of fermentation, GABA was produced at the conversion rate of 69.32% from glutamate. The alcohol dehydrogenase activation rate, acetaldehyde dehydrogenase activation activity and hydroxyl inhibition rate of fermented persimmon juice were 38.91%, 34.72%, and 16.79%, respectively, which displayed higher anti-hangover activity compared to unfermented persimmon. Moreover, the angiotensin-converting enzyme inhibition ability of fermented persimmon juice was 42.34% and the expression level of human endothelin-1 was reduced to 34.64 μg/mL. These findings indicated GABA-enriched fermented persimmon juice had potential anti-hangover and anti-hypertensive properties.
RESUMEN
El presente estudio tuvo como objetivo desarrollar y caracterizar un jugo de caqui fermentado con posibles efectos antiresaca y antihipertensivos utilizando bacterias ácido lácticas productoras de ácido gamma-aminobutírico (GABA). Los resultados revelan que la fermentación conjunta de Lactobacillus plantarum C17 y Lactobacillus pentosus Lp-B produce un rendimiento más alto de GABA, de 2.102 mmol/L. Transcurridas 48 horas de fermentación se produjo GABA a partir de glutamato a una tasa de conversión del 69.32%. La tasa de activación de alcohol deshidrogenasa, la tasa de activación de acetaldehído deshidrogenasa y la tasa de inhibición de hidroxilo del jugo de caqui fermentado registraron niveles de 38.91%, 34.72% y 16.79%, respectivamente. Estos resultados dan cuenta de una mayor efecto antiresaca en comparación con el jugo de caqui no fermentado. Además, se constató que la capacidad de inhibición de la enzima convertidora de angiotensina del jugo de caqui fermentado fue de 42.34% y que el nivel de expresión de endotelina-1 humana se redujo a 34.64 μg/ml. Estos hallazgos indican que el jugo de caqui fermentado enriquecido con GABA tiene propiedades que potencialmente permiten reducir la resaca y la hipertensión.
1. Introduction
Persimmon (Diospyros kaki L.) is a deciduous fruit that consumed widely in many countries around the world, being applied for curing various diseases, such as hypertension, coughs, frostbite, paralysis, burns, and bleeding in ancient medicine (Liu et al., Citation2019). Today, Asia continues to be the main producer worldwide (90.3%), followed by Europe (6.7%). In the case of countries, the main production comes from China (Pérez-Burillo, Oliveras, Quesada, Rufián-Henares, & Pastoriza, Citation2018) and there are many persimmon varieties of which Mopan persimmon is one kind. Persimmon is full of nutrients like protein, vitamin C, abundant carbohydrates, and amino acids required by the human body (Pérez-Burillo et al., Citation2018) which give persimmon more biological activity. Persimmon is an excellent source of bioactive compounds such as ascorbic acid, carotenoids, and condensed tannins (Gu et al., Citation2008) and has been confirmed to have beneficial effects against diabetes as well as a number of degenerative and cardiovascular diseases (George & Redpath, Citation2008). Meanwhile, the antioxidant activity of persimmon has been widely known (Hossain, Moon, & Kim, Citation2018). These evidence make very attractive the use and value of persimmon as potential functional ingredients (Lucas-González, Viuda-Martos, Álvarez, & Fernández-López, Citation2018). Although the high yield of persimmon guarantees the raw material conditions for persimmon processing and production, the bioavailability of persimmons is currently not high. However, due to the high contents of moisture, sugars, and polyphenols, persimmon fruits are easily decayed and browned after harvesting, leading to short shelf life and difficulty in handling, transportation and storage (Zhou, Zhao, Sheng, Tao, & Yang, Citation2011). Moreover, persimmon contains more tannins and has astringent taste which is not suitable for direct consumption. In addition, there are not many types of processed products of persimmon, mainly dried persimmon, persimmon vinegar and persimmon wine, and they do not have advantages in the market. This may be due to the increasingly saturated or supersaturated market of fresh fruit. So new ways and products for persimmon processing become the primary problem that needs to be solved urgently.
