 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Sorghum’s biological potential can be exploited to formulate healthy foods. The objective of this work was to optimize the germination process of sorghum to maximize the content of free phenolic compounds and antioxidant activity. According to the response surface methodology, the maximum content of total phenols and ferulic acid was obtained at 35°C and 28 h of germination, while the optimal antioxidant activity was obtained at 33.6°C and 28 h and 27.4°C 28 h measured as DPPH and TEAC respectively. The porridges obtained from germinated sorghum flours showed a higher content of total phenols and antioxidant activity compared to non-germinated ones. Porridges formulated with germinated sorghum flour showed an increase of 12% as compared with porridge formulated with flour non-germinated sorghum. Ferulic acid showed a significant reduction in both evaluated porridges.
RESUMEN
El potencial biológico de sorgo, puede ser aprovechado para formular alimentos saludables. El objetivo de este trabajo fue optimizar el proceso de germinación del sorgo para maximizar el contenido de compuestos fenólicos libres y actividad antioxidante. De acuerdo con la metodología de superficie de respuesta, el máximo contenido de fenoles totales y ácido ferúlico se obtuvo a 35°C y 28 h de germinación, mientras que la máxima actividad antioxidante se obtuvo a los 33.6°C y 28 h y 27.4°C 28 h medida como DPPH y TEAC respectivamente. Las papillas obtenidas a partir de harinas de sorgo germinadas mostraron un mayor contenido de fenoles totales y actividad antioxidante con respecto a las no germinadas. Las papillas formuladas con harina de sorgo germinado mostraron un aumento del 12% en comparación con las papillas formuladas con harina de sorgo no germinado. El ácido ferúlico mostró una reducción significativa en ambas papillas evaluadas.
1. Introduction
Sorghum [Sorghum bicolor (L.) Moench] is the fifth most important commercial cereal in the world being USA, India, Mexico, Nigeria, Sudan and Ethiopia the major producers with a production of 57.6 million tons (FAO, Citation2017). It is considered a staple food of 30 underdeveloped countries (Africa and Asia). Sorghum is used for the preparation of steamed, boiled, baked and fried products such as couscous, fermented porridges, pancakes, sorghum omelettes and alcoholic and fermented beverages. Meanwhile, in developed countries such as the United States, Japan and Australia, sorghum is used as livestock feed, however, there is currently an interest in this grain due to its nutritional and antioxidant characteristics (Afify et al., Citation2012; Ashok Kumar et al., Citation2011; Rakshit & Wang, Citation2016). Regular consumption of whole grains reduces the risk of heart disease and diabetes by 20–30%, improves the regulation of blood glucose levels and the control of weight, and decreases the risk of certain types of cancer (Flight & Clifton, Citation2006; Sahyoun et al., Citation2006). The mechanisms that could explain these effects are still unclear, but special interest has been paid to the study of phenolic compounds (Villa-Rodriguez et al., Citation2019). Sorghum has proven to be an excellent source of phenolic compounds, mainly ferulic acid, that exhibit beneficial impacts on human health according to in vitro and in vivo studies (Awika et al., Citation2009; Yang et al., Citation2009). However, their beneficial potential is limited because 80% of this compound is covalently bound to non-starch polysaccharides present in the cell walls of grain or structurally no available to digestion and absorption processes. Therefore, to improve the accessibility of this component, different methodologies, such as microwave treatment (Hithamani & Srinivasan, Citation2014), extrusion (Salazar-López et al., Citation2016) and fermentation (Zaroug et al., Citation2014), among others, have been performed, obtaining satisfactory results. Germination is a biological process that begins with the imbibition process (water absorption) by inactive grains and ends with the emergence of the embryonic axis, resulting in structural modifications and the novo synthesis of biomolecules, including phenolic compounds (Benincasa et al., Citation2015). Factors such as temperature and time can significantly influence several biochemical aspects during the germination process. Although the results of studies of the germination of sorghum on the release of bioactive compounds have varied due to different experimental conditions, to date, there are no optimization studies of the germination process in white sorghum grain that involve phenolic compounds, particularly, the ferulic acid content and antioxidant activity, as variable responses.
