 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Umami peptides were derived from enzymatic hydrolysis of sunflower seed protein, followed by isolation and identification. The flavourzyme-enzymatic hydrolysates exhibited the highest umami intensity value of 10.72 ± 0.05 and the lowest bitterness, when pH was adjusted to 7.0, the flavorzyme was added at enzyme-to-substrate (E/S) ratio of 1:25 (w/w), then incubated in a temperature-controlled water bath at 55°C, and the hydrolysis time was 480 min. Ultrafiltration, gel filtration chromatography, and reversed-phase high-performance liquid chromatography (RP-HPLC) were used to isolate the umami peptides of sunflower seed. Five fractions were obtained after ultra-filtration, fraction with molecular weight 1-3 kDa showed highest umami intensity value 13.93 ± 0.06. Fraction F1, which was purified via sephadex gel filtration chromatography, exhibited high umami value (12.21 ± 0.06), and low bitterness (4.57 ± 0.06). Seven sub-fractions were purified by RP-HPLC, and the umami taste value of F1-4 was the highest (14.16 ± 0.05), which was 1.32 times stronger than that of sunflower seed protein hydrolysates without any purification. Furthermore, the structure of fraction F1-4 was characterized by mass spectrometry, and three peptide fractions were identified, with amino acid sequences of DVNNPANQLD, NNENQLDEYQR, and EFEGGSIEH, respectively.
RESUMEN
En este estudio se derivaron péptidos umami de la hidrólisis enzimática de la proteína de la semilla de girasol, realizándose posteriormente su aislamiento e identificación. Cuando el pH se ajustó a 7.0, los hidrolizados enzimáticos flavourzyme produjeron el valor más alto de intensidad umami (10.72 ± 0.05) y el amargor más bajo. La enzima flavourzyme fue añadida en una relación enzima-sustrato (E/S) de 1:25 (p/p); luego se incubó en un baño de agua a temperatura controlada de 55°C, constatándose que el tiempo de hidrólisis fue de 480 minutos. Para aislar los péptidos umami de la semilla de girasol se utilizó ultrafiltración, cromatografía de filtración en gel y cromatografía líquida de alto rendimiento en fase inversa (RP-HPLC). Una vez aplicada la ultrafiltración se obtuvieron cinco fracciones. La fracción con peso molecular 1-3 kDa mostró el valor más alto de intensidad umami (13.93±0.06). La fracción F1, que se purificó mediante cromatografía de filtración en gel sephadex, registró un alto valor umami (12.21±0.06) y amargor bajo (4.57±0.06). Mediante RP-HPLC se purificaron siete subfracciones, evidenciándose que el valor más alto de sabor umami fue el de F1-4 (14.16±0.05), 1.32 veces más fuerte que el de los hidrolizados de proteína de semillas de girasol sin ninguna purificación. Además, empleando espectrometría de masas se caracterizó la estructura de la fracción F1-4, identificándose tres fracciones de péptidos con secuencias de aminoácidos DVNNPANQLD, NNENQLDEYQR y EFEGGSIEH, respectivamente.
1. Introduction
Umami can enhance the continuity, harmony, and mellow taste of food which make one have a joyful emotion. Nowadays, researchers found that besides amino acids, nucleotides and organic acids, plant protein hydrolysates also contain a large number of umami peptides (Kim et al., Citation2017). Zhang et al. (Citation2019) isolated six novel peptides from peanut protein hydrolysate and found that all peptides were perceived umami with threshold values from 0.39 to 1.11 mM, with umami-enhancing abilities simultaneously with threshold values from 0.33 to 0.82 mM. By analyzing the relationship between flavor characteristics and structure, it was found that umami peptides contain amino acid residues with acidic, polar, and hydrophilic properties (Zhang et al., Citation2019). Four peptides were identified from umami deamidated wheat gluten hydrolysates, ApGlu-Pro-Ser, pGlu-Pro, pGlu-Pro-Glu and pGlu-Pro-Ser, respectively. The N-terminal of above-mentioned peptides were found all glutamine residues, and these peptides were considered to be formed by cyclization of N-terminal glutamine residues during hydrolysates preparation (Schlichtherle-Cerny & Amadò, Citation2002). Wheat gluten was deamidated, therefore, glutamine existed in the form of glutamic acid residue in hydrolysate. Later, flavor components of two kinds of Japanese soy sauce were studied and found the umami ingredients contained a number of peptides; then, the amino acid composition of these peptides was analyzed and it was noticed that most of them contained glutamine residues. In addition, free glutamic acid, free aspartic acid and several sweet free amino acids in the presence of sodium salt were considered to be the main factors causing the umami taste of two kinds of Japanese soy sauce (Lioe et al., Citation2006). The major source of umami found in rice bran hydrolyzed by flavorzyme and umamyzyme was the hydrolysates with molecular weight smaller than 3000 Da (Bagnasco et al., Citation2013). Some researchers enzymatically hydrolyzed wheat gluten protein and isolated the umami peptide Asp-Cys-Gly (Cui et al., Citation2015). It was suggested, the umami peptides released by enzymatic hydrolysis of plant proteins, containing more umami amino acids such as acidic amino acid, glutamic acid (Glu) and aspartic acid (Asp), have molecular weight less than 3 kDa. Based on the findings above, the reasons for umami taste of peptide are very complicated, its amino acid composition, N-terminal amino acid residue type, molecular weight, amino acid sequence, that the peptide structure are all the influencing factors. However, little is known what the structure–activity relationship of umami peptides is, therefore, there is still wide area to study.
