ABSTRACT
The main aim of this study was to transform bovine colostrum into a tribiotic product through a metabolic conversion with a microbial consortium based on artisanal and starter microorganisms. As such, the bovine colostrum was firstly transformed enzymatically for 48 h with a selected strain of a non-pathogenic Candida lipolytica strain, and then lactic acid fermentation was performed for 48 h, by using a co-culture of 25 g/L fresh kefir grains and a 10 g/L commercial FreshQ® culture (Chr. Hansen, Denmark). The obtained product is recommended to be used as an ingredient for the milk-derived nutraceuticals’ formulation. As such, the product displayed increased antioxidant potential of 3.15 mM Trolox equivalent/g. Furthermore, the peptide fractions with a molecular weight lower than 3 kDa showed a notable in vitro ABTS radical scavenging activity, similar to a concentration of 2 nM captopril, hence proving a high potential in reducing the blood pressure.
RESUMEN
El objetivo principal de este estudio fue transformar calostro bovino en un producto tribiótico mediante una conversión metabólica con un consorcio microbiano conformado por microorganismos artesanales e iniciadores. En primer lugar, el calostro bovino fue transformado enzimáticamente durante 48 horas, empleando para ello una cepa seleccionada de una cepa no patógena de Candida lipolytica. Luego, utilizando un co-cultivo de 25 g/L de granos frescos de kéfir y un cultivo comercial de 10 g/L de FreshQ® (Chr. Hansen, Dinamarca), se realizó la fermentación del ácido láctico durante 48 horas. Se recomienda el uso del producto obtenido como ingrediente para la formulación de nutracéuticos derivados de la leche. En este sentido, el producto mostró mayor potencial antioxidante de 3.15 mM de equivalente de Trolox/g. Además, las fracciones peptídicas con peso molecular inferior a 3 kDa mostraron una notable actividad de eliminación de radicales ABTS in vitro, similar a una concentración de 2 nM de captopril, lo que da cuenta de su alto potencial de reducción de la presión arterial.
1. Introduction
Kefir, an ancient fermented milk product with a refreshing sour taste and aroma, that originated in the Balkans (Arslan, Citation2015), Eastern Europe and the North Caucasus Mountains of Russia, is still consumed for its nutritive and functional properties (Seo et al., Citation2018; Shi et al., Citation2018). The kefir not only has a unique taste but also possesses many human health benefits due to its probiotic, prebiotic, antimicrobial, anticarcinogenic, antidiabetic and antiallergic activities (Shi et al., Citation2018).
Known as artisanal cultures, the kefir grains represent a complex of polysaccharides, proteins and beneficial microorganisms (lactic acid bacteria, acetic bacteria and yeasts) associated in a natural consortium (Dallas et al., Citation2016).
Nonetheless, the bovine colostrum is rich in proteins, fats, lactose, vitamins, minerals, immunoglobulins, antimicrobial proteins and enzymes (lactoferrin and lactoperoxidase) (Ayar et al., Citation2016; Playford et al., Citation2000). The fermentation of colostrum with kefir grains represents a suitable approach to improve the functionality in accordance with the metabolic activity of multiple cultures (bacteria and yeasts) which act in symbiosis, with a great impact on the consumer’s health. Also, the fermentation improves the colostrum’s preservation, due to the fact that it is very susceptible to microbial contamination and also to heating lability due to its high protein content (Windayani et al., Citation2019).
Among various protein sources, milk and colostrum are considered a good source of bioactive peptides.
A large diversity of naturally formed bioactive peptides has been found in the fermented dairy products. The incidence, specific activity and quantity of bioactive peptides present in fermented products are correlated to many factors, such as the substrate’s composition, the biochemical properties of the starter cultures, the bioprocess parameters and the storage conditions. It is remarkable that in these fermented products, peptides with different bioactivities, e.g., calcium-binding, antihypertensive, antioxidant, immunomodulatory and antimicrobial, can be found simultaneously (Korhonen, Citation2012).
The peptides obtained from milk and the colostrum proteins revealed antimicrobial activity, cholesterol-lowering capacity, reducing blood pressure ability, mainly due to the angiotensin conversion enzyme (ACE) inhibition (Gandhi & Shah, Citation2014; Rahimi et al., Citation2016), antioxidant activity (Hafeez et al., Citation2014), and cyto-and immunomodulatory effects (Mohanty et al., Citation2016). Some peptides revealed prebiotic activities (Figueroa-Gonzalez et al., Citation2019; Vasile et al., Citation2016). Prebiotic compounds act as substrates for the growth and multiplication of probiotic bacteria and therefore improve the gastrointestinal functions and the immune system, increase the absorption of calcium and magnesium, influence the blood’s glucose levels and improve the plasmatic lipids (Fazilah et al., Citation2018).