2. Materials and methods
White sorghum grain was provided by the Produce Nayarit Foundation, Mexico. The grain was cleaned manually for the elimination of impurities, and then seeds that did not have cracks or tears in the coat were selected. Once selected, the material was stored at 4–8°C.
2.1. Germination procedure
Prior to the optimization process, it was necessary to carry out a preliminary study to determine the effect of soaking time on germination time and phenolic content. Once the relevance of soaking the sorghum seeds was established, the germination study began. Briefly, sorghum seeds (25 g/treatment) were sanitized with 0.1% sodium hypochlorite at a ratio of 1:3 (w/v) for 5 min. Then, the sodium hypochlorite was removed, and the grains were rinsed with distilled water. The seeds were distributed into germination trays and placed in automatic germination equipment (Model 1000FAT, SEEDBURO). Germination was carried out with photoperiods of 8 h/light and 16 h/darkness and a relative humidity of 98% at different germination temperatures and times (). The sorghum seeds were watered every day with 2 mL of distilled water to maintain the moisture content. Once germination was carried out, the sorghum seeds were dried by lyophilization. The freeze-dried material was milled at a particle size <0.5 mm mesh and stored at 4°C until extraction.
Table 1. Experimental data for total phenols content (TPC), ferulic acid content (FA) and antioxidant activity (DPPH and TEAC) of sorghum germinated under different conditions1,2,3.
Tabla 1. Datos experimentales para contenido de fenoles totales (TPC), contenido de ácido ferúlico (FA) y actividad antioxidante (DPPH y TEAC) en sorgo germinado bajo diferentes condiciones1,2,3
2.2. Extraction of free phenolic compounds
To solubilize the free phenolic compounds contained in the sorghum samples, the procedure described by Salazar-López et al. (Citation2016) was used. Briefly, 1 g of each of the samples was weighed into 25 mL centrifuged tubes and homogenized with 15 mL of 80% methanol. The tubes were closed and sonicated for 1 h and then centrifuged at 100 × g for 15 min. The supernatants were removed, and the residues were extracted once again under the same conditions. Both supernatants for each sample were pooled and filtered with filter paper (Whatman No. 1). Then, the supernatants were evaporated to dryness in a rotary evaporator under vacuum and resuspended in 7 mL of 50% methanol. The concentration of the methanolic extracts was 0.14 g/mL.
2.3. Total phenolic compound determination
The total phenolic compounds of the germinated sorghum were quantified following the methodology proposed by Singleton and Rossi (Citation1965), with modifications to adapt the assay to a microplate reader (FluoStar Omega, BMG Labtech Inc., Ortenberg, Germany) (Salazar-López et al., Citation2016).
2.4. Quantification of free ferulic acid by UHPLC-DAD
Ferulic acid was quantified using a UHPLC (Ultra Performance Liquid Chromatography) system (Agilent Technologies 1260, USA) with a diode array detector (DAD). The separation was carried out on a Zorbax Eclipse Plus rapid resolution column (50 mm × 2.1 mm i.d.). A binary elution system consisting of water:acetic acid (99.9:0.1, phase A) and methanol:acetic acid (99.9:0.1, phase B) was implemented, and the gradient elution program was as follows: 0–9 min, 91% phase A; 9–11 min, 86% phase A; and 11–15 min, 84% phase A. The flow rate was 0.7 mL/min, and ferulic acid was detected at 280 nm according to the retention time. Quantification was achieved with a ferulic acid reference curve of several concentrations. The results were expressed as μg ferulic acid/g of sample (Salazar-López et al., Citation2016).
2.5. Antioxidant capacity determination
The TEAC and DPPH assays are based on the ability of antioxidant molecules to capture the cationic radical ABTS●+ and DPPH respectively and both TEAC and DPPH assays were adapted to a microplate reader (FluoStar Omega, BMG Labtech Inc.) following the procedure reported by Salazar-López et al. (Citation2017), (Citation2018)).
2.6. Preparation procedure of porridge from germinated sorghum flour
For porridge preparation, flour produced from sorghum grain that was germinated under optimal conditions (35°C and 28 h of germination) was used. In a bowl, 35 g of flour was placed and homogenized in cow’s milk (50 mL). After this, 200 mL of previously heated cow’s milk was added to the mixture and then cooked at 80°C for 7 min with constant stirring. The same procedure was used to prepare a porridge with flour from non-germinated sorghum. Samples were cooled at room temperature, after which the phenolic content, ferulic acid and antioxidant activity analyses were evaluated as described above.