Sunflower seed protein is also an important plant protein resource, which contains as high as 30% of glutamic acid and aspartic acid which belong to umami amino acids (Feng, Citation2015). However, it has not been reported yet whether sunflower seed protein can release umami peptides after hydrolysis. Thus, the objective of the present work is to purify and characterize umami peptides from sunflower seed protein hydrolysates. It may provide scientific basis for elucidating the structure–activity relationship of umami peptides, furthermore, suggest a novel way for further processing and utilization of sunflower seed protein.
2. Materials and methods
2.1. Materials and reagents
Low-temperature sunflower defatted seed meal was provided by Inner Mongolia Luhua Co., Ltd. Flavorzyme (enzyme activity 20 × 104 U/g) was purchased from Novozymes (China) Biotechnology Co., Ltd. Analytical grade anhydrous ethanol, hydrochloric acid, and sodium hydroxide were purchased from Tianjin Damao Chemical Reagent Factory. Tartaric acid and potassium chloride were purchased from Sinopharm Chemical Reagent Co., Ltd. HPLC-grade trifluoroacetic acid (TFA) and methanol were purchased from Sigma, USA, HPLC-grade acetonitrile were purchased from Merck, Germany, and Sephadex G-25 was purchased from Sigma, USA.
2.2. Protein extraction
500 g of the sunflower seed meal (through 60 mesh sieve and defatted) was fully dispersed in 5000 mL of anhydrous ethanol and stirred for 60 min at 25°C water bath. The mixture was centrifuged at 2,683 g for 15 min to remove the supernatant, and the precipitate was stirred with deionized water at a ratio of 1:15 (w/v). The pH was adjusted to 7.0 using 5% NaOH, followed by stirring for 90 min at 50°C and centrifuging at 2,683 g for 15 min. The protein extract was precipitated from the resultant supernatant by adjusting the pH value to 4.0 using 2% HCl. The protein extract was obtained after centrifuging at 2,683 g for 15 min, dissolved in deionized water at a ratio of 1:2 (w/v), adjusted pH to 7, then freeze-dried for further enzymatic hydrolysis (Hu et al., Citation2018).
2.3. Degree of hydrolysis (DH)
The degree of hydrolysis of the sample was evaluated based on the testing methodology published by Sharma et al. (Citation2016) using the following equation:
where VNaOH represents the volume consumption of NaOH in mL, NNaOH stand for NaOH solution molarity, “α”represents the average degree of dissociation of α-NH2 group in protein isolate sample, Mp stands for the hydrolyzed protein mass in g, and Htot stands for peptide bonds (7.5 mM/g sunflower seed protein).
2.4. Enzymatic hydrolysis
2500 mg of sunflower seed protein was dispersed in 100 ml of deionized water and uniformly distributed, then the pH was adjusted to 7.0. The flavorzyme was added at enzyme-to-substrate (E/S) ratio of 1:25 (w/w), which was then incubated in a temperature-controlled water bath at 55°C for 480 min. The mixture was incubated in 90°C water bath for 10 min to deactivated enzyme activity, then cooled to room temperature and centrifuged at 2,683 g for 20 min. The supernatant was collected, and the pH was adjusted to 7.0 using 1 mol/L NaOH; then, it was freeze-dried for further use.
