ABSTRACT
Fresh chicken meat is a highly perishable product. The effect of immersing chicken breast in an aqueous hop extract (2 g/L) for 18 h at 2°C prior to being vacuum packed, on the growth of spoilage bacteria during a 35-day refrigerated storage period was evaluated in triplicate. The hop extract immersion treatment resulted in a significant decrease (p < 0.05) in lactic acid bacteria counts during the first storage weeks and in Brochothrix thermosphacta counts from day 21 to 35. No effect could be observed on the Enterobacteriaceae growth. A triangle test analysis (54 consumers) tasting hop-extract-immersed vs water-immersed chicken breast showed no differences in cooked chicken flavor. The use of hop extract could increase the shelf life of vacuum-packed fresh chicken without affecting palatability.
RESUMEN
La carne de pollo es altamente perecedera. Este estudio evalúa por triplicado el efecto de sumergir pechuga de pollo en un extracto acuoso de lúpulo (2 g/L), frente a sumergirlo en agua durante 18 h a 2°C, antes de su envasado al vacío, sobre el crecimiento de la microbiota alterante durante su posterior enfriamiento a refrigeración. La exposición al lúpulo disminuyó significativamente (p < 0.05) los recuentos de bacterias ácido-lácticas durante las primeras semanas de almacenamiento y de Brochothrix thermosphacta en los días 21 a 35. No se observó efecto sobre el crecimiento de Enterobacteriaceae. Mediante la prueba triangular (54 consumidores), se demostró que la inmersión en el extracto de lúpulo no modificó el sabor de la pechuga de pollo cocinada. El tratamiento con extracto de lúpulo puede aumentar la vida útil del pollo refrigerado envasado al vacío sin afectar su palatabilidad.
Introduction
Microbial growth is the main cause of spoilage in refrigerated fresh meat causing deleterious quality changes (Nychas, Skandamis, Tassou, & Koutsoumanis, Citation2008). Chicken meat is considered to be highly perishable (Jiménez et al., Citation1997) as it offers a suitable environment for the growth of spoilage microorganisms due to its pH of 5.6–6.5 and high content of nutrients which are easily assimilable (Silva, Domingues, & Nerín, Citation2018). The spoilage microbiota of chicken meat associated with the refrigerated storage consists of strict aerobic microorganisms such as Pseudomonas spp. and facultative anaerobic microorganism as lactic acid bacteria (LAB), Brochothrix thermosphacta, Enterobacteriaceae and yeasts (Doulgeraki, Ercolini, Villani, & Nychas, Citation2012; Pothakos, Devlieghere, Villani, Björkroth, & Ercolini, Citation2015; Vasconcelos, Saraiva, & de Almeida, Citation2014). When chicken meat is vacuum packed, the growth of aerobic spoilage microorganisms is inhibited owing to the low concentration of oxygen on the meat surface resulting in the proliferation of facultative anaerobes (Signorini, Citation2007; Silva et al., Citation2018).
A well-known approach to increase the shelf life of meat is the use of antimicrobials. Nowadays, there is an increasing trend to use natural sources of antimicrobials (Zhang, Wu, & Guo, Citation2016). Hops, the Humulus lupulus L. flowers, are widely used in breweries for its antimicrobial properties and characteristic flavor and aroma. Hops contain compounds such as prenylated acylphloroglucinol derivatives (bitter acids) and xanthohumol, which have been proved to inhibit microbial growth mainly of Gram-positive bacteria (Bogdanova et al., Citation2018; Kramer et al., Citation2015). The use of β-bitter acids from hops as antimicrobials for cooked meat and casings for meat products has been approved by the USDA Food Safety and Inspection Service (Singh, Smith, & Bailey, Citation2014).