Many strains of lactic acid bacteria (LAB), generally regarded as safe (GRAS), that can decrease cholesterol, decrease lactose intolerance, enhance intestinal function, and improve immunity (Kaprasob, Kerdchoechuen, Laohakunjit, Sarkar, & Shetty, Citation2017; Kim et al., Citation2018). Lactobacillus species have received tremendous attention due to their potential biological activity (Zhang, Ma, He, Lu, & Ren, Citation2018). Therefore, LAB are widely used in the production of fermented foods for fruits and vegetables (Cagno, Coda, Angelis, & Gobbetti, Citation2013). LAB isolated from traditional fermented food can produce a variety of biologically active compounds during fermentation (Mozzi, Ortiz, Bleckwedel, Vuyst, & Pescuma, Citation2013). GABA is a non-protein amino acid that acts as one of the major inhibitory neurotransmitters in the central nervous system (Xie et al., Citation2017) which can be produced by LAB. GABA has been authenticated as new resource food by China Food and Drug Administration (Kim, Lee, Ji, Lee, & Hwang, Citation2009) in 2009 due to its various physiological functions in animals and humans such as hypotensive action and improving conditions like insomnia, depression, chronic alcoholism, and diabetes (Das & Goyal, Citation2015). In addition, GABA is produced by decarboxylation of glutamate or its salts by glutamate decarboxylase (GAD) (Ko, Lin, & Tsai, Citation2013). At present, there are many studies on the enrichment of GABA by LAB. However, most of them produced GABA by exogenous addition of glutamic acid and its salts (Kwon, Garcia, Song, & Lee, Citation2016; Song & Yu, Citation2018). Exogenous additions are prone to contamination compared to natural media and are not suitable for the development of functional food. This requires us to find natural cultures such as grains, fruits, and vegetables which is suitable for GABA production. Meanwhile, high-performance production, optimization through different biotechnological techniques, and the discovery of new high-GABA producing strains will remain a focus of interest in research into GABA as a health-related novel biological active compound (Oh, Jeong, Velmurugan, Park, & Jeong, Citation2017; Quílez & Diana, Citation2017).
To the best of our understanding, limited studies have been reported on the fermenting persimmon juice with LAB. However, no report has yet been made on enriching GABA of fermented persimmon juice. This study was to choose GABA-producing lactic acid bacteria for persimmon juice fermentation and then explored its anti-hangover and anti-hypertensive effects in vitro. This will provide a new way for the persimmon processing industry.
2. Materials and methods
2.1. Materials and persimmon juice
Ripe persimmons (Diospyros kaki L. cv. Mopan) were collected in Baoding, Hebei province, China. After cleaning and peeling, persimmon and deionized water were mixed in a ratio of 1:2 (w:v) and then homogenized with a tissue mincer. Pectinase was added to hydrolyze with a ratio of 0.015% (w:w) and then persimmon juice was obtained by centrifuging at 9520 × g for 10 min at 4°C using high speed refrigerated centrifuge. (TGL 16M, Yida Instrument, China)
2.2. LAB strains and inoculum preparation
Lactobacillus plantarum C17 (CICC 22194), Lactobacillus pentosus Lp-B (CICC 22159) and Lactobacillus rhamnosus R11 (CICC 22173) were isolated from fermented cabbage pickles, fermented cucumber kimchi, Xinjiang camel milk, respectively. All of them are deposited in the China Center of Industrial Culture Collection. The three LAB strains were activated with MRS solid medium at 37°C for 24 h, to give approximately 109 viable colony forming units (CFU)/ml.
2.3. Persimmon juice fermentation
Single or mixed bacterial cultures (the ratio of mixed strains was 1:1) were added to 100 g of pasteurized persimmon juice with inoculum concentration of 5%. The initial fermentation temperature and fermentation time were 30°C and 48 h, respectively.
2.4. Determination of GABA
GABA contents were determined by Berthelot colorimetry (Zhang, Dai, Wang, & Li, Citation2018) with modification. The supernatant was obtained after centrifugation at 2380 × g for 20 min at 4°C from the fermentation broth. 0.1 mol/L of sodium carbonate solution and pH 10 of borate buffer solution were added to 0.4 mL pretreated fermentation broth. Then, 1 mL 6% phenol and 1 mL sodium hypochlorite were added to the mixture, respectively. Rested for 4–8 min after mixing. Then put the test tube into boiling water for 10 min, and cooled down for 20 min immediately. After the color became blue-green, 2 mL of 60% ethanol was added to the reaction solution. The absorbance was measured at 640 nm.
2.5. Determination of glutamate
The glutamate in persimmon juice and fermented broth were determined by ninhydrin colorimetry method (Wang, Wang, Hong, & Zhao, Citation2005). 5 mL of sample with appropriate dilution and 1 mL of ninhydrin solution were mixed together, then the mixture was heated in a water bath at 90°C for 20–25 min. When the mixture cooled to room temperature, measured absorbance at 569 nm.
