ABSTRACT
In this study, propolis extract emulsions were used in chitosan coatings to keep the storage quality of crayfish meat during shelf-life (16 days). Crayfish meats were coated with a solution of chitosan containing propolis extract emulsions (0.3 and 0.6% v/v) and then stored at 4 C. Coatings protective effectiveness was determined by chemical analyses (pH, Thiobarbituric acid, Peroxide value, Total volatile basic nitrogen, and K values), microbiological (Total aerobic mesophilic bacteria, Psychrotrophic bacteria H2S-producing bacteria, Yeasts- Moulds), and sensorial attributes (Odor, Taste, Firmness and Overall acceptance). Results demonstrate that chitosan coatings containing propolis extract are effective in controlling the growth of bacteria and chemical indices. These results can be beneficial for seafood processing sectors, as well as for food technologists.
RESUMEN
En el presente estudio se utilizaron emulsiones de extracto de propóleo en recubrimientos de quitosano destinados a mantener la calidad de la carne de cangrejo de río durante su almacenamiento en su tiempo de vida útil (16 días). Para ello, se recubrió la carne de cangrejo de río con una solución de quitosano que contenía emulsiones de extracto de propóleo (0.3 y 0.6% v/v) y posteriormente se la almacenó a 4°C. La eficacia protectora de los recubrimientos se determinó realizando análisis químicos (pH, ácido tiobarbitúrico, valor de peróxido, nitrógeno básico volátil total y valores K), microbiológicos (bacterias mesófilas aeróbicas totales, bacterias psicrotróficas productoras de H2S, levaduras y mohos) y sensoriales (olor, sabor, firmeza y aceptación general). Los resultados permiten constatar que los recubrimientos de quitosano que contienen extracto de propóleo son eficaces para controlar el crecimiento de bacterias y los índices químicos. Estos resultados pueden ser beneficiosos para el sector de procesamiento de mariscos, así como para los tecnólogos de alimentos.
1. Introduction
Astacus leptodactylus are commonly found in lakes, ponds, and rivers in Turkey and low-fat, low-calorie, and rich in protein. However, it is susceptible to spoilage just like fish and other seafood and should be consumed fresh immediately after harvest, or measures should be taken to preserve its original freshness as much as possible. Therefore, adequate packaging technologies need to be developed for the preservation of fish or other foods (Bahadır Koca & Argun Uzunmehmetoğlu, Citation2018; Ojagh et al., Citation2010).
Synthetic preservatives are reported to delay the lipid peroxidation process and increase the shelf life of foods. But, synthetic additives can be affected by consumer health, so consumers tend to avoid foods prepared with chemical preservatives (Yazgan et al., Citation2020). Propolis is a natural product collected by honeybees from plants especially flowers and buds. Withal, it is included in the GRAS list and is used as a bioactive food Even though the composition of propolis changes depending on its source, it generally consists of 50% resin, 30% wax, 10% essential and aromatic oils, 5% pollen, 5% other organic compounds and mineral substances supplement. The main active ingredients of propolis are benzoic acid, cinnamic acid, phenols, ketophenols, hydroquinone, coumarins, and naphthalene (Galeotti et al., Citation2018).
Some researchers have reported that propolis can be used as a resource natural antioxidant and antimicrobial in meat and fish products (Gutierrez-Cortes & Suarez Mahecha, Citation2014; Hascik et al., Citation2015; Uçak, Citation2018). Moreover, many studies have reported that chitosan films or coatings enriched with plant extracts or essential oils improve the quality and shelf life of foods (Cai et al., Citation2018; Fadıloğlu & Emir Çoban, Citation2018; Fan et al., Citation2009; Shahbazi & Shavisi, Citation2018). However, according to our literature seek, there is not found any information on the application of propolis extract/chitosan coating to crayfish meat. Therefore, this study was planned to evaluate the effect of chitosan/propolis extract emulsion coating on the refrigerated storage quality and shelf life of crayfish (Astacus leptodactylus) meat.