Nevertheless, up until now there are very few reports regarding the bovine colostrum fermentation with kefir grains. Our previous study reported and assessed the possibility to obtain bioactive peptides by bovine colostrum enzymatic hydrolysis using a non-pathogenic Candida lipolytica selected strain (Cotârleț et al., Citation2019).
Therefore, the aims of the study were to obtain a tribiotic (pre, pro and postbiotic) product derived from bovine colostrum, by two biotransformation steps (enzymatic hydrolysis and fermentation), through the use of selected and artisanal starter cultures (bacteria and yeasts). The characterization of the functional product in regards to the antioxidative, antimicrobial and antihypertensive properties was also targeted. Furthermore, the colostrum-derived peptides separated from the fermented product were investigated for their prebiotic effect, radical scavenging and ACE-inhibitory activities.
2. Materials and methods
2.1. Starter microorganisms
The kefir grains were provided by an artisanal manufacturer from Romania and preserved as a stock culture in 400 g/L glycerol at −80°C. For their use as the inoculum, the kefir grains were inoculated (25 g/L) and propagated in pasteurized whole milk (35 g/L fat) at room temperature for 24 h. The grains were retrieved by sieving, afterwards being washed with sterile distilled water and then used as inoculum (Chen et al., Citation2009).
The non-pathogenic Candida lipolytica MIUG D67 yeast strain was previously selected as a lipase and protease producer (Cotârleț et al., Citation2019). The yeast strain belongs to the Microorganisms Collection of the Bioaliment Research Platform (acronym MIUG), Faculty of Food Science and Engineering,”Dunărea de Jos” University of Galați, Romania. The stock culture was preserved in 400 g/L glycerol at −80ºC, for further analysis. For the reactivation, the cultivation took place on a Yeast Extract Chloramphenicol Agar (YGC) medium, at 25°C for 3 days. The commercial lactic acid bacteria, namely FreshQ® (Lactobacillus rhamnosus and Lactobacillus paracasei) culture and the L. casei 431 (Lactobacillus casei ssp. paracasei) probiotic culture were provided by Chr. Hansen, Denmark as lyophilized cultures.
2.2. Bovine colostrum biotransformation
One hundred g/L bovine colostrum (Axyar, Belgium) diluted in distilled water, was heat treated at 105°C, for 10 min. The colostrum’s chemical composition was as follows (g/kg): 736 proteins, 27 fat, 169 carbohydrates, 207 total immunoglobulins, 51 water content, pH 6.4. The substrate’s biotransformation was achieved in two steps. In the first step, the bovine colostrum was incubated with Candida lipolytica MIUG D67 strain inoculum (1·108 CFU/mL), on a rotary shaker, at 30°C and 150 rpm for 48 h. In the second step, 25 g/L kefir grains and 1 g/L of FreshQ® culture were added and the fermentation was continued for 48 h at 30°C, in a stationary system. After fermentation, the kefir grains were separated by using a sterilized plastic sieve. One part of the fermented product was stored at 4°C for further analysis (fresh sample) and another part was lyophilized with the Alpha 1–4 equipment (Martin Christ, Germany).
2.3. Separation of the peptide fractions
The lyophilized sample was solubilized in distilled water, in the concentration of 10 g/L and preserved overnight (4ºC, at 135 x g) and then centrifuged at 8000 x g, at 4°C, for 15 min. The supernatants were fractionated by centrifugal ultrafiltration through membrane filter units (AmiconUltracel YM-10, Millipore, Germany, cut-off at 3 kDa and 10 kDa) and the resulted supernatants were preserved at 4ºC for further analysis. The peptides concentration was determined by using the bicinchoninic acid assay kit (Thermo Fisher, Germany) (Gaspar-Pintiliescu et al., Citation2019).
2.4. pH and acidity assay
The pH of the fermented samples was measured with the MP 2000 pH meter (Mettler Toledo, Switzerland). The total titratable acidity (TTA) assay was performed according to Cotârleț et al. (Citation2019). The TTA was expressed in ºThorner (ºTh).
2.5. Antimicrobial activity assay
The antimicrobial potential of the fresh fermented product was tested against two spoilage microorganisms, such as Bacillus subtilis MIUG B1 (aerobic sporulating bacteria) and Aspergillus niger MIUG M5 (common mould), by using the well-radial diffusion method (Cotârleț et al., Citation2019; Kim et al., Citation2016). The indicator strains were preserved within the MIUG Collection of microorganisms in 400 g/L glycerol at −80ºC. For the reactivation of the cultures, they were cultivated under specific microbiological conditions, as follows: the bacteria on a Plate Count Agar (PCA) medium, at 37°C for 24 h and the moulds on a Yeast Extract Chloramphenicol Agar (YGC) medium, at 25°C for 3–5 days.