2.6. Determination of anti-hangover efficacy
2.6.1. Determination of alcohol dehydrogenase (ADH) activity
ADH activity was determined following the Valle & Hoch method (Blair & Bodley, Citation1969). In brief, 1.5 mL pyrophosphate buffer (0.1 M, pH 8.8), 0.1 mL of 0.25 U mL−1 ADH, 0.5 mL ethanol (11.5%, v/v), and 0.1 mL of the beverage sample were mixed at 25°C, and then 1.0 mL 0.01 M NAD+ was added to initiate the reaction. The absorbance was determined at 340 nm by a microplate reader and was measured again after 15 min.
2.6.2. Determination of acetaldehyde dehydrogenase (ALDH) activity
ALDH activity was determined by Blair & Bodley method with modification (Moreb, Gabr, Vartikar, & Gowda, Citation2005). In brief, 1.6 mL pyrophosphate buffer (0.1 M, pH9.5), 0.1 mL of 0.25 U mL−1 ADH, 0.1 mL of 0.1 M acetaldehyde, 0.1 mL of 0.01 M pyrazole, and 0.1 mL of the beverage sample were mixed at 30°C, and then 1.0 mL 0.01 M NAD+ was added to initiate the reaction. The absorbance was immediately measured at 340 nm, and was measured again after the mixture was warmed at 30°C for 15 min.
2.6.3. Determination of hydroxyl scavenging ability
Hydroxyl scavenging ability was determined by the deoxyribose (DR) method with slight modification (Halliwell, Gutteridge, & Aruoma, Citation1987). Briefly, 0.1 mL sample was added with 0.4 mL phosphate buffer, 0.1 mL EDTA solution, 0.1 mL H2O2, deoxyribose 0.1 mL, ascorbic acid 0.1 mL, 0.1 mL FeCl3 solution were mixed, then incubated at 37°C for 1 h. Add 1.0 mL hydrochloric acid to terminate the reaction, thiobarbituric acid solution was used for color. The mixture was boiled for 15 min in water. The absorbance was immediately measured at 523 nm when it was cooled. If turbidity occurred, 3.0 mL of n-butanol for colorimetric extraction could solve this cause.
2.7. Determination of anti-hypertensive efficacy
2.7.1. Determination of ACE inhibition ability by high-performance liquid chromatography (HPLC)
ACE inhibition ability was determined by HPLC (Waters 2489, Massachusetts, USA), accordance with the methodology of (Zhao, Citation2009). In this study, the reduction in hippuric acid was used to indicate the inhibition rate of ACE. A standard curve for hippuric acid was established. Twenty uL sample and 50 uL Hepes buffer were mixed together, then preheat for 3 min at 37°C. Add 10 uL ACE enzyme solution (0.1 U/mL) and react at 37°C for 20 min. One hundred uL 1 mol/L hydrochloric acid was to terminate the reaction. The mixture was filtered through a 0.45 um filter to be tested. The chromatographic conditions were as follows: column:C18; mobile phase:methanol; flow rate: 1 mL/min; column temperature 30°C; wavelength 228 nm; injection volume 25 uL; elution conditions: methanol/phosphate buffer (10 mmol/L pH = 2.4 PBS) = 25:75.
2.7.2. Expression of human endothelin-1 (ET-1)
This study used a commercial kit by ELASA method to detect the expression of ET-1. (Beijing Donggeboye Biological Technology Corporation, Beijing, China)
2.8. Statistical analysis
All experiments were carried out in triplicate, and each sample was analyzed in duplicate. The results are expressed as mean ± S.D. SPSS Statistics 22 was used for Analysis of variance.
3. Results and discussion
3.1. Selection of LAB strains
Through the previous experimental results, the reducing sugar in the persimmon juice can be utilized to reduce the pH of persimmon juice and produce lactic acid, whether it is single or mixed strains fermentation (The data were shown in Supplementary Table S1 and Supplementary Figure S1). This is consistent with the changes in pH and TTA in fermented cashew apple juice (Kaprasob et al., Citation2017). The persimmon fermented with both single strain and mixed strains can consume glucose. Moreover, the mixed strains consumed more glucose than single strain, which can be interpreted as the synergistic effect between mixed strains to promote the absorption and utilization of glucose. In summary, all three strains can be grown in persimmon, using the glucose to produce lactic acid, decreasing the pH of fermentation broth by the results.