2.7. Experimental design and statistical analysis
To evaluate the conditions of sorghum germination for optimizing the total phenol and ferulic acid content and antioxidant activity, a two-factor and three-level, face-centered central composite design that included five central points was used. The factors evaluated were the germination temperature (X1, 20, 27.5 and 35°C) and time (X2, 10, 19 and 28 h); the maximum and minimal coded values were assigned at each factor with corresponding true values. Thirteen experiments were performed according to the experimental design, and a repetition of the experiment was considered (). These combinations of treatments are based in the central composite design for optimization. It is a “face centered” design which means that there are no axial points due to logistics of experimental conditions. The range for each factor (time and temperature) are critical since the purpose is to obtain a germinated seed which favors the maximum of phenolic compounds that have antioxidant properties. There is a constraint to germination phase, thus a limit to time and also temperature. To analyze the experimental data, regression analysis by response surface methodology was used. Experimental data were fitted to a second-order polynomial model, and regression coefficients were obtained; the model is shown in the following Equationequation (1)(1)
(1) :
where Y is the predicted response variable (total phenolic compound content, ferulic acid content and antioxidant activity), β0 (intercept), β1 and β2 (linear terms) are regression coefficients, β11 and β22 (quadratic terms) and β12 (interaction term), and X1 and X2 are the independent variables. All experimental runs were carried out in triplicate. The statistical significance of the terms in the regression equations was examined by variance analysis (ANOVA) for each response variable. The goodness of fit of the obtained models was evaluated by calculating the coefficients of multiple determination (R2). The data were analyzed by Minitab statistical software (Minitab LLC, State College, Pennsylvania, USA), and statistical software (STATISTICA 10, TIBCO, Palo Alto, California, USA) was used to generate the response surface and contour plots.
A validation test was carried out using the optimal conditions obtained by the prediction models, using the variables of total phenolic compound content, ferulic acid and antioxidant activity.
3. Results and discussion
3.1. Optimal germination conditions
Many studies have been conducted to optimize germination conditions by evaluating phenolic compounds as a response, these studies have been carried out on fruits and several cereals, such as wheat, corn and barley, but not on sorghum. To optimize the germination conditions, the maximal and minimal levels of two factors were established: time and temperature (). In the same the average values obtained from the evaluated treatments for all response variables are shown. The results of ANOVA showed that the selected quadratic models adequately represented the data obtained for all responses (). Multiple regression coefficients were determined using the least squares technique to predict the quadratic polynomial models for the TPC content, FA content, DPPH radical scavenging activity and TEAC of the germinated sorghum. The maximum predictable response for the variables was obtained based on a total of 13 experiments required for determining 6 regression coefficients; these coefficients indicate the goodness of fit of the predictive model. The results suggested that the quadratic term of temperature had a negligible effect on the total phenolic content (p > .05). The model of the ferulic acid content showed that all terms of the second-order polynomial equation had a significant influence on this response variable, indicating that both time and temperature strongly influence the ferulic acid content in germinated sorghum. The value observed for the determination coefficient (R2 = 0.95) and the adjusted determination coefficient (R2 = 0.94) demonstrated the adequacy of the quadratic model for explaining the variation in the content of this phytochemical as a result of the germination conditions.
Table 2. Coefficients of equations (prediction models), analysis of variance and determination coefficients, showing the relationship among the factors (Temperature and Time) and the total phenols content (TPC), ferulic acid content (FA) and antioxidant activity (DPPH and TEAC) of germinated sorghum.
Tabla 2. Coeficientes de las ecuaciones (modelos de predicción), análisis de varianza y coeficientes de determinación que muestran la relación entre los factores (Temperatura y tiempo) y contenido de fenoles totales (TPC), contenido de ácido ferúlico (FA) y actividad antioxidante (DPPH y TEAC) en sorgo germinado
The regression analysis applied to the DPPH and TEAC results showed that the models were significant (p < .001), the lack of fit was significant at p < .001, and the models could explain 88% and 90% respectively.
