 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, curcumin nanosuspensions (Cur-NSs) were prepared using D-alpha-tocopheryl polyethylene glycol succinate (vitamin E TPGS), and their physical properties (i.e., particle size, dispersion stability) were investigated. Also, the antioxidant activity of Cur-NSs in emulsion systems was evaluated by peroxide value change and the oxidation products were monitored by nuclear magnetic resonance spectroscopy. The initial particle size of Cur-NSs containing a high curcumin concentration (0.30–0.50%) was smaller than that at low concentration (0.05–0.15%). When Cur-NSs were stored at 25°C, aggregation of curcumin particles was observed with increasing storage period. The aggregation rate decreased as the curcumin concentration in Cur-NSs increased. These observations were supported by changes in the transmission intensity profile using Turbiscan. Regarding the antioxidant activity of Cur-NSs, the emulsions with curcumin additions of 200 mg/kg or higher showed improved antioxidant effects compared to the control, but a lower activity than the positive control with 200 mg/kg catechin.
RESUMEN
En este estudio se prepararon nanosuspensiones de curcumina (Cur-NSs) utilizando succinato de polietilenglicol de D-alfa-tocoferil (vitamina E TPGS), investigándose sus propiedades físicas (es decir, tamaño de las partículas y estabilidad de la dispersión). Además, mediante el cambio del valor de peróxido se evaluó la actividad antioxidante de la Cur-NSs en sistemas de emulsión y posteriormente se vigilaron los productos de la oxidación empleando espectroscopia de resonancia magnética nuclear. El tamaño inicial de las partículas de Cur-NSs que contienen una alta concentración de curcumina (0.30-0.50%) era menor que el de las de baja concentración (0.05-0.15%). Al almacenarse las Cur-NSs a 25°C, se observó la agregación de partículas de curcumina cuando el periodo de almacenamiento era cada vez mayor. La tasa de agregación disminuyó al aumentar la concentración de curcumina en la Cur-NSs. Estas observaciones fueron respaldadas por cambios en el perfil de la intensidad de transmisión usando Turbiscan. En cuanto a la actividad antioxidante de la Cur-NSs en comparación con el control, las emulsiones que contenían adiciones de curcumina de 200 mg/kg o más mostraron mejores efectos antioxidantes pero su actividad fue menor que el control positivo con 200 mg/kg de catequina.
1. Introduction
The increase in functional foods have made the food industry to produce foods containing biological active or functional ingredients (Chen et al., Citation2006; Joye et al., Citation2014; Kaialy & Shafiee, Citation2016; Weiss et al., Citation2006). However, most functional ingredients have poor water solubility and permeability (Frank et al., Citation2014; Junyaprasert & Morakul, Citation2015; Keck & Müller, Citation2006; Peltonen & Strachan, Citation2015), resulting in lower body bioavailability and absorption compared to dosage (Peltonen & Strachan, Citation2015). These drawbacks are a major challenge in the manufacture of functional foods and several approaches (e.g., nanosuspensions, nanoemulsions, nanoparticles, polymer micelles, and liposomes) to mitigate these challenges were considered and developed (Chen et al., Citation2011; Frank et al., Citation2014). Most of these concepts are based on particle size reduction, therefore, nanoparticle size and distribution are important factors in the design of such approaches (Liu et al., Citation2016; McClements, Citation2014; McClements & McClements, Citation2016).
According to literature, if the particle size is reduced to the nanometer range (i.e., the average particle size is generally less than 1 μm), the surface area increases, and with it the saturated solubility and dissolution rate, improving bioavailability and absorption (Gao et al., Citation2008; Junyaprasert & Morakul, Citation2015). In this regard, previous studies (Ahmed et al., Citation2012; Hu et al., Citation2012; Jang et al., Citation2014; X. Wang et al., Citation2008; Yu & Huang, Citation2012) reported that the bioavailability of curcumin improved using an emulsion-based delivery system. Ji et al. (Citation2016) observed that the solubility and bioavailability of solid curcumin lipid nanoparticles were improved with TPGS and polyoxyethylene 20 stearylether (Brij78). In addition, Acosta (Citation2009) and Kesisoglou et al. (Citation2007) reported that when encapsulating lipophilic functional ingredients using nanoemulsions and other colloidal systems, bioavailability increased as droplet size decreased.
To achieve nanonization, top-down or bottom-up techniques are used. Top-down techniques include disintegration (e.g., pearl milling, ball milling, high-pressure homogenization, and microfluidization) and bottom-up techniques are solvent-based precipitation methods (Gao et al., Citation2008; Junyaprasert & Morakul, Citation2015; Keck & Müller, Citation2006). Particle properties of nanosuspensions depend on the technology and conditions of the manufacturing process, where crystalline nanoparticles are mainly produced through top-down technology, whereas amorphous nanoparticles are mainly produced using the bottom-up technology (McClements & McClements, Citation2016; Peltonen & Strachan, Citation2015). In general, amorphous particles obtained through the latter method show high solubility (Bi et al., Citation2015; Junyaprasert & Morakul, Citation2015; Peltonen & Strachan, Citation2015), but in practice, the top-down technique is preferred over the bottom-up technique because the latter uses toxic solvents during the manufacturing process and provides insufficient storage stability (Keck & Müller, Citation2006).
Curcumin extracted from turmeric rhizome (Curcuma longa L.) has several biologically beneficial functions, such as antioxidant activity (Ahsan et al., Citation1999; AK & Gülçin, Citation2008; Joung et al., Citation2016), anti-carcinogenicity (Basniwal et al., Citation2014; Duvoix et al., Citation2005; Ruby et al., Citation1995), anti-inflammatory (Huang et al., Citation1992; X. Wang et al., Citation2008), antibacterial (Apisariyakul et al., Citation1995; Basniwal et al., Citation2011; Moghadamtousi et al., Citation2014), and liver protection (Cutrin et al., Citation2013; Ghosh et al., Citation2012; Naksuriya et al., Citation2014), and is considered an attractive candidate for producing functional foods.
