 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Edible insects have been proposed as a good source of different nutrients including protein. However, the nutritional value of edible insects could be affected by several factors that must be considered in order to enhance their potential application in food. In this work, the effect of feeding two different diets, alfalfa and maize green fodder, on the chemical composition of edible grasshopper (Sphenarium purpurascens) consumed in Mexico was assessed. The dry matter, crude protein content, amino acid profile, in vitro protein digestibility, crude fat, and insoluble fiber content differed significantly between grasshoppers fed with alfalfa and maize (p-value < 0.05). Grasshoppers fed with alfalfa showed an increment of 10% in essential amino acid index and biological value compared to grasshopper fed with maize green fodder. Our results demonstrate that the nutritional composition of edible grasshopper S. purpurascens can be modified through diet resulting in an increase in its nutritional value.
RESUMEN
Los insectos comestibles son considerados una gran fuente de nutrientes. Sin embargo, la composición nutricional de los insectos comestibles puede verse afectada por diferentes factores que deben ser considerados para aumentar su consumo. En este trabajo, se revisó el efecto de dos diferentes dietas, alfalfa y forraje de maíz, en la composición nutricional del chapulín comestible (Sphenarium purpurascens) consumido en México. Se obtuvieron valores significativamente diferentes (valor p < 0.05) en el contenido de proteína, el perfil de aminoácidos, la digestibilidad de proteína in vitro, y en el contenido de grasa y de fibra insoluble. El chapulín alimentado con alfalfa mostró un incremento del 10% en el índice de aminoácidos esenciales y el valor biológico comparado con el chapulín alimentado con maíz. Estos resultados indican que la composición nutricional de chapulín S. purpurascens puede ser modificada a través de la dieta para obtener un mayor valor nutricional en este alimento.
PALABRAS CLAVE:
1. Introduction
The interest in edible insects in the last few decades has rapidly increased. They are considered as promising candidates for food production due to their highly efficient metabolism, short generation times, generation of lower amounts of greenhouse gases, low water consumption and as a healthy source of food (Wegier et al., Citation2018). In different regions of the world including Africa, Latin America, and Asia, insects have been consumed for centuries (Bukkens, Citation1997). In Mexico, the edible grasshoppers (Shenarium purpurascens, Pyrgomorphidae family, Orthoptera order) have been consumed traditionally as condiment, snack, or main gourmet dish. Grasshoppers were considered as damaging pests for diverse crops, farmers either collect them for sale as food or as fried snacks (Cerritos & Cano-Santana, Citation2008). Typically, the grasshoppers are collected during the rainy season in early winter mainly from fields planted with beans, maize, and alfalfa.
Although insects seem to be one of the most attractive options for human consumption, the acceptance of the insects as food is influenced by several factors such as sensory properties, social environment, personal beliefs, contamination risks, amongst others (Menozzi et al., Citation2017; Zielińska et al., Citation2015). Different studies have reported analysis of macronutrients, amino acid profile, protein content, lipids, vitamins, and techno-functional properties of various insects (González et al., Citation2019; Ramos-Elorduy et al., Citation2012; Schmidt et al., Citation2019; Soares de Castro et al., Citation2018; Torruco-Uco et al., Citation2019; Zielińska et al., Citation2018). In this context, different biomolecules contained in edible insects have been studied to demonstrate the health benefits of insect consumption or its specific use. Specifically, several authors have reported protein content in a range from 43.9% to 77.1% for different species of grasshoppers and crickets (Ramos-Elorduy et al., Citation2012; Rutaro et al., Citation2018; Rutaro et al., Citation2018; Rutaro et al., Citation2018; Ssepuuya et al., Citation2016; Torruco-Uco et al., Citation2019; Zielińska et al., Citation2015). These investigations affirm that these insects have potential as food due to their high content of protein, and in some cases, edible insects satisfy the level of essential amino acids recommended by FAO (Nowak et al., Citation2016; Zielińska et al., Citation2015). For this reason, insects can be used as a source of protein. In the case of fiber content, Zielińska et al. (Citation2015) reported low fiber content in edible insects, a higher percentage of 3.65% found in Grillodes sigillatus and the lowest (1.97%) found in Tenebrio molitor. Chitin is the most common form of insoluble fiber in insects contained mainly in the exoskeleton. It is considered indigestible fiber composed of β-1,4-linked 2-acetamido-2-deoxy-d-glucose, it is the second most abundant polymer in nature and it is present in a range from 11.6 to 137.2 mg per kg of dry matter (Finke, Citation2007). Chitin has proven to be beneficial for human health because it is a good source of insoluble fiber with potential prebiotic properties although the prebiotic mechanism in the intestine is not as yet well understood (Stull et al., Citation2018). On the other hand, some studies of larvae of T. molitor have shown that they exhibit different polyunsaturated fatty acid composition, which is beneficial to human health (Paul et al., Citation2017; Raksakantong et al., Citation2010). Therefore, several biomolecules contained in insects are of interest to researchers for different applications.
It has been shown that the chemical composition of insects can be modified by diverse factors including sex, environmental factors (temperature, day length, humidity, light intensity), stage of life, and diet (Haber et al., Citation2019; Kulma et al., Citation2019; Lehtovaara et al., Citation2017; Rutaro et al., Citation2018). Therefore, each potential application or use of an edible insect must be analyzed considering a specific context. In this context, it is commonly known in the Central and South of Mexico that there is a difference in the taste between grasshoppers hatched from alfalfa fields and those from maize fields, grasshoppers from alfalfa field have a sweetener taste while those from maize fields have a slightly bitter taste (Cohen et al., Citation2009). This change in taste could be related to changes in their composition affected by diet. However, the effect of feeding alfalfa and maize on the chemical composition and nutritional value of edible grasshoppers has not been explored. Therefore, the objective of this study was to elucidate whether there is a difference in the nutritional composition of grasshoppers fed with two different crops, alfalfa and maize green fodder. Grasshopper samples were analyzed for their proximal composition, in vitro protein digestibility, chitin content, amino acid profile, and mineral composition.