2.5. Electronic tongue (E-tongue) analysis
Analysis was performed with a commercial E-tongue to detect the taste bitter and umami, Taste-Sensing System 5000Z (AIRSENSE Analytics, Germany) equipped with an array of chemical sensors. For the E-tongue analysis, the sample was dissolved in deionized water to prepare solution of 80 ml with a concentration of 1 mg/ml. The E-tongue analysis system needed self-inspection, diagnosis and correction step before data acquisition, to ensure the reliability and stability of data. Four replicated samples, the first data was removed, and average values of the last three were used for subsequent analysis (Cai et al., Citation2019).
2.6. Purification of umami peptide
2.6.1. Ultra-filtration
The sunflower seed protein hydrolysates were filtered sequentially using an ultra-filtration unit (8200, Millipore, USA). Five fractions were obtained with molecular weights of ≤1 kDa, 1–3 kDa, 3–5 kDa, 5–10 kDa and ≥10 kDa, respectively. Each fraction was concentrated and freeze-dried for further use.
2.6.2. Sephadex gel filtration chromatography
Sephadex G-25 was soaked in twofold volume of deionized water for 24 h at room temperature. After pre-equilibrated, the supernatant and upper layer of fine particles was discarded. The remaining matter was then soaked in 50% ethanol for 24 h and the ethanol was discarded, followed by immersing in deionized water to complete pretreatment, then swelled in a water bath for 30 min, cooled and degassed, loaded to column (5 cm × 40 cm) at last. The column was equilibrated with deionized water and absorbance was measured at 220 nm, wait until the absorbance showed 0, 1 mL of the strongest umami taste fraction obtained from ultra-filtration with concentration of 30 mg/mL was added. Eluted the sample with deionized water at a constant speed (1 mL/min), and the absorbance was measured at 220 nm using an inline spectrophotometer to determine the elution profile of the sample. Fractions with the same peak were collected, concentrated, and freeze-dried for further use (Feng et al., Citation2018).
2.6.3. Reverse-phase high-performance liquid chromatography (RP-HPLC)
The strongest umami taste fraction separated on Sephadex G-25 was dissolved in ultrapure water to make solution of 10 mg/mL, and further isolated using RP-HPLC on a Hypersil GOLD C18 analytical column (ID 250 mm × 4.6 mm, 5 μm) at a flow rate of 0.8 mL/min, with a linear gradient of aqueous solution (A) and acetonitrile containing 0.05% TFA (B), as follows: 0 min, 1% B; 0–15 min, 1–5% B; and 15–30 min, 5–10% B. The total eluting time was 35 min, with an injection volume 100 μL, and detection temperature at 25°C. The eluent peaks were monitored at 220 nm using a UV-Vis variable wavelength detector, and the fractions were collected and freeze-dried (Ma et al., Citation2017).
2.7. Characterization of purified peptides using mass spectrometry
The peptide fraction with the strongest umami taste after RP-HPLC was dissolved in ultrapure water which containing 0.1% TFA, and mixed in ratio of 1:1 with the substrate solution (5 mg/mL α-cyano-4-hydroxycinnamic acid and 0.1% TFA in 50% acetonitrile). After mixed uniformly, 1 μL of the mixture was applied to the Scorce 384 target plate, followed by drying naturally. The sample plate was then sent to the ion source for detection. After initial mass spectrometry scanning, the ions detected from mass spectrogram were selected, and analyzed by mass spectrometry (Fu, Citation2018).
2.8. Statistical analysis
Experiments were conducted in triplicate, and data were expressed as means ± standard deviation (SD). One-way analysis of variance (ANOVA) and Duncan’s new multiple range test were used to determine significant differences among these means at p < .05.
3. Results and discussion
3.1. Sensory characteristics of sunflower seed protein hydrolysates
At present, a large number of studies have found that flavorzyme can be used to hydrolyze plant-derived proteins to prepare small molecular peptides with strong umami (Bagnasco et al., Citation2013; Kong et al., Citation2018b; Normah & Nurdalila Diyana, Citation2018). The present work treated sunflower seed protein with flavorzyme to produce umami hydrolysates. Sunflower seed protein was derived from defatted low-temperature sunflower seed meal, and the hydrolysates were obtained from the flavorzyme hydrolysis of the protein. Sunflower seed protein was hydrolyzed to 600 min, in this process, samples were taken after hydrolysis to 0 min, 60 min, 120 min, 240 min, 360 min, 480 min and 600 min, respectively. Umami taste of each sample was analyzed by electronic tongue. As shown in , DH reached 10.53 ± 0.63% when hydrolyzed to 480 min, and the hydrolysates exhibited the highest umami intensity value 10.72 ± 0.05, with the lowest bitterness. DH tended to be stable followed by further hydrolysis, and the flavor is no longer improved. Therefore, the hydrolysate was further separated after enzymatic hydrolysis for 480 min.