As previously mentioned, the generation of nanosuspensions is one of the few strategies that can be considered to improve bioavailability and absorption. However, since nanosuspensions are a thermodynamically unstable system, destabilization of emulsions such as agglomeration may occur over time. Therefore, it is important to use an appropriate type and concentration of stabilizers to formulate stable nanosuspensions (Y. Wang et al., Citation2013). Vitamin E TPGS is a known surfactant in the pharmaceutical industry and can be used to increase the oral bioavailability of curcumin (Ji et al., Citation2016). This study aimed to prepare curcumin nanosuspensions with vitamin E TPGS as a surfactant using the top-down technology (high-pressure homogenization), characterize its physicochemical properties, and evaluate the antioxidant activity in emulsion systems.
2. Materials and methods
2.1. Materials
Curcumin (≥80% curcumin, from Curcuma longa L.), D-α-tocopherol polyethylene glycol 1000 succinate (vitamin E TPGS), 2-[bis(2- hydroxyethyl)amino]-2-(hydroxymethyl) propane-1,3-diol (bis-tris), polyoxyethylene (20) sorbitan monolaurate (Tween 20), (+)-catechin hydrate (catechin), barium chloride, iron (II) sulfate, ammonium thiocyanate, chloroform-d (CDCl3, 99.8 atom% D, contains 0.1% (v/v) TMS) were all obtained from Sigma-Aldrich Co. (St. Louis, MO). Commercial refined soybean oil (SBO) produced by CJ Cheiljedang was purchased from a local market in Korea and then further refined according to the method of X. Y. Wang et al. (Citation2014) to prepare stripped soybean oil (SSBO). The tocopherol content in SBO and SSBO was determined according to the method of Lee et al. (Citation2015), presented in . All other chemicals used in this study were of analytical grade.
Table 1. Total tocopherol content in soybean oil (SBO) and stripped soybean oil (SSBO).
Tabla 1. Contenido total de tocoferol en el aceite de soya [soja] (SBO) y en el aceite de soya despojado (SSBO)
2.2. Preparation of curcumin nanosuspensions (Cur-NSs)
The curcumin nanosuspension (Cur-NS) was prepared by a modified method of Y. Gao et al. (Citation2010). Briefly, vitamin E TPGS was dissolved in 20 mM bis-tris buffer (0.02 wt% sodium azide, pH 7.0) to prepare an aqueous phase, and then curcumin (0.05‒0.5 wt%) was added to the aqueous phase and dissolved completely by stirring and sonication. This solution was then premixed at 8,000 rpm for 5 min using a Silverson mixer (L4RT, Silverson Machines Ltd., Chesam, UK), followed by a final homogenization using a high-pressure homogenizer (MN400BF, Micronox, Seongnam, Korea) to prepare the nanosuspension containing curcumin. The high-pressure homogenization conditions were as follows: two cycles at 20.7 MPa followed by 5 cycles at 51.7 MPa and 15 cycles at 172.4 MPa. The composition of the nanosuspensions containing curcumin is shown in .
Table 2. Composition of curcumin nanosuspensions.
Tabla 2. Composición de las nanosuspensiones de curcumina
2.3. Physicochemical properties of the curcumin nanosuspensions (Cur-NSs)
2.3.1. Measurement of the particle size of Cur-NSs
The particle size of Cur-NSs was measured using the dynamic light scattering (DLS) method and a particle size analyzer (Zetasizer Nano ZS, Malvern Instruments Ltd., Worcestershire, UK). The DLS method is most suitable and widely used for measuring particle sizes in the range of 1–3000 nm.
In the present study, each Cur-NS was diluted 10-fold with 20 mM bis-tris buffer (0.02 wt% sodium azide, pH 7.0) to avoid multiple scattering effects. Measurements were performed every 7 days in triplicate for samples stored at 25°C for 28 days.
2.3.2. Determination of aggregation rate
Nanosuspensions with high surface areas are thermodynamically unstable, which promotes aggregation and crystal growth (Gao et al., Citation2007). This phenomenon is called Ostwald ripening, so aggregation can be an intrinsic property of nanosuspensions (Y. Gao et al., Citation2010; Y. Wang et al., Citation2013). In this study, the aggregation rate was calculated based on the Lifshitz-Slyozov-Wagner (LSW) equation (Kabalnov & Shchukin, Citation1992; Kim et al., Citation2016; Verma et al., Citation2011) below:
where d(t) is the mean droplet size at time = t, d(0) is the initial droplet size, ω is the aggregation rate, α is the characteristic length scale (α = 2γVm/RT), S∞ is the bulk solubility of the dispersed phase for continuous phase, D is the translation diffusion coefficient of the dispersed phase. According to this equation, the cube of size [d(t)3] represents a linear relationship with time (t), so the aggregation rate (ω) of the Cur-NS is the slope of the straight line of the linear function.
2.3.3. Zeta-potential measurement
The zeta-potential is the one of many factors that have a great impact on the long-term stability of colloid dispersions or emulsion systems (Y. Gao et al., Citation2010; McClements & McClements, Citation2016; Shin et al., Citation2016). The zeta-potential was also measured using a zetasizer (Zetasizer Nano ZS, Malvern Instruments Ltd., Worcestershire, UK). In the present study, each Cur-NS was diluted 10 times with 20 mM bis-tris buffer (0.02 wt% sodium azide, pH 7.0) before measurements to avoid multiple scattering effects. The measurements were carried out in triplicate at room temperature.
2.3.4. Dispersion stability determined by Turbiscan
Turbiscan ( Lab., Formulaction, L’Union, France) is an instrument that can assess the stability of dispersions or emulsion through multiple light-scattering phenomena without sample dilution. In this system, the dispersion stability is measured while the 880 nm near-infrared light scans up and down the glass vial containing the dispersion sample. If emulsion instability such as creaming or changes in particle size (flocculation or coalescence) occurs, it changes the near-infrared transmittance or backscattering intensity. Based on these results it was possible to estimate the dispersion or emulsion stability (Mengual et al., Citation1999). In the present study, the dispersion stability was monitored for 3 days similar to the previous curcumin nanoemulsion study (Kim et al., Citation2016).
2.4. Antioxidant activity measurements
2.4.1. Preparation of emulsions containing stripped soybean oil (SSBO emulsion)
SSBO emulsions were prepared by the following procedure. First, an aqueous phase was prepared by mixing a hydrophilic emulsifier (0.3 wt% Tween 20) in 20 mM bis-tris buffer solution (0.02 wt% sodium azide, pH 7.0). Secondly, after adding the mixture to the oil phase (10 wt% SSBO), this immiscible solution was premixed for 2 min at 5,000 rpm using a Silverson mixer (L4RT, Silverson Machines Ltd., Bucks, UK). Thirdly, this premix was then homogenized by a microfluidizer (M-110Y, Microfluidics, MA, USA) at 20.7 MPa for 2 cycles to prepare the final SSBO emulsion (0.3 wt% Tween 20, 10 wt% oil, 20 mM bis-tris, pH 7).
