?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In order to isolate antioxidant peptides from oats globulin hydrolyzed alcalase, an antioxidant peptide was isolated and purified using ultra-filtration and ion-exchange chromatography. Hydroxyl and 1,1-diphenyl-2-pycrylhydrazyl (DPPH) radical scavenging capacity was analyzed to evaluate the antioxidant activity of peptide. Five fractions were obtained after ultra-filtration, fraction with molecular weight >10KDa showed the strongest hydroxyl and DPPH radical scavenging ability of 58.38 ± 0.87% and 24.53 ± 0.53% (p < 0.05). Fraction C which was purified via ion-exchange chromatography, exhibited the maximum antioxidant activity (IC50 of hydroxyl and DPPH radical scavenging ability was 1.83 ± 0.03 mg mL−1 and 4.11 ± 0.07 mg mL−1) among other fractions (p < 0.05). Subsequently, five antioxidant peptides were identified by electrospray ionization mass spectrometry (ESI-MS/MS), with the amino acid sequence of IRIPIL, FLKPMT, NSKNFPTL, LIGRPIIY, and FNDILRRGQLL, with a molecular weight of 723.50, 735.40, 919.48, 943.59, and 1343.76 Da, respectively.
RESUMEN
Con el fin de aislar péptidos antioxidantes de subtilisina hidrolizada de globulina de avena, se aisló y se purificó un péptido antioxidante usando ultrafiltración y cromatografía de intercambio iónico. Para evaluar la actividad antioxidante del péptido se analizó su capacidad de eliminación de radicales hidroxilo y 1,1-difenil-2-picrilhidrazilo (DPPH). Una vez realizada la ultrafiltración se obtuvieron cinco fracciones; la fracción con peso molecular >10KDa mostró una capacidad más fuerte de eliminación de radicales hidroxilo y DPPH de 58.38 ± 0.87% y 24.53 ± 0.53% (p < 0.05). Entre las demás fracciones, la fracción C, purificada empleando cromatografía de intercambio iónico, exhibió la actividad antioxidante máxima (p < 0.05) (la capacidad de eliminación de radicales hidroxilo y DPPH de IC50 fue de 1.83 ± 0.03 mg mL−1 y 4.11 ± 0.07 mg mL−1). Posteriormente, utilizando espectrometría de masas por ionización y electrospray (ESI-MS/MS), se identificaron cinco péptidos antioxidantes con la secuencia de aminoácidos IRIPIL, FLKPMT, NSKNFPTL, LIGRPIIY y FNDILRRGQLL, con un peso molecular de 723.50, 735.40, 919.48, 943.59 y 1343.76 Da, respectivamente.
1. Introduction
Oxidative stress leads to many chronic complaints ranging from tumorigenesis to age-related diseases (Kris-Etherton et al., Citation2002). The generation of free radicals during normal physiology contributed to cellular damage and producing physiological varieties (Tovar-pérez et al., Citation2017). Natural and synthetic antioxidants can protect the human body from developing various diseases besides oxidative damage in foods have been reported (Ketnawa, Wickramathilaka, & Liceaga, Citation2018). However, vitamins or artificial antioxidants in food products are strictly regulated for the potential hazards to human health. Thus, natural antioxidants attract the researchers’ note in recent years.
Natural source can provide many antioxidants, such as antioxidant peptides, flavones, and polyphenol, have been reported as a substitute to man-made antioxidants (Wang et al., Citation2017). Food and pharmaceutical industries are specially interested in natural antioxidant peptides with low molecular weight, high activity, and easy absorption (Agrawal, Joshi, & Gupta, Citation2016). Peptides and protein hydrolysates are the latest sources of natural antioxidants (Ayim, Ma, Ali, Alenyorege, & Donkor, Citation2018; Feng, Ruan, Jin, Xu, & Wang, Citation2018). Antioxidant peptides from flaxseed (Franck et al., Citation2019), chickpeas (Serrano-Sandoval, Guardado-Félix, & Gutiérrez-Uribe, Citation2019), sunflower (Dabbour, He, Mintah, Xiang, & Ma, Citation2019) and Castanea mollissima Blume (Feng et al., Citation2018) have been isolated. However, rare information is available on globulin peptides from oats.