2. Materials and methods
2.1. Materials
Chitosan (medium molecular weight: 190–310 kDa; deacetylation degree: 75–85%, viscosity of 200–800 cPs) and Tween-80 were purchased from Sigma-Aldrich. Propolis liquid extract (≥98%, food-grade, FDA approved) was obtained from a commercial company (Talya herbal product Co., Turkey). All other reagents were procured in analytical grade.
2.2. Preparation of chitosan-based coating-forming emulsions
The propolis emulsion was prepared using the method of Wu et al. (Citation2018). Propolis extract (0.3 and 0.6% v/v) and 2 g of surfactant Tween 80 were mixed and added 50 mL of deionized water, and then stirred for 1 h at 25°C. Afterward, again deionized water was added 50 mL. Propolis emulsions were acquired by stirring 6 h at 25°C
Chitosan/propolis extract emulsion coating was prepared according to the method of Sun et al. (Citation2019) a minor change. 2% (w/v) chitosan and 1% glycerol were dissolved in prepared propolis emulsions (100 mL) at 25 °C and stirred for 2 h to obtain a homogeneous coating solution. The chitosan coatings were coded as Ch, Ch-0.3% PE and Ch-0.6% PE.
2.3. Preparation sample treatments
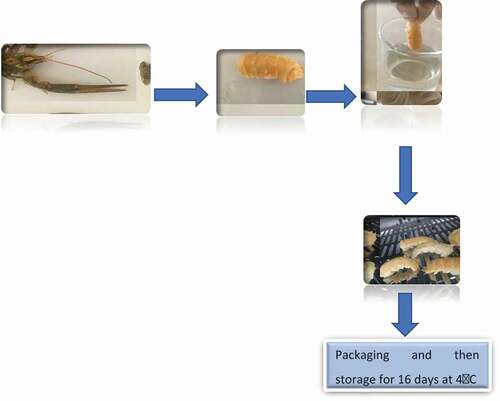
A total of 5 kg of live Crayfish of the same size (8–9 cm) obtained from Keban Dam Lake in Elazığ were transferred to the laboratory under aseptic and cold conditions in 1 h. They were washed with tap water and boiled for 10 minutes at 100°C. After boiling, crayfishes were cooled and separated from shells. The deshelled crayfish were randomly divided into four groups: Cont (uncoated), Ch, Ch-0.3% PE and Ch-0.6% PE. The crayfish were individually immersed in the coating solutions for 2 min (crayfish/coating solution ratio: 1/3). Then, the coated crayfish were dried for 1 h in a cold air cabinet set at 10°C to form the edible coating and were stored at 4°C. All samples were separately packed in a sterile polyethylene bag and stored for 16 days (). The present study was conducted as two parallels.
2.4. Chemical analysis
Ten grams of crayfish meat were homogenized in 100 mL of sterile distilled water. The pH was measured in triplicate at room temperature, according to the method reported by Gokalp et al. (Citation2001). The total volatile base nitrogen (TVB-N) value was determined by using an automatic Kjeldahl apparatus according to the micro-titration methodology reported by Gharibzahedi and Mohammadnabi (Citation2017). The TVB-N value was stated as mg/100 g fish muscle thiobarbituric acid (TBA) value was measured using the method reported by Kilic and Richards (Citation2003). Spectrophotometric measurements were made based on the principle of malondialdehyde in samples to react with TBA reagent. The TBA values were described as mg MDA/kg seafood flesh. The PV value was assigned using the method of Shantha and Decker (Citation1994). 2 g sample was mixed in 30 ml chloroform-glacial acetic acid solution (chloroform/glacial acetic acid, 3/2). Then 1 ml of saturated potassium iodide (KI) solution was added and mixed again. Subsequently, it was kept in the dark for 5 minutes, and 75 ml of pure water and 1 ml of starch solution were added and titrated with 0.1 M sodium thiosulphate (Na2S2O3) solution. The K-value was determined by an HPLC using 150 × 4.60 mm column (a Merck-Hitachi Model D-6500). The extraction procedure was based on that by Fan et al. (Citation2008).