Scientific literature on the antimicrobial effect of hop extracts added to the meat is fairly scarce. To our knowledge, the only studies found in the literature are those by Shen, Geornaras, Kendall, and Sofos (Citation2009) and Kramer et al. (Citation2015), who showed the antimicrobial effect of β-acids against Gram-positive foodborne pathogens in frankfurters and marinated pork, respectively. No studies have been found regarding the inhibition of spoilage microbiota in vacuum-packed fresh meat where Gram-positive LAB and B. thermosphacta are predominant spoilage microorganisms (Doulgeraki et al., Citation2012; Gribble & Brightwell, Citation2013). The aim of this study was to evaluate the effect of immersing fresh chicken meat into an aqueous hop extract on the growth of spoilage microorganisms during vacuum-packed refrigerated storage.
Materials and methods
Hops extract, breast chicken samples, and sample preparation
One kg of freshly cropped hops of the Nugget variety was generously provided by a local producer (Orbigo Valley S.L., Madrid, Spain). The hops, based on the supplier information, contained α-acid, β-acid, and co-humulone composition of 4.8–5.3%, 12–16%, and 22–28%, respectively. In order to obtain an extract with the same amount of hops and extraction conditions as those commonly used during the brewing process (so as to infuse hops oils and aroma into the hot wort), a 2 g sample (in triplicate) of hops was boiled into 1 L of water (c.a. 97.4°C) for 30 min and the final volume was filled up to 1 L. The boiled mix was filtered through Whatman no. 1 filter paper (GE Healthcare Europe GmbH, Barcelona, Spain) and the aqueous extract was frozen in high-density polyethylene bottles, where it was kept at −18°C until further use.
A pair of chicken carcasses of less than 3 days post-mortem were purchased weekly for 3 weeks at the local markets, then placed into plastic bags and transported to the lab in isothermal packaging (expanded polyethylene boxes) for no more than 2 h. The pH of the breasts was immediately measured using a Basic C20 pH meter equipped with a puncture 52–32 electrode (Crison Instruments, Barcelona, Spain). Afterward, for each of the pair of carcasses purchased on the same day, the breasts (with the skin) were removed from the carcasses under aseptic conditions and then halved. Two breast halves (one from each of the carcasses) were used as controls (ConB) and the other two as hops-treated (HopB). ConB and HopB halves were, respectively, immersed into distilled water or into hop extract (previously thawed at 5°C for 24 h), for 18 h at 2 ± 1.5°C and at a breast/liquid ratio of 1/2 (w/w). The breast halves were then removed from the liquid. One half breast per treatment was used for sensory evaluation after cooking, and the other used for microbial quantification, which was carried out weekly during a 35-day storage period at 2 ± 1.5°C.
Sensory evaluation of immersed breasts after cooking
The similarity or, in contrast, difference in taste between the chicken breasts immersed in water or in hop infusion was evaluated using the triangle test following the BS ISO (Citation2004). The α and β risks and the proportion of distinguishers (pd) were established as 0.1%, 0.05% and 30% respectively, which resulted in an amount of no less than 54 responses required (Sinkinson, Citation2017).
After being removed from the liquid (water or aqueous hop extract), the corresponding chicken breast halves were skinned and then cooked in a convection oven at 150 ± 2°C for 35 min until reaching a core temperature of 70 ± 2°C. The chicken breasts were allowed to cool to room temperature (20 ± 2°C) and then transversely cut into 3-mm thick slices; the first 5 slices (proximal side) and the three-cm final portion (distal side) were discarded so approximately 20 slices from the central portion of each half-breast were obtained for the analysis.