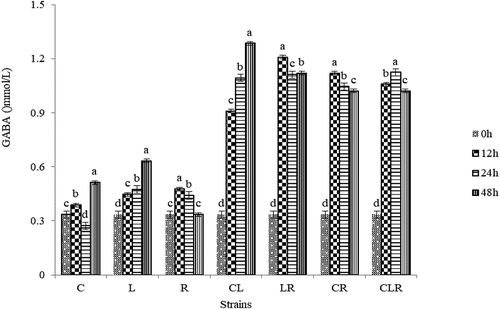
The changes of GABA contents in 48 h during single or mixed bacteria fermentation are shown in . We can see the production of GABA increased by fermentation whether it was produced by single or mixed strains. The fermentation with persimmon by mixed strains produced more GABA than single strain. During fermentation by single strain, L. pentosus Lp-B produced more GABA than other strains, and the trend of production was always rising in 48 h. It indicated L. pentosus Lp-B had a good ability to produce GABA. During fermentation by mixed strains, the GABA produced by the fermentation of L. plantarum C17 and L. pentosus Lp-B had been on the rise, and reached the highest after 48 h. While the other three mixed strains also produced more GABA than before, but there was no regularity in the concent of GABA produced in the fermentation of 12–48 h, and there were different changes among them. The production by L. pentosus Lp-B with fermentation of persimmon after 48 h was 0.632 mg/mL, but that by the co-fermentation with L. plantarum C17 and L. pentosus Lp-B was 1.395 mg/mL, this can explain there is a synergistic effect (Yingxuan, Erni, Feng, & Xing, Citation2008) between the two strains that can increase the activity of GAD, thereby improving the biosynthesis of GABA. Combined with the above results, this experiment selected L. plantarum C17 and L. pentosus Lp-B to ferment persimmon juice. Another study reported the generation of GABA by fermenting Gastrodia elata, a medicinal herb, with a co-culture of Lactobacillus brevis GABA 100 and Bifidobacterium bifidum BGN4, and also achieved an increased GABA production (Kim, Park, Kang, & Ji, Citation2014). Co-culturing with LAB seems to be a promising methodology to generate GABA (Kwon et al., Citation2016).
Figure 1. The changes of GABA contents in 48 h during single or mixed bacteria fermentation. C, L.plantarum C17; L, L.pentosus Lp-B; L.rhamnosus R11; CL, L.plantarum C17 and L.pentosus Lp-B; LR, L.pentosus Lp-B and L.rhamnosus R11; CR, L.plantarum C17 and L.rhamnosus R11; M, three LAB strains. Bars represent standard errors of the means (n = 3). The letters indicated the changes in GABA in the same strain at different fermentation times. Data points indicated with different letters are significantly different from each other at p < 0.05.
Figura 1. Cambios detectados en el contenido de GABA a lo largo de 48 horas durante la fermentación de bacterias simples o mixtas. C, L.plantarum C17; L, L.pentosus Lp-B; L.rhamnosus R11; CL, L.plantarum C17 y L.pentosus Lp-B; LR, L.pentosus Lp-B y L.rhamnosus R11; CR, L.plantarum C17 y L.rhamnosus R11; M, tres cepas LAB. Las barras representan errores estándar de las medias (n = 3). Las letras indican los cambios en GABA en la misma cepa en diferentes tiempos de fermentación. Los puntos de datos indicados con letras diferentes presentan diferencias significativas entre sí en p < 0.05.
3.2. Conditions of GABA production
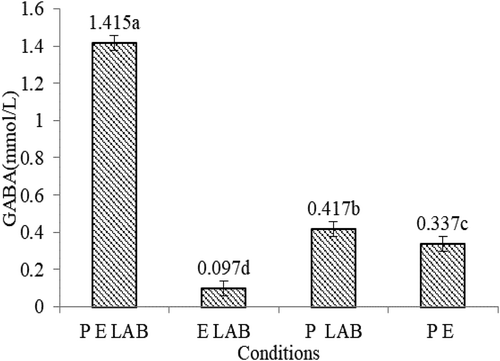
The most GABA was produced by co-fermentation of L. plantarum and L. pentosus with persimmon juice which was treatment by pectinase from . While without the conditions of strains, persimmon or enzyme treatment, the content of GABA was 0.097, 0.289, 0.417, respectively. LAB can more easily use persimmon after enzymatic hydrolysis, and the growth of LAB also improved the production of GAD. In addition, LAB strains producing GABA are mostly cultured in a synthetic or semi-synthetic medium, and purification of GABA after fermentation is indispensable, which greatly increases the time and cost of production. But the precursor substance of GABA, glutamic acid, is derived from the persimmon rather than exogenous sources, which also avoids contamination of materials and ensures safety. The results indicated that the production of GABA in this experiment was related to strains, persimmon juice, and pectinase treatment. This experiment is consistent with the result that cellular activity of L. brevis JCM 1059T and the treatment of the date residue with the carbohydrate-degrading enzymes were critical for the GABA production (Hasegawa, Yamane, Funato, Yoshida, & Sambongi, Citation2017). In this experiment, the fermented persimmon juice riched in GABA content of 2.102 mmol/L was obtained by optimizing the pectinase treatment conditions and fermentation conditions. The optimization process was shown in Supplementary Table S2-4 and Supplementary Figure S2-3.