For the antimicrobial activity evaluation, a cells suspension of each indicator microorganism was obtained by transferring the fresh biomass with sterile loops into a sterile saline solution (9 g/L NaCl) at o cells’ concentration of 106 CFU/mL. One mL of each suspension was homogenized with 15 mL of the specific culture medium (PCA and YGC), in sterile Petri dishes. Then, several wells with a 5 mm diameter were made and 100 μL of the fresh product were placed into the wells, followed by incubation at a specific temperature for each microorganism. The test was considered positive when the inhibition zone appeared around the well while the diameter of the inhibition was measured and expressed in mm.
2.6. Antioxidant potential evaluation
The antioxidant activity of the freshly fermented samples was determined by using the DPPH assay (Yuan et al., Citation2013). The antioxidant activity was expressed as mM Trolox equivalents (TE)/g sample by using Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), under the same conditions.
The radical scavenging activity of the peptide samples was determined by the 2, 2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay (Crăciunescu et al., Citation2012). A calibration curve was done using different concentrations of Trolox, as a standard antioxidant. The radical scavenging activity was expressed as mM Trolox/mg protein.
2.7. ACE-inhibitory activity assay
The ACE inhibition was measured using an experimental in vitro model, as previously described (Papadimitriou et al., Citation2007). Captopril, a well-known drug for lowering blood pressure, was used as the standard in the concentration range of 1–25 nM (Ahmad et al., Citation2017). The ACE-inhibitory activity of the peptide samples was calculated according to the following formula:
ACE-inhibitory activity (%) = (1 – ODsample/ODcontrol) x 100
where ODsample = the absorbance of the sample, ODcontrol = the absorbance of the control.
2.8. Prebiotic effect of the bioactive peptides assay
The L. casei 431 probiotic culture was used as a test culture for the in vitro evaluation of the prebiotic effect. The inoculum was prepared from 1 g of freeze-dried stock culture (1· 1011 CFU/g), mixed with 50 mL of MRS broth (Carl Roth, Germany) and incubated for 15 min, at 37ºC. Then, 10 mL/L of the inoculum was cultivated on an MRS broth supplemented with 20 g/L peptides’ fractions and incubated for 24–48 h, at 37ºC, under stationary conditions. The control samples were prepared following a similar protocol without the addition of the bioactive peptides. After cultivation, all the samples were kept at 4ºC and the cells’ viability was monitored, during storage, after 7 and 14 days. The cells’ viability was quantified through the indirect counting method (Parra et al., Citation2013). The prebiotic index (Ipreb), as the lactic acid bacteria growth ratio in the presence of prebiotics and of the control without prebiotics, was calculated according to the following formula (Figueroa-Gonzalez et al., Citation2019):
I preb = CFU in medium with prebiotic/CFU in control medium
2.9. Statistical analysis
All the experiments were performed in triplicate. The results were expressed as mean value ± standard deviation (SD) for three independent samples (n = 3). The statistical analysis was performed using one-tailed paired Student’s t-test on each pair of interest. The statistical differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Bovine colostrum biotransformation
A new strategy was performed in order to obtain a tribiotic fermented product containing prebiotics, probiotics and postbiotics from the bovine colostrum that was metabolized by enzymatic transformation and fermentation using a multiple consortium of microorganisms (artisanal and selected starter cultures). Firstly, the substrate was enzymatically transformed by the Candida lipolytica MIUG D67 yeast, which has protease and lipase activity (Cotârleț et al., Citation2019). Then, in the second stage, the transformed substrate was fermented with fresh milk kefir grains and Fresh Q® cultures. During the proposed two stages of the substrate’s biotransformation, the pH and the acidity gradually increased from 6.9 up to 7.2, after 48 h of incubation during the action of the yeast. Then, after the kefir grains and Fresh Q® culture’s addition, the pH values decreased at the end of the fermentation process and reached the value of 4.3 ().
Table 1. The pH and acidity variation during the bovine colostrum biotransformation.
Tabla 1. The Variación del pH y acidez durante la biotransformación del calostro bovino
Gaspar-Pintiliescu et al. (Citation2019) reported similar results for the bovine colostrum‘s biotransformation by using multiple cultures. The pH values of the fermentation medium gradually increased from 6.77 to 7.26, after 48 h of incubation of the C. lipolytica MIUG D67. Furthermore, after the kefir grains’ inoculation, the pH values decreased up to 4.84, after another 48 h of fermentation.