Recently, there is a growing desire to use oats as a component of new food products or human nutrition, due to its health efficacy and nutrients. Oats usually contain 9 and up to 20 percent of protein (McMullen, Citation1991). Oat protein is regarded as a high-quality grain protein, and globulin is the main component of oat protein. Compared to other cereals, oat not only has high protein content but also with a more favorable composition of essential amino acids. The present study evaluated the antioxidant capacity of oat globulin alcalase-enzymatic hydrolysates. Isolated peptides through ultra-filtration and ion-exchange chromatography, then identified the purified peptides with the maximum antioxidant capacity by ESI-MS/MS.
2. Materials and methods
2.1. Materials
Oats (Meng Yan No.2) were from Inner Mongolia Academy of Agricultural & Animal Husbandry Sciences which harvested in 2017 and stored at 0°C ~ 4°C. Grounded the oats into powder (sieve with a diameter of 80 mesh screen size) and defatted. High-performance liquid chromatography (HPLC)-grade acetonitrile and trifluoroacetic acid (TFA) were purchased from Sigma (Beijing, China). Alcalase purchased from Novozymes (Haidian distract, 100085, Beijing, China) and other analytical-grade reagents were purchased from Chemical Reagents Co., Ltd. of China Pharmaceutical Group (NO.52 Ningbo road, Shanghai, China).
2.2. Protein extraction
Sifted the grounded Oats flour through 80-mm mesh, 500 g of them was taken to defatted using Hexane at a ratio of 1:3 (w/v) for 6 h, followed by stirring and changing Hexane every 2 h. Defatted Oats flour was diluted in 6250 mL of 10% sodium chloride, and adjusted pH to 8.0 with stirring and incubation for 2.5 h at 4°C. Centrifuged the combination at 10000 g for 15 min, and the pH of the gathered supernatant was adjusted to 4.3 using 1 M hydrochloric acid. After that, centrifuged at 10000 g for 10 min after resting 20 min, used deionized water to rinse the precipitates. Lyophilizated and kept protein isolate at −20°C (Hu et al., Citation2018).
2.3. Degree of hydrolysis (DH)
Evaluated the samples based on testing methodology published by Sharma et al. (Citation2016) and use the following equation:
where VNaOH represented the volume consumption of NaOH in mL, NNaOH stand for NaOH solution molarity, “a”represented the average degree of dissociation of α-NH2 group in protein isolate sample, Mp stands for the hydrolyzed protein mass in g, and Htot stands for peptide bonds (7.31 mM/g oats protein).
2.4. Enzyme hydrolysis
Oats globulin isolate was hydrolyzed by using alcalase, enzyme-to-substrate (E/S) ratio of 1:40, 1:20 and 1:10 (v/w), respectively. Hydrolyzed with E/S ratio 1:20 to explain enzyme ratio result on DH at a substrate concentration of 100 mg mL−1. Other conditions of hydrolysis kept were as listed below: hydrolysis temperature of 55°C, pH at 8.5 and hydrolysis time of 0–180 min. The DHs calculating 30 min interval of oats globulin isolate.
2.5. Determination of peptide yield
Formaldehyde titration was taken to determine peptide yield, determine total nitrogen and ammonia-based nitrogen content in enzymatic supernatant by the Kjeldahl (GB/T5009.5–2003) and formaldehyde titration method (Liu, Citation2018).
where N1 represented total nitrogen content of enzymatic supernatant/g, N2 stand for ammonia-based nitrogen content in enzymatic supernatant/g, N3 represented total nitrogen content of raw material.
2.6. Antioxidant activity assay
2.6.1. Hydroxyl radical (·OH) scavenging activity
OH scavenging activity was determined based on the method published by Ma et al. (Citation2017).
2.6.2. DPPH radical (DPPH·) scavenging ability
DPPH· scavenging ability was measured in accordance with the method described by Fan, Yi, Zhang, Wen, and Zhao (Citation2017).