Figure 2. GABA production under different conditions. P, persimmon; E, pectinase; LAB, L.plantarum C17 and L.pentosus Lp-B. Bars represent standard errors of the means (n = 3). Data points indicated with different letters are significantly different from each other at p < 0.05.
Figura 2. Producción de GABA en diferentes condiciones. P, caqui; E, pectinasa; LAB, L.plantarum C17 y L.pentosus Lp-B. Las barras representan errores estándar de las medias (n = 3). Los puntos de datos indicados con letras diferentes presentan diferencias significativas entre sí en p < 0.05.
3.3. The conversion rate of GABA from glutamate
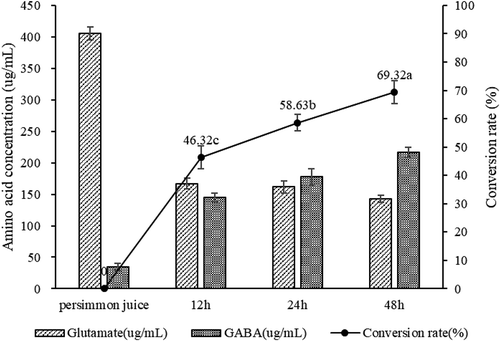
shows the conversion rate of GABA. The GABA produced by LAB during fermentation was converted by glutamate with glutamate decarboxylase. Among them, glutamic acid decarboxylase was produced during the growth of lactic acid bacteria. Glutamate was derived from the fermentation broth and could be derived from the fermentation substrate itself on the one hand and from the fermentation broth on the other hand. In contrast, exogenous addition of glutamate was prone to contamination. Persimmon juice itself had a large amount of glutamic acid, which could be used as a natural medium for lactic acid bacteria. In this experiment, the persimmon juice contained in the enzymatic hydrolysis contained 405.28 ug/mL. During the 48 h fermentation period, the content of glutamic acid was always decreasing, indicating that the lactic acid bacteria utilized persimmon juice in their own growth and production of GABA. Glutamate. During the 48 h fermentation, the conversion of GABA from glutamic acid reached a maximum of 69.32%. It was indicated that the GABA produced during the fermentation comes from glutamic acid in the persimmon juice.
Figure 3. The conversion rate of GABA. Bars represent standard errors of the means (n = 3). Data points indicated with different letters are significantly different from each other at p < 0.05.
Figura 3. Tasa de conversión de GABA. Las barras representan errores estándar de las medias (n = 3). Los puntos de datos indicados con letras diferentes presentan diferencias significativas entre sí en p < 0.05.
3.4. Anti-hangover activity
It is shown in that the ADH activation activity of persimmon, fermented persimmon, and GABA standards was 4.84%, 38.91%, 25.25%, respectively. The ADH activation rate of fermented persimmon and GABA standard increased by 34.07% and 20.41% compared to persimmon. ADH, one of the key enzymes for the metabolism of alcohol in the human body, reduces its activity in the case of long-term heavy drinking or excessive drinking which causes alcohol damage to the stomach and liver and other organs (Lee et al., Citation2011). The results of this experiment showed that fermented persimmon juice and GABA could increase the activity of ADH and promote the metabolism of ethanol. Fermented persimmon had higher ADH activity than GABA, probably because of the existence of other biologically active compounds which promote the metabolism of ethanol.
Table 1. The ADH activation activity, ALDH activity and hydroxyl inhibition ability of persimmon, fermented persimmon and GABA.
Tabla 1. Actividad de activación de ADH, actividad de ALDH y capacidad de inhibición de hidroxilo de caqui, caqui fermentado y GABA.
The ALDH activation activity of persimmon was 7.96 U/ml, while the ALDH activation activity of fermented persimmon and GABA standards was 34.72 U/ml and 14.79 U/ml. The ALDH activation activity of fermented persimmon was improved which could be explained that GABA played an important role in this aspect. This promoted the conversion of acetaldehyde and the metabolism of ethanol, which is conducive to anti-hangover. ALDH is a key enzyme in the major pathway of alcohol metabolism, and it has a critical role in the metabolism of ethanol (Yuncao Fan, Ye, Lin, Wang, & Lin, Citation2018). The effect of ALDH is to convert acetaldehyde to acetic acid. The results indicated that one of the mechanisms of GABA’s anti-hangover activity was to increase the activity of ALDH, promote the metabolism of alcohol, and inhibit the absorption of alcohol in the human body.