During the colostrum’s fermentation, the microorganisms present in the kefir grains and the commercial starter culture transform lactose into organic acids that decrease the pH of the fermented sample (Ayar et al., Citation2016; Suriasih et al., Citation2012). Furthermore, the simple compounds that are formed during the first stage are valuable nutrients for the microorganisms involved in the fermentation.
Regarding the titratable acidity, in the first 24–48 h of colostrum hydrolysis no variations were detected (25°Th). When the kefir grains and FreshQ® culture were added, the titratable acidity of the fermented colostrum reached values between 150° and 168°Th, after 24–48 h of fermentation, at 30ºC. In the literature, there are no reported data regarding the colostrum’s fermentation with milk kefir grains. Our previous study demonstrated this ability of the artisanal multi-strains consortium (Cotârleț et al., Citation2019). These data were in agreement with previous reports that indicated similar TTA values (25°Th), results obtained 48 h after the yeast culture’s action upon the bovine colostrum substrate, and comparable values up to 162.5°Th, after another 48 h of fermentation with the yeast and the kefir grain consortia (Gaspar-Pintiliescu et al., Citation2019).
Shi et al. (Citation2018) reported that the fermentation of goat milk by the K1 kefir grain was obtained at a pH of 4.09 ± 0.02 and an acidity of 89.42 ± 1.02°Th, after the incubation with 20 g/L inoculum at 25ºC for 22 h. Afterwards, the pasteurized goat milk was inoculated with 20 g/L kefir grains and incubated at 25°C for 24 h. The obtained fermented products had a pH of 4.6–4.8 and an acidity of 90-100ºTh (Nacheva et al., Citation2017).
3.2. Antimicrobial activity of the fermented product
The antimicrobial potential of the fermented sample, obtained through the colostrum’s biotransformation with an enhanced consortium of selected and artisanal microorganisms (kefir grains, selected yeast and commercial LAB culture), was assessed against two food spoilage microorganisms (). The fermented sample showed an acceptable antimicrobial activity against Bacillus subtilis MIUG B1 (5.0 mm), while for Aspergillus niger MIUG M64 only the spore-forming inhibition was observed (the white zone was compared to the rest of the media which was black) (12.0 mm).
Figure 1. Antimicrobial activity of the colostrum fermented sample against Aspergillus niger (left) and Bacillus subtilis (right).
Figura 1. Actividad antimicrobiana de la muestra fermentada con calostro contra el Aspergillus niger (izquierda) y el Bacillus subtilis (derecha)
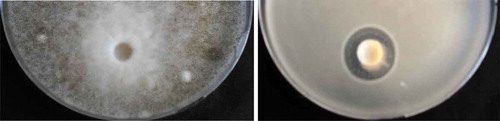
The antimicrobial potential of the fermented product could be associated with the biosynthesis of the organic acids, peptides, carbon dioxide, hydrogen peroxide, ethanol and diacetyl through the metabolic activity of the microorganisms implied in the substrate biotransformation (Muhialdin et al., Citation2011). Therefore, the multiple strains that co-habit within the kefir grains microbiota work in perfect symbiosis and possess a synergic competitive action against nonspecific food microbiota.
Ahmed et al. (Citation2011), assessed the antimicrobial activity of the fermented cow milk kefir and kefir grains by using the disk diffusion method. The fermented kefir suspension showed a significant antibacterial activity (13.0 mm) compared to the fresh kefir grains (8.0 mm), against Bacillus subtilis BW2 strain. The growth of the Aspergillus flavus AH3 was not affected by the fermented kefir suspension whereas the sporulation was clearly inhibited.
3.3. Antioxidant and antihypertensive activity of the fermented sample and peptide fractions
The antioxidant activity of the colostrum-fermented sample was assessed by the DPPH method. The fermentation process conducted with a multiple microbial consortium (1·106 CFU/mL Candida lipolytica MIUG D67, 25 g/L kefir grains and 1 g/L of Fresh Q®) determined an antioxidant potential of 3.15 mM TE/g at the end of the fermentation period (96 h), at 37ºC ().
Table 2. Antioxidant activity of the fermented product based on the bovine colostrum.
Tabla 2. Actividad antioxidante del producto fermentado basado en el calostro bovino
Yilmaz-Ersan et al. (Citation2018) reported that by fermenting different types of milk with kefir grains, a different antioxidant activity (by DDPH assay) can be obtained, namely between 8.70 and 5.03 mg TE/100 mL for raw and heat-treated ewe milk and between 3.14 and 2.66 mg TE/100 mL for raw and heat-treated cow milk.