2.7. Purification of anti-oxidative peptide
2.7.1. Ultra-filtration
Use ultra-filtration unit (8200, Millipore, USA) to filtered sequentially the oats globulin hydrolysates. Five fragments with molecular weights of <1KDa, 1–3KDa, 3–5KDa, 5–10KDa, and >10KDa were obtained. Evaluated the antioxidant activity of each fragments under the equal reaction conditions as described earlier. Then lyophilized the functional antioxidant fraction, in the next step, followed ion-exchange chromatography.
2.7.2. Ion exchange chromatography
The most active antioxidant fraction obtained after ultrafiltration was subjected to 732 cation exchange column (2.6 cm × 60 cm) for purification. Dissolved freeze-dried fraction Ⅴ in ammonia acetate buffer (pH4.5), the concentration was 24 mg mL−1. Eluted the sample with 0.2 mol L−1 ammonia water at a constant speed (1.0 mL min−1) and gathered elute at 4 min intervals, and determined absorbance at 220 nm. Evaluated the antioxidant activity under the equal reaction conditions as described earlier. Then lyophilized the functional antioxidant fraction, and for subsequent use.
2.8. Characterization of purified peptides
Evaluated the molecular weight and amino acid sequence of the chosen peptide through ESI-MS/MS, the experimental parameters were as follows: capillary temperature of 360°C, flow rate of 0.4 μL min−1, the ion source voltage with 1800 V. In the mass range from m/z 350–2000, obtained mass spectra. Then, detection of cationic peptide ions using data-related acquisition Automatic Mass Spectrometry (n) mode. Repeated analysis of each sample. Combined all MS/MS spectra of each duplicate, then submitted to the database search.
2.9. Statistical analysis
All tests were conducted at least in duplicates. Results are showed as mean ± standard deviation and were analyzed through SPSS 9.0 software. A P-value of less than 0.05 was deemed significant.
3. Result and discussion
3.1. Enzymatic hydrolysis of oats globulin
In order to obtain peptides with high bioactivity, many factors must be considered. Antioxidative peptides can be released through single enzyme hydrolysis. (Tu, Cheng, Lu, & Du, Citation2018) shows the DH changes with different oats globulin hydrolyzing time at different E/S ratio. DH grew with the increasing hydrolysis time, indicating a gradual release of peptide fragments during the hydrolyzing process (Tang, Peng, Zhen, & Chen, Citation2009). In the first 30 min, the DH or release of peptide fragments of oats globulin was higher, and from 30 to 120 min, the value decreased gradually (p < 0.05), which was consistent with pony seed protein hydrolysis (Zhang et al., Citation2019) and oat glutelin hydrolyzing process (Ma, Zhang, Bao, & Dong, Citation2014). At the initial stage, the sensitive peptide bond breaks rapidly, while the insensitive peptide bond breaks later. Therefore, the hydrolysis speed is very fast at the beginning of the reaction, and after a period of time, the speed decreases and reaches equilibrium (Hou et al., Citation2012). With extend hydrolyzing process, e.g., >2 h, the hydrolysis rate kept almost constant.
Figure 1. Oats globulin DH changes during hydrolysis by alcalase at different E/S ratios.
Figura 1. Cambios de DH de globulina de avena durante la hidrólisis por subtilisina a diferentes ratios E/S
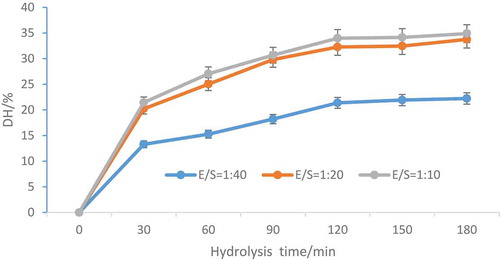
The DH of oats globulin was intimately related to the enzyme concentration used, which named E/S ratio. The DH is lower with E/S ratio of 1:40 (p < 0.05), when the E/S ratio was 1:20 and 1:10, respectively, DH between the two was statistically insignificant (). Eventually, the optimum hydrolysis condition was found as follows: 120 min and 1:20(v/w), based on hydrolysis time and E/S ratio.