2. Materials and methods
We collected individual grasshoppers (Sphenarium purpurascens) hatched at the same season from two different fields, alfalfa and maize in Acajete, Puebla (parallels 19° 00ʹ 30” and 19° 11ʹ 06” of north latitude and meridians 97° 53ʹ 54” and 98° 00ʹ 00” of western longitude). Around 250 g of grasshoppers per each sample were processed. The average size of the grasshopper was 2.4 cm. Both batches were collected early hours of the same day and processed 2 h later. The samples were frozen at −80°C and freeze-dried for further use.
2.1. Chemical composition
Chemical analyses were performed using AOAC (Citation2000) methods. Moisture, crude protein, crude fat, and ash were obtained according to the approved AOAC methods 925.09, 920.87, 945.38 F, and 923.03, respectively. Dietary fibers (soluble and insoluble) were performed using the commercial kits of Megazyme (AOAC method 996.11, AACC method 76–31.01, and AOAC method 2002.02, respectively). Chitin was estimated following the method of Black and Schwartz (Citation1950) with few modifications reported by González et al. (Citation2019). Briefly, samples (500 mg) were treated with 10 mL of 1 M HCl at 85°C for 50 min under constant stirring. Then, the samples were centrifuged at 3000 x g for 5 min and washed with distilled water to remove the excess of HCl. Sediment from the previous step was suspended in 10 mL of 1 M NaOH and kept at 90°C for 35 min under constant stirring to remove proteins completely. The mixture was vacuum filtrated in a Buchner funnel with filter paper (pore size 20–25 μm), washed three times with deionized water to remove the excess of NaOH, and dried at 100°C overnight. The residue obtained was designated as a purified insect chitin in the form of a very light brown powder, whose mass was estimated by gravimetry.
2.2. Mineral determination
Mineral composition of samples was done in two steps: (1) acid digestion of samples and (2) inductively coupled plasma mass spectrometry (ICP/MS) analysis. For the acid microwave-assisted digestion of insects, 0.5 g sample was mixed with 10 mL of HNO3 77% (v/v). The resulting mixture was placed in a closed microwave system (Mars 5 CEM, Matthews, NC, USA) executing the following the program sequence: the temperature was increased from room temperature to 180ºC during 15 min; maintained at 180ºC for 10 min and then the temperature was lowered to 50ºC in 20 min. After digestion, the sample volume was adjusted to 20 mL with double-deionized water and stored at 4ºC until analysis. The mineral content from acid digestion was measured using a Xseries 2 inductively coupled to a plasma mass spectrometer (ICP/MS) (Thermo Scientific, Waltham, MA, USA) with a Type C glass concentric nebulizer (Meinhard, Golden, CO, USA). Helium containing 7% hydrogen was used as the reaction gas to prevent possible interferences, and Ho and Tb were used as the internal standards.
2.3. In vitro protein digestibility
In vitro protein digestibility was performed using a multienzymatic technique (Hsu et al., Citation1977), using the following enzymes: (1) pancreatic porcine trypsin type IX-S (T4799, Sigma Aldrich); (2) α-chymotrypsin type II from bovine pancreas (C4129, Sigma Aldrich); and (3) S. griseus protease type XIV (P5147, Sigma Aldrich) which substituted peptidase from porcine intestinal (Hervera et al., Citation2009).
2.4. Amino acid composition
Samples were defatted with hexane (1:4 w/v) in constant agitation at 50ºC (Thermo Scientific SHKE5000, Massachusetts, USA) during 12 h; the solvent was replaced every 6 h. Subsequently, the defatted insect powder was air-dried at 35ºC overnight and stored at −20ºC. Protein extraction was performed based on the method for alkaline protein solubilization followed by acid precipitation (Föste et al., Citation2015) with some modifications (Mishyna et al., Citation2019). The amino acid analysis of insects was evaluated by the acid hydrolysis method (Chavan et al., Citation2001) with some modifications (Mohapatra et al., Citation2019). Samples containing 6 mg of protein were hydrolyzed in a reflux glass-tube by adding 0.7 mL of 6 N HCl (0.1% v/v phenol). The tube was then placed in a heating plate at 100°C for 60 h for hydrolysis completion. Thereafter, the sample was diluted with 17 mL of HPLC-grade water and dried in an evaporator. The remaining powder was reconstituted in 2 mL of 20 mM HCl. Derivatization was conducted by using a Waters AccQ•Tag Ultra Derivatization Kit (cat. 186003836). Briefly, 20 µL of the sample and 60 µL of borate buffer were vortexed for a few seconds. Then, 20 µL of AccQ•Flour reagent was added to the mixture and incubated for 1 min. The final mixture was kept in a heat block at 55°C for 10 min. Amino acid quantification was performed in a Waters UPLC-FL system. The separation of amino acids was carried out in an AccQ•Tag (3.9x150 mm) column (Waters, Ireland, UK) at a temperature of 30°C, and at a flow rate of 1 mL/min. Two microliters of sample were injected and amino acids were detected using a fluorescence detector (λ excitation/emission 250/395 nm). Amino acid elution was performed by using a gradient method created with two different mobile phases: A: 100% AccQ•Tag Ultra eluent A concentrate and B: 60% HPLC-grade acetonitrile. The elution was carried out as follows: 0–0.5 min, from 0% to 2% B; 0.5–6 min, from 4% to 6% B; 6–10 min, from 6% to 10% B; 10–20 min, from 10% to 34% B; 20–22 min, from 34% to 100% B; 22–25 min, from 100% B isocratic elution; 25–26 min, from 100% to 0% B; 26–33 min, from 0% B isocratic elution. The EAAI was obtained considering the following essential amino acids: threonine, methionine, histidine, isoleucine, leucine, valine, and phenylalanine and comparing with whole egg protein as standard (FAO, Citation1970). The essential amino acid index (EAAI) was calculated using the method of Labuda et al. (Citation1982). Biological value (BV) was obtained using EquationEquation (1)(1)
(1) (Oser, Citation1959):
2.5. Statistical analysis
Each experiment was performed in triplicate. Data were reported as mean ± standard deviations. Results were subjected to analysis differences among means by t-student test in Minitab18 (State College, PA, USA).