2.4.2. Oxidation stability measurements of SSBO emulsions containing curcumin nanosuspensions
In this study, to investigate the antioxidant activity of Cur-NS, Cur-NS was added to SSBO emulsions at various concentrations, and 200 mg/kg catechin was used as a positive control. Cur-NS containing 0.5 wt% curcumin was added to SSBO emulsions to obtain concentrations of 100, 200, 300, and 400 mg/kg, respectively.
A volume of 50 mL of the SSBO emulsions containing Cur-NS was transferred into a vial, and the remainder poured into a 100 mL Erlenmeyer flask. The vials were stored in an incubator at 45°C to measure the peroxide values, and the Erlenmeyer flasks containing the emulsions were placed in a dark area at 25°C to evaluate the emulsion stability.
2.4.3. Measurements of droplet size in SSBO emulsions
The droplet size in the SSBO emulsions was measured to monitor the emulsion stability during storage with a static light scattering (SLS) method using a particle size analyzer (Mastersizer S, Malvern Instrument, Worcestershire, UK). In the present study, the results were expressed as volume-surface mean diameter (d32) described as below:
where ni is the number of particles of diameter di. The measurements were performed in duplicate, storing all the samples at 25°C for 60 days.
2.4.4. Measurements of peroxide values (POV)
Peroxide values are measured to determine the amount of hydroperoxides produced as primary oxidation compounds at the initial lipid oxidation stage. In the present study, the peroxide values were measured according to the method of Mei et al. (Citation1999). First, a Fe2+/thiocyanate solution was freshly prepared by mixing 1 vol of Fe2+ solution with 1 vol of thiocyanate solution (3.94 M ammonium thiocyanate in H2O). The Fe2+ solution was the supernatant obtained from centrifuging a mixture of 3 mL of 0.144 M BaCl2 and 3 mL of freshly prepared 144 M FeSO4. Three mL of methanol/1-butanol (2/1, v/v) was then mixed with 30 μL of the thiocyanate/Fe2+ solution and 20 μL of emulsion sample. After adding Fe2+ and standing for 20 minutes in a dark place, the absorbance was measured at 510 nm with a spectrophotometer (Optizen 2120UV, Duksan Mecasys Co., Seoul, Korea). Lipid peroxides were quantified from a standard curve using H2O2 and expressed as mM peroxides/L emulsion.
2.4.5. Measurements of 1H nuclear magnetic resonance (1H NMR) spectra
Primary oxidation products (i.e., hydroperoxides) are easily converted to secondary oxidation products. The amounts of secondary oxidation products (aldehydes) as well as other primary oxidation products (conjugated dienes) were measured with 1H NMR spectroscopy. The degree of lipid oxidation in the SSBO emulsion was also determined by 1H NMR (Jia et al., Citation2015), which was carried out with a 600 MHz Bruker Avance III 600 spectrometer (Bruker Biospin Co., Billerica, MA). The parameters were: 12,335.5 Hz of spectral width, 16 scans, 2.656 s of acquisition time, 25°C of analysis temperature.
Briefly, chloroform was added to the emulsion to extract the oil. The mixture was then centrifuged at 3,000 rpm for 5 min to collect its lower layer (chloroform layer). Chloroform was removed from the lower layer to obtain the oil. Finally, 600 μL of chloroform-d was added to 50–100 mg of extracted oil, and this mixture placed in an NMR tube.
Oxidation products were quantified following the procedure of X. Y. Wang et al. (Citation2014), and all NMR data treated with ACD Labs NMR Processer (version 10.0). The integrated 1H-NMR signals proportional proton numbers (Holzgrabe, Citation2010) was normalized to quantify the amount of oxidation products, such as Z,E an E,E conjugated dienes in chains having hydroperoxy groups and aldehydes. The amounts of these products were expressed as mmol/L based on the reference of tetramethylsilane at δ = 0 ppm.
2.5. Statistical analysis
Measurements in this study were performed in duplicate or triplicate. Experimental data were treated by analysis of variance (ANOVA) using SAS 9.4 for Windows, and the results presented as mean values and standard deviation. Significant differences in means were determined by Duncan’s multiple range test.
3. Results and discussion
3.1. Changes of particle size in curcumin nanosuspensions during storage time
and show the changes in particle size and polydispersity index (PDI) in Cur-NSs of varying curcumin concentrations during storage, and presents the aggregation rates. In general, the initial particle size of Cur-NSs was similar to that reported by Y. Gao et al. (Citation2010), but decreased with increasing curcumin concentration. The initial particle size of Cur-NSs at a relatively low curcumin concentration (0.05–0.15 wt%) was larger than that of Cur-NSs with 0.30–0.50 wt% curcumin (i.e., 0.05–0.15 wt% curcumin: ~240 nm and 0.3–0.5 wt% curcumin: ~215 nm). This resulted from the high collision frequency between particles at higher curcumin concentrations during high-pressure homogenization (McClements, Citation2015). As with particle size changes, PDI decreased as curcumin concentration increased. PDI represents the width of the size distribution (Kalang & Valenta, Citation2011), and PDI values lower than 0.2 indicate good quality of dispersion with a narrow size distribution.
Table 3. Particle size and polydispersity of curcumin nanosuspensions containing various concentrations of curcumin during storage at 25°C.
Tabla 3. Tamaño de las partículas y polidispersidad de las nanosuspensiones de curcumina que contienen diversas concentraciones de curcumina durante el almacenamiento a 25°C
Table 4. Aggregation rates (ω) of curcumin nanosuspensions containing various concentrations of curcumin during storage at 25°C.
Tabla 4. Tasas de agregación (ω) de nanosuspensiones de curcumina que contienen varias concentraciones de curcumina durante el almacenamiento a 25°C
Figure 1. Changes in particle size of curcumin nanosuspensions with various curcumin concentrations during storage time at 25°C. The curcumin nanosuspensions were made with TPGS.