A total of 54 volunteers (31 females and 23 males) were recruited for the test. The panelists were students and staff from the University of León (Spain) and the Instituto de Ganadería de Montaña, Consejo Superior de Investigaciones Científicas (Spain) who were used to eating chicken. They were previously instructed on the triangle test methodology and the use of the corresponding questionnaire. The test was carried out in three sessions. The slices from a ConB and HopB half-breasts obtained from the same chicken carcass were tested by 18 panelists per session. A white opaque plastic dish containing three slices of the same size identified with three-digit random codes and at room temperature was offered to each panelist (2 ConB + 1 HopB, or 1 ConB + 2 HopB, for panelist assigned odd and even numbers, respectively), asking them to taste the slices in a pre-set order (left to right) and indicate which was the odd sample. The sensory evaluations were conducted under artificial white light in individual booths. A cup with still mineral water was also given to the participants to cleanse their palate between samples. The panelists were allowed to swallow the samples if they wished to do so.
Microbial changes in chicken during storage
Having being removed from the distilled water or the hop extract, the breast halves used for microbiological analysis were skinned and transversely cut into six portions of similar size under aseptic conditions. Five portions were vacuum packed using 150 μm plastic film bags with oxygen permeability of 30 cm3/(m2 × bar × 24 h) at 23°C and 0% relative humidity at 0.03 bar, with the vacuum maintained for 10 s before sealing the vacuum bag, and stored at 4°C for up to 35 days. During the storage period, one of the portions was weekly sampled for microbiological analysis of relevant specific spoilage microbial groups in vacuum-packed poultry (Corry, Citation2007): LAB, Enterobacteriaceae, and B. thermosphacta counts. The remaining portion was analyzed on the cutting day (day 0).
The chicken portions were removed from their packages. From each portion, a 10-g (±0.1 g) sample was aseptically placed into a sterile Stomacher bag with 90 mL of sterilized peptone water (0.1%), containing NaCl (0.85%), and homogenized for 2 min with a Stomacher-400 circulator (Seward, West Sussex, UK). Serial decimal dilutions were then prepared, and plated on relevant media in duplicate (Oxoid Ltd., Basingstoke, Hampshire, U.K.) and incubated as follows: LAB, 1-mL aliquots from adequate dilutions were poured into De Man-Rogosa-Sharpe (MRS; CM 0361) agar covered with a double layer and incubated at 30°C for 72 h; Enterobacteriaceae, 1-mL aliquots were poured into violet red bile glucose (VRBGA; CM 1082) agar covered with a double layer agar and incubated at 37°C for 48 h (red-purple colonies were counted); and B. thermosphacta, 0.1-mL aliquots were surface poured onto STAA Agar Base (CM 0881) supplemented with STA Selective Supplement (Oxoid, SR162E; 0.4 mL/100 mL) and sterilized glycerol (1.5 g/100 mL), and incubated at 22°C for 48 h (straw-colored oxidase-negative colonies were counted).
Statistical analysis
For the sensory evaluation, the maximum and minimum number of correct responses needed to conclude similarity or difference were obtained from the corresponding statistical tables (Sinkinson, Citation2017). Differences in microbial counts, expressed as Log of colony forming units (CFU) per g of chicken between ConB and HopB treatments, were assessed using repeated-measures analysis of variance (six time levels). The experimental unit was the chicken carcass (n = 3) and the degrees of freedom were 1 for treatment and time, and 4 for the error. For the microbial counts showing significance regarding treatment, count differences within each sampling day were analyzed using the Student's t-test with the level of significance set at p < 0.05. The analysis of variance and the Student's t-test were carried out using the SPSS version 24 software (SPSS Inc., IBM Corp.; Armonk, NY). DMFit web edition (Institute of Food Research, Norwich, UK; Baranyi & Roberts, Citation1994) was also used to model and graph the modeled growth curves from the microbial counts.