3. Results and discussion
Two samples of grasshoppers fed with different crops, alfalfa (Medicago sativa) and maize green fodder (Zea mays), were studied. Chemical analyses, chitin quantification, mineral determination, amino acid composition, and in vitro protein digestibility results are presented. Significant differences (p-value < 0.05) among the means of samples were found in the analyses for crude protein, insoluble dietary fiber and fat contents, as well as in in vitro digestibility, and amino acid profile of analyzed proteins.
3.1. Dietary fiber and chitin content
In the proximal composition of grasshoppers fed with alfalfa (ALF) or maize green fodder (MGF) is presented. The content of insoluble dietary fiber (IDF) was 31.1% and 22.2%, for ALF and MGF samples, respectively, and differed significantly (tdf = 9.97, p-value < 0.05). While soluble dietary fiber (SDF) was found to be 0.70% and 0.93% for ALF and MGF samples, respectively, with non-significant difference (tdf = −0.26, p-value > 0.05). IDF mainly refers to cellulose, hemicellulose, and lignin molecules, and in the case of insects, it is thought to include chitin due to its similarity in structure to cellulose (Finke, Citation2007). The observed difference in IDF between insects fed alfalfa and maize could be related to differences in the contents of polysaccharides between these foods. Alfalfa leaves contain 26.5-40% of neutral detergent fiber (NDF) (OECD, Citation2005), whereas maize leaves contain 63.6% of NDF (Heuzé et al., Citation2017). NDF includes cellulose, hemicellulose, and lignin molecules. Different compositions of NDF in alfalfa and maize leaves and their digestibility might affect their content in grasshopper. However, further information on the carbohydrate metabolism of S. purpurascens is needed in order to better understand these differences.
Table 1. Chemical composition, chitin determination, in vitro protein digestibility and protein indicators of grasshopper samples fed with alfalfa (ALF) or maize green fodder (MGF) presented in g/100 g of dry weight.
Tabla 1. Composición química, determinación de quitina, digestibilidad de proteína in vitro e indicadores de calidad proteica de las muestras de chapulín alimentado con alfalfa (ALF) o forraje de maíz (MGF) en g/100 g de peso seco
For this work, chitin content is not influenced by diet of the insect. There were no significant differences in chitin content of grasshoppers fed with alfalfa (24.9%) and MGF (21.5%) (tdf = 1.32, p-value >0.05, ). These values were higher than those reported for several species of grasshoppers (range of 5.3%-14%, for grasshopper Dociosaurus aroccanus) (Erdogan & Kaya, Citation2016; Kaya et al., Citation2015). In this study, chitin, which is the main component of insoluble dietary fiber, represented up to 95% of the IDF of grasshopper samples fed with MGF and 75% of those fed with ALF (). The high content of chitin in this grasshopper suggests that it can be an alternative chitin source for several applications (Erdogan & Kaya, Citation2016). Considering total dietary fiber (TDF), there was a significant difference of 10.4% in the IDF content, this difference might correspond to other molecules of IDF different from chitin.
3.2. Crude fat and ash content
Results of fat content presented a significant difference (tdf = 6.48, p-value <0.05), 14.86% for ALF sample and 10.37% for MGF sample (). This range of values are in concordance with those reported by several authors, Ramos-Elorduy et al. (Citation2012) found a similar value (10.8%) for S. purpurascens, while Melo-Ruiz et al. (Citation2015) reported 6.02%, and Torruco-Uco et al. (Citation2019) reported 8.98% for the same grasshopper. According to these results, there was a significant difference in fat content between grasshoppers fed different diet. In the case of fat content, maize leaves and alfalfa correspond to 2% and 2.4–3.8% of dry matter, respectively (Goossen et al., Citation2018; Heuzé et al., Citation2017; Yari et al., Citation2017). Besides, 78% of fatty acids found in alfalfa are unsaturated, and these include 58% of α-linolenic acid and 17.5% of linoleic acid. Unsaturated fatty acids play an important role in human health. For example, α-linolenic acid can reduce inflammation during cardiovascular problems, prevent blood clotting, and decrease triglyceride levels (Paul et al., Citation2016). Therefore, a higher content in alfalfa could result in higher accumulation of fatty acids in grasshoppers.