Ozcan et al. (Citation2018) observed that the maximum DPPH radical scavenging activity of the buffalo milk fermented with a commercial starter culture was 3.14 mg Trolox/100 mL while the activity of the buffalo milk fermented with kefir grains was only 2.36 mg Trolox/100 mL. Therefore, the observed differences regarding the antioxidant activity of fermented products are correlated to the qualitative and quantitative proteins’ composition of the milk and to the enzymatic activity of the starter cultures so that the bioactive peptides with functional properties would be released (Niero et al., Citation2018).
Afterwards, the bioactive peptides separated from the lyophilized-fermented sample were analysed by using the ABTS assay. The peptide fractions with an MW ˂ 3 kDa revealed a high ABTS radical scavenging activity (2.28 mM Trolox/mg protein) compared to the peptide fractions with an MW ˂ 10 kDa (1.85 mM Trolox/mg protein) ().
Table 3. Antioxidant activity (ABTS assay) and ACE inhibition of the peptide fractions separate from the fermented colostrum.
Tabla 3. Actividad antioxidante (ensayo ABTS) e inhibición de la ECA de las fracciones peptídicas separadas del calostro fermentado
Gaspar-Pintiliescu et al. (Citation2019) compared the antioxidant activity (ABTS method) of two fermented bovine colostrum samples and their peptide-enriched fractions. The first sample was inoculated with Candida lipolytica MIUG D99 strain (C1) and the second one with C. lipolytica MIUG D67 strain (C2). Concerning the free radicals scavenging activity, the study demonstrated that in the C1 sample, the peptides were more active compared to C2 sample. The difference is attributed mainly to the yeast strains’ enzymatic capacity to break down the proteins during the substrate’s biotransformation.
Yilmaz-Ersan et al. (Citation2018) reported for the ABTS method, after 1 day of storage, values, between 11.05 and 13.62 mg TE/100 mL for the cow milk kefir that was obtained using kefir grains and the ewe milk kefir made with a starter culture. A significant increase of the ABTS values was observed until 14 days of storage for all the samples followed by a decrease thereafter until 4 weeks of storage. Among the samples, the ewe milk kefir, made using the starter culture, exhibited the highest ABTS inhibition rate ().
Further, the ACE-inhibitory activity of the peptides’ fractions separated by ultrafiltration from the lyophilized-fermented sample was assessed using a known substrate. A high ACE inhibition rate was observed for both peptide fractions, the values varying between 53.36% for the peptide fractions with an MW ˂10 kDa and 61.12% for the peptide fractions with an MW ˂ 3 kDa (). Furthermore, the inhibition percentages of 31.70% and 64.42% were attained using 1 nM and 2 nM captopril, respectively, hence indicating a high potential in reducing the blood pressure (data not shown).
Gaspar-Pintiliescu et al. (Citation2019) compared the ACE inhibition activity of two fermented colostrum extracts (obtained with two different yeast strains) and peptide-enriched fractions (MW ˂ 10 kDa). Both of the fermented products showed a high ACE inhibition of 78.52% and 72.85% for C1 and C2 extracts, respectively. The significantly increased (p < 0.05) inhibitory activities of the P1 (94.40%) and P2 (88.62%) peptide-enriched fractions were analysed compared to the corresponding fermented colostrum extracts. These values were close to the one exerted by 5 ng/ml captopril (96.48%), hence indicating the antihypertensive effect of the colostrum fermented with the kefir enhanced consortia.
A great number of bioactive peptides have been identified over the years, peptides that showed various activities including antioxidative, antihypertensive, antimicrobial, immunomodulatory (Korhonen & Pihlanto, Citation2007), thus inspiring a lot of interest among scientists since these compounds offer many other therapeutic properties to the fermented dairy products (Shori & Baba, Citation2015).
3.4. Prebiotic effect of bioactive peptides
Prebiotics must be able to stimulate the growth of probiotics in the colon by passing through the upper intestinal tract without being metabolized (Figueroa-Gonzalez et al., Citation2019).
The prebiotic effect of the peptide fractions separated by ultrafiltration from the freeze-dried fermented sample was analysed, at a concentration of 20 g/L, on L. casei 431 probiotic strain, during the in vitro fermentation (48 h, at 37°C) and during storage (after 7 and 14 days at 4°C) (). The peptide fraction with MW ˂ 10 kDa registered a higher protein concentration (273 µg/mL), while the peptide fraction with MW ˂ 3 kDa had a lower protein concentration (173 µg/mL).
Table 4. The prebiotic effect of peptide fractions (20 g/L) upon L. casei 431 culture during fermentation and storage at 4°C.