2.5. Microbiological analysis
Ten grams of crayfish meat were taken, transferred aseptically to a sterile stomacher bag containing 90 ml of buffered peptone water, and mixed for 1 min using a Stomacher blender (Lab Stomacher Blender 400-BA 7021 Seward Medical, UK). Then, decimal dilutions (1:10, diluent: 0.1% peptone water) were prepared and inoculated on agar plates to determine:
the total mesophilic aerobe bacteria (TMAB) and psychrotrophic bacteria (PB) counts on pour plates of plate count agar incubated for 2 days at 30°C and 10 days at 5°C, respectively;
b) H2S-producing bacteria count assigned using iron agar plates incubated for 2 days at 30°C;
yeasts and molds determined using potato dextrose agar (PDA) and incubated for 5 days at 22°C. All counts were expressed as log CFU/g (Halkman, Citation2005).
2.6. Sensory evaluation
The sensorial attributes of crayfish meat were determined according to the method reported by Cai et al. (Citation2015). Seven experienced panelists measured important quality parameters, such as odor, taste, firmness, and overall acceptable using a ten-grade hedonic score system about crayfish meat: 10.0–9.0 (excellent), 8.9–7.0 (good), 6.9–4.9 (fair), and 3.0–1.0 (rejectable).
2.7. Statistical evaluation
The IBM SPSS®26 (SPSS Inc., Chicago, IL, USA) statistical package was used for data processing. The statistical significance of the differences between the groups and storage days of the chemical and microbiological data determined as a result of the analyses carried out in the study was determined using variance analysis (ANOVA)and Duncan’s multiple range tests. Differences were regarded as statistically significant at p < .05. All experiments were replicated two times for all groups.
3. Results and discussion
3.1. Chemical quality
The pH of the crayfish meat used in this study was 5.72. In all groups, the pH value reduced at first but then increased throughout storage (). The reason reduced in the pH value of samples can be caused by the formation of lactic acid from glycogen in fish muscles (Fan et al., Citation2008; Manju et al., Citation2007). In contrast, the rising pH value was reported to adhere to the release of protein metabolites, like ammonia and tri-methylamine by the activity of deterioration bacteria. While for the Ch coatings containing propolis liquid extract samples, the pH value observed was low compared to control samples during the storage period (p < .05). Furthermore, lower pH changes were determined in the Ch+0.3% PE and Ch+0.6% PE samples. It can be deduced that the lower pH values of the Ch+0.3% PE and Ch+0.6% PE samples combined with chilling could increase microbial prevention and cause prolongation of the quality of crayfish meat (Abd El-halim El-Sherif & Abd El-Ghafour, Citation2015)
Figure 2. Changes in chemical quality a) Ph, b) TVB-N, c) TBA, d)PV and e) K-value crayfish coated with Ch coatings throughout storage at 4°C . (Cont: without coated; Ch: coated with Chitosan; Ch+0.3% PE: coated with Chitosan+0.3% Propolis extract; Ch+0.6% PE: coated with Chitosan+0.6% Propolis extract).