Results and discussion
Sensory evaluation of immersed breasts after cooking
The triangular test was conducted to determine whether immersing the chicken breast in hops aqueous extract (at a ratio of 2 g of hops equivalent/L) for 18 h has or does not have any effect on cooked chicken flavor. The analysis showed that 18 out of the 54 participants were right in their response. This amount of correct answers pointed to ConB samples being similar to HopB samples in taste, i.e. a significance for similarity with an estimated pd of 30% (β) <0.05; and a significance for difference (α) >0.1 (Sinkinson, Citation2017). Thus, the typical bitterness and aroma produced by hops resins and essential oils in beer (Oladokun et al., Citation2016) should to not be noticeable in the hop-extract-immersed chicken breasts. These compounds probably would not have diffused into the meat in significant amount from the surface in contact with the hop extract and their concentration in meat would have been under their flavor threshold, which in beer appeared to be about 5 ppm (Hughes & Baxter, Citation2001). Supporting this finding, a trained panel was not capable of detecting the ‘bitter’ attribute in fresh pork loin slices marinated in brine containing 5 g/L of a commercial 40%-β-acid hop extract (Kramer et al., Citation2015); a concentration presumably higher than that expected in the extract used in this study.
Breast pH and microbial changes in chicken during storage
The mean (±standard deviation) value of pH value of breast at arrival to the lab (n = 6) was 5.75 (±0.06), which could be considered as a normal pH for fresh poultry (Swatland, Citation2008). The LAB counts in the chicken breast during refrigerated storage under vacuum for ConB and HopB treatments are shown in . Lactic acid bacteria counts, regardless of treatment, increased during the first 3 weeks of storage until reaching and maintaining maximum levels of about 7–8 Log CFU/g, indicating depletion of glucose and other substrates (Jones, Citation2004). LAB and specifically Lactobacilli spp., Carnobacterium spp., and Leuconostoc spp. have been recognized as predominant spoilage microorganisms in refrigerated-stored non-high pH vacuum-packed fresh meat, generating off flavors and slime (Doulgeraki et al., Citation2012; Pothakos et al., Citation2015). The results from this study confirm that LAB microorganisms are capable of growing more on refrigerated-stored vacuum-packed meat than the other facultative anaerobic meat spoilage microorganisms (Narasimha Rao & Sachindra, Citation2002), i.e. more than Enterobacteriaceae and B. thermosphacta ( and , respectively).
Figure 1. Changes in lactic acid bacteria counts (mean values, n = 3, and standard error: vertical bars) in chicken breast previously immersed in water or hop aqueous extract, ConB (▲) and HopB (●) treatments, respectively (18 h, 2°C), during refrigerated storage (2°C) under vacuum. CFU: Colony forming units. *: significant difference between treatments within each storage time (p < 0.05; Student's t-test). Growth curves were modeled using the DMFit Web Edition (Baranyi & Roberts, Citation1994); lag time and maximum growth rate were 0.71 h and 0.24 Log CFU/(g x h) (R2 = 0.997) for ConB, and 5.55 and 0.26 (R2 = 0.984) for HopB.
Figura 1. Cambios en los recuentos de bacterias ácido-lácticas (valores promedio, n = 3, y error estándar: barras verticales) en pechuga de pollo refrigerada (2°C) a vacío, que fue previamente sumergida en agua o en un extracto acuoso de lúpulo, tratamientos ConB (▲) y HopB (●), respectivamente (18 h, 2°C). CFU: Unidades formadoras de colonias. *: diferencia significativa entre tratamientos para cada día de almacenamiento (p < 0.05; Student's t-test). Las curvas de crecimiento fueron modeladas usando el software DMFit Web Edition (Baranyi & Roberts, Citation1994); la fase de latencia y la velocidad máxima de crecimiento fueron 0.71 h y 0.24 Log CFU/(g x h) (R2 = 0.997) para ConB, y 5.55 y 0.26 (R2 = 0.984) para HopB
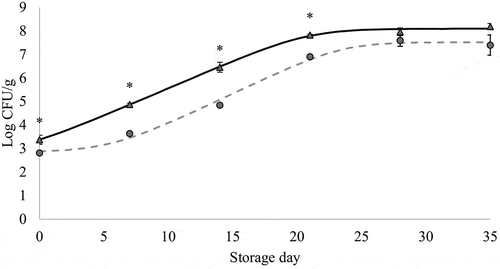
Figure 2. Changes in Enterobacteriaceae counts (mean values, n = 3, and standard error: vertical bars) in chicken breast previously immersed in water or hop aqueous extract, ConB (▲) and HopB (●) treatments, respectively (18 h, 2°C), during refrigerated storage (2°C) under vacuum. CFU: Colony forming units. *: significant difference between treatments within each storage time (p < 0.05; Student's t-test). Growth curves were modeled using the DMFit Web Edition (Baranyi & Roberts, Citation1994); lag time and maximum growth rate were 0.0 h and 0.31 Log CFU/(g x h) (R2 = 0.983) for ConB, and 0.54 and 0.23 (R2 = 0.981) for HopB.