Figura 1. Cambios en el tamaño de las partículas de las nanosuspensiones de curcumina con diversas concentraciones de curcumina durante el tiempo de almacenamiento a 25°C. Las nanosuspensiones de curcumina se elaboraron con TPGS
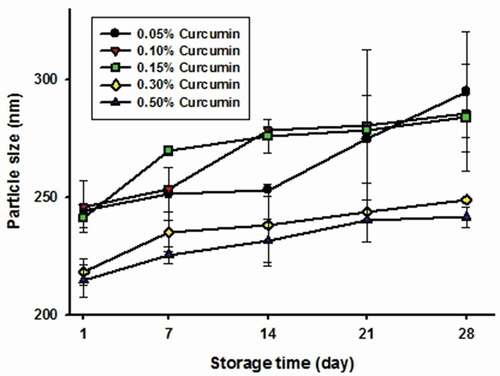
In addition, as shown in , the aggregation rate during the storage period decreased as the curcumin concentration in Cur-NSs increased, which was the result of the initial particle size. According to Stokes’ law, the square of the particle radius is directly related to the creaming or sedimentation rate (McClements, Citation2015). Therefore, the larger the particle size, the faster the movement of the particles, resulting in an increase in aggregation.
3.2. Zeta-potential of curcumin nanosuspensions
The Cur-NSs used in this study showed a zeta potential value of −18.20 ± 0.46 mV regardless of the curcumin concentration in Cur-NSs. This value (data in detail not shown) is comparable to those of other studies [−14.84 ± 1.68 mV in the study of Y. Gao et al. (Citation2010), −16.4 to −23.3 mV in the study of Shin et al. (Citation2016), −12.50 mV in the study of Rachmawati et al. (Citation2013)], and it is believed that this is due to the ionizable group of vitamin E TPGS. It is known that the stabilization by electrostatic repulsion is effective only when the zeta potential value is ± 30 mV or more (Y. Gao et al., Citation2010; Mengual et al., Citation1999), and thus stabilization of Cur-NSs by the zeta potential only is questionable. On the other hand, it was reported that vitamin E TPGS has a hydrophilic part (tocopherol succinate) and a hydrophobic part (polyethylene glycol) in the molecule, it can therefore suppress the aggregation of particles through a steric mechanism (Mengual et al., Citation1999; Yue et al., Citation2013). In addition, Mu and Seow (Citation2006) also used vitamin E TPGS in drug nanoparticle delivery systems and reported that vitamin E TPGS has considerable surface activity.
In conclusion, these results suggest that the main stabilization mechanism of Cur-NSs by vitamin E TPGS is a steric stabilization rather than an electrostatic stabilization. Stability evaluation of curcumin nanosuspensions by Turbiscan.
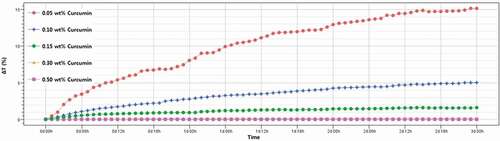
The result of the dispersion stability test of Cur-NSs is shown in , and the changes in transmittance intensity (ΔT) are expressed as a function of time (Qi et al., Citation2017). The ΔT (%) values of Cur-NSs with various curcumin concentrations were 15.12 at 0.05 wt%, 5.00 at 0.10 wt%, 1.60 at 0.15 wt%, 0.09 at 0.30 wt%, and 0 at 0.50 wt%, respectively. The TPGS addition was the same (1%) for all Cur-NSs but the dispersion stability determined by Turbiscan varied, which was attributed to the particle size difference as the curcumin concentration changed. That is, the higher the curcumin concentration in Cur-NSs, the smaller the particle size, and the more stable the dispersion. As described above, these results are in good agreement with the results of particle size change and aggregation rate.
Figure 2. Changes in the transmission profiles of the curcumin nanosuspensions using the Turbiscan system. (ΔT = Tt ‒ T0, Tt: transmission intensity at time = t, T0: transmission intensity at time = 0). Measurements were made at 25°C for 3 days.
Figura 2. Cambios en los perfiles de transmisión de las nanosuspensiones de curcumina utilizando el sistema Turbiscan (ΔT = Tt – T0. Tt: intensidad de transmisión en el tiempo = t, T0: intensidad de transmisión en el tiempo = 0). Las mediciones se hicieron a 25°C durante 3 días
3.3. Oxidation stability of SSBO emulsions containing curcumin nanosuspensions
3.3.1. Changes of droplet size in SSBO emulsions during storage time
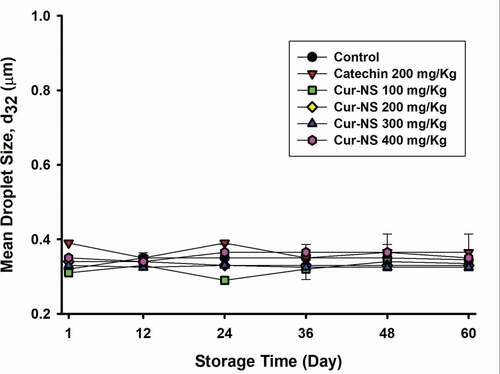
shows the droplet size change of the SSBO emulsions during storage at 25°C. The initial droplet size of each SSBO emulsion ranged from 0.33 to 0.37 μm, and there were no significant changes in droplet size during the storage period (Jia et al., Citation2015). This means that the prepared SSBO emulsions were stable for the duration of the experiment.
Figure 3. Changes in droplet size of SSBO emulsions containing curcumin nanosuspensions during storage time at 25°C. The curcumin nanosuspensions were added to SSBO emulsions at various concentrations.
Figura 3. Cambios en el tamaño de las gotas de las emulsiones de SSBO que contienen nanosuspensiones de curcumina durante el tiempo de almacenamiento a 25°C. Las nanosuspensiones de curcumina se añadieron a las emulsiones SSBO en varias concentraciones
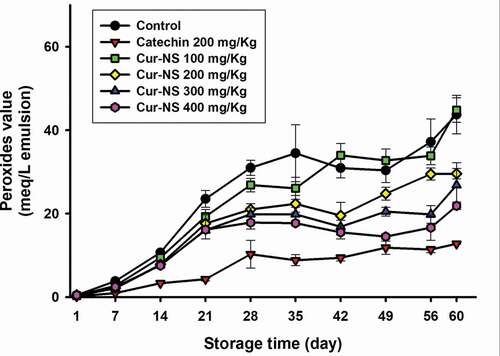
3.3.2. Behavior of the peroxide values during storage time
shows the change in peroxide values in SSBO emulsions stored at 45°C for 60 days. All emulsions containing more than 200 mg/kg curcumin (i.e., Cur-NS 200) showed significantly lower POV compared to the control (i.e., SSBO emulsion without Cur-NS), and higher POV than the positive control with 200 mg/kg catechin added. In addition, POV of the emulsion samples containing Cur-NS decreased as the amount of curcumin added to the SSBO emulsion increased.