We can see the hydroxyl inhibition activity of persimmon, fermented persimmon and GABA standards were 14.26%, 16.79%, 14.97%, respectively. Excessive drinking can hurt the liver. There are many factors that cause liver cell damage in ethanol, and the free radical mechanism is one of the incentives among them. The active oxygen species is responsible for the occurrence of lipid peroxidation in the body and causes many diseases such as inflammation, cancer, aging, atherosclerosis, etc. (Halliwell et al., Citation1987). Among them, the activity of ·OH is the strongest. Therefore, this experiment investigated the inhibitory effect of fermented persimmon juice on ·OH, which helped to reveal its mechanism of the hangover. From the results, the hydroxyl inhibition rate of fermented persimmon and GABA standards was not significantly higher than persimmon. The reason might be unfermented persimmon contained many phenolic substances which played a certain role in hydroxyl inhibition.
3.5. Anti-hypertensive activity
The ACE inhibitory activity of persimmon was 6.88%, while fermented persimmon and GABA standards were 42.14% and 27.16%, respectively (). The ACE inhibitory activity of persimmon was increased by fermentation, which is may be due to the action of GABA produced during fermentation, and partly because the production of some bioactive peptides also inhibited ACE activity. Moreover, ACE inhibitor is one of the drugs used to treat hypertension (Bao et al., Citation2016). The results indicated that one of the anti-hypertensive mechanisms of GABA was to inhibit the ACE activity, thereby preventing the conversion of angiotensin 1 to angiotensin 2 for the purpose of blood pressure lowering.
Figure 4. The ACE inhibition rate and expression of ET-1 in persimmon, fermented persimmon and GABA standards. Bars represent standard errors of the means (n = 3). Values with different letters presented significant differences. Lowercase letters indicated differences in ET-I expression levels, and uppercase letters indicated differences in ACE inhibition rates. The content of GABA standard was 2 mmol/L.
Figura 4. Tasa de inhibición de la ECA y expresión de ET-1 en los estándares de caqui, caqui fermentado y GABA. Las barras representan errores estándar de las medias (n = 3). Los valores con letras diferentes presentan diferencias significativas. Las letras minúsculas indican diferencias en los niveles de expresión de ET-I, y las letras mayúsculas indican diferencias en las tasas de inhibición de la ECA. El contenido del estándar GABA fue de 2 mmol/L.
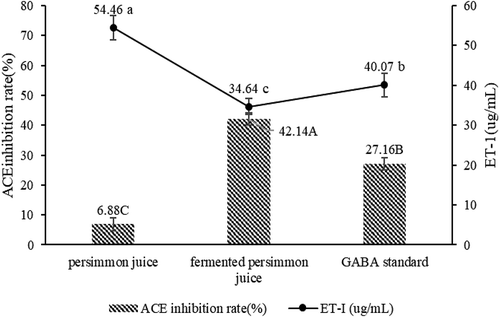
The concentrations of ET-1 in persimmon, fermented persimmon, and GABA standards were 54.46 μg/mL, 34.64 μg/mL, and 40.07 μg/mL, respectively (). Fermented persimmon and GABA standards reduced the concentration of ET-1 compared with persimmon and decreased the expression of ET-1, which was reduced by 36.39% and 26.42%, respectively. ET-1, produced by vascular endothelial cells is a potent vasoconstrictor that is upregulated in the presence of CVD risk factors (Yang, Liu, Meng, & Hu, Citation2018). Therefore, reducing the concentration of ET-1 can be one of the regulation mechanisms of hypotension. Fermented persimmon and GABA standard suppressed the expression of ET-1 in blood vessels which resulted in vasodilation and lowering the blood pressure. From the experimental results, one of the anti-hypertensive mechanisms of GABA is to act as an expression inhibitor of ET-1, thereby achieving the purpose of lowering blood pressure. Fermented persimmon can reduce the concentration of ET-1 more than GABA standards, which may be the presence of other bioactive substances. In line with this study, wheat-based fermented rice enriched with GABA (Zareian, Oskoueian, Forghani, & Ebrahimi, Citation2015) has been reported to potentially suppress the expression of vascular ET-1 protein and subsequently regulate the blood pressure.