Figura 2. Cambios en los recuentos de Enterobacteriaceae (promedio, n = 3, y error estándar: barras verticales) en pechuga de pollo refrigerada (2°C) a vacío, que fue previamente sumergida en agua o en un extracto acuoso de lúpulo, tratamientos ConB (▲) y HopB (●), respectivamente (18 h, 2°C). CFU: Unidades formadoras de colonias. *: diferencia significativa entre tratamientos para cada día de almacenamiento (p < 0.05; Student's t-test). Las curvas de crecimiento fueron modeladas usando el software DMFit Web Edition (Baranyi & Roberts, Citation1994); la fase de latencia y la velocidad máxima de crecimiento fueron 0.0 h y 0.31 Log CFU/(g x h) (R2 = 0.983) para ConB, y 0.54 y 0.23 (R2 = 0.981) para HopB
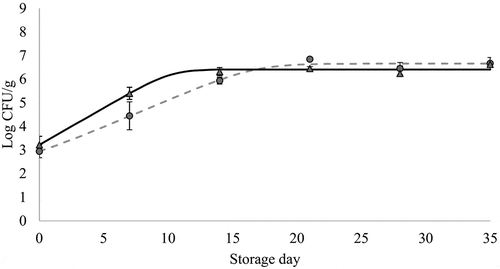
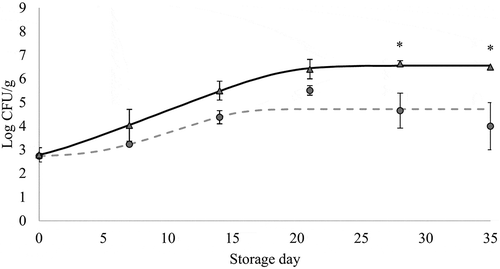
Figure 3. Changes in Brochothrix thermosphacta counts (mean values, n = 3, and standard error: vertical bars) in chicken breast previously immersed in water or hop aqueous extract, ConB (▲) and HopB (●) treatments, respectively (18 h, 2°C), during refrigerated storage (2°C) under vacuum. CFU: Colony forming units. *: significant difference between treatments within each storage time (p < 0.05; Student's t-test). Growth curves were modeled using the DMFit Web Edition (Baranyi & Roberts, Citation1994); lag time and maximum growth rate were 1.01 h and 0.21 Log CFU/(g x h) (R2 = 0.997) for ConB, and 4.66 and 0.18 (R2 = 0.423) for HopB.