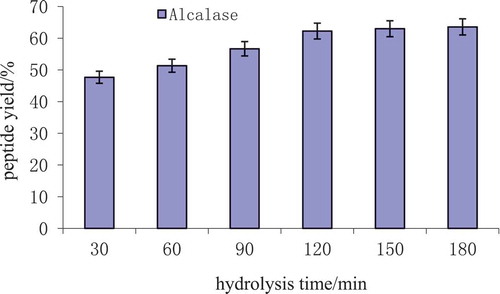
3.2. Effect of different hydrolysis time on peptide yield
As shown in , the yield of oat globulin peptide hydrolyzed by alcalase increased with increasing hydrolysis time. Peptide yield was the maximum at 180 min (63.56 ± 2.03) %. Also, with higher peptide yield (62.25 ± 1.52) %, when hydrolysis time reached 120 min.
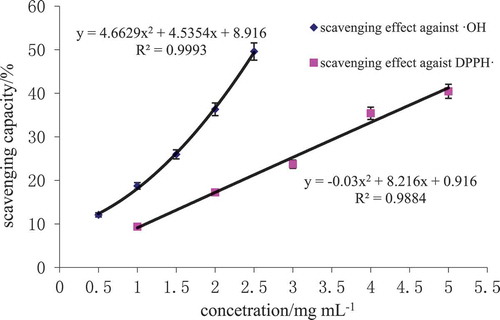
3.3. Free radical scavenging activity of oats globulin hydrolysates
The scavenging effect of hydroxyl and DPPH radical of oats globulin hydrolysates was showed in . With the increasing concentration of hydrolysates, scavenging effect on two kinds of free radicals exhibited quadratic upward trend. The 50% inhibitory concentration (IC50) of the scavenging effect of hydroxyl and DPPH radical was 2.52 ± 0.07 mg mL−1 and 5.85 ± 0.13 mg mL−1. This indicated oats globulin hydrolysates exhibited good free radical scavenging activity, which agreed with a previous report (Lin, Zhang, & Zhang, Citation2011).
3.4. Ultra-filtration of oats globulin hydrolysates
One of the effective ways to obtain bioactive peptides is the hydrolysis of proteins, which can conduct molecules scope from free amino acids to various peptides with different molecular weight. Antioxidant activity is considered to be closely associated with peptide length, and hard to assign which amino acid residues are critical to antioxidant activity. Ultra-filtration is a common way for the isolation of different molecular weight peptide fractions. Correspondingly, obtained five fractions after ultra-filtration: Ⅰ (MW<1KDa), Ⅱ (1< MW<3KDa), Ⅲ (3< MW<5KDa), Ⅳ (5< MW<10KDa) and Ⅴ (MW>10KDa); antioxidant activities were searched through scavenging abilities of hydroxyl and DPPH radical.
In five fractions separated by ultra-filtration, fraction Ⅴ showed the highest hydroxyl and DPPH radical scavenging ability of 58.38 ± 0.87% and 24.53 ± 0.53% with a concentration of 2 mg mL−1 (p < 0.05) as shown in . Two kinds of free radical scavenging abilities of fraction Ⅲ and Ⅳ are comparable. The free radical scavenging ability of fraction Ⅰ was the lowest (p < 0.05). These results were in accordance with the study by Kim et al. (Citation2013), who isolated Mytilus coruscus antioxidant peptides by ultra-filtration and discovered that the fraction with MW>30 ku had the highest hydroxyl radical absorptive activity in comparison with rest fractions. Thus, fractionⅤ was subsequently selected for, further evaluating its radical scavenging activity.
Figure 4. Antioxidant activity of ultrafiltration components.
Figura 4. Actividad antioxidante de los componentes de ultrafiltración
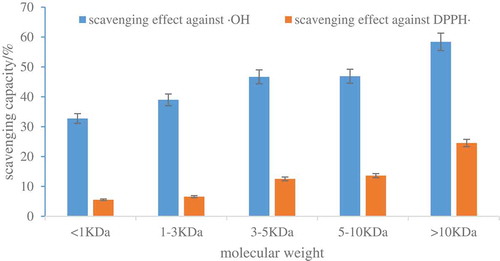
As oat globulin hydrolysates showed 36.33 ± 0.65% and 17.24 ± 0.42%, scavenging efficiency on two kinds of free radical at a concentration of 2 mg mL−1 (p < 0.05). The antioxidant activity of oat globulin hydrolysates was improved obviously by ultra-filtration.