Tabla 4. Efecto prebiótico de las fracciones de péptidos (20 g/L) sobre el cultivo de L. casei 431 durante la fermentación y almacenamiento a 4 °C
The results indicated that the commercial probiotic strain grew well in the MRS media supplemented with 20 g/L peptide fractions, as prebiotics. The supplementation effects of various peptide fractions on the viable cells depend on the fermentation time and prebiotic concentration. As it can be seen from , the viable cells number increased with the fermentation time, for both of the added peptide fractions. The prebiotic effect increased in the MRS broth supplemented with 20 g/L peptide fractions (MW ˂ 3 kDa) compared to the control (), although no significant effect was observed between the experiments (p > 0.05). After 48 h of fermentation in the MRS media supplemented with 20 g/L peptide fractions with a molecular weight lower than 3 kDa, the prebiotic effect was clearly displayed. From the obtained data it can be observed that the prebiotic effect increased with the fermented product’s storage period. Thus, after 14 days of storage, the following prebiotic effect values of 1.077 and 1.104 were registered for both of the peptide fractions.
It is known that a prebiotic index higher than 1.0 means that the peptides have a positive effect on the probiotics’ growth. If the prebiotic index is close to 1.0, this indicates a low effectiveness of the evaluated peptide fractions (Figueroa-Gonzalez et al., Citation2019).
The obtained results are in agreement with Gandhi and Shah (Citation2014), who reported an increased cell counts for L. acidophilus and S. thermophiles strains used as starters, when the reconstituted skimmed milk was supplemented with a peptides fraction (MW ˂ 3 kDa) compared to the control sample. Although no significant effect was observed, the supplementation with the fraction with MW ˂ 3 kDa promoted the cells’ growth more than the fraction with MW > 10 kDa for the studied lactic acid bacteria strains. This could be explained by the increased availability of the additional nitrogen sources delivered by the peptides and amino acids in the reconstituted skim milk supplemented with the fraction with MM ˂ 3 kDa.
Yu et al. (Citation2016) investigated the effect of whey peptide extract with MW ˂ 1 kDa on the growth of probiotic bacteria (Lactobacillus acidophilus and Bifidobacterium animalis).
The results demonstrated that the whey peptide extracts (10 g/L) had the capacity to stimulate the proliferation of both probiotic bacteria.
Vasiljevic et al. (Citation2007), showed that the mixed yogurt culture, Lactobacillus bulgaricus and Streptococcus thermophilus, appeared unaffected by the addition of prebiotics (5 g/L β-glucan) during the cold storage. The supplementation of yogurt with selected prebiotics improved the viability and stability of the Bifidobacterium animalis ssp. lactis Bb-12TM strain during 4 weeks of cold storage. The addition of the oat’s β-glucan resulted in an effect comparable to that of the proven prebiotic, inulin.
4. Conclusions
The current study was designed to obtain and characterize a tribiotic-fermented product, through two steps of biotransformation (enzymatic conversion and fermentation) of the bovine colostrum, using several artisanal and starter cultures which act in synergism and symbiosis. The obtained fermented product has functional properties due to its complex content of prebiotics, probiotics and postbiotics. The results offer a new perspective in the development of valuable fermented foods and ingredients for the nutraceuticals, pharmaceuticals and cosmeceuticals’ production based on colostrum by exploiting the excellent properties of a wild consortium of microorganisms associated within the kefir grains microbiota and selected starters.
Disclosure
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmad, I., Yanuar, A., & Munim, A. (2017). Review of angiotensin-converting enzyme inhibitory assay: Rapid method in drug discovery of herbal plants. Pharmacognosy Reviews, 11(21), 1–7. https://doi.org/10.4103/phrev.phrev_45_16
- Ahmed, A. I., Ghaly, M. F., Ayman, K., & El-N.. (2011). Milk Kefir: Ultrastructure, antimicrobial activity and efficacy on aflatoxin B1 production by Aspergillus flavus. Current Microbiology, 62(5), 1602–1609. https://doi.org/10.1007/s00284-011-9901-9
- Arslan, S. (2015). A review: Chemical, microbiological and nutritional characteristics of kefir. CyTA –journal of Food, 13(3), 340–345. https://doi.org/10.1080/19476337.2014.981588
- Ayar, A., Sıçramaz, H., & Çetin, İ. (2016). The effect of bovine colostrum on the lactic flora of yogurt and kefir. JSM Biotechnology and Biomedical Engineering, 3(4), 1063. https://www.jscimedcentral.com/Biotechnology/vol3issue4.php
- Chen, T. H., Wang, S. Y., Chen, K. N., Liu, J. R., & Chen, M. J. (2009). Microbiological and chemical properties of kefir manufactured by entrapped microorganisms isolated from kefir grains. Journal of Dairy Science, 92(7), 3002–3013. https://doi.org/10.3168/jds.2008-1669
- Cotârleț, M., Vasile, A. M., Cantaragiu, A. M., Gaspar-Pintiliescu, A. G., Crăciunescu, O., Oancea, A., Moraru, A., Moraru, I., & Bahrim, G. E. (2019). Colostrum-derived bioactive peptides obtained by fermentation with kefir grains enriched with selected yeasts. The Annals of the University Dunarea de Jos of Galati, Fascicle VI – Food Technology, 43, 54–68.