Figura 3. Cambios en los recuentos de Brochothrix thermosphacta (valores promedio, n = 3, y error estándar: barras verticales) en pechuga de pollo refrigerada (2°C) a vacío, que fue previamente sumergida en agua o en un extracto acuoso de lúpulo, tratamientos ConB (▲) y HopB (●), respectivamente (18 h, \C). CFU: Unidades formadoras de colonias. *: diferencia significativa entre tratamientos para cada día de almacenamiento (p < 0.05; Student's t-test). Las curvas de crecimiento fueron modeladas usando el software DMFit Web Edition (Baranyi & Roberts, Citation1994); la fase de latencia y la velocidad máxima de crecimiento fueron 1.01 h y 0.21 Log CFU/(g x h) (R2 = 0.997) para ConB, y 4.66 y 0.18 (R2 = 0.423) para HopB
Immersing the meat in hop extract resulted in a significant decrease in the growth rate of LAB during the first 3 weeks of storage, i.e. days 0, 7, 14, and 21 (p < 0.05). However, no differences were observed between treatments on days 28 and 35. The delay in LAB growth under the HopB treatment could be attributed to hop compounds such as α- and β-acids and xanthohumol with proven antimicrobial effects against Gram-positive bacteria in food, especially in low fat and low pH food, during refrigerated storage (Kramer et al., Citation2015).
The shelf life of refrigerated vacuum-packed chicken meat, as assessed by edible quality deterioration, appeared to be limited to a few weeks (Jiménez et al., Citation1997). The end of shelf life has been related to LAB levels in meat reaching 7 Log CFU/g (Kreyenschmidt et al., Citation2010). The chicken breast immersed in hop extract showed those levels 6 days later than breast immersed in water did, i.e. 22 days vs 16 days, as estimated from the modeled growth curves (). The difference in LAB growth between treatments leads to the suggestion that hops compounds slightly inhibit LAB growth in the surface of refrigerated-stored vacuum-packed meat, and that the immersion in hop extract could be relevant for chicken retailing due to its capability to delay spoilage and thus to prolong the shelf life of fresh chicken refrigerated stored under vacuum. Hops have also been shown to be capable of inhibiting LAB species in beer (Sakamoto & Konings, Citation2003).
As can be seen in , for both ConB and HopB treatments Enterobacteriaceae showed an exponential growth during the first 2 weeks of refrigerated storage before starting the stationary phase with counts of 6–7 Log CFU/g, with no significant differences between them. This pattern agrees with that found by Jiménez et al. (Citation1997) reporting values close to 6 Log CFU/g in vacuum-packed 1-week refrigerated-stored fresh chicken breast. Previous studies stated that psychrotrophic Enterobacteriaceae species contribute to spoilage in refrigerated-stored vacuum-packed meat (Doulgeraki et al., Citation2012) causing spoilage at levels of 7 Log CFU/g (Samelis, Citation2006). Compared with LAB, both the maximum counts and the time elapsed until the appearance of the stationary phase in Enterobacteriaceae appeared to be lower. This could denote a competitive effect of LAB against Enterobacteriaceae in the vacuum-packed chicken, which might partly be explained by the higher ability of LAB for the consumption of limiting nutrients under specific environmental conditions (Narasimha Rao & Sachindra, Citation2002).
With regard to the effect of hops on Enterobacteriaceae counts in refrigerated-stored vacuum-packed chicken breast, the Student's t-test showed no significant differences due to the treatment on any sampling day (p > 0.05). This lack of significant effect agrees with previous findings reporting that the antimicrobial effect of hop compounds is mainly exerted towards Gram-positive bacteria, being slight or non-existent against Gram-negatives, which can develop resistance mechanisms (Bocquet, Sahpaz, & Rivière, Citation2018).
B. thermosphacta grows in refrigerated-stored vacuum-packed meat and contributes to its spoilage. According to Gribble and Brigthweel (Citation2013), counts of between 4 and 5 Log CFU/cm2 could be indicative of spoilage. In this study, higher counts of B. thermosphacta were observed in ConB than in HopB, and were significantly different (p < 0.05) on storage days 28th and 35th (). The highest levels of B. thermosphacta found in the refrigerated-stored ConB breast were comparable to those reported by Gribble and Brightwell (Citation2013) in vacuum-packed beef and lamb, i.e. 5–6 Log CFU/g. Comparing both immersion treatments, our results suggest that hop-extract exposure could induce a quicker beginning of the cell stationary phase (and lower final counts) since the B. thermosphacta in HopB had decreased growth rates compared to ConB counts.