Figura 2. Cambios en la calidad química a) Ph, b) TVB-N, c) TBA, d) PV y e) valor K del cangrejo de río recubierto con recubrimientos de Ch durante el almacenamiento a 4°C. (Cont: sin recubrimiento; Ch: recubierto con chitosán; Ch+0.3% PE: recubierto con chitosán+0.3% de extracto de propóleo; Ch+0.6% PE: recubierto con chitosán+0.6% de extracto de propóleo)
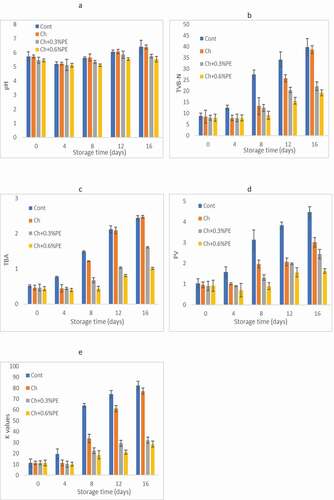
Alterations in the TVB-N of the samples with storage are demonstrated in ). Coated groups demonstrated a tendency of decreasing TVB-N value throughout the first 4 days of storage. Subsequently, a rise was determined until the end of storage. These results were also similar to the findings of other researchers. The rise in TVB-N value was particularly clear in Control and Ch crayfish samples, on the other hand, crayfish samples with Ch+PE coatings supplemented with PE significantly decreased the value of TVB-N during storage. Gimenez et al. (Citation2002) reported a value of 25 mg/100 g fish meat as the beginning of spoilage. In this study, TVB-N values remained below this limit of admissibility until at the end of storage in Ch+PE coated groups, meanwhile, the limit of admissibility was exceeded on day 8 and day 12 for control and group coated with Ch, respectively. These conclusions are in parallel with the findings of Martínez-Álvarez et al. (Citation2008), Tsironi et al. (Citation2009), and Nekuie Fard et al. (Citation2015). Ojagh et al. (Citation2010) declared that chitosan coatings combined with cinnamon oil effectively suppress the rise in TVB-N value and protect refrigerated rainbow trout fillets.
The changes in the PV of all the groups during chilled storage are presented in ). At the start of the storage period, the PVs were determined as 0.96 meqO2/kg, 0.90 meqO2/kg, and 0.92 meqO2/kg for the crayfish coated with Ch, Ch+0.3% PE and Ch+0.6% PE, respectively. The PV increased during storage time, as awaited, the control group had the highest PV values and PV might be significantly affected (p < .05) by PE concentration in the coating solutions; however, which were lower than that of Ch+0.3% PE. Similar results were also revealed for the yellowfin tuna meat (Thunnus albacares) coated with chitosan/lemon peel extract (Sabu et al., Citation2020) who mentioned that supplemented coating with lemon peel extract was efficient in restraining the formation of lipid oxidation products. A study conducted by Viji et al. (Citation2015) determined similar findings in Indian mackerel coated with a combination of citrus peel and mint leaf extracts.
The variations in the TBA value of crayfish coated along storage time are indicated in ). The initial TBA values were in the range of 0.52± 0.12 to 0.44 ± 0.07 mg MDA/kg (p< .05); however, the TBA values increased as gradually until the end of storage time in all groups. This increase was slower observed in crayfish samples coated with Ch containing PE when compared with other samples (p< .05). Similar results were reported by Thaker et al. (Citation2017), Fadıloğlu and Emir Çoban (Citation2018), and Sun et al. (Citation2019), in meat and fish coated with coatings containing essential oil. After day 12, the control group and there were no significant differences among the Ch group (p> .05). According to these results, propolis extract might be used to delay lipid oxidation in the active coatings for shellfish and fish products. Yu et al. (Citation2017) reported that the chitosan coating combined with essential oils (clove, cinnamon, and lemongrass) has strong antioxidant activities and preservative effects for antioxidant enzyme activities.
The initial K-value for fresh crayfish were determined as 11.35%. In all the groups, the K- value rose during storage at 4°C (). During storage, the K-values of the control and Ch groups were found to be higher than the groups containing propolis (p < .05). Many researchers have reported that the K-value is a good freshness index for determining quality during storage (Fan et al., Citation2009). Besides, the K-value varies according to the processing, the season in which the fish was caught, and the fish species. Dong et al. (Citation2019) reported that Allium sativum EO can prevent the degradation of ATP and keep the quality of Pseudosciaena crocea. Cinnamon bark EO might prevent the degradation of ATP and retain the freshness of grass carp fillet (Huang et al., Citation2017). Chitosan at 2% was determined to be effective in lower the K-value (Hassoun et al., Citation2020). According to our results, it might be supposed that the Ch+PE coatings prohibited the degradation of ATP and keep on quality of crayfish during refrigerated storage. Similar results were reported by Liu et al. (Citation2019) for crayfish coated by a gelatin-containing red pitaya peel methanol extract.