The values predicted by the polynomial equations were optimized for each of the dependent variables. In the case of the total phenolic and ferulic acid contents, 35°C and 28 hr were the conditions that maximized their content. In the case of the antioxidant activity measured by DPPH and TEAC, the optimal conditions were 33.6°C and 28 hr and 27.4°C and 28 hr, respectively. The critical values in terms of uncoded variables for total phenolic compound content, ferulic acid content and antioxidant activity are given in . In this table, it is also possible to observe the values for each response variable obtained from the validation test. The relationship between independent and dependent variables is illustrated in a three-dimensional representation on the response surface and two-dimensionally in contour plots generated by the prediction models. shows the quadratic effect of time on the total phenolic compound content. The total phenolic compound content showed low values during the first hours of germination until reaching maximum values at increased germination times. Since the quadratic term of temperature was not significant, the total phenolic compound content increased linearly with increasing time. it was observed that ferulic acid content and antioxidant activity (DPPH and TEAC) increased at low and high germination times and temperature. For these response variables, maximal values were found at a high germination time and temperature.
Table 3. Comparison between predicted values and experimental values for total phenols content (TPC), ferulic acid content (FA) and antioxidant activity (DPPH and TEAC).
Tabla 3. Comparación entre los valores predichos y los valores experimentales para contenido de fenoles totales (TPC), contenido de ácido ferúlico (FA) y actividad antioxidante (DPPH y TEAC)
Figure 1. Response surfaces and contour plots for TPC (Total phenols content) (a), Ferulic acid (b), antioxidant activity determined by the DPPH (c), and TEAC (d) assays in sorghum as a function of germination time and temperature.
Figura 1. Superficies de respuesta y diagramas de contorno para contenido de fenoles totales (a), Ácido Ferúlico (b), actividad antioxidante determinada por los ensayos de DPPH (c), y y TEAC (d) en sorgo en función del tiempo y la temperatura de germinación
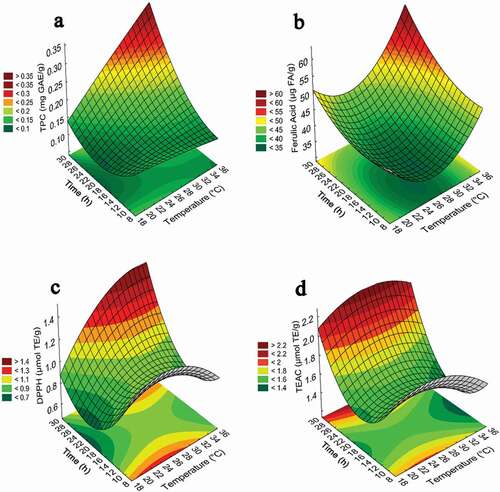
Regarding the optimal temperature and time as conditions to increase phytochemical content, our results were different from those reported by other authors (Paucar-Menacho et al., Citation2017a; Paucar‐Menacho et al., Citation2018; Paucar-Menacho et al., Citation2017b), who reported germination studies for several grains in which germination temperature and time were evaluated as factors that maximize the antioxidant activity, phenolic compound content and GABA (γ-amino butyric acid) content. The results were as follows for amaranth, quinoa and blue corn: 26°C and 63 hr, 20°C and 42 hr, and 26°C and 63 hr, respectively. For these grains, it was found that a low temperature combined with a long germination time were the optimal conditions to obtain maximal phytochemical values. Several authors mention that during seed germination, dramatic changes in their chemical composition can occur, reflecting the dynamic and complex flow of nutrients and phytochemicals, including mobilization, degradation and accumulation (Barnes & Anderson, Citation2018). Therefore, it is difficult to establish comparisons with other studies regarding factors that could affect phenolic and other bioactive compounds among different cereal grains. Donkor et al. (Citation2012) found results in sorghum germinated at 16°C for 120 hr; in this case, the ferulic acid content showed no significant changes in comparison with that of the non-germinated sorghum. Dicko et al. (Citation2005) reported a study of the germination of 50 different varieties of sorghum and found that the phenolic content varied in response to germination conditions (27°C/72 hr), showing increases of up to 65% and 75% in some varieties and decreases of 24% and 37% in others. An optimized amaranth germination study at 30°C and 28 hr concluded that the antioxidant activity (ORAC and TEAC) and total phenolic compound content increased by up to 300%, 470% and 829%, respectively, compared with those of non-germinated amaranth (Perales-Sánchez et al., Citation2014). Several situations can contribute to the observed increase in free total phenolic compound content and ferulic acid content in germinated sorghum. One of them could be that the metabolic activation of grain by the imbibition process promotes the increase of the respiration rate in grains, which gives rise to energy demand and, consequently, the utilization of structures such as starch, lipids and proteins by activation of degradative enzymes, such as α-amylase, lipases and proteases; this results in a degradation of the food matrix and, likely, the release of bioactive substances, among them, phenolic compounds and ferulic acid. Barnes and Anderson (Citation2018) also mentioned that during the development of the seed, there is a process of building and rebuilding structures; in particular, the cell wall is rich in xylans and galactomannans, which are modified, lost or changed in terms of extensibility to promote or stop germination, causing the combination or release of phenols. Temperature is one of the factors that can regulate the biosynthesis and content of phenols, and the increase in these factors could be associated with seed adaptation to temperature regimes, resulting in synergistic biochemical compositions (Tesfay et al., Citation2016). Although cereals form a relevant part of the human diet, their importance regarding their biological potential has not yet been emphasized. First, fruits have traditionally been studied extensively over several decades for their influence on health aspects, likely because they are mostly consumed raw, whereas cereals should be processed before eaten. Processing, such as milling, decortication, baking, cooking, and sprouting, among others, could markedly modify the bioactive properties of grains, making it harder for phenolic profile characterization. However, scientific evidence has shown that cereals can be promoters of beneficial changes in several pathologies even more so than those shown by the consumption of some fruits (Fardet, Citation2010; Gong et al., Citation2018). The content of total phenolic compounds in sorghum is low compared with that in berries, which have the highest content of total phenols, with values ranging between 3.05 and 8.50 mg GAE/g (Nile & Park, Citation2014; De Souza et al., Citation2014). These values are 27–77 times higher than those of non-germinated sorghum and 11–27 times higher than those of sorghum germinated under optimized conditions. However, cereals, such as sorghum, could compete with fruits other than berries, which exhibit total phenolic compound contents ranging from 0.11 to 5.85 mg GAE/g (Fu et al., Citation2011). In particular, the cereal group is characterized by a moderate total phenolic compound content (<1.0 mg GAE/g) (Deng et al., Citation2012), but it is relevant to consider that the synergistic action of the compounds mainly contained in the bran and germ of cereals could act as protective agents (Fardet, Citation2010; Liu, Citation2007).
3.2. Germinated sorghum flour porridges
To evaluate the biological potential of germinated sorghum-based products, thin porridges were prepared from germinated and non-germinated sorghum flours. As shown in , it is possible to observe that, with the exception of ferulic acid content and independent of grain condition (non-germinated or germinated), the cooking process improved the total phenolic compound content and antioxidant activity. It was observed that germinated porridge showed a higher total phenolic compound content than that of the non-germinated sorghum porridge (0.43 ± 0.02 mg GAE/g vs 0.47 ± 0.008 mg GAE/g + 10%), a higher DPPH radical scavenging activity (3.22 ± 0.20 µmolTE/g vs 3.66 0.10 ± 0.57 0.04 µmolTE/g + 12%) and a higher TEAC (2.42 ± 0.18 µmolTE/g vs 2.77 ± 0.15 µmolTE/g + 12%) (). It is possible that the cooking process could have enhanced the extraction of phenols from the food matrix. Cooking has been reported to soften the cell wall and other components of cells, such as vacuoles, releasing phenolic compounds (Chinedum et al., Citation2018).
Figure 2. Total phenols (a); Ferulic acid (b); DPPH (c) and TEAC (d) of porridges prepared from non-germinated and germinated sorghum flours. Bars with different letters between porridges are significantly different (p < .05). Black bars corresponding to non-germinated sorghum flours and germinated sorghum flours (predicted values).
Figura 2. Fenoles totales (a); Ácido Ferúlico (b); DPPH (c) y TEAC (d) en papillas preparadas a partir de harinas de sorgo germinado y no germinado. Letras diferentes sobre las barras entre papillas son significativamente diferentes (p < .05). Barras negras corresponden a las harinas de sorgo no germinado y germinado (valores predichos)
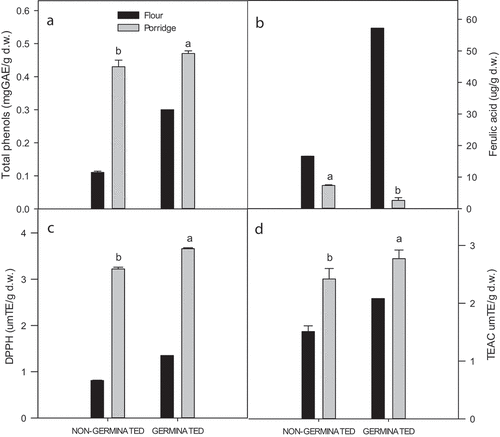
This is the first time that changes in bioactive compounds in germinated sorghum intended for thin porridges have been reported. As already described above, the process of the germination of sorghum improved the content of ferulic acid. However, this effect was not reflected in the cooked product from both non-germinated and germinated grains, and lower values of this phenol were found in the porridge prepared from the germinated grain than in that prepared from the non-germinated grain. It is possible to attribute this fact to the germination process in sorghum grain releasing a high amount of ferulic acid that could be available to interact with other components, such as proteins, lipids or carbohydrates, the interactions of which were favored in the cooking process. These interactions could be chemical (for example, conjugation, covalent bonding or hydrogen bonding) or electrostatic. On the other hand, it is possible that dimerization reactions of ferulic acid were promoted by the cooking process.
3.3. Conclusion
The optimization of the germination process at 35°C and 28 hr in sorghum increased the contents of phenolic compounds and ferulic acid, while the optimum antioxidant activity, as determined by DPPH and TEAC assays, was achieved at 33.6°C and 28 hr and 27.4°C and 28 hr, respectively. Additionally, the conventionally prepared germinated sorghum thin porridge had a higher total phenolic compound content and antioxidant activity than those of the porridge prepared from non-germinated sorghum, but the ferulic acid content was lower. It appeared that other phenolic compounds were released during the cooking process that could contribute to the antioxidant activity. We concluded that optimization of sorghum germination is a viable option for the development of foods with improved potential biological properties. Studies on the bio-accessibility of phenolic compounds that are affected by germination and cooking processes are in progress.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Ruiz Hernández, A.A. received a scholarship from CONACyT (National Research and Technology Council), Mexico.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Afify, A. E. M. M., El-Beltagi, H. S., Abd El-Salam, S. M., & Omran, A. A. (2012). Biochemical changes in phenols, flavonoids, tannins, vitamin E, β–carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pacific Journal of Tropical Biomedicine, 2(3), 203–209. https://doi.org/https://doi.org/10.1016/S2221-1691(12)60042-2
- Ashok Kumar, A., Reddy, B. V. S., Sharma, H. C., Hash, C. T., Srinivasa Rao, P., Ramaiah, B., & Reddy, P. S. (2011). Recent advances in sorghum genetic enhancement research at ICRISAT. American Journal of Plant Sciences, 2(4), 589–600. https://doi.org/http://dx.doi.org/10.4236/ajps.2011.24070
- Awika, J. M., Yang, L., Browning, J. D., & Faraj, A. (2009). Comparative antioxidant, antiproliferative and phase II enzyme inducing potential of sorghum (Sorghum bicolor) varieties. LWT - Food Science and Technology, 42(6), 1041–1046. https://doi.org/https://doi.org/10.1016/j.lwt.2009.02.003
- Barnes, W. J., & Anderson, C. T. (2018). Release, recycle, rebuild: Cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Molecular Plant, 11(1), 31–46. https://doi.org/https://doi.org/10.1016/j.molp.2017.08.011
- Benincasa, P., Galieni, A., Manetta, A. C., Pace, R., Guiducci, M., Pisante, M., & Stagnari, F. (2015). Phenolic compounds in grains, sprouts and wheatgrass of hulled and non‐hulled wheat species. Journal of the Science of Food and Agriculture, 95(9), 1795–1803. https://doi.org/https://doi.org/10.1002/jsfa.6877
- Chinedum, E., Sanni, S., Theressa, N., & Ebere, A. (2018). Effect of domestic cooking on the starch digestibility, predicted glycemic indices, polyphenol contents and alpha amylase inhibitory properties of beans (Phaseolis vulgaris) and breadfruit (Treculia africana). International Journal of Biological Macromolecules, 106(2018), 200–206. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2017.08.005
- de Souza, V. R., Pereira, P. A. P., Da Silva, T. L. T., de Oliveira Lima, L. C., Pio, R., & Queiroz, F. (2014). Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chemistry, 156(2014), 362–368. https://doi.org/https://doi.org/10.1016/j.foodchem.2014.01.125
- Deng, G. F., Xu, X. R., Guo, Y. J., Xia, E. Q., Li, S., Wu, S., Chen, F., Ling, W. H., & Li, H. B. (2012). Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. Journal of Functional Foods, 4(4), 906–914. https://doi.org/https://doi.org/10.1016/j.jff.2012.06.008
- Dicko, M. H., Gruppen, H., Traoré, A. S., van Berkel, W. J., & Voragen, A. G. (2005). Evaluation of the effect of germination on phenolic compounds and antioxidant activities in sorghum varieties. Journal of Agricultural and Food Chemistry, 53(7), 2581–2588. https://doi.org/https://doi.org/10.1021/jf0501847
- Donkor, O. N., Stojanovska, L., Ginn, P., Ashton, J., & Vasiljevic, T. (2012). Germinated grains–Sources of bioactive compounds. Food Chemistry, 135(3), 950–959. https://doi.org/https://doi.org/10.1016/j.foodchem.2012.05.058
- FAO. (2017). FAOSTAT: Crops.Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data/QC
- Fardet, A. (2010). New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutrition Research Reviews, 23(1), 65–134. https://doi.org/https://doi.org/10.1017/S0954422410000041
- Flight, I., & Clifton, P. (2006). Cereal grains and legumes in the prevention of coronary heart disease and stroke: A review of the literature. European Journal of Clinical Nutrition, 60(10), 1145–1159. https://doi.org/https://doi.org/10.1038/sj.ejcn.1602435
- Fu, L., Xu, B. T., Xu, X. R., Gan, R. Y., Zhang, Y., Xia, E. Q., & Li, H. B. (2011). Antioxidant capacities and total phenolic contents of 62 fruits. Food Chemistry, 129(2), 345–350. https://doi.org/https://doi.org/10.1016/j.foodchem.2011.04.079
- Gong, L., Cao, W., Chi, H., Wang, J., Zhang, H., Liu, J., & Sun, B. (2018). Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Research International, 103(84–102), 84–102. https://doi.org/https://doi.org/10.1016/j.foodres.2017.10.025
- Hithamani, G., & Srinivasan, K. (2014). Bioaccessibility of polyphenols from wheat (Triticum aestivum), sorghum (Sorghum bicolor), green gram (Vigna radiata), and chickpea (Cicer arietinum) as influenced by domestic food processing. Journal of Agricultural and Food Chemistry, 62(46), 11170–11179. https://doi.org/https://doi.org/10.1021/jf503450u
- Liu, R. H. (2007). Whole grain phytochemicals and health. Journal of Cereal Science, 46(3), 207–219. https://doi.org/https://doi.org/10.1016/j.jcs.2007.06.010
- Nile, S. H., & Park, S. W. (2014). Edible berries: Bioactive components and their effect on human health. Nutrition, 30(2), 134–144. https://doi.org/https://doi.org/10.1016/j.nut.2013.04.007
- Paucar-Menacho, L. M., Martinez-Villaluenga, C., Dueñas, M., Frias, J., & Peñas, E. (2017a). Optimization of germination time and temperature to maximize the content of bioactive compounds and the antioxidant activity of purple corn (Zea mays L.) by response surface methodology. LWT-Food Science and Technology, 76(2017), 236–244. https://doi.org/https://doi.org/10.1016/j.lwt.2016.07.064
- Paucar‐Menacho, L. M., Martínez‐Villaluenga, C., Dueñas, M., Frias, J., & Peñas, E. (2018). Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. International Journal of Food Science & Technology, 53(2), 516–524. https://doi.org/https://doi.org/10.1111/ijfs.13623
- Paucar-Menacho, L. M., Peñas, E., Dueñas, M., Frias, J., & Martinez-Villaluenga, C. (2017b). Optimizing germination conditions to enhance the accumulation of bioactive compounds and the antioxidant activity of kiwicha (Amaranthus caudatus) using response surface methodology. LWT-Food Science and Technology, 76(2017), 245–252. https://doi.org/https://doi.org/10.1016/j.lwt.2016.07.038
- Perales-Sánchez, J. X., Reyes-Moreno, C., Gómez-Favela, M. A., Milán-Carrillo, J., Cuevas-Rodríguez, E. O., Valdez-Ortiz, A., & Gutiérrez-Dorado, R. (2014). Increasing the antioxidant activity, total phenolic and flavonoid contents by optimizing the germination conditions of amaranth seeds. Plant Foods for Human Nutrition, 69(3), 196–202. https://doi.org/https://doi.org/10.1007/s11130-014-0430-0
- Rakshit, S., & Wang, Y.-H. (2016). The sorghum genoma (1st ed.). Springer.
- Sahyoun, N. R., Jacques, P. F., Zhang, X. L., Juan, W., & McKeown, N. M. (2006). Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. The American Journal of Clinical Nutrition, 83(1), 124–131. https://doi.org/https://doi.org/10.1093/ajcn/83.1.124
- Salazar-López, N. J., Astiazarán-García, H., González-Aguilar, G. A., Loarca-Piña, G., Ezquerra-Brauer, J. M., Domínguez Avila, J. A., & Robles-Sánchez, M. (2017). Ferulic acid on glucose dysregulation, dyslipidemia, and inflammation in diet-induced obese rats: An integrated study. Nutrients, 9(7), 675. https://doi.org/https://doi.org/10.3390/nu9070675
- Salazar-López, N. J., González-Aguilar, G. A., Rouzaud-Sández, O., & Robles-Sánchez, M. (2018). Bioaccessibility of hydroxycinnamic acids and antioxidant capacity from sorghum bran thermally processed during simulated in vitro gastrointestinal digestion. Journal of Food Science and Technology, 55(6), 2021–2030. https://doi.org/https://doi.org/10.1007/s13197-018-3116-z
- Salazar-López, N. J., Loarca-Piña, G., Campos-Vega, R., Gaytán-Martínez, M., Morales-Sánchez, E., Esquerra-Brauer, J. M., & Robles-Sánchez, M. (2016). The extrusion process as an alternative for improving the biological potential of sorghum bran: Phenolic compounds and antiradical and anti-inflammatory capacity. Evidence-Based Complementary and Alternative Medicine, 2016. Article ID 8387975. https://doi.org/https://doi.org/10.1155/2016/8387975
- Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158. https://doi.org/https://doi.org/10.1007/s11101-009-9145-5
- Tesfay, S. Z., Modi, A. T., & Mohammed, F. (2016). The effect of temperature in moringa seed phytochemical compounds and carbohydrate mobilization. South African Journal of Botany, 102(2016), 190–196. https://doi.org/https://doi.org/10.1016/j.sajb.2015.07.003
- Villa-Rodriguez, J. A., Ifie, I., Gonzalez-Aguilar, G. A., & Roopchand, D. E. (2019). The gastrointestinal tract as prime site for cardiometabolic protection by dietary polyphenols. Advances in Nutrition, 10(6), 999–1011. https://doi.org/https://doi.org/10.1093/advances/nmz038
- Yang, L., Browning, J. D., & Awika, J. M. (2009). Sorghum 3-deoxyanthocyanins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. Journal of Agricultural and Food Chemistry, 57(5), 1797–1804. https://doi.org/https://doi.org/10.1021/jf8035066
- Zaroug, M., Orhan, I. E., Senol, F. S., & Yagi, S. (2014). Comparative antioxidant activity appraisal of traditional Sudanese kisra prepared from two sorghum cultivars. Food Chemistry, 156(2014), 110–116. https://doi.org/https://doi.org/10.1016/j.foodchem.2014.01.069