Table 1. DH and taste intensity with different hydrolysis time of sunflower seed protein hydrolysates.
Tabla 1. DH e intensidad del sabor con diferente tiempo de hidrólisis de los hidrolizados de proteína de semillas de girasol.
The intensity of umami increased as the degree of enzymatic hydrolysis increased when preparing the sunflower seed umami peptides. It indicated that umami small peptides were released continuously with increased hydrolysis time until 480 min. As the enzymatic hydrolysis proceeded, the bitterness increased significantly; however, the umami taste did not increase significantly after 480 min. It may be because a large number of peptides with hydrophobic residues were cleaved and released, the umami taste of the hydrolysate was covered up by increasing bitterness (Liu, Citation2017; Xie, Citation2015). Therefore, it is important to control the degree of enzymatic hydrolysis (Wang & Guo, Citation2016).
3.2. Ultra-filtration of sunflower seed protein hydrolysates
The sunflower seed hydrolysates were filtered sequentially, and five fractions with molecular weights of ≥10 kDa, 5–10 kDa, 3–5 kDa, 1–3 kDa and ≤1 kDa were obtained. shows that fraction with molecular weight of 1–3 kDa had the strongest umami taste and the weakest bitterness, which was significantly different from other fractions (P < .05). Umami gradually weakened while bitterness gradually increased as the molecular weight increased (Liu, Citation2017; Song, Citation2006). Kong et al. (Citation2018b) isolated the umami peptides and found low-molecular-weight fractions (MW<3 KDa), possessed the strongest umami taste. The present results were consistent with the above report, indicating that the low-molecular-weight peptide from the sunflower protein possesses umami taste. Hence, the fraction of peptides with molecular weight of 1–3 kDa was collected and freeze-dried for further purification.
Table 2. Taste intensity value of each fraction after ultra-filtration.
Tabla 2. Valor de la intensidad del sabor de cada fracción después de la ultrafiltración.
3.3. Sephadex gel filtration chromatography
Sephadex G-25 was used as gel chromatography filler to isolate fraction 1–3 kDa with the strongest umami, the result is shown in . Three peaks, namely F1, F2, and F3, were detected. The taste intensity of each fraction was measured and the result is shown in . Fraction F1 and F2 had the higher umami intensity of 12.21 ± 0.06 and 12.53 ± 0.08, respectively; however, the bitterness of F2 was significantly higher than that of F1 (P < .05). Therefore, fraction F1 was selected for further purification.
Table 3. Taste intensity value of each fraction which isolated by Sephadex G-25 gel filtration chromatography.
Tabla 3. Valor de la intensidad del sabor de cada fracción que se aisló mediante cromatografía de filtración en gel Sephadex G-25.
Figure 1. Purification of fraction 1-3 kDa through Sephadex G-25 gel filtration chromatography.
Figura 1. Purificación de la fracción 1-3 kDa mediante cromatografía de filtración en gel Sephadex G-25.
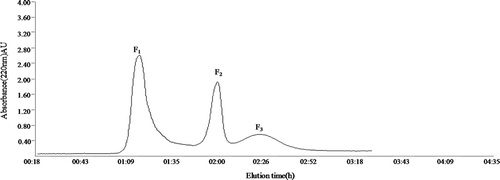
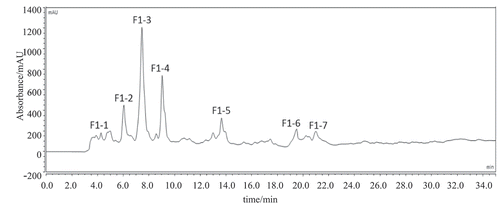
Figure 2. RP-HPLC pattern from a C18 column (4.6 mm × 250 mm) of fraction F1. (RP-HPLC represents reverse-phase high-performance liquid chromatography).