These results clearly indicated that the antioxidant activity of Cur-NS is due to the presence of curcumin. On the other hand, the POV of the control was lower than that in the study of Jia et al. (Citation2015), which was due to the higher tocopherol content of SSBO in this study. It was reported that the hydrogen atom of the phenolic group in the molecule is responsible for the antioxidant activities in lipid emulsion systems (Ak & Gülçin, Citation2008; Kharat & McClements, Citation2019).
3.3.3. Monitoring the amount of oxidation products by 1H NMR
To confirm the previous PV results, 1H-NMR was used to monitor the formation of primary oxidized products (conjugated dienes) and secondary oxidized products (aldehydes). As shown in a, b, the 1H-NMR signal was observed at 5.40 to 6.60 ppm (), which was assigned to (Z,E) and E,E) conjugated dienes (i.e., hydroperoxide), and aldehydes observed between 9.40 and 9.80 ppm (). These assignments are consistent with previous results (Chang et al., Citation2019; Jia et al., Citation2015, Citation2016). For conjugated dienes and aldehydes, the signal intensity was highest in the control and lowest in the sample with catechin added. In addition, in the case of the same experimental treatment, the overall signal intensity corresponding to the secondary oxide aldehydes was low compared to the signal intensity of the primary oxide conjugated dienes. This indicates that overall oxidation has not progressed to a significant extent.
Figure 4 a, b. Expanded region between 5.45 and 6.60 ppm of the 1H-NMR spectra for conjugated dienes (Figure 4a) and 9.40 and 9.80 ppm for aldehydes (Figure 4b). Signals in : (a-d) Z,E-conjugated double groups, δa 5.55 ppm, δb 6.55 ppm, δc 6.00 ppm, δd 5.50 ppm; (e-h) E,E-conjugated double groups, δe 5.75 ppm, δf 6.25 ppm, δg 6.05 ppm, δh 5.45 ppm. Signals in : (1) n-alkanals, δ1 9.76 ppm, (2) 4-hydroperoxy-(E)-2–alkenals, δ2 9.59 ppm, (3) 4-hydroxy-(E)-2-alkenals, δ3 9.57 ppm, (4) 4,5-epoxy-(E)-2-alkenals, δ4 9.55 ppm, (5) (E,E)-alkadienals, δ5 9.53 ppm, (6) (E)-2-alkenals, δ6 9.49 ppm 1H-NMR spectroscopy was carried out for samples after 60 days of storage.
Figura 4a, b. Región expandida entre 5.45 y 6.60 ppm del espectro 1H-NMR para los dienos conjugados (Figure 4a) y 9.40 y 9.80 ppm para los aldehídos (Figura 4b). Notas en la : (a-d) Z,E-grupos dobles conjugados, δa 5.55 ppm, δb 6.55 ppm, δc 6.00 ppm, δd 5.50 ppm; (e-h) E,E-grupos dobles conjugados, δe 5.75 ppm, δf 6.25 ppm, δg 6.05 ppm, δh 5.45 ppm. Notas de la : (1) n-alcanales, δ1 9.76 ppm, (2) 4- hidroperoxi -(E)-2-alcanales, δ2 9.59 ppm, (3) 4-hidroxi-(E)-2-alcanales, δ3 9.57 ppm, (4) 4,5-epoxi-(E)-2-alcanales, δ4 9.55 ppm, (5) (E,E)-alcadienales, δ5 9,53 ppm, (6) (E)-2-alquenal, δ6 9,49 ppm. Se realizó una espectroscopia de 1H-NMR para las muestras después de 60 días de almacenamiento
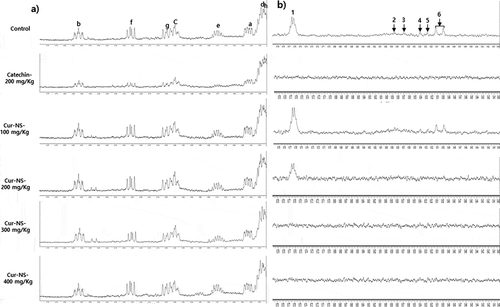
Figure 5. Changes in peroxides values (POV) of SSBO emulsions containing curcumin nanosuspensions during storage time at 45°C. The curcumin nanosuspensions were added to SSBO emulsions at various concentrations.
Figura 5. Cambios en los valores de peróxidos (POV) de las emulsiones de SSBO que contienen nanosuspensiones de curcumina durante el tiempo de almacenamiento a 45°C. Las nanosuspensiones de curcumina se añadieron a las emulsiones SSBO en varias concentraciones
shows the changes in the amount of primary oxidized products (conjugated dienes) and secondary oxidized products (aldehydes) during storage of SSBO emulsion samples at 45°C. Aldehydes were not observed until 20 days of storage, whereas the amount of conjugated dienes varied depending on the emulsion samples. It was the highest in the control group, and lowest in the catechin-containing group. It was observed for Cur-NSs samples that the higher the amount of curcumin added, the lower the number of dienes produced. With increasing storage period, the total amount of conjugated dienes and aldehydes gradually increased in all emulsion samples, but in general, these amounts were lower than those reported by Jia et al. (Citation2015). Similar to the POV results, it was determined over the entire storage period that the amount of oxidation products was more efficiently reduced for the SSBO emulsions containing higher curcumin amounts.
Table 5. Changes in the amount of primary oxidation products (conjugated dienes) and secondary oxidation products (aldehydes) in SSBO emulsions during storage at 45°C by 1H-NMR (unit: mmol oxidation products/L emulsion).