- Crăciunescu, O., Constantin, D., Gaspar, A., Toma, L., Utoiu, E., & Moldovan, L. (2012). Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chemistry Central Journal, 6(1), 97–107. https://doi.org/10.1186/1752-153X-6-97
- Dallas, D. C., Citerne, F., Tian, T., Silva, V. L. M., Kalanetra, K. M., Frese, S. A., Robinson, R. C., Mills, D. A., & Barile, D. (2016). Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chemistry, 197, 273–284. https://doi.org/10.1016/j.foodchem.2015.10.116
- Fazilah, N. F., Ariff, A. B., Khayat, M.,. E., Rios-Solis, L., & Halim, M. (2018). Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. Journal of Functional Food, 48, 387–399. https://doi.org/10.1016/j.jff.2018.07.039
- Figueroa-Gonzalez, I., Rodriguez-Serrano, G., Gomez-Ruiz, L., Garcia-Garibay, M., & Cruz-Guerrero, A. (2019). Prebiotic effect of commercial saccharides on probiotic bacteria isolated from commercial products. Food Science and Technology, 39(3), 747–753. https://doi.org/10.1590/fst.07318
- Gandhi, A., & Shah, N. (2014). Cell growth and proteolytic activity of Lactobacillus acidophilus, Lactobacillus helveticus, Lactobacillus delbrueckii ssp. bulgaricus, and Streptococcus thermophiles in milk as affected by supplementation with peptide fractions. International Journal of Food Sciences and Nutrition, 65(8), 937–941. https://doi.org/10.3109/09637486.2014.945154
- Gaspar-Pintiliescu, A., Oancea, A., Cotârleţ, M., Vasile, A. M., Bahrim, E. G., Shaposhnikov, S., Crăciunescu, O., & Oprita, E. I. (2019). Angiotensin-converting enzyme inhibition, antioxidant activity and cytotoxicity of bioactive peptides from fermented bovine colostrum. International Journal of Dairy Technology, 3(1), 108–116. https://doi.org/10.1111/1471-0307.12659
- Hafeez, Z., Cakir-Kiefer, C., Roux, E., Perrin, C., Miclo, L., & Dary-Mourot, A. (2014). Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Research International, 63(Part A), 71–80. https://doi.org/10.1016/j.foodres.2014.06.002
- Kim, D. H., Jeong, D., Kim, H., Kang, I. B., Chon, J. W., Song, K. Y., & (2016). Antimicrobial activity of kefir against various food pathogens and spoilage bacteria. Korean Journal for Food Science of Animal Resources, 36(6), 787–790. https://doi.org/10.5851/kosfa.2016.36.6.787
- Korhonen, H., & Pihlanto, A. (2007). Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Current Pharmaceutical Design, 13(8), 829–843. https://doi.org/10.2174/138161207780363112
- Korhonen, H. J. (2012). Production and properties of health-promoting proteins and peptides from bovine colostrum and milk. Cellular and Molecular Biology, 58(1), 26–38. doi:10.1170/T915.