Figura 2. Patrón RP-HPLC de una columna C18 (4.6 mm × 250 mm) de la fracción F1. (RP-HPLC representa la cromatografía líquida de alto rendimiento de fase inversa).
3.4. RP-HPLC
Fraction F1 isolated from Sephadex G-25 with the highest umami value was further purified by RP-HPLC. Seven sub-fractions were purified, namely F1-1, F1-2, F1-3, F1-4, F1-5, F1-6 and F1-7, respectively. Peaks which was eluted at the same retention time were collected and freeze-dried. The samples were accurately formulated as solution with concentration of 1 mg/mL to measure umami intensity.
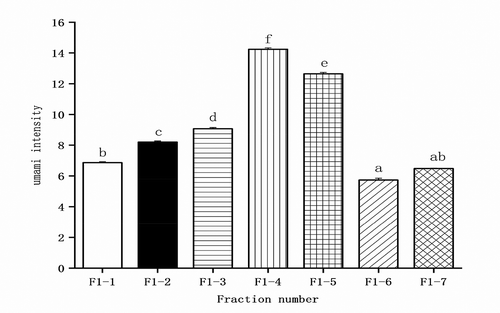
The umami intensity of each fraction is shown in , the umami taste value of F1-4 was the highest (14.16 ± 0.05), which was 1.32 times stronger than that of sunflower seed protein hydrolysates without any purification. Therefore, fraction F1-4 was subjected to mass spectrometry for identification and characterization.
Figure 3. Umami intensity value of main fractions after separated by RP-HPLC. (RP-HPLC represents reverse-phase high-performance liquid chromatography).
Figura 3. Valor de la intensidad del umami de las fracciones principales después de ser separadas por el RP-HPLC. (RP-HPLC representa la cromatografía líquida de alto rendimiento de fase inversa).
3.5. Identification and characterization of umami peptides
Among many different molecular weight sunflower seed peptides contained in fraction F1-4, three of them, which showed higher response values, namely peptide I, II, and III. Each of these three peptides had molecular weights of 1099.50, 1422.63, and 1004.43, respectively. The MS/MS spectrum of these three peptides are illustrated in .
Figure 4. MS/MS spectrum of the peptide DVNNPANQLD. (MS/MS represents secondary mass spectrometry).
Figura 4. Espectro MS/MS del péptido DVNNPANQLD. (MS/MS representa la espectrometría de masas secundaria).
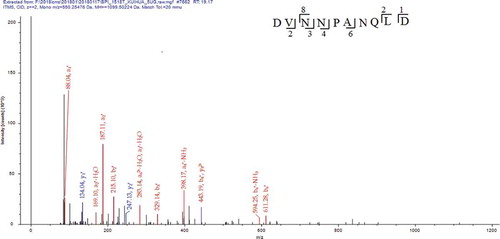
Figure 5. MS/MS spectrum of the peptide NNENQLDEYQR. (MS/MS represents secondary mass spectrometry).
Figura 5. Espectro MS/MS del péptido NNENQLDEYQR. (MS/MS representa la espectrometría de masas secundaria).
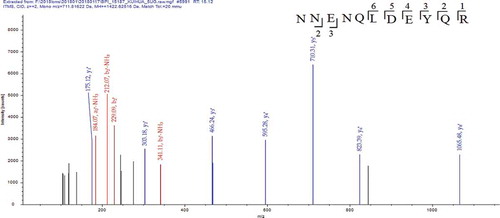
Figure 6. MS/MS spectrum of the peptide EFEGGSIEH. (MS/MS represents secondary mass spectrometry).
Figura 6. Espectro MS/MS del péptido EFEGGSIEH. (MS/MS representa la espectrometría de masas secundaria).
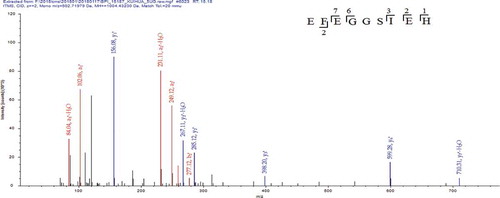
After matching with the database of sunflower seed protein, it was found that DVNNPANQLD and EFEGGSIEH were originated from the AOA251UYX1 sequence fragment of sunflower seed 11S globulin, while NNENQLDEYQR was originated from the AOA251UCD5 sequence fragment of sunflower seed 11S globulin and the AOA251TAA6 sequence fragment of sunflower seed glutelin. As listed in , structural characterization of sunflower seed umami peptides was identified by mass spectrometry as DVNNPANQLD, EFEGGSIEH, and NNENQLDEYQR.