3.5. Ion exchange chromatography
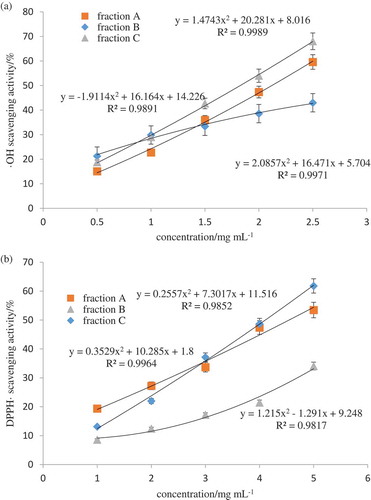
Freeze-dried fraction Ⅴ was dissolved in a solution with final content of 24 mg mL−1, and eluted on a 732 cation exchange column (2.6 cm ×60 cm) with 0.2 mol L−1 ammonia water at a flow rate of (1.0 mL min−1). Detected absorbance in real-time at 220 nm. shows the elution profile, three peaks, namely, A, B, C were detected. Fraction C has the longest retention time, and delegated the basic amino acid. Faction A delegated the acidic amino acid. Based on the hydroxyl and DPPH radical scavenging results in , the highest peak fraction C exhibited the highest radical scavenging ability with an IC50 of 1.83 ± 0.03 mg mL−1 and 4.11 ± 0.07 mg mL−1 respectively, which was statistically higher than that of fraction A (IC50 of 2.72 ± 0.05 mg mL−1 and 4.94 ± 0.09 mg mL−1), and fraction B (IC50 of 4.23 ± 0.08 mg mL−1 and 6.35 ± 0.13 mg mL−1) (p < 0.05) (). Consequently, the antioxidant peptides of fraction C were concentrated. As such, identify the peptide sequences through ESI-MS/MS in this fraction.
Table 1. IC50 of three fractions purified by ion-exchange chromatography in free radical scavenging assays.
Tabla 1. IC50 de tres fracciones purificadas por cromatografía de intercambio iónico en ensayos de eliminación de radicales libres
Figure 5. Purification of oats globulin hydrolysates through ion-exchange chromatography.
Figura 5. Purificación de hidrolizados de globulina de avena mediante cromatografía de intercambio iónico
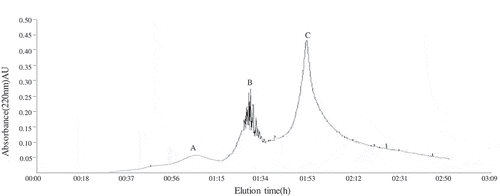
Figure 6. (a) Hydroxyl radical scavenging activity of three fractions after ion exchange purification. (b) DPPH radical scavenging activity of three fractions after ion exchange purification.
Figura 6. (a) Actividad de eliminación de radicales de hidroxilo de tres fracciones después de la purificación por intercambio iónico. (b) Actividad de eliminación de radicales DPPH de tres fracciones después de la purificación por intercambio iónico
3.6. Identification and characterization of antioxidant peptides
As antioxidant activities of fraction C were the strongest, we further determined the amino acid sequences by ESI-MS/MS. As displayed by , five peptides were detected, namely, Ile-Arg- Ile-Pro- Ile-Leu (IRIPIL, peptide 1) with MW of 723.50 Da; Phe-Leu-Lys-Pro-Met-Thr (FLKPMT, peptide 2) with MW of 735.40 Da; Asn-Ser-Lys-Asn-Phe-Pro-Thr-Leu (NSKNFPTL, peptide 3) with MW of 919.48 Da; Leu-Ile-Gly-Arg-Pro-Ile-Ile-Tyr (LIGRPIIY, peptide 4) with MW of 943.59 Da and Phe-Asn-Asp-Ile-Leu-Arg-Arg-Gly-Gln-Leu-Leu (FNDILRRGQLL, peptide 5) with MW of 1343.76 Da.