4. Conclusion
In this study, the persimmon juice was successfully enriched GABA through the co-fermentation of L. plantarum C17 and L.pentosus Lp-B. The highest content of GABA was 2.102 mg/mL. Meanwhile, the potential anti-hangover activity and anti-hypertensive activity of GABA-enriched fermented persimmon juice were demonstrated by in vitro experiments. It can be seen that persimmon is an attractive fermentation substrate, but today persimmon still needs to be deepened in its development and utilization to enhance its nutritional value and enrich new processed products. This study will provide a new way of thinking for the persimmon processing industry and inject new vitality into the food industry.
Conflict of interest
The authors have declared no conflict of interest.
Supplemental Material
Download MS Word (1.1 MB)Acknowledgments
The authors gratefully appreciated the financial support from the following research funds: National Key Research and Development Plan “Study on Key Technologies for Multi-dimensional Identification Detection of Important Food Authenticity” (2017YFC160089); Development and Demonstration of Special Agriculture Product Processing Technology (16227109D).
Additional information
Funding
References
- Bao, X., Zhu, W., Zhang, R., Wen, C., Wang, L., & Yan, Y. (2016). Synthesis and evaluation of novel angiotensin II receptor 1 antagonists as anti-hypertension drugs. Bioorganic & Medicinal Chemistry, 24(9), 2023–2031.
- Blair, A. H., & Bodley, F. H. (1969). Human liver aldehyde dehydrogenase: Partial purification and properties. Canadian Journal of Biochemistry, 47(3), 265.
- Cagno, R. D., Coda, R., Angelis, M. D., & Gobbetti, M. (2013). Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiology, 33(1), 1–10. doi:10.1016/j.fm.2012.09.003
- Das, D., & Goyal, A. (2015). Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT-food Science and Technology, 61(1), 263–268. doi:10.1016/j.lwt.2014.11.013
- George, A. P., & Redpath, S. (2008). Health and medicinal benefits of persimmon fruit: A review. Advances in Horticultural Science, 22(4), 244–249.
- Gu, H. F., Li, C. M., Xu, Y. J., Hu, W. F., Chen, M. H., & Wan, Q. H. (2008). Structural features and antioxidant activity of tannin from persimmon pulp. Food Research International, 41(2), 0–217.
- Halliwell, B., Gutteridge, J. M., & Aruoma, O. I. (1987). The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemistry, 165(1), 215–219. doi:10.1016/0003-2697(87)90222-3
- Hasegawa, M., Yamane, D., Funato, K., Yoshida, A., & Sambongi, Y. (2017). Gamma-aminobutyric acid fermentation with date residue by a lactic acid bacterium, Lactobacillus brevis. Journal of Bioscience and Bioengineering, 125, S1389172317306357.
- Hossain, A., Moon, H. K., & Kim, J. K. (2018). Antioxidant properties of korean major persimmon (diospyros kaki) leaves. Food Science and Biotechnology, 27(1), 177–184. doi:10.1007/s10068-017-0195-y
- Kaprasob, R., Kerdchoechuen, O., Laohakunjit, N., Sarkar, D., & Shetty, K. (2017). Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochemistry, 50, S1359511317302301.
- Kim, D. H., Dasagrandhi, C., Park, S. K., Eom, S. H., Man, K. H., Mok, J. S., & Kim, Y. M. (2018). Optimization of gamma-aminobutyric acid production using sea tangle extract by lactic acid bacterial fermentation. LWT, 90, S0023643818300112.
- Kim, J. A., Park, M. S., Kang, S. A., & Ji, G. E. (2014). Production of γ-aminobutyric acid during fermentation of Gastrodia elata Bl. by co-culture of Lactobacillus brevis GABA 100 with Bifidobacterium bifidum BGN4. Food Science and Biotechnology, 23(2), 459–466.
- Kim, J. Y., Lee, M. Y., Ji, G. E., Lee, Y. S., & Hwang, K. T. (2009). Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. International Journal of Food Microbiology, 130(1), 12–16.
- Ko, C. Y., Lin, H.-T. V., & Tsai, G. J. (2013). Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochemistry, 48, 559–568.
- Kwon, S. Y., Garcia, C. V., Song, Y. C., & Lee, S. P. (2016). GABA-enriched water dropwort produced by co-fermentation with Leuconostoc mesenteroides SM and Lactobacillus plantarum K154. LWT, 73, 233–238.
- Lee, S., Kim, A., Lee, J. H., Kim, M. H., Lee, B. S., & Jee, Y. T. (2011). Effects of minerals added to medicinal plant extracts on alcohol-induced oxidative stress and alcohol metabolism in rats. Journal of the Korean Society of Food Science and Nutrition, 40(3), 393–400.