Table 4. Structural characterization of sunflower seed umami peptides.
Tabla 4. Caracterización estructural de los péptidos umami de las semillas de girasol.
In 1908, Japanese scholars discovered amino acids that compose natural proteins all belong to L-form, and most of these amino acids have certain tastes. For example, glutamic acid (Glu), glutamine (Gln), aspartic acid (Asp), and asparagine (Asn) taste umami, glycine (Gly), serine (Ser), glutamine (Gln), threonine (Thr), alanine (Ala), and proline (Pro) taste sweet, and hydrophobic amino acids are mostly bitter (Dang et al., Citation2015; Su et al., Citation2012). Among them, isoleucine (Ile), leucine (Leu), valine (Val), phenylalanine (Phe), tryptophan (Trp), and tyrosine (Tyr) are strong hydrophobic amino acids, usually result in bad taste characteristics (Kong et al., Citation2018a; Miyanaga et al., Citation2002).
As presented in , the ratio of umami amino acids to the total amino acids was high in peptides I, II, and III, with the umami amino acid contents of 60.00%, 72.73% and 66.67%, respectively. The glutamic acid and aspartic acid contents were also high in peptides I, II, and III, 20.00%, 27.27% and 33.33%, respectively. Three peptides are low content with hydrophobic amino acids, 40.00%, 18.18%, and 22.22% in peptides I, II, and III, respectively. Among them, strong hydrophobic amino acids content in three peptides are less than 25%. Glutamic acid and aspartic acid are typical amino acids with umami characteristics, commonly acting as umami enhancer in peptides and play important role in determination of umami taste (Kong et al., Citation2018b). Noguchi et al. (Citation1975) isolated four umami peptides from fish protein hydrolysates, all of which contained aspartic acid and glutamic acid residues. Many studies also have found that the intensity of bitterness is closely related to the ratio of hydrophobic amino acids (Ishibashi et al., Citation1987). The present study also found consistent result indicating that peptides with high umami amino acid contents and the lower hydrophobic amino acid contents show weak bitterness and intense umami.
Table 5. The presentation rate of each category amino acid in sunflower seed umami peptides.
Tabla 5. Tasa de presentación de cada categoría de aminoácidos en los péptidos umami de las semillas de girasol.
The taste characteristics of polypeptides are not only related to the amino acid composition, but also related to the position of amino acids in peptide chain. Normah et al. (Citation2004) found that the position of hydrophobic amino acids affected the bitterness of peptides. When the C-terminus of polypeptide is hydrophobic amino acid, peptides are mostly bitter. Some researchers studied umami peptides isolated from beef bouillon and found peptide with acidic amino acid at N-terminal and basic amino acid at C-terminal showed umami, but lost umami taste if the terminal structure were reversed (Masahiro et al., Citation1989). N-terminal and C-terminal of the three peptides identified in the current work were all hydrophilic amino acids. Furthermore, N-terminal of peptides II and III were acidic amino acids, and C-terminal were basic amino acids. The results mentioned above indicated that the characteristics of the three umami peptides which isolated from sunflower seed hydrolysates were consistent with previous reports. The chemical solid-phase method was used to synthesize these peptides, and their taste was evaluated and validated by electronic tongue in further study.
Moreover, the studies of umami peptides have confirmed that the mechanism of umami taste of peptide is that umami peptide and umami receptor T1R1/T1R3 is combined by hydrogen bond and hydrophobic interaction (Xie, Citation2019; Yu, Citation2017). Therefore, in the further study, the molecular binding effect of purified umami sunflower seed peptide with umami receptor T1R1/T1R3 is being analyzed and explored, which helps clarify the mechanism of its umami taste.
4. Conclusions
The current work isolated and purified umami peptides from sunflower seed protein after hydrolyzed by flavorzyme. The hydrolysates can afford the highest umami intensity value 10.72 ± 0.05, also with the lowest bitterness, when the hydrolysis time was 480 min. Then, the peptides were isolated gradually and identified by mass spectrometry. Three umami proteolytic peptides of sunflower seed were purified, with sequences identified as DVNNPANQLD, NNENQLDEYQR, and EFEGGSIEH. The three peptides had relative molecular mass of 1000–1500 Da and their umami amino acid contents were higher than 60%.