Figure 7. (a) MS/MS spectrum of the peptide IRIPIL. (b) MS/MS spectrum of the peptide FLKPMT. (c) MS/MS spectrum of the peptide NSKNFPTL. (d) MS/MS spectrum of the peptide LIGRPIIY. (e) MS/MS spectrum of the peptide FNDILRRGQLL.
Figura 7. (a) Espectro MS/MS del péptido IRIPIL. (b) Espectro MS/MS del péptido FLKPMT. (c) Espectro MS/MS del péptido NSKNFPTL. (d) Espectro MS/MS del péptido LIGRPIIY. (e) Espectro MS/MS del péptido FNDILRRGQLL
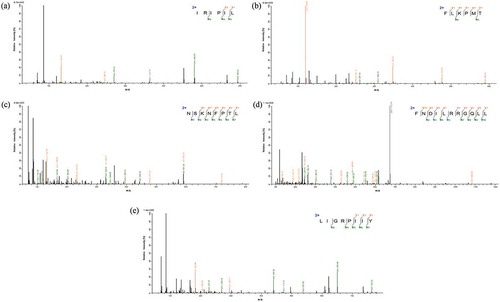
The content of hydrophobic amino acids in anti-oxidative peptides was higher, which included leucine (L), isoleucine (Ile), and proline (P) in IRIPIL peptide, phenylalanine (F), leucine (L), proline (P), and sulphur-amino acid ethionine (M) in FLKPMT peptide, serine (S), leucine (L), phenylalanine (F), and proline (P) in NSKNFPTL, leucine (L), isoleucine (Ile), glycine (G), proline (P), and aromatic amino acids Tyrosine (Y) in LIGRPIIY, phenylalanine (F), isoleucine (Ile), leucine (L), glycine (G), and aspartic acid (D) in FNDILRRGQLL, five peptides identified from oats globulin.
The molecular weight of anti-oxidative peptides was small or low, which was indicated in previous studies. Especially, compared to others, oligopeptides contain 2–10 amino acids that have exhibited maximum antioxidant activity (Samaranayaka & Li-Chan, Citation2011). The amino acid composition characterization of the peptide also confirmed the importance of its antioxidant properties. Researchers assessed the flaxseed protein alcalase-enzymatic peptides and generalized that the antioxidant potential of peptide fraction with the higher MW was lower than a peptide fragment of MW <1500 Da. It is considered that the exists of hydrophobic amino acids (Ile and Leu), acidic (Asp) and basic (His) amino acids in the peptide sequences can make a great contribution to the fermented anchovy fish (Budu) extract showed high antioxidant activity (Najafian & Babji, Citation2019). Hydrophobic amino acids play a significant part in improving the antioxidant properties of peptides, on account of they can enhance the approachability of the antioxidant peptides to hydrophobic polyunsaturated chain of fatty acids in biofilms to restrict oxidative damage. Aromatic amino acids (Phe, Tyr, and His) could transform radicals into stable molecules by donating electrons, which was reported by Sarmadi and Ismail (Sarmadi & Ismail, Citation2010). Antioxidant activity of all amino acids were compared by ORAC assay, and noticed that Trp, Tyr, Met, Cys, His, and Phe showed the higher antioxidant activity (Hernándezledesma, Dávalos, Begoña Bartolomé, & Amigo, Citation2005). Met, Pro, Cys, Ala, Gly, Val, and Leu with a higher radical-scavenging ability (Giménez, Alemán, Montero, & Gómez-Guillén, Citation2009).
4. Conclusion
The current work, isolated and purified five antioxidant peptides from oats globulin after hydrolyzed by alcalase. Their sequences were identified as Ile-Arg-Ile-Pro-Ile-Leu (IRIPIL, peptide 1), Phe-Leu-Lys-Pro-Met-Thr (FLKPMT, peptide 2), Asn-Ser-Lys-Asn-Phe-Pro-Thr-Leu (NSKNFPTL, peptide 3), Leu-Ile-Gly-Arg-Pro-Ile-Ile-Tyr (LIGRPIIY, peptide 4) and Phe-Asn-Asp-Ile-Leu-Arg-Arg-Gly-Gln-Leu-Leu (FNDILRRGQLL, peptide 5). The amino acid sequence of peptides possibly contributed to its antioxidant activities. Moreover, this work confirmed that the oat globulin peptide from fraction C had higher antioxidant activity than other fractions. Hydrophobic amino acid residues, Leu, Ile, Pro, Phe, Ser and Gly, aromatic amino acid Tyr, and sulphur-amino acid Met are likely the key factors contributing its antioxidant activity.