It has been reported grasshoppers have a desirable fat composition when used as food source due to the high level of polyunsaturated fatty acids (PUFAs) (Paul et al., Citation2016; Torruco-Uco et al., Citation2019). PUFA concentration of 69.3% for S. purpurascens has been found, depicting a higher concentration compared to 30.6% saturated fatty acids (Torruco-Uco et al., Citation2019). Although the lipid content is relatively low in S. purpurascens when compared with other species (e.g., R. differens) or food sources, the ability to provide a rich content of unsaturated fatty acids (67-75%) over saturated fatty acids (29-31%) (Hyun et al., Citation2012; Torruco-Uco et al., Citation2019) makes it a possible source of high-quality oil. Furthermore, a study on the influence of diet on fatty acid content in grasshopper in East Africa concluded that the fatty acid composition in R. differens can be influenced through diet (Rutaro et al., Citation2018). Hence, further studies are needed to determine the influence of diet on fat profile composition in S. purpurascens.
Regarding ash content, values of 4.05% in alfalfa samples and 4.08% in MGF samples were found, with a non-significant difference (tdf = −0.62, p-value >0.05). These values are in concordance with those previously reported for S.purpurascens by other authors ranging from 1.42% to 4.87% (Ramos-Elorduy et al., Citation2012; Torruco-Uco et al., Citation2019).
3.3. Mineral composition
In the results of mineral content are presented, and nonsignificant differences (p-value >0.05) between samples were found. The minerals with the higher content in both samples were K, Ca, and Mg, with values around 1018, 218, and 127 mg/100 g of sample, respectively. Minerals like Na, Zn, and Fe presented values of 36, 17, and 15 mg/100 g of sample, respectively, for both samples. According to these results, the mineral content was not influenced by diet. Both samples of grasshoppers presented high levels of nutritional valued minerals such as Ca (higher than 125 mg/100 g found in milk), Se (similar to 0.038 mg/100 g found in beef bottom round steak), and Fe (higher than 5.8 mg/100 g found in beef liver), representing an alternative source of these minerals (ODS, Citation2020).
Table 2. Mineral composition of grasshopper fed with alfalfa (ALF) or maize green fodder (MGF) presented in mg/100 g db.
Table 2. Composición mineral de chapulín alimentado con alfalfa (ALF) o forraje de maíz (MGF) en mg/100 g de peso seco
3.4. Protein analysis
The crude protein content of the MGF sample presented 63.9% (w/w) dry basis, which is significantly higher value than that of the ALF sample, which presented 60.0% (w/w) (tdf = −3.68, p-value <0.05). These values are in concordance with those reported in the literature for a mixture of Acrididae ranging from 43.93% to 77.13% that includes locusts, grasshopper, and crickets (Ramos-Elorduy et al., Citation2012). Similarly, values of 53.57%, 65.2%, and 75.87% of protein content were reported for Sphenarium purpuracens (Melo-Ruiz et al., Citation2015; Ramos-Elorduy et al., Citation2012; Torruco-Uco et al., Citation2019). A higher content of protein in grasshopper was found as compared to the protein content of other foods such as soybean (41.3%), lentil (27.2%), beans (24.8%), and in the range of protein content of beef (45.4%), chicken (58.8%) and eggs (47.7%) (FAO, Citation1970).
Intrinsic factors such as environment, origin, and stage of the life cycle, as well as feeding of the insects, might affect the insect protein content. These, along with extrinsic factors such as the method used to determine protein content can affect the estimation of protein. In the case of insects, an important element in the composition that contains nitrogen is chitin, which is part of the insoluble fiber present in the exoskeleton of the insect. Therefore, chitin content must be considered in the determination of protein content in order to not overestimate this value since Kjeldahl method, which determines the total nitrogen content in the sample, was used to obtain total protein. The results presented in corresponding to crude protein were corrected considering the presence of chitin nitrogen (factor of conversion of 5.6).
Regarding the significant difference of 3.9% obtained between ALF and MGF samples, it is important to consider the different diet of grasshoppers. MGF is mainly composed of stalks, leaves, and ears of the maize plant. Even when the values of protein (between 15.3% and 25.8%) in alfalfa (OECD, Citation2005) are higher than that (11.4%) for the leaves of MGF (Heuzé et al., Citation2017) grasshoppers fed with MGF presented a higher content of crude protein. The results of this study show that the diet of the insect has an impact on the content of crude protein of the insect as initially hypothesized. Results of amino acid profile and in vitro digestibility are further presented in order to extend this discussion.
3.5. In vitro protein digestibility
The in vitro protein digestibility is presented in and resulted in 90.0% and 87.9% for ALF and MGF samples, respectively. There was a significant difference (tdf = 10.29, p-value < 0.05) between the samples. These values were higher than those reported for grasshoppers S. purpurascens and R. differens (85.4% and 85.67%, respectively) (Aragón-García et al., Citation2018; Kinyuru et al., Citation2010). In vitro protein digestibility of meat and casein was found to be 89.65% and 95.69%, respectively (Queiroz Mendes et al., Citation2016), whereas soybean and chickpea flours depicted digestibility values of 59.4% and 62.3%, respectively (Avilés-Gaxiola et al., Citation2018). In this case, ALF and MGF samples presented an in vitro protein digestibility similar to meat.
It has been reported that chitin is the main factor affecting the in vitro protein digestibility since it is not absorbed in the small intestine and interferes with net protein utilization (Longvah et al., Citation2011; Marono et al., Citation2015). However, in this case, a non-significant difference was found in chitin content (), but a slight significant difference was obtained in in vitro protein digestibility. This result might be related to different structural components of the insect.