3.2. Microbiological quality
The microbial number of all groups gradually increased with storage time.In accordance with the International Standards (ICMSF [International Commisson on Microbiological Spescifications for Foods], Citation1986), the maximum admissible limit of TMAB in raw fish is 7 log CFU/g. In the present study, the initial TMAB counts of crayfish meat were determined to be 3.22 log CFU/g. The TMAB counts of samples were significantly different between uncoated group and chitosan/propolis emulsions coated groups (p < .05). On day 4, the TMAB in all groups was below 5 log CFU/g; however, on day 12 the control group reached a count of 8.40 log CFU/g. This count was greater than the maximal admissible limit of ICMSF. Ucak et al. (Citation2020) reported lower TMAB numbers in gelatin-coated trout fillets containing propolis. The lower TVC of samples may be attributed to the antimicrobial activity of propolis. These results can be explained by the phenolic compounds contained in propolis and polyphenolic fractions such as flovonoids (Pobiega et al., Citation2019; Spinelli et al., Citation2015).
The main microorganisms that cause spoilage of fish and fish products stored in cold conditions are gram-negative psychrophilic bacteria. As displayed in ), the rise in the psychrotrophic bacteria (PB) count in the control group was greater than in the coated groups. Initially, the psychrotrophic bacteria count was 2.88 log CFU/g, which increased to 8.00 log CFU/g at day 20 of the storage for control groups. While the psychrotrophic bacteria counts for groups coated with 0.3 and 0.6% PE containing were 6.12 and 5.40 log CFU/g, at the end of storage. Similar results were found in studies for chitosan/cinnamon oil and fish gelatine/GL-βCD-curcumin for fresh fish protection (Jouki et al., Citation2014; Sun et al., Citation2019). Ucak et al. (Citation2020) reported that during the storage period, PB of fillets coated with gelatin films containing propolis were lower than those of the gelatin film coated samples and control samples.
Figure 3. Changes in microbial quality a) TMAB, b) PB, c) H2S, d) Yeast-Mold and e) Pseudomonas spp. crayfish coated with Ch coatings throughout storage at 4 °C . (Cont: without coated; Ch: coated with Chitosan; Ch+0.3% PE: coated with Chitosan+0.3% Propolis extract; Ch+0.6% PE: coated with Chitosan+0.6% Propolis extract).
Figura 3. Cambios en la calidad microbiana a) TMAB, b) PB, c) H2S, d) levadura-moho, y e) pseudomonas spp. cangrejos de río recubiertos con Ch durante el almacenamiento a 4°C . (Cont: sin recubrimiento; Ch: recubierto con chitosan; Ch+0.3% PE: recubierto con chitosan+0.3% extracto de propóleo; Ch+0.6% PE: recubierto con chitosan+0.6% extracto de propóleo)
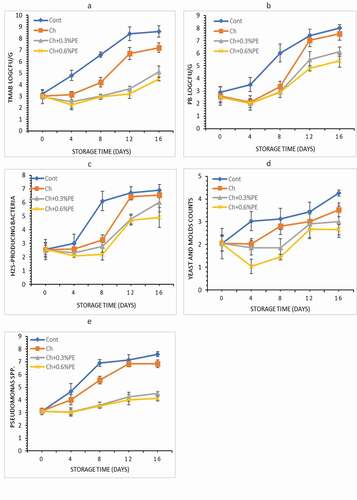
The varieties of H2S-producing bacteria (), yeast and mold bacteria (), and Pseudomonas spp. bacteria () populations indicated a similar tendency with TAMB and psychrophilic bacteria. On day 20, groups coated with Ch containing PE obtained lower counts.