Further studies on individual oat globulin compounds, and its antioxidant activity in vivo, and dissimilar antioxidant mechanisms are necessary.
Disclosure statement
The authors did not report any potential conflict of interest.
Additional information
Funding
References
- Agrawal, H., Joshi, R., & Gupta, M. (2016). Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chemistry, 204, 365–372. doi:10.1016/j.foodchem.2016.02.127
- Ayim, I., Ma, H. L., Ali, Z., Alenyorege, E. A., & Donkor, P. O. (2018). Preparation of antioxidant peptides from tea (Camellia sinensis L.) residue. Journal of Food Measurement and Characterization, 12, 2128–2137. doi:10.1007/s11694-018-9828-y
- Dabbour, M., He, R. H., Mintah, B., Xiang, J. H., & Ma, H. L. (2019). Changes in functionalities, conformational characteristics and antioxidative capacities of sunflower protein by controlled enzymolysis and ultrasonication action. Ultrasonics - Sonochemistry, 58, 104625. doi:10.1016/j.ultsonch.2019.104625
- Fan, Y., Yi, J., Zhang, Y., Wen, Z., & Zhao, L. (2017). Physicochemical stability and in vitro bioaccessibility of β-carotene nanoemulsions stabilized with whey proteindextran conjugates. Food Hydrocolloids, 63, 256–264. doi:10.1016/j.foodhyd.2016.09.008
- Feng, Y. X., Ruan, G. R., Jin, F., Xu, J., & Wang, F. J. (2018). Purification, identification, and synthesis of five novel antioxidant peptides from Chinese chestnut (Castanea mollissima Blume) protein hydrolysates. LWT - Food Science and Technology, 92, 40–46. doi:10.1016/j.lwt.2018.01.006
- Franck, M., Perreault, V., Suwal, S., Marciniak, A., Bazinet, L., & Doyen, A. (2019). High hydrostatic pressure-assisted enzymatic hydrolysis improved protein digestion of flaxseed protein isolate and generation of peptides with antioxidant activity. Food Research International, 115, 467–473. doi:10.1016/j.foodres.2018.10.034
- Giménez, B., Alemán, A., Montero, P., & Gómez-Guillén, M. C. (2009). Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chemistry, 114(3), 976–983. doi:10.1016/j.foodchem.2008.10.050
- Hernándezledesma, B., Dávalos, A., Begoña Bartolomé, A., & Amigo, L. (2005). Preparation of antioxidant enzymatic hydrolysates from α-Lactalbumin and β-Lactoglobulin. Identification of active peptides by HPLC-MS/MS. Journal of Agricultural & Food Chemistry, 53(3), 588. doi:10.1021/jf048626m
- Hou, Y. K., Wang, S., Huang, K., Wang, W. J., Li, D., & Wang, J. Z. (2012). Optimization of walnut enzymatic hydrolysis technology and antioxidant activity analysis of hydrolysates. Production and Scientific Research Experience, 38(4), 99–103.
- Hu, F., Ci, A. T., Wang, H., Zhang, Y. Y., Zhang, J. G., Thakur, K., & Wei, Z. J. (2018). Identification and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chemistry, 261, 301–310. doi:10.1016/j.foodchem.2018.04.025
- Ketnawa, S., Wickramathilaka, M., & Liceaga, A. M. (2018). Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chemistry, 254, 36–46. doi:10.1016/j.foodchem.2018.01.133
- Kim, E. K., Oh, H. J., Kim, Y. S., Hwang, J. W., Ahn, C. B., Lee, J. S., … Park, P. J. (2013). Purification of a novel peptide derived from Mytilus coruscus and in vitro/in vivo evaluation of its bioactive properties. Fish & Shellfish Immunology, 34, 1078–1084. doi:10.1016/j.fsi.2013.01.013
- Kris-Etherton, P., Hecker, K., Bonanome, A., Coval, S., Binkoski, A., Hilpert, K., … Etherton, T. (2002). Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. The American Journal of Medicine, 133, 71S–88S. doi:10.1016/S0002-9343(01)00995-0
- Lin, R., Zhang, M. L., & Zhang, J. C. (2011). Purification and antioxidant activity of globulins from naked oat seeds. Chinese Food Chemistry, 32(1), 31–34.