Conclusion
The results of this study show that a previous immersion of fresh chicken in a hop aqueous extract obtained by boiling 2 g of hops per L of water can exert a significant antimicrobial activity against facultative anaerobe Gram-positive spoilage microorganisms in refrigerated-stored vacuum-packed chicken breast. The growth rate of lactic acid bacteria seems to be delayed and B. thermosphacta counts at the stationary growth phase appear to be reduced. In spite of hops being bitter, the immersion in the hop extract did not affect the chicken flavor. Further studies are needed to gain additional knowledge on the antimicrobial effect of hop extracts on the surface of fresh chicken to be vacuum packed, i.e. dose dependency of microbial inhibition, influence of different extraction methods of hops antimicrobial compounds, effect of exposure procedure.
Conflict of interest
None of the authors has a financial or personal relationship with other people organizations that could inappropriately influence or bias the paper or present other types of conflict interest.
Acknowledgments
D.E. Carballo is grateful for a doctoral grant from CONACYT (MEX/Ref. 288189). The authors would like to thank Mr. José Manuel Martínez Fernández from Orbigo Valley SL for providing the hops.
Additional information
Funding
References
- Baranyi, J., & Roberts, T. A. (1994). A dynamic approach to predicting bacterial growth in food. International Journal of Food Microbiology, 23, 277–294. doi:10.1016/0168-1605(94)90157-0
- Bocquet, L., Sahpaz, S., & Rivière, C. (2018). An overview of the antimicrobial properties of hop. In J. M. Mérillion & C. Riviere (Eds.), Natural antimicrobial agents (pp. 31–54). Cham, Switzerland: Springer.
- Bogdanova, K., Kolar, M., Langova, K., Dusek, M., Mikyska, A., Bostikova, V., … Olsovska, J. (2018). Inhibitory effect of hop fractions against Gram-positive multi-resistant bacteria. A pilot study. Biomedical Papers, 162, 276–283. doi:10.5507/bp.2018.026
- Corry, J. E. L. (2007). Spoilage organism in fresh meat and poultry. In G. C. Mead (Ed.), Microbial analysis of red meat, poultry and eggs (pp. 101–122). Boca Raton, FL, USA: Woodhead Publishing Limited.
- Doulgeraki, A. I., Ercolini, D., Villani, F., & Nychas, G.-J. E. (2012). Spoilage microbiota associated to the storage of raw meat in different conditions. International Journal of Food Microbiology, 157, 130–141. doi:10.1016/j.ijfoodmicro.2012.05.020
- Gribble, A., & Brightwell, G. (2013). Spoilage characteristics of Brochothrix thermosphacta and campestris in chilled vacuum packaged lamb, and their detection and identification by real time PCR. Meat Science, 94, 361–368. doi:10.1016/j.meatsci.2013.03.016
- Hughes, P. S., & Baxter, E. D. (2001). Beer: Quality, safety and nutritional aspects. Cambridge, UK: Royal Society of Chemistry.
- ISO, B. S. (2004). Sensory analysis—methodology—triangle test, BS ISO 4120. London, UK: Brithish Standard Institution.