- Liu, M., Yang, K., Wang, J., Zhang, J., Qi, Y., & Wei, X. (2019). Young astringent persimmon tannin inhibits methicillin-resistant Staphylococcus aureus isolated from pork. LWT, 100, 48–55. doi:10.1016/j.lwt.2018.10.047
- Lucas-González, R., Viuda-Martos, M., Álvarez, J. A. P., & Fernández-López, J. (2018). Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chemistry, 256, 252–258.
- Moreb, J., Gabr, A., Vartikar, G. R., & Gowda, S. (2005). Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroperoxycyclophosphamide and acetaldehyde. The Journal of Pharmacology and Experimental Therapeutics, 312(1), 339–345. doi:10.1124/jpet.104.072496
- Mozzi, F., Ortiz, M. E., Bleckwedel, J., Vuyst, L. D., & Pescuma, M. (2013). Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Research International, 54(1), 1152–1161.
- Oh, B. T., Jeong, S. Y., Velmurugan, P., Park, J. H., & Jeong, D. Y. (2017). Probiotic-mediated blueberry (Vaccinium corymbosum L.) fruit fermentation to yield functionalized products for augmented antibacterial and antioxidant activity. Journal of Bioscience and Bioengineering, 124, S1389172317302669.
- Pérez-Burillo, S., Oliveras, M. J., Quesada, J., Rufián-Henares, J. A., & Pastoriza, S. (2018). Relationship between composition and bioactivity of persimmon and kiwifruit. Food Research International, 105, 461–472. doi:10.1016/j.foodres.2017.11.022
- Quílez, J., & Diana, M. (2017). Chapter 5 – Gamma-aminobutyric acid-enriched fermented foods. In Juana Frias, Cristina Martinez-Villaluenga, Elena Peñas (Eds.), Fermented foods in health and disease prevention (pp. 85–103). Academic Press.
- Song, H. Y., & Yu, R. C. (2018). Optimization of culture conditions for gamma-aminobutyric acid production in fermented adzuki bean milk. Journal of Food and Drug Analysis, 26(1), 74.
- Wang, A., Wang, L. L., Hong, Y. I., & Zhao, Z. H. (2005). Research of determination of glutamic acid by colorimetry. Chinese Condiment, 8, 50–52+35.
- Xie, M., Chen, H., Nie, S., Tong, W., Yin, J., & Xie, M. (2017). Gastroprotective effect of gamma-aminobutyric acid on ethanol-induced gastric mucosal injury. Chemico-biological Interactions, 272, 125–134. doi:10.1016/j.cbi.2017.04.022
- Yang, S., Liu, L., Meng, L., & Hu, X. (2018). Capsaicin alleviates hyperlipidemia, oxidative stress, endothelial dysfunction, and atherosclerosis in Guinea pigs fed on a high-fat diet. Chemico-biological Interactions, 297, 1–7.
- Yingxuan, X. U., Erni, X. U., Feng, N., & Xing, F. (2008). Research progress on application of microbial mixed fermentation. China Brewing, 9, 1–4.
- Yuncao Fan, Z. C., Ye, T., Lin, W., Wang, Q., & Lin, B. (2018). Aldehyde dehydrogenase II rs671 polymorphism in essential hypertension. Clinica Chimica Acta, 487, 153–160. doi:10.1016/j.cca.2018.09.037
- Zareian, M., Oskoueian, E., Forghani, B., & Ebrahimi, M. (2015). Production of a wheat-based fermented rice enriched with γ-amino butyric acid using Lactobacillus plantarum MNZ and its antihypertensive effects in spontaneously hypertensive rats. Journal of Functional Foods, 16, 194–203.
- Zhang, J., Dai, T., Wang, D., & Li, P. (2018). Optimization of process conditions of γ-amino butyric acid-enriched fermented milk with sprouted soybeans. doi:10.6041/j.issn.1000-1298.2018.03.047
- Zhang, Z. P., Ma, J., He, Y. Y., Lu, J., & Ren, D. F. (2018). Antioxidant and hypoglycemic effects of diospyros lotus fruit fermented with microbacterium flavum and lactobacillus plantarum. Journal of Bioscience and Bioengineering, 125(6), 682–687.
- Zhao, S. J. (2009). Studies on ACE inhibitory activity and γ-aminobutyric acid in fermented milk by L. helveticus ND01. Journal of Chinese Institute of Food Science and Technology, 6, 54–60.
- Zhou, C., Zhao, D., Sheng, Y., Tao, J., & Yang, Y. (2011). Carotenoids in fruits of different persimmon cultivars. Molecules, 16(12), 624–636. doi:10.3390/molecules16010624