3.3. Sensorial quality
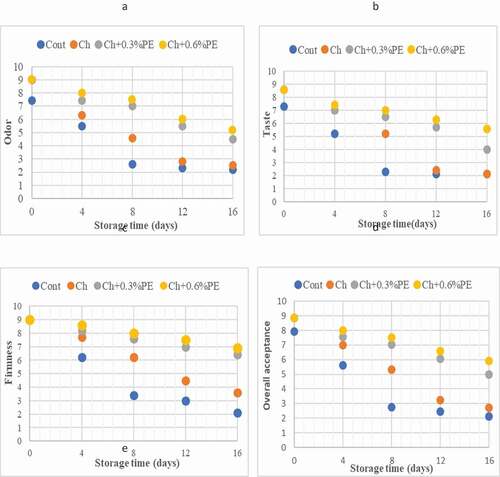
The sensory evaluation results are shown in . At the beginning of the storage, a glossy and smooth layer of Ch-based coatings was seen on the crayfish sample surfaces, resulting in increased scoring. Therefore, the sensory score of the control group was lower than the coated groups (p< .05). With the progress of storage, the sensory scores of all groups decreased. A faster decrease was detected in the control group (p< .05). Control and Ch were reported inadmissible by the panelists in terms of odor and taste on day 12 and day16, respectively. Also, the Ch+0.6% PE group even on day 16 was considered to be consumable by panelists. These data suggest that 0.6% of PE concentration is more effective in Ch based coatings. Spinelli et al. (Citation2015) reported that the sensory quality of fresh fish burgers containing microencapsulated propolis improved. Ucak et al. (Citation2020) found similar results. They reported that combining PE with gelatin films had a positive effect on trout fillet.
Figure 4. Changes in sensorial quality a) odor, b) taste, c) firmness and d) overall acceptance of crayfish coated with Ch coatings throughout storage at 4 °C . (Cont: without coated; Ch: coated with Chitosan; Ch+0.3% PE: coated with Chitosan+0.3% Propolis extract; Ch+0.6% PE: coated with Chitosan+0.6% Propolis extract).
Figura 4. Cambios en la calidad sensorial a) olor, b) sabor, c) firmeza y d) aceptación global de los cangrejos de río recubiertos con Ch durante el almacenamiento a 4°C . (Cont: sin recubrimiento; Ch: recubierto con chitosán; Ch+0.3% PE: recubierto con chitosán+0.3% de extracto de propóleo; Ch+0.6% PE: recubierto con chitosán+0.6% de extracto de propóleo)
4. Conclusion
The incorporation of propolis liquid extract into a crayfish chitosan coating increased the effectiveness of the coating. Ch+PE treatment increased the crayfish shelf life by ~7 days compared to the control group. Chemical, microbiological and sensorial analyses showed that the Ch+0.6%PE coating had an obvious effect on the quality of crayfish 4°C storage and their shelf lives. Therefore, PE combined with coatings might be useful as a safe natural protective for seafood.
Conflicts of interest
The authors declare no conflict of interest
Additional information
Funding
References
- Abd El-halim El-Sherif, S. A. E. H., & Abd El-Ghafour, S. (2015). Nutritive value of canned River Nile Crayfish (Procambarus clarkii) products. The Egyptian Journal of Aquatic Research, 41(3), 265–272. https://doi.org/https://doi.org/10.1016/j.ejar.2015.06.002
- Bahadır Koca, S., & Argun Uzunmehmetoğlu, E. (2018). Interactions of season, sex and size on nutrient composition of freshwater crayfish (Astacus leptodactylus Eschscholtz, 1823) from Lake Eğirdir. Food Science and Technology, 38(Suppl.1), 44–49. https://doi.org/https://doi.org/10.1590/1678-457x.15817
- Cai, L., Cao, A., Bai, F., & Li, J. (2015). Effect of ε-polylysine in combination with alginate coating treatment on physicochemical and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) during refrigerated storage. LWT - Food Science and Technology, 62(2), 1053–1059. https://doi.org/https://doi.org/10.1016/j.lwt.2015.02.002
- Cai, L., Leng, L., Cao, A., Cheng, X., & Li, J. (2018). The effect of chitosan-essential oils complex coating on physicochemical, microbiological, and quality change of grass carp (Ctenopharyhgodon idella) fillets. Journal of Food Safety, 38(1), e12399. https://doi.org/https://doi.org/10.1111/jfs.12399