Credit author statement
Xiaolan Bao: Investigation, Writing-Original draft preparation, [email protected]
Sarina Ma: Investigation, Writing-Original draft preparation, [email protected]
Yanan Fu: Methodology, Writing-Reviewing and Editing, [email protected]
Jiale Wu: Methodology, Writing-Reviewing and Editing, [email protected]
Meili Zhang: Conceptualization, Methodology, Writing-Reviewing and Editing
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bagnasco, L., Pappalardo, V. M., Meregaglia, A., Kaewmanee, T., & Ubiali, D. (2013). Use of food-grade proteases to recover umami protein–peptide mixtures from rice middlings. Food Research International, 50(1), 420–427. https://doi.org/10.1016/j.foodres.2012.11.007
- Cai, W. C., Tang, F. X., Zhao, X. X., Guo, Z., Zhang, Z. D., Dong, Y., & Shan, C. H. (2019). Different lactic acid bacteria strains affecting the flavor profile of fermented jujube juice. Journal of Food Processing and Preservation, 43(9), 1–14. https://doi.org/10.1111/jfpp.14095
- Cui, C., Qian, Y. P., Peng, J., Wang, H., & Zhao, H. (2015). Isolation, identification and taste analysis of flavor peptides in gluten fermentation broth. Modern Food Science and Technology, 31(9), 175–179. https://doi.org/10.13982/j.mfst.1673-9078.2015.9.029
- Dang, Y., Gao, X., Ma, F., & Wu, X. (2015). Comparison of umami taste peptides in water-soluble extractions of Jinhua and Parma hams. LWT – Food Science and Technology, 60(2), 1179–1186. https://doi.org/10.1016/j.lwt.2014.09.014
- Feng, W. J. (2015). Characteristics and structure identification of sunflower seed septides and peanut peptides during calcium binding. Inner Mongolia Agricultural University, 34–39.
- Feng, Y. X., Ruan, G. L., Jin, F., Xu, J., & Wang, F. J. (2018). Purification, identification, and synthesis of five novel antioxidant peptides from Chinese chestnut (Castanea mollissima Blume) protein hydrolysates. LWT – Food Science and Technology, 92, 40–46. https://doi.org/10.1016/j.lwt.2018.01.006
- Fu, Y. N. (2018). Structure identification and preparation of umami peptide from sunflower protein. Inner Mongolia Agricultural University, 11–31.
- Hu, F., Ci, A. T., Wang, H., Zhang, Y. Y., Zhang, J. G., Thakur, K., & Wei, Z. J. (2018). Identification and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chemistry, 261, 301–310. https://doi.org/10.1016/j.foodchem.2018.04.025
- Ishibashi, N., Fukui, S., Okai, H., Kikuchi, E., & Kouge, K. (1987). Studies on flavored peptides. Part II. Bitterness of phenylalanine- and tyrosine-containing peptides. Agricultural and Biological Chemistry, 51(12), 3309–3313. https://doi.org/10.1080/00021369.1987.10868574
- Kim, Y., Kim, E. Y., Son, H. J., Lee, J. J., Choi, Y. H., & Rhyu, M. R. (2017). Identification of a key, umami-active fraction in modernized Korean soy sauce and the impact thereof on bitter-masking. Food Chemistry, 233, 256–262. https://doi.org/10.1016/j.foodchem.2017.04.123
- Kong, Y., Zhang, L. L., Zhang, Y. Y., Sun, B. G., Sun, Y., Zhao, J., & Chen, H. T. (2018a). Evaluation of non-volatile taste components in commercial soy sauces. International Journal of Food Properties, 21(1), 1854–1866. https://doi.org/10.1080/10942912.2018.1497061
- Kong, Y., Zhang, L. L., Zhao, J., Zhang, Y. Y., Sun, B. G., & Chen, H. T. (2018b). Isolation and identification of the umami peptides from shiitake mushroom by consecutive chromatography and LC-Q-TOF-MS. Food Research International, 11, 0963–9969. https://doi.org/10.1016/j.foodres.2018.11.060
- Lioe, H. N., Takara, K., & Yasuda, M. (2006). Evaluation of peptide contribution to the intense umami taste of Japanese soy sauces. Journal of Food Science, 71(3), S277–S283. https://doi.org/10.1111/1365-2621.2006.tb15654.x
- Liu, B. Y. (2017). Study on preparation of low-bitterness peptide powders from gluten and debittering mechanism. Jiangnan University, 14–25.