- Mohanty, D. P., Mohapatra, S., Misra, S., & Sahu, P. S. (2016). Milk derived bioactive peptides and their impact on human health – A review. Saudi Journal of Biological Sciences, 23(5), 577–583. https://doi.org/10.1016/j.sjbs.2015.06.005
- Muhialdin, B. J., Hassan, Z., & Sadon, S. K. (2011). Biopreservation of food by lactic acid bacteria against spoilage fungi. Annals of Food Science and Technology, 12(1), 44–57. http://www.afst.valahia.ro/issues/14-annals-food-science-and-technology-2011
- Nacheva, I., Doneva, M., Petya Metodieva, P., & Loginovska, K. (2017). Tracing some quality and biochemical parameters of kefir from goat milk during storage. Journal of Mountain Agriculture on the Balkans, 20(1), 1–9. http://rimsa.eu/index.php/journal
- Niero, G., Curro, S., Costa, A., Penasa, M., Cassandro, M., Boselli, C., Giangolini, G., & De Marchi, M. (2018). Short communication: Phenotypic characterization of total antioxidant activity of buffalo, goat, and sheep milk. Journal of Dairy Science, 101(6), 4864–4868. https://doi.org/10.3168/jds.2017-13792
- Ozcan, T., Sahin, S., Akpinar-Bayizit, A., & Yilmaz-Ersan, L. (2018). Assessment of antioxidant capacity by method comparison and aminoacid characterization in buffalo milk kefir. International Journal of Dairy Technology, 72(1), 67–73. https://doi.org/10.1111/1471-0307.12560
- Papadimitriou, C. G., Vafopoulou-Mastrojiannaki, A., Silva, S. V., Gomes, A. M., Malcata, F. X., & Alichanidis, E. (2007). Identification of peptides in traditional and probiotic sheep milk yoghurt with angiotensin I-converting enzyme (ACE)-inhibitory activity. Food Chemistry, 105(2), 647–656. https://doi.org/10.1016/j.foodchem.2007.04.028
- Parra, K., Ferrer, M., Pinero, M., Barboza, Y., & Medina, L. M. (2013). Use of Lactobacillus acidophilus and Lactobacillus casei for a potential probiotic legume-based fermented product using Pigeon Pea (Cajanus cajan). Journal of Food Protection, 76(2), 265–271. https://doi.org/10.4315/0362-028X.JFP-12-138
- Playford, R. J., Macdonald, C. E., & Johnson, W. (2000). Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. The American Journal of Clinical Nutrition, 72(1), 5–14. https://doi.org/10.1093/ajcn/72.1.5
- Rahimi, M., Ghaffari, S. M., Salami, M., Mousavy, S. J., Niasari-Naslaji, A., Jabanbani, R., Yousefinejad, S., Khalesi, M., & Moosavi-Movahedi, A. A. (2016). ACE-inhibitory and radical scavenging activities of bioactive peptides obtained from camel milk casein hydrolysis with proteinase K. Dairy Science and Technology, 96(4), 489–499. https://doi.org/10.1007/s13594-016-0283-4
- Seo, M. K., Park, E. J., Ko, S. Y., Choi, E. W., & Kim, S. (2018). Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. Journal of Dairy Science, 101(10), 8662–8671. https://doi.org/10.3168/jds.2018-15014
- Shi, X., Chen, H., Li, Y., Huang, J., & He, Y. (2018). Effects of kefir grains on fermentation and bioactivity of goat milk. Acta Universitatis Cibiniensis. Series E: FOOD TECHNOLOGY, XXII, 22(1), 43–50. https://content.sciendo.com/view/journals/aucft/22/1/aucft.22.issue-1.xml
- Shori, A. B., & Baba, A. S. (2015). Fermented milk derives bioactive peptides with antihypertensive effects. Integrative Food, Nutrition and Metabolism, 2(3), 180–183. doi:10.15761/IFNM.1000126
- Suriasih, K., Aryanta, W. A., Mahardika, G. M., & Astawa, N. M. (2012). Microbiological and chemical properties of kefir made of Bali cattle milk. Food Science and Quality Management, 6, 12–22. https://www.iiste.org/Journals/index.php/FSQM/article/view/2485/2507
- Teijeiro, M., Pérez, P. F., De Antoni, G. L., & Golowczyc, M. A. (2018). Suitability of kefir powder production using spray drying. Food Research International, 112, 169–174. https://doi.org/10.1016/j.foodres.2018.06.023
- Vasile, A., Carcionivoschi, N., & Bahrim, G. (2016). The prebiotic and protective effects of buckwheat flour and oat bran on Lactobacillus acidophilus. The Annals of the University Dunarea de Jos of Galati, Fascicle VI – Food Technology, 40, 40–50.
- Vasiljevic, T., Kealy, T., & Mishra, V. K. (2007). Effects of β-glucan addition to a probiotic containing yogurt. Journal of Food Science, 72(7), 405–411. https://doi.org/10.1111/j.1750-3841.2007.00454.x
- Windayani, N., Turniati, T., & Listiawati, M. (2019). Psychochemical and organoleptic characteristics of colostrum kefir as antibacterial. Journal of Physics: Conf. Series, 1175, 1–6 https://doi.org/10.1088/1742-6596/1175/1/012016
- Yilmaz-Ersan, L., Ozcan, T., Akpinar-Bayizit, A., & Sahin, S. (2018). Comparison of antioxidant capacity of cow and ewe milk kefirs. Journal of Dairy Science, 101(5), 1–11. https://doi.org/10.3168/jds.2017-13871
- Yu, Y. J., Amorim, M., Marques, C., Calhau, C., & Pintado, M. (2016). Effects of whey peptide extract on the growth of probiotics and gut microbiota. Journal of Functional Foods, 21, 507–516. https://doi.org/10.1016/j.jff.2015.10.035
- Yuan, C., Du, L., Jin, Z., & Xu, X. (2013). Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydrate Polymers, 91(1), 385–389. https://doi.org/10.1016/j.carbpol.2012.08.059