- Jiménez, S. M., Salsi, M. S., Tiburzi, M. C., Rafaghelli, R. C., Tessi, M. A., & Coutaz, V. R. (1997). Spoilage microflora in fresh chicken breast stored at 4 degrees C: Influence of packaging methods. Journal of Applied Microbiology, 83, 613–618. doi:10.1046/j.1365-2672.1997.00276.x
- Jones, R. J. (2004). Observations on the succession dynamics of lactic acid bacteria populations in chill-stored vacuum-packaged beef. International Journal of Food Microbiology, 90, 273–282. doi:10.1016/S0168-1605(03)00310-6
- Kramer, B., Thielmann, J., Hickisch, A., Muranyi, P., Wunderlich, J., & Hauser, C. (2015). Antimicrobial activity of hop extracts against foodborne pathogens for meat applications. Journal of Applied Microbiology, 118, 648–657. doi:10.1111/jam.2015.118.issue-3
- Kreyenschmidt, J., Hübner, A., Beierle, E., Chonsch, L., Scherer, A., & Petersen, B. (2010). Determination of the shelf life of sliced cooked ham based on the growth of lactic acid bacteria in different steps of the chain. Journal of Applied Microbiology, 108, 510–520. doi:10.1111/j.1365-2672.2009.04451.x
- Narasimha Rao, D., & Sachindra, N. M. (2002). Modified atmosphere and vacuum packaging of meat and poultry products. Food Reviews International, 18, 263–293. doi:10.1081/FRI-120016206
- Nychas, G.-J. E., Skandamis, P. N., Tassou, C. C., & Koutsoumanis, K. P. (2008). Meat spoilage during distribution. Meat Science, 78, 77–89. doi:10.1016/j.meatsci.2007.06.020
- Oladokun, O., Tarrega, A., James, S., Cowley, T., Dehrmann, F., Smart, K., … Hort, J. (2016). Modification of perceived beer bitterness intensity, character and temporal profile by hop aroma extract. Food Research International, 86, 104–111. doi:10.1016/j.foodres.2016.05.018
- Pothakos, V., Devlieghere, F., Villani, F., Björkroth, J., & Ercolini, D. (2015). Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Science, 109, 66–74. doi:10.1016/j.meatsci.2015.04.014
- Sakamoto, K., & Konings, W. N. (2003). Beer spoilage bacteria and hop resistance. International Journal of Food Microbiology, 89, 105–124. doi:10.1016/S0168-1605(03)00153-3
- Samelis, J. (2006). Managing microbial spoilage in the meat industry. In C. W. de Blackburn (Ed.), Food spoilage microorganisms (pp. 213–286). Cambridge, United Kingdom: Woodhead Publishing.
- Shen, C., Geornaras, I., Kendall, P. A., & Sofos, J. N. (2009). Antilisterial activities of salad dressings, without or with prior microwave oven heating, on frankfurters during simulated home storage. International Journal of Food Microbiology, 132, 9–13. doi:10.1016/j.ijfoodmicro.2009.03.008
- Signorini, M. (2007). Microbiología de carnes envasadas al vacío y la biopreservación como medio para prolongar la vida de anaquel [Microbiology of vacuum packed meat and biopreservation as approarch to prolong meat shelf life]. Nacameh, 1, 26–40.
- Silva, F., Domingues, F. C., & Nerín, C. (2018). Trends in microbial control techniques for poultry products. Critical Reviews in Food Science and Nutrition, 58, 591–609. doi:10.1080/10408398.2016.1206845
- Singh, M., Smith, J., & Bailey, M. (2014). Using natural antimicrobials to enhance the safety and quality of poultry. In T. M. Taylor (Ed.), Handbook of natural antimicrobials for food safety and quality (pp. 375–401). Cambridge, United Kingdom: Woodhead Publishing.
- Sinkinson, C. (2017). Triangle test. In R. Lauren (Ed.), Discrimination testing in sensory science : A practical handbook (pp. 153–170). Duxford, UK: Woodhead Publishing.
- Swatland, H. J. (2008). How pH causes paleness or darkness in chicken breast meat. Meat Science, 80, 396–400. doi:10.1016/j.meatsci.2008.01.002
- Vasconcelos, H., Saraiva, C., & de Almeida, J. M. M. M. (2014). Evaluation of the spoilage of raw chicken breast fillets using Fourier transform infrared spectroscopy in tandem with chemometrics. Food and Bioprocess Technology, 7, 2330–2341.
- Zhang, H., Wu, J., & Guo, X. (2016). Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Science and Human Wellness, 5(1), 39–48. doi:10.1016/j.fshw.2015.11.003