Tabla 5. Cambios en la cantidad de productos de oxidación primaria (dienos conjugados) y productos de oxidación secundaria (aldehídos) en las emulsiones de SSBO durante el almacenamiento a 45°C por 1H-NMR (unidad: productos de oxidación mmol/emulsión L)
After 60 days of storage, the total conjugated diene concentrations in the control, positive control (catechin 200 mg/kg), Cur-NS 100 mg/kg, Cur-NS 200 mg/kg, Cur-NS 300 mg/kg, and Cur-NS 400 mg/kg samples were 11.54, 4.43, 12.09, 8.04, 9.04, and 8.57 mmol oxidation products/L emulsion, respectively. The total aldehyde concentrations in the control, positive control, Cur-NS 100 mg/kg, Cur-NS 200 mg/kg, Cur-NS 300 mg/kg, and Cur-NS 400 mg/kg were 0.943, trace, 0.888, 0.486, 0.317, and 0.162 mmol oxidation products/L emulsion, respectively. The amount of oxidized products generated in the SSBO emulsion during the storage period was highest in the control and the lowest in the sample with added catechin. With respect to SSBO emulsions with curcumin addition, the higher the amount added, the less oxidized products were produced. These results indicated that Cur-NSs provide antioxidant activity to lipid emulsion (Jia et al., Citation2015).
4. Conclusions
In this study, nanosuspensions (Cur-NSs) with various curcumin concentrations were prepared using a high-pressure homogenizer. Their physicochemical properties and antioxidant activities were evaluated using an emulsion system. The initial particle sizes of Cur-NSs containing high curcumin concentrations (0.30–0.50%) were smaller than those at low concentrations (0.05–0.15%), which was due to the higher collision frequency and efficiency between particles at higher concentrations during high-pressure homogenization. Storing Cur-NSs at 25°C, aggregation of curcumin particles was observed with increasing storage period. The aggregation rate decreased as the curcumin concentration increased, which was related to the initial particle size. This result was in good agreement with the Turbiscan analysis evaluating the dispersion stability. POV and 1H NMR results showed that depending on the curcumin concentration, Cur-NSs exhibited antioxidant activities in the lipid emulsion systems, especially when 200 mg/kg or more curcumin was added. The amount of peroxides, conjugated dienes, and aldehydes produced was low compared to the control. Presently, there are only few studies on the antioxidant activity of curcumin in emulsion systems reported, therefore there is a need to initiate more studies using alternative emulsion systems.
Author contributions
Sun-Hyung Kim: Data curation, Writing–Original draft preparation, Eui-Seok Lee: Writing–Reviewing and Editing, Ki-Teak Lee: Manuscript Reviewing, Soon-Taek Hong: Conceptualization, Methodology
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Acosta, E. (2009). Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Current Opinion in Colloid & Interface Science, 14(1), 3–15. https://doi.org/https://doi.org/10.1016/j.cocis.2008.01.002
- Ahmed, K., Li, Y., McClements, D. J., & Xiao, H. (2012). Nanoemulsion-and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chemistry, 132(2), 799–807. https://doi.org/https://doi.org/10.1016/j.foodchem.2011.11.039
- Ahsan, H., Parveen, N., Khan, N. U., & Hadi, S. M. (1999). Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chemico-biological Interactions, 121(2), 161–175. https://doi.org/https://doi.org/10.1016/S0009-2797(99)00096-4
- Ak, T., & Gülçin, İ. (2008). Antioxidant and radical scavenging properties of curcumin. Chemico-biological Interactions, 174(1), 27–37. https://doi.org/https://doi.org/10.1016/j.cbi.2008.05.003
- Apisariyakul, A., Vanittanakom, N., & Buddhasukh, D. (1995). Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae). Journal of Ethnopharmacology, 49(3), 163–169. https://doi.org/https://doi.org/10.1016/0378-8741(95)01320-2
- Basniwal, R. K., Buttar, H. S., Jain, V. K., & Jain, N. (2011). Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. Journal of Agricultural and Food Chemistry, 59(5), 2056–2061. https://doi.org/https://doi.org/10.1021/jf104402t
- Basniwal, R. K., Khosla, R., & Jain, N. (2014). Improving the anticancer activity of curcumin using nanocurcumin dispersion in water. Nutrition and Cancer, 66(6), 1015–1022. https://doi.org/https://doi.org/10.1080/01635581.2014.936948
- Bi, Y., Liu, J., Wang, J., Hao, J., Li, F., Wang, T., Sun H. W., & Guo, F. (2015). Particle size control and the interactions between drug and stabilizers in an amorphous nanosuspension system. Journal of Drug Delivery Science and Technology, 29, 167–172. https://doi.org/https://doi.org/10.1016/j.jddst.2015.07.012
- Chang, H. J., Shin, K. S., & Lee, J. H. (2019). Effects of emulsifier type on physical and oxidative stabilities of algae oil-in-water emulsions. International Journal of Food Science and Technology, 54(5), 1530–1540. https://doi.org/https://doi.org/10.1111/ijfs.13981
- Chen, H., Khemtong, C., Yang, X., Chang, X., & Gao, J. (2011). Nanonization strategies for poorly water-soluble drugs. Drug Discovery Today, 16(7), 354–360. https://doi.org/https://doi.org/10.1016/j.drudis.2010.02.009
- Chen, H., Weiss, J., & Shahidi, F. (2006, February, 28). Nanotechnology in nutraceuticals and functional foods. Food Technology, 60(3), 30–36. https://www.ift.org/news-and-publications/food-technology-magazine/issues/2006/march/features/nanotechnology-in-nutraceuticals-and-functional-foods
- Cutrin, J. C., Crich, S. G., Burghelea, D., Dastrù, W., & Aime, S. (2013). Curcumin/Gd loaded apoferritin: A novel “theranostic” agent to prevent hepatocellular damage in toxic induced acute hepatitis. Molecular Pharmaceutics, 10(5), 2079–2085. https://doi.org/https://doi.org/10.1021/mp3006177
- Duvoix, A., Blasius, R., Delhalle, S., Schnekenburger, M., Morceau, F., Henry, E., & Diederich, M. (2005). Chemopreventive and therapeutic effects of curcumin. Cancer Letters, 223(2), 181–190. https://doi.org/https://doi.org/10.1016/j.canlet.2004.09.041
- Frank, K. J., Westedt, U., Rosenblatt, K. M., Hölig, P., Rosenberg, J., Mägerlein, M., Brandl, M., & Brandl, M. (2014). What is the mechanism behind increased permeation rate of a poorly soluble drug from aqueous dispersions of an amorphous solid dispersion? Journal of Pharmaceutical Sciences, 103(6), 1779–1786. https://doi.org/https://doi.org/10.1002/jps.23979