3.6. Amino acid profile
The amino acid profile of analyzed proteins is presented in , grasshopper fed with ALF and MGF in comparison to the amino acid profile of whole hen egg (FAO, Citation1970) is shown. Considering essential amino acids (EAA), threonine, with a ratio of 1.12 and 1.0 (mg of amino acid found in the sample divided by mg of amino acid in the reference) compared with the same content of amino acid in egg, was found in a higher level in ALF and MGF samples, respectively. While phenylalanine (ratio of 0.96) in the ALF sample was the second EAA found with similar levels than the reference (see ). In the same way, the limiting EAA is histidine with a ratio of 0.53 and 0.35, respectively, in ALF and MGF samples. The rest of the essential amino acids were found in a ratio range between 0.65 and 0.75.
Figure 1. Amino acid profile of proteins analyzed from grasshopper fed with alfalfa or maize green fodder (MGF). The reference refers to the whole hen egg (FAO, Citation1970). * Significant difference (p-value < 0.05).
Figura 1. Perfil de aminoácidos de las proteínas analizadas de chapulín alimentado con alfalfa o forraje de maíz. Se utilizó la información del huevo de gallina como referencia (FAO, Citation1970). *Differencia significativa (valor p < 0.05)
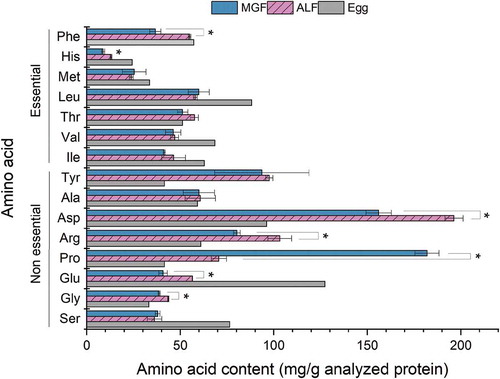
Non-essential amino acids (NAA) found in a higher level in the samples compared to their content in the whole egg were tyrosine, aspartate, glycine, proline, alanine, and arginine (with ratios ranging between 1.0 and 2.34) in ALF sample. In the case of MGF sample, the same amino acids were found in a higher level (with ratio in a range between 1.0 and 2.24), except for proline that presented the highest ratio of 4.37. The NAA with lower content compared with the reference is glutamate and serine in both samples (ratio between 0.32 and 0.49). The amino acid profile compared with the reference presented some differences mainly in NAA contents.
The total content of EAA is 300.86, and 269.50 mg/g of analyzed protein (see ), in ALF and MGF samples, respectively, where the grasshoppers fed with ALF resulted with a non-significant higher content of EAA compared to those fed with MGF (p-value >0.05). On the other hand, the sum of NAA is 664.81 and 689.27 mg/g of analyzed protein, in ALF and MGF samples, respectively, with a non-significant difference (p-value >0.05). However, the sum of EAA and NAA resulted in non-significant differences, significant differences were found in the contents of amino acids individually.
In , amino acids with significant differences (p-value <0.05) can be observed. Seven amino acids of a total of 15 resulted in a significant difference between the samples. Histidine (tdf = 6.19, p-value <0.05) and phenylalanine (tdf = 8.56, p-value <0.05) are EAA, and the rest are NAA. Phenylalanine was found with a value of 55.02 and 36.64 mg/g of analyzed protein in ALF and MGF samples; and histidine with values of 12.99 and 8.71 mg/g of analyzed protein in ALF and MGF samples. Meanwhile, aspartate (tdf = 6.83, p-value <0.05) was found to be the most abundant NAA with 196.3 and 156.09 mg/g of analyzed protein in ALF and MGF samples, respectively. Second, a high content of proline (tdf = −20.8, p-value <0.05) with values of 70.66 and 181.94 mg/g of analyzed protein in ALF and MGF samples was also observed. Another amino acid that presented a high content was arginine (tdf = 4.94, p-value <0.05) with a value of 103.2 and 80.24 mg/g of analyzed protein in ALF and MGF samples. Then, glutamate (tdf = 9.48, p-value <0.05) with values of 56.52 and 40.81 mg/g of analyzed protein for ALF and MGF samples and glycine (tdf = 10.4, p-value <0.05) with a value of 43.62 and 38.73 mg/g of analyzed protein in ALF and MGF samples were found with significant differences.
These differences can be attributed to the diet of the grasshopper. In the case of alfalfa leaves, amino acids such as phenylalanine (up to 79.5 vs. 59 mg/g of protein), arginine (up to 77 vs. 61 mg/g of protein), glycine (up to 73.5 vs. 55 mg/g of protein) and histidine (up to 37 vs. 23 mg/g of protein) are contained in higher levels than reported in maize leaves (Heuzé et al., Citation2017; OECD, Citation2005). Information for the rest of amino acids, aspartate, proline, and glutamate in maize leaves was not reported. According to these differences in the profile of amino acids which resulted with higher values in the ALF sample, it is possible to see an effect of the diet of the insect in its nutritional value.
The essential amino acid index (EAAI) values were calculated (see ): 75.19% and 65.44% for ALF and MGF samples, and resulted in a non-significant difference between the samples (tdf = 3.08, p-value >0.05) with a p-value of 0.091. The value of the ALF sample was similar to those previously reported (72-77%) for samples of house cricket Acheta domestica (Kulma et al., Citation2019). In general, values of EAAI between 70% and 90% are considered as a useful source of protein. Additionally, the biological value (BV) of the ALF sample is 70.75% and 60.12% in the MFG sample. A good quality of the product is in the range of 70-100%. Then, the grasshopper obtained from alfalfa fields could be considered as a useful protein intake unlike the MGF grasshopper.