- Liu, L. (2018). Effect of probiotics on the production mechanism of cheese bioactive peptides and their structural characteristics (Doctoral dissertation). Northeast Agricultural University of China, 21–32.
- Ma, S., Zhang, M. L., Bao, X. L., & Dong, T. (2014). The purification of free radical scavenging peptides from naked oats. Journal of Food and Nutrition Research, 2(10), 675–680. doi:10.12691/jfnr-2-10-4
- Ma, S., Zhang, M. L., Beta, T., Dong, T., Bao, X. L., & Li, Z. Q. (2017). Purification and structural identification of glutelin peptides derived from oats. CyTA - Journal of Food, 15(4), 508–515. doi:10.1080/19476337.2017.1301555
- McMullen, M. S. (1991). Oats. In K. J. Lorenz & K. Kulp (Eds.), Handbook of cereal science and technology (pp. 199–232). New York, NY: Marcel Dekker.
- Najafian, L., & Babji, A. S. (2019). Purification and identification of antioxidant peptides from fermented fish sauce (Budu). Journal of Aquatic Food Product Technology, 28(1), 14–24. doi:10.1080/10498850.2018.1559903
- Samaranayaka, A. G. P., & Li-Chan, E. C. Y. (2011). Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. Journal of Functional Foods, 3(4), 229–254. doi:10.1016/j.jff.2011.05.006
- Sarmadi, B. H., & Ismail, A. (2010). Antioxidative peptides from food proteins: A review. Peptides, 31, 1949–1956. doi:10.1016/j.peptides.2010.06.020
- Serrano-Sandoval, S. N., Guardado-Félix, D., & Gutiérrez-Uribe, J. A. (2019). Changes in digestibility of proteins from chickpeas (Cicer arietinum L.) germinated in presence of selenium and antioxidant capacity of hydrolysates. Food Chemistry, 285, 290–295. doi:10.1016/j.foodchem.2019.01.137
- Sharma, J. G., Kumar, A., Saini, D., Targay, N. L., Khangembam, B. K., & Chakrabarti, R. (2016). In vitro digestibility study of some plant protein sources as aquafeed for carps Labeo rohita and Cyprinus carpio using pH-Stat method. Indian Journal of Experimental Biology, 54(9), 606–611.
- Tang, C. H., Peng, J., Zhen, D. W., & Chen, Z. (2009). Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chemistry, 115, 672–678. doi:10.1016/j.foodchem.2008.12.068
- Tovar-pérez, E. G., Guerrero-becerra, L., Lugo-cervantes, E., Guerrero-becerra, L., Lugo-cervantes, E., & Tovar-pérez, E. G. (2017). Antioxidant activity of hydrolysates and peptide fractions of glutelin from cocoa (Theobroma cacao L.) seed. CyTA- Journal of Food, 15, 489–496. doi:10.1080/19476337.2017.1297963
- Tu, M. L., Cheng, S. Z., Lu, W. H., & Du, M. (2018). Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. TrAC Trends in Analytical Chemistry, 105, 7–17. doi:10.1016/j.trac.2018.04.005
- Wang, Q., Zhao, Y. Y., Guan, L., Zhang, Y. P., Dang, Q. F., Dong, P., … Liang, X. G. (2017). Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chemistry, 227, 9–15. doi:10.1016/j.foodchem.2017.01.081
- Zhang, F., Qu, J., Thakur, K., Zhang, J. G., Mocan, A., & Wei, Z. J. (2019). Purification and identification of an antioxidative peptide from peony (Paeonia suffruticosa Andr.) seed dreg. Food Chemistry, 285, 266–274. doi:10.1016/j.foodchem.2019.01.168