- Ma, S., Zhang, M. L., Beta, T., Dong, T., Bao, X. L., & Li, Z. Q. (2017). Purification and structural identification of glutelin peptides derived from oats. CyTA-Journal of Food, 15(4), 508–515. https://doi.org/10.1080/19476337.2017.1301555
- Masahiro, T., Tohru, N., Makoto, T., Kawasaki, Y., & Kikuchi, E. (1989). The relationship between taste and primary structure of “delicious peptide” (Lys-Gly-Asp-Glu-Glu-Ser-Leu-Ala) from beef soup. Agricultural and Biological Chemistry, 53(2), 319–325. https://doi.org/10.1080/00021369.1989.10869317
- Miyanaga, Y., Tanigake, A., Nakamura, T., Kobayashi, Y., Ikezaki, H., & Taniguchi, A. (2002). Prediction of the bitterness of single, binary- and multiple-component amino acid solutions using a taste sensor. International Journal of Pharmaceutics, 248(1–2), 207. https://doi.org/10.1016/s0378-5173(02)00456-8
- Noguchi, M., Arai, S., Yamashita, M., Yamashita, M., Kato, H., & Fujimaki, M. (1975). Isolation and identification of acidic oligopeptides occurring in a flavor potentiating fraction from a fish protein hydrolysate. Journal of Agricultural and Food Chemistry, 23(1), 49–53. https://doi.org/10.1021/jf60197a003
- Normah, I., Jamilah, B., Saari, N., & Che Man, Y. B. (2004). Chemical and taste characteristics of threadfin bream (Nemipterus japonicus) hydrolysate. Journal of the Science of Food and Agriculture, 84(11), 1290–1298. https://doi.org/10.1002/jsfa.1743
- Normah, I., & Nurdalila Diyana, M. R. (2018). Evaluation of umaminess in green mussel hydrolysate (Perna viridis) produced in the presence of sodium tripolyphosphate and NaCl. International Food Research Journal, 25(6), 2524–2530.
- Schlichtherle-Cerny, H., & Amadò, R. (2002). Analysis of taste-active compounds in an enzymatic hydrolysate of deamidated wheat gluten. Journal of Agricultural and Food Chemistry, 50(6), 1515–1522. https://doi.org/10.1021/jf010989o
- Sharma, J. G., Kumar, A., Saini, D., Targay, N. L., Khangembam, B. K., & Chakrabarti, R. (2016). In vitro digestibility study of some plant protein sources as aquafeed for carps Labeo rohita and Cyprinus carpio using pH-Stat method. Indian Journal of Experimental Biology, 54(9), 606–611.
- Song, M. (2006). Study on both preparation and characterization of hydrolysate of soybean protein isolate with umami taste. China Agricultural University, 14–21.
- Su, G. W., Cui, C., Zheng, L., Yang, B., Ren, J. Y., & Zhao, M. M. (2012). Isolation and identification of two novel umami and umami-enhancing peptides from peanut hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS. Food Chemistry, 135(2), 479–485. https://doi.org/10.1016/j.foodchem.2012.04.130
- Wang, Y. Y., & Guo, X. F. (2016). The tasting mechanism and the preparing methods of umami peptides. Grain Processing, 41(6), 36–41.
- Xie, M. (2015). Separation and purification of bitter peptides and debittering methods in hydrolysates of cod fish. Ocean University of China, 33–42.
- Xie, X. X. (2019). Flavor characteristics of umami peptides from Meretrix meretrix (Linnaeus) and Aloididae aloidi and their interactions with umami receptor T1R1/T1R3. Bohai University, 39–48.
- Yu, X. Q. (2017). Structural simulation of umami receptor T1R1/T1R3 and the interaction with umami hexapeptides. Shanghai Ocean University, 20–30.
- Zhang, J. N., Zhao, M. M., Su, G. W., & Lin, L. Z. (2019). Identification and taste characteristics of novel umami and umami-enhancing peptides separated from peanut protein isolate hydrolysate by consecutive chromatography and UPLC–ESI–QTOF–MS/MS. Food Chemistry, 278, 674–682. https://doi.org/10.1016/j.foodchem.2018.11.114