4. Conclusion
The results in this study confirm that there is an effect of diet on the chemical composition and nutritional value of grasshopper. Grasshoppers fed alfalfa presented a higher nutritional value than those fed maize. The diet of grasshoppers could be controlled to change their chemical composition towards designing insect-based food with increased nutritional value as alternative food. It is noteworthy to mention that this is the first attempt in evaluating whether or not diet affects the nutritional composition of grasshopper Sphenarium purpurascens. More detailed studies of the diet effect on nutritional components of edible insects must be performed.
Acknowledgments
The authors would like to thank the Nutriomics Focus Group of Tecnologico de Monterrey at the FEMSA-Biotechnology Center and the National Council on Science and Technology of Mexico (CONACyT).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- AOAC. (2000). Official methods of analysis.
- Aragón-García, A., Rodríguez-Lima, D. R., Pino-Moreno, J. M., Aragón-Sánchez, M., Carlos-Ángeles, S., & García-Pérez, A. (2018). Valor nutritivo de la harina del chapulín Sphenarium purpurascens Charpentier, 1845 (Orthoptera: Pyrgomorphidae) tostado y natural. Entomología Mexicana, 5(1), 106–112. http://www.entomologia.socmexent.org/revista/2018/BHN/BHN106-112.pdf
- Avilés-Gaxiola, S., Chuck-Hernández, C., Rocha-Pizaña, M., del, R., García-Lara, S., López-Castillo, L. M., & Serna-Saldívar, S. O. (2018). Effect of thermal processing and reducing agents on trypsin inhibitor activity and functional properties of soybean and chickpea protein concentrates. LWT - Food Science and Technology, 98(August), 629–634. https://doi.org/10.1016/j.lwt.2018.09.023
- Black, M. M., & Schwartz, H. M. (1950). The estimation of chitin and chitin nitrogen in crawfish waste and derived products. Analyst, 75(889), 185–189. https://doi.org/10.1039/AN9507500185
- Bukkens, S. G. F. (1997). The nutritional value of edible insects. Ecology of Food and Nutrition, 36(2–4), 287–319. https://doi.org/10.1080/03670244.1997.9991521
- Cerritos, R., & Cano-Santana, Z. (2008). Harvesting grasshoppers Sphenarium purpurascens in Mexico for human consumption: A comparison with insecticidal control for managing pest outbreaks. Crop Protection, 27(3–5), 473–480. https://doi.org/10.1016/j.cropro.2007.08.001
- Chavan, U. D., McKenzie, D. B., & Shahidi, F. (2001). Protein classification of beach pea (Lathyrus maritimus L.). Food Chemistry, 75(2), 145–153. https://doi.org/10.1016/S0308-8146(01)00122-4
- Cohen, J. H., Mata Sánchez, N. D., & Montiel-Ishino, F. (2009). Chapulines and food choices in rural Oaxaca. Gastronomica, 9(1), 61–65. https://doi.org/10.1525/gfc.2009.9.1.61
- Erdogan, S., & Kaya, M. (2016). High similarity in physicochemical properties of chitin and chitosan from nymphs and adults of a grasshopper. International Journal of Biological Macromolecules, 89(8), 118–126. https://doi.org/10.1016/j.ijbiomac.2016.04.059
- FAO. (1970). Amino-acid content of foods and biological data on proteins. Retrieved July 30, 2019 from http://www.fao.org/3/AC854T/AC854T00.htm#TOC
- Finke, M. D. (2007). Estimate of chitin in raw whole insects. Zoo Biology, 26(2), 105–115. https://doi.org/org/doi:10.1002/zoo.20123
- Föste, M., Elgeti, D., Brunner, A., Jekle, M., & Becker, T. (2015). Isolation of quinoa protein by milling fractionation and solvent extraction. Food and Bioproducts Processing, 96(4), 20–26. https://doi.org/10.1016/j.fbp.2015.06.003
- González, C. M., Garzón, R., & Rosell, C. M. (2019). Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innovative Food Science and Emerging Technologies, 51(1), 205–210. https://doi.org/10.1016/j.ifset.2018.03.021
- Goossen, C. P., Bosworth, S. C., Darby, H. M., & Kraft, J. (2018). Microwave pretreatment allows accurate fatty acid analysis of small fresh weight (100 g) dried alfalfa, ryegrass, and winter rye samples. Animal Feed Science and Technology, 239(February), 74–84. https://doi.org/10.1016/j.anifeedsci.2018.02.014
- Haber, M., Mishyna, M., Itzhak Martinez, J. J., & Benjamin, O. (2019). Edible larvae and pupae of honey bee (Apis mellifera): Odor and nutritional characterization as a function of diet. Food Chemistry, 292(23), 197–203. https://doi.org/10.1016/j.foodchem.2019.04.041
- Hervera, M., Baucells, M. D., González, G., Pérez, E., & Castrillo, C. (2009). Prediction of digestible protein content of dry extruded dog foods: Comparison of methods. Journal of Animal Physiology and Animal Nutrition, 93(3), 366–372. https://doi.org/10.1111/j.1439-0396.2008.00870.x
- Heuzé, V., Tran, G., Edouard, N., & Lebas, F. (2017). Feedipedia. Retrieved June 30, 2019 from https://www.feedipedia.org/node/358