- Dong, Z., Luo, C., Guo, Y., Ahmed, I., Pavase, T. R., Lv, L., Li, Z., & Lin, H. (2019). Characterization of new active packaging based on PP/LDPE composite films containing attapulgite loaded with Allium sativum essence oil and its application for large yellow croaker (Pseudosciaena crocea) fillets. Food Packaging and Shelf Life, 20(2), 100320. https://doi.org/https://doi.org/10.1016/j.fpsl.2019.100320
- Fadıloğlu, E. E., & Emir Çoban, Ö. (2018). Effects of chitosan edible coatings enriched with sumac on the quality and the shelf life of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) fillets. Journal of Food Safety, 38(6), e12545. https://doi.org/https://doi.org/10.1111/jfs.12545
- Fan, W., Chi, Y., & Zhang, S. (2008). The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chemistry, 108(1), 148–153. https://doi.org/https://doi.org/10.1016/j.foodchem.2007.10.057
- Fan, W., Sun, J., Chen, Y., Qiu, J., Zhang, Y., & Chi, Y. (2009). Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chemistry, 115(1), 66–70. https://doi.org/https://doi.org/10.1016/j.foodchem.2008.11.060
- Galeotti, F., Maccari, F., Fachini, A., & Volpi, N. (2018). Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods, 7(3), 41. https://doi.org/https://doi.org/10.3390/foods7030041
- Gharibzahedi, S. M. T., & Mohammadnabi, S. (2017). Effect of novel bioactive edible coatings based on jujube gum and nettle oil-loaded nanoemulsions on the shelf-life of Beluga sturgeon fillets. International Journal of Biological Macromolecules, 95(2), 769–777. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2016.11.119
- Gimenez, B., Roncales, P., & Beltran, J. A. (2002). Modified atmosphere packaging of filleted rainbow trout. Journal of the Science of Food and Agriculture, 82(10), 1154–1159. https://doi.org/https://doi.org/10.1002/jsfa.1136
- Gokalp, H. Y., Kaya, M., Zorba, O., & Tulek, Y. (2001). Quality control and laboratory practice guidelines in meat and meat products. Ataturk University Agriculture Faculty Publications.
- Gutierrez-Cortes, C., & Suarez Mahecha, H. (2014). Antimicrobial activity of propolis and its effect on the physicochemical and sensoral characteristics in sausages. Vitae, 21(2), 90–96.
- Halkman, A. K. (2005). Merck food microbiology applications. Başak Publications.
- Hascik, P., Elimam, I. O., Krocko, M., Bobko, M., Kacniova, M., Garlík, J., & Saleh, A. A. (2015). The influence of propolis as supplement diet on broiler meat growth performance, carcass body weight, chemical composition and lipid oxidation stability. Acta Universitatis Agriculturae Silviculturae Mendelianae Brunensis, 63(2), 411–418. https://doi.org/https://doi.org/10.11118/actaun201563020411
- Hassoun, A., Carpena, M., Prieto, M. A., Simal-Gandara, J., Özogul, F., Özogul, Y., Çoban, Ö. E., Guðjónsdóttir, M., Barba, F. J., Marti-Quijal, F. J., Jambrak, A. R., Maltar-Strmečki, N., Kljusurić, J. G., & Regenstein, J. M. (2020). Use of spectroscopic techniques to monitor changes in food quality during application of natural preservatives: A review. Antioxidants (Basel, Switzerland), 9(9), 882. https://doi.org/https://doi.org/10.3390/antiox9090882
- Huang, Z., Liu, X., Jia, S., & Luo, Y. (2017). Antimicrobial effects of cinnamon bark oil on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Food Control, 82, 316–324. https://doi.org/https://doi.org/10.1016/j.foodcont.2017.07.017
- ICMSF (International Commisson on Microbiological Spescifications for Foods). (1986). Microorganisms in Foods 2. sampling for microbiological analysis (2nd ed.). University of Toronto Press.