- Gao, L., Zhang, D., & Chen, M. (2008). Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. Journal of Nanoparticle Research, 10(5), 845–862. https://doi.org/https://doi.org/10.1007/s11051-008-9357-4
- Gao, L., Zhang, M., Chen, T., Zheng, S., & Wang, S. (2007). Preparation and characterization of an oridonin nanosuspension for solubility and dissolution velocity enhancement. Drug Development and Industrial Pharmacy, 33(12), 1332–1339. https://doi.org/https://doi.org/10.1080/03639040701741810
- Gao, Y., Li, Z., Sun, M., Li, H., Guo, C., Cui, J., Zhai, G., Cao, F., Xi, Y., Lou, H., & Zhai, G. (2010). Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Development and Industrial Pharmacy, 36(10), 1225–1234. https://doi.org/https://doi.org/10.3109/03639041003695139
- Ghosh, D., Choudhury, S. T., Ghosh, S., Mandal, A. K., Sarkar, S., Ghosh, A., & Das, N. (2012). Nanocapsulated curcumin: Oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chemico-biological Interactions, 195(3), 206–214. https://doi.org/https://doi.org/10.1016/j.cbi.2011.12.004
- Holzgrabe, U. (2010). Quantitative NMR spectroscopy in pharmaceutical applications. Progress in Nuclear Magnetic Resonance Spectroscopy, 57(2), 229–240. https://doi.org/https://doi.org/10.1016/j.pnmrs.2010.05.001
- Hu, L., Jia, Y., Niu, F., Jia, Z., Yang, X., & Jiao, K. (2012). Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. Journal of Agricultural and Food Chemistry, 60(29), 7137–7141. https://doi.org/https://doi.org/10.1021/jf204078t
- Huang, H. C., Jan, T. R., & Yeh, S. F. (1992). Inhibitory effect of curcumin, an anti-inflammatory agent, on vascular smooth muscle cell proliferation. European Journal of Pharmacology, 221(2), 381–384. https://doi.org/https://doi.org/10.1016/0014-2999(92)90727-L
- Jang, D. J., Kim, S. T., Oh, E. C., & Lee, K. Y. (2014). Enhanced oral bioavailability and antiasthmatic efficacy of curcumin using redispersible dry emulsion. Bio-medical Materials and Engineering, 24(1), 917–930. https://doi.org/https://doi.org/10.3233/BME-130886
- Ji, H., Tang, J., Li, M., Ren, J., Zheng, N., & Wu, L. (2016). Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Delivery, 23(2), 459–470. https://doi.org/https://doi.org/10.3109/10717544.2014.918677
- Jia, C. H., Shin, J. A., & Lee, K. T. (2015). Effects of caffeic acid phenethyl ester and 4-vinylcatechol on the stabilities of oil-in-water emulsions of stripped soybean oil. Journal of Agricultural and Food Chemistry, 63(47), 10280–10286. https://doi.org/https://doi.org/10.1021/acs.jafc.5b02423
- Jia, C. H., Wang, X. Y., Qi, J. F., Hong, S. T., & Lee, K. T. (2016). Antioxidant properties of caffeic acid phenethyl ester and 4-vinylcatechol in stripped soybean oil. Journal of Food Science, 81(1), C35–C41. https://doi.org/https://doi.org/10.1111/1750-3841.13160
- Joung, H. J., Choi, M. J., Kim, J. T., Park, S. H., Park, H. J., & Shin, G. H. (2016). Development of food‐grade curcumin nanoemulsion and its potential application to food beverage system: Antioxidant property and in vitro digestion. Journal of Food Science, 81(3), N745–N753. https://doi.org/https://doi.org/10.1111/1750-3841.13224
- Joye, I. J., Davidov-Pardo, G., & McClements, D. J. (2014). Nanotechnology for increased micronutrient bioavailability. Trends in Food Science & Technology, 40(2), 168–182. https://doi.org/https://doi.org/10.1016/j.tifs.2014.08.006
- Junyaprasert, V. B., & Morakul, B. (2015). Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian Journal of Pharmaceutical Sciences, 10(1), 13–23. https://doi.org/https://doi.org/10.1016/j.ajps.2014.08.005
- Kabalnov, A. S., & Shchukin, E. D. (1992). Ostwald ripening theory: Applications to fluorocarbon emulsion stability. Advances in Colloid and Interface Science, 38, 69–97. https://doi.org/https://doi.org/10.1016/0001-8686(92)80043-W
- Kaialy, W., & Shafiee, M. A. (2016). Recent advances in the engineering of nanosized active pharmaceutical ingredients: Promises and challenges. Advances in Colloid and Interface Science, 228, 71–91. https://doi.org/https://doi.org/10.1016/j.cis.2015.11.010
- Kalang, V., & Valenta, C. (2011). Lecithin-based nanoemulsions. Journal of Drug Delivery Science and Technology, 21(1), 55–76. https://doi.org/https://doi.org/10.1016/S1773-2247(11)50006-1
- Keck, C. M., & Müller, R. H. (2006). Drug nanocrystals of poorly soluble drugs produced by high pressure homogenization. European Journal of Pharmaceutics, 62(1), 3–16. https://doi.org/https://doi.org/10.1016/j.ejpb.2005.05.009
- Kesisoglou, F., Panmai, S., & Wu, Y. (2007). Nano sizing—oral formulation development and biopharmaceutical evaluation. Advanced Drug Delivery Reviews, 59(7), 631–644. https://doi.org/https://doi.org/10.1016/j.addr.2007.05.003
- Kharat, M., & McClements, D. J. (2019). Recent advances in colloidal delivery systems for nutraceuticals: A case study – Delivery by design of curcumin. Journal of Colloid and Interface Science, 557, 506–518. https://doi.org/https://doi.org/10.1016/j.jcis.2019.09.045
- Kim, S. H., Ji, Y. S., Lee, E. S., & Hong, S. T. (2016). Ostwald ripening stability of curcumin-loaded MCT nanoemulsion: Influence of various emulsifiers. Preventive Nutrition and Food Science, 21(3), 289–295. https://doi.org/https://doi.org/10.3746/pnf.2016.21.3.289
- Lee, J. H., Lee, H. N., Shin, J. A., Chun, J. Y., Lee, J. S., & Lee, K. T. (2015). Content of fat-soluble nutrients (cholesterol, retinol, and α-tocopherol) in different parts of poultry meats according to cooking method. Journal of the Korean Society of Food Science and Nutrition, 44(2), 234–241. https://doi.org/https://doi.org/10.3746/jkfn.2015.44.2.234
- Liu, W., Zhai, Y., Heng, X., Che, F. Y., Chen, W., Sun, D., & Zhai, G. (2016). Oral bioavailability of curcumin: Problems and advancements. Journal of Drug Targeting, 24(8), 694–702. https://doi.org/https://doi.org/10.3109/1061186X.2016.1157883
- McClements, D. J. (2014). Particle characteristics and their impact on physicochemical properties of delivery systems. In D. J. McClements (Ed.), Nanoparticle-and microparticle-based delivery systems: Encapsulation, protection and release of active compounds (pp. 79–121). CRC Press.
- McClements, D. J. (2015). Emulsion stability. In D. J. McClements (Ed.), Food emulsions: Principles, practice, and techniques (3rd ed., pp. 289–382). CRC Press.
- McClements, J., & McClements, D. J. (2016). Standardization of nanoparticle characterization: Methods for testing properties, stability, and functionality of edible nanoparticles. Critical Reviews in Food Science and Nutrition, 56(8), 1334–1362. https://doi.org/https://doi.org/10.1080/10408398.2014.970267
- Mei, L., McClements, D. J., & Decker, E. A. (1999). Lipid oxidation in emulsions as affected by charge status of antioxidants and emulsion droplets. Journal of Agricultural and Food Chemistry, 47(6), 2267–2273. https://doi.org/https://doi.org/10.1021/jf980955p
- Mengual, O., Meunier, G., Cayré, I., Puech, K., & Snabre, P. (1999). TURBISCAN MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta, 50(2), 445–456. https://doi.org/https://doi.org/10.1016/S0039-9140(99)00129-0
- Moghadamtousi, S. Z., Kadir, H. A., Hassandarvish, P., Tajik, H., Abubakar, S., & Zandi, K. (2014). A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Research International. 2014. Article ID 186864. https://doi.org/https://doi.org/10.1155/2014/186864
- Mu, L., & Seow, P. H. (2006). Application of TPGS in polymeric nanoparticulate drug delivery system. Colloids and Surfaces. B, Biointerfaces, 47(1), 90–97. https://doi.org/https://doi.org/10.1016/j.colsurfb.2005.08.016
- Naksuriya, O., Okonogi, S., Schiffelers, R. M., & Hennink, W. E. (2014). Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials, 35(10), 3365–3383. https://doi.org/https://doi.org/10.1016/j.biomaterials.2013.12.090
- Peltonen, L., & Strachan, C. (2015). Understanding critical quality attributes for nanocrystals from preparation to delivery. Molecules, 20(12), 22286–22300. https://doi.org/https://doi.org/10.3390/molecules201219851
- Qi, X., Dong, Y., Wang, H., Wang, C., & Li, F. (2017). Application of turbiscan in the homoaggregation and heteroaggregation of copper nanoparticles. Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 535(2), 96–104. https://doi.org/https://doi.org/10.1016/j.colsurfa.2017.09.015
- Rachmawati, H., Shaal, L. A., Müller, R. H., & Keck, C. M. (2013). Development of curcumin nanocrystal: Physical aspects. Journal of Pharmaceutical Sciences, 102(1), 204–214. https://doi.org/https://doi.org/10.1002/jps.23335
- Ruby, A. J., Kuttan, G., Babu, K. D., Rajasekharan, K. N., & Kuttan, R. (1995). Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Letters, 94(1), 79–83. https://doi.org/https://doi.org/10.1016/0304-3835(95)03827-J
- Shin, G. H., Li, J., Cho, J. H., Kim, J. T., & Park, H. J. (2016). Enhancement of curcumin solubility by phase change from crystalline to amorphous in Cur‐TPGS nanosuspension. Journal of Food Science, 81(2), N494–N501. https://doi.org/https://doi.org/10.1111/1750-3841.13208
- Verma, S., Kumar, S., Gokhale, R., & Burgess, D. J. (2011). Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. International Journal of Pharmaceutics, 406(1), 145–152. https://doi.org/https://doi.org/10.1016/j.ijpharm.2010.12.027
- Wang, X., Jiang, Y., Wang, Y. W., Huang, M. T., Ho, C. T., & Huang, Q. (2008). Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chemistry, 108(2), 419–424. https://doi.org/https://doi.org/10.1016/j.foodchem.2007.10.086
- Wang, X. Y., Yang, D., Zhang, H., Jia, C. H., Shin, J. A., Hong, S. T., & Lee, K. T. (2014). Antioxidant activity of soybean oil containing 4-vinylsyringol obtained from decarboxylated sinapic acid. Journal of the American Oil Chemists’ Society, 91(9), 1543–1550. https://doi.org/https://doi.org/10.1007/s11746-014-2492-4
- Wang, Y., Zheng, Y., Zhang, L., Wang, Q., & Zhang, D. (2013). Stability of nanosuspensions in drug delivery. Journal of Controlled Release, 172(3), 1126–1141. https://doi.org/https://doi.org/10.1016/j.jconrel.2013.08.006
- Weiss, J., Takhistov, P., & McClements, D. J. (2006). Functional materials in food nanotechnology. Journal of Food Science, 71(9), R107–R116. https://doi.org/https://doi.org/10.1111/j.1750-3841.2006.00195.x
- Yu, H., & Huang, Q. (2012). Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. Journal of Agricultural and Food Chemistry, 60(21), 5373–5379. https://doi.org/https://doi.org/10.1021/jf300609p
- Yue, P. F., Wan, J., Wang, Y., Li, Y., Ma, Y. Q., Yang, M., & Wang, C. H. (2013). D-Alpha-tocopherol acid polyethylene glycol 1000 succinate, an effective stabilizer during solidification transformation of baicalin nanosuspensions. International Journal of Pharmaceutics, 443(1), 279–287. https://doi.org/https://doi.org/10.1016/j.ijpharm.2012.12.036