- Hsu, H. W., Vavak, D. L., Satterlee, L. D., & Miller, G. A. (1977). A multienzyme technique for estimating protein digestibility. Journal of Food Science, 42(5), 1269–1273. https://doi.org/10.1111/j.1365-2621.1977.tb14476.x
- Hyun, S. H., Kwon, K. H., Park, K. H., Jeong, H. C., Kwon, O., Tindwa, H., & Han, Y. S. (2012). Evaluation of nutritional status of an edible grasshopper. Oxya Chinensis Formosana. Entomological Research, 42(5), 284–290. https://doi.org/10.1111/j.1748-5967.2012.00469.x
- Kaya, M., Erdogan, S., Mol, A., & Baran, T. (2015). Comparison of chitin structures isolated from seven orthoptera species. International Journal of Biological Macromolecules, 72(1), 797–805. https://doi.org/10.1016/j.ijbiomac.2014.09.034
- Kinyuru, J. N., Kenji, G. M., Njoroge, S. M., & Ayieko, M. (2010). Effect of processing methods on the in vitro protein digestibility and vitamin content of edible winged termite (Macrotermes subhylanus) and grasshopper (Ruspolia differens). Food and Bioprocess Technology, 3(5), 778–782. https://doi.org/10.1007/s11947-009-0264-1
- Kulma, M., Kouřimská, L., Plachý, V., Božik, M., Adámková, A., & Vrabec, V. (2019). Effect of sex on the nutritional value of house cricket. Acheta Domestica L. Food Chemistry, 272(3), 267–272. https://doi.org/10.1016/j.foodchem.2018.08.049
- Labuda, J., Kacerovský, O., Kováè, M., & Štìrba, A. (1982). Výživa a krmenie hospodárských zvierat. Príroda. 164s.
- Lehtovaara, V. J., Valtonen, A., Sorjonen, J., Hiltunen, M., Rutaro, K., Malinga, G. M., Nyeko, P., & Roininen, H. (2017). The fatty acid contents of the edible grasshopper Ruspolia differens can be manipulated using artificial diets. Journal of Insects as Food and Feed, 3(4), 253–262. https://doi.org/10.3920/JIFF2017.0018
- Longvah, T., Mangthya, K., & Ramulu, P. (2011). Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chemistry, 128(2), 400–403. https://doi.org/10.1016/j.foodchem.2011.03.041
- Marono, S., Piccolo, G., Loponte, R., Di Meo, C., Attia, Y. A., Nizza, A., & Bovera, F. (2015). In vitro crude protein digestibility of tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Italian Journal of Animal Science, 14(3), 943. https://doi.org/10.4081/ijas.2015.3889
- Melo-Ruiz, V., Sandoval-Trujillo, H., Quirino-Barreda, T., Sánchez-Herrera, K., Díaz-García, R., & Calvo-Carrillo, C. (2015). Chemical composition and amino acids content of five species of edible grasshoppers from Mexico. Emirates Journal of Food and Agriculture, 27(8), 654–658. https://doi.org/10.9755/ejfa.2015.04.093
- Menozzi, D., Sogari, G., Veneziani, M., Simoni, E., & Mora, C. (2017). Eating novel foods: An application of the theory of planned behaviour to predict the consumption of an insect-based product. Food Quality and Preference, 59(5), 27–34. https://doi.org/10.1016/J.FOODQUAL.2017.02.001
- Mishyna, M., Martinez, -J.-J. I., Chen, J., & Benjamin, O. (2019). Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Research International, 116(2), 697–706. https://doi.org/10.1016/J.FOODRES.2018.08.098
- Mohapatra, D., Patel, A. S., Kar, A., Deshpande, S. S., & Tripathi, M. K. (2019). Effect of different processing conditions on proximate composition, anti-oxidants, anti-nutrients and amino acid profile of grain sorghum. Food Chemistry, 271(2), 129–135. https://doi.org/10.1016/j.foodchem.2018.07.196
- Nowak, V., Persijn, D., Rittenschober, D., & Charrondiere, U. R. (2016). Review of food composition data for edible insects. Food Chemistry, 193(4), 39–46. https://doi.org/10.1016/j.foodchem.2014.10.114
- ODS. (2020). Fact sheet. Retrieved February 7, 2020 from National Institutes of Health https://ods.od.nih.gov/factsheets/
- OECD. (2005). Consensus document on compositional considerations for new varieties of alfalfa and other temperate forage legumes: Key feed nutrients, anti-nutrients and secondary plant metabolites (Vol. 13). https://read.oecd-ilibrary.org/economics/series-on-the-safety-of-novel-foods-and-feeds-no-13-consensus-document-on-compositional-considerations-for-new-varieties-of-alfalfa-and-other-temperate-forage-legumes_oecd_papers-v5-art31-en#page5
- Oser, B. L. (1959). An integrated essential amino acid index for predicting the biological value of proteins. In A. A. Albanese, (Ed.), Protein and amino acid nutrition (pp. 281–295). New York: Academic Press. https://doi.org/org/10.1016/B978-0-12-395683-5.50014-6
- Paul, A., Frederich, M., Caparros Megido, R., Alabi, T., Malik, P., Uyttenbroeck, R., Francis, F., Blecker, C., Haubruge, E., Lognay, G., & Danthine, S. (2017). Insect fatty acids: A comparison of lipids from three orthopterans and Tenebrio molitor L. larvae. Journal of Asia-Pacific Entomology, 20(2), 337–340. https://doi.org/10.1016/j.aspen.2017.02.001
- Paul, A., Frederich, M., Uyttenbroeck, R., Malik, P., Filocco, S., Richel, A., Heuskin, S., Alabi, T., Caparros Megido, R., Franck, T., Bindelle, J., Maesen, P., Francis, F., Lognay, G., Blecker, C., Haubruge, E., & Danthine, S. (2016). Nutritional composition and rearing potential of the meadow grasshopper (Chorthippus parallelus Zetterstedt). Journal of Asia-Pacific Entomology, 19(4), 1111–1116. https://doi.org/10.1016/j.aspen.2016.09.012
- Queiroz Mendes, F., De Almeida Oliviera, M. G., Brunoro Costa, N. M., Vieira Pires, C., & Passos, F. R. (2016). Capability of in vitro digestibility methods to predict in vivo digestibility of vegetal and animal proteins. Archivos Latinoamericanos de Nutrición, 66(1), 5–16. https://www.alanrevista.org/ediciones/2016/1/art-1/
- Raksakantong, P., Meeso, N., Kubola, J., & Siriamornpun, S. (2010). Fatty acids and proximate composition of eight Thai edible terricolous insects. Food Research International, 43(1), 350–355. https://doi.org/10.1016/j.foodres.2009.10.014
- Ramos-Elorduy, B. J., Pino Moreno, J. M., & Martínez Camacho, V. H. (2012). Could grasshoppers be a nutritive meal? Food and Nutrition Sciences, 3(2), 164–175. https://doi.org/10.4236/fns.2012.32025
- Rutaro, K., Malinga, G. M., Lehtovaara, V. J., Opoke, R., Nyeko, P., Roininen, H., & Valtonen, A. (2018). Fatty acid content and composition in edible Ruspolia differens feeding on mixtures of natural food plants. BMC Research Notes, 11(1), 1–6. https://doi.org/10.1186/s13104-018-3792-9
- Rutaro, K., Malinga, G. M., Lehtovaara, V. J., Opoke, R., Valtonen, A., Kwetegyeka, J., Nyeko, P., & Roininen, H. (2018). The fatty acid composition of edible grasshopper Ruspolia differens (Serville) (Orthoptera: Tettigoniidae) feeding on diversifying diets of host plants. Entomological Research, 48(6), 490–498. https://doi.org/10.1111/1748-5967.12322
- Rutaro, K., Malinga, G. M., Opoke, R., Lehtovaara, V. J., Omujal, F., Nyeko, P., Roininen, H., & Valtonen, A. (2018). Artificial diets determine fatty acid composition in edible Ruspolia differens (Orthoptera: Tettigoniidae). Journal of Asia-Pacific Entomology, 21(4), 1342–1349. https://doi.org/org/10.1016/j.aspen.2018.10.011
- Schmidt, A., Call, L. M., Macheiner, L., & Mayer, H. K. (2019). Determination of vitamin B 12 in four edible insect species by immunoaffinity and ultra-high performance liquid chromatography. Food Chemistry, 281(2), 124–129. https://doi.org/10.1016/j.foodchem.2018.12.039
- Soares de Castro, R. J., Ohara, A., Gonçalves Dos Santos Aguilar, J., & Fontenele Domingues, M. A. (2018). Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends in Food Science and Technology, 76(April), 82–89. https://doi.org/10.1016/j.tifs.2018.04.006
- Ssepuuya, G., Mukisa, I. M., & Nakimbugwe, D. (2016). Nutritional composition, quality, and shelf stability of processed Ruspolia nitidula (edible grasshoppers). Food Science and Nutrition, 5(1), 103–112. https://doi.org/10.1002/fsn3.369
- Stull, V. J., Finer, E., Bergmans, R. S., Febvre, H. P., Longhurst, C., Manter, D. K., Patz, J. A., & Weir, T. L. (2018). Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Scientific Reports, 8(1), 10762. https://doi.org/10.1038/s41598-018-29032-2
- Torruco-Uco, J. G., Hernández-Santos, B., Herman-Lara, E., Martínez-Sánchez, C. E., Juárez-Barrientos, J. M., & Rodríguez-Miranda, J. (2019). Chemical, functional and thermal characterization, and fatty acid profile of the edible grasshopper (Sphenarium purpurascens Ch.). European Food Research and Technology, 245(2), 285–292. https://doi.org/10.1007/s00217-018-3160-y
- Wegier, A., Alavez, J., Pérez-López, L., Calzada, L., & Cerritos, R. (2018). Beef or grasshopper hamburgers: The ecological implications of choosing one over the other. Basic and Applied Ecology, 26(1), 89–100. https://doi.org/10.1016/j.baae.2017.09.004
- Yari, M., Valizadeh, R., Ali Nnaserian, A., Jonker, A., & Yu, P. (2017). Carbohydrate and lipid spectroscopic molecular structures of different alfalfa hay and their relationship with nutrient availability in ruminants. Asian-Australasian Journal of Animal Sciences, 30(11), 1575–1589. https://doi.org/10.5713/ajas.16.0756
- Zielińska, E., Baraniak, B., Karaś, M., Rybczyńska, K., & Jakubczyk, A. (2015). Selected species of edible insects as a source of nutrient composition. Food Research International, 77(16), 460–466. https://doi.org/10.1016/j.foodres.2015.09.008
- Zielińska, E., Karaś, M., & Baraniak, B. (2018). Comparison of functional properties of edible insects and protein preparations thereof. LWT - Food Science and Technology, 91(5), 168–174. https://doi.org/10.1016/j.lwt.2018.01.058