- Jouki, M., Yazdi, F. T., Mortazavi, S. A., Koocheki, A., & Khazaei, N. (2014). Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. International Journal of Food Microbiology, 174(3), 88–97. https://doi.org/https://doi.org/10.1016/j.ijfoodmicro.2014.01.001
- Kilic, B., & Richards, M. P. (2003). Lipid oxidation in poultry Doner Kebabi pro-oxidative and anti-oxidative factors. Journal of Food Science, 68(2), 690–696. https://doi.org/https://doi.org/10.1111/j.1365-2621.2003.tb05732.x
- Liu, W., Shen, Y., Li, N., Mei, J., & Jing, X. (2019). Application of gelatin ıncorporated with red pitaya peel methanol extract as edible coatingfor quality enhancement of crayfish (Procambarus clarkii) during refrigerated storage. Journal of Food Quality, 1715946(Special Issue), 8. https://doi.org/https://doi.org/10.1155/2019/1715946
- Manju, S., Jose, L., Srinivasa Gopal, T. K., Ravishankar, C. N., & Lalitha, K. V. (2007). Effects of sodium acetate dip treatment and vacuum-packaging on chemical, microbiological, textural and sensory changes of Pearlspot (Etroplus suratensis) during chill storage. Food Chemistry, 102(1), 27–35. https://doi.org/https://doi.org/10.1016/j.foodchem.2006.04.037
- Martínez-Álvarez, Ó., Gomez‐Guillen, M. D. C., & Montero, P. (2008). Chemical and microbial quality indexes of Norwegian lobsters (Nephrops norvegicus) dusted with sulphites. International Journal of Food Science & Technology, 43(6), 1099–1110. https://doi.org/https://doi.org/10.1111/j.1365-2621.2007.01576.x
- Nekuie Fard, A., Seidgar, M., & Azadikhah, D. (2015). The study of frozen Astacus leptodactylus tail fillet quality changes. AACL Bioflux, 8(6), 6.
- Ojagh, S. M., Rezaei, M., Razavi, S. H., & Hosseini, S. M. H. (2010). Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chemistry, 120(1), 193–198. https://doi.org/https://doi.org/10.1016/j.foodchem.2009.10.006
- Pobiega, K., Kraśniewska, K., & Gniewosz, M. (2019). Application of propolis in antimicrobial and antioxidative protection of food quality – A review. Trends in Food Science & Technology, 83(47), 53–62. https://doi.org/https://doi.org/10.1016/j.tifs.2018.11.007
- Sabu, S., Ashıta, T., & Stephy, S. (2020). Chitosan and lemon peel extract coating on quality and shelf life of yellowfin tuna (Thunnus albacares) meat stored under refrigerated condition. Indian Journal of Fisheries, 67(1), 114–122. https://doi.org/https://doi.org/10.21077/ijf.2019.67.1.91361-15
- Shahbazi, Y., & Shavisi, N. (2018). Chitosan coatings containing mentha spicata essential oil and zinc oxide nanoparticle for shelf life extension of rainbow trout fillets. Journal of Aquatic Food Product Technology, 27(9), 986–997. https://doi.org/https://doi.org/10.1080/10498850.2018.1518945
- Shantha, N. C., & Decker, E. A. (1994). Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. Journal of AOAC International, 77(2), 421–424. https://doi.org/https://doi.org/10.1093/jaoac/77.2.421
- Spinelli, S., Conte, A., Lecce, L., Incoronato, A. L., & Nobile, M. A. (2015). Microencapsulated propolis to enhance the antioxidant properties of fresh fish burgers. Journal of Food Process Engineering, 38(6), 527–535. https://doi.org/https://doi.org/10.1111/jfpe.12183
- Sun, X., Guo, X., Ji, M., Wu, J., Zhu, W., Wang, J., Cheng, C., Chen, L., & Zhang, Q. (2019). Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chemistry, 272(30), 643–652. https://doi.org/https://doi.org/10.1016/j.foodchem.2018.08.040
- Thaker, M., Hanjabam, M. D., Gudipati, V., & Kannuchamy, N. (2017). Protective effect of fish gelatin‐based natural antimicrobial coatings on quality of ındian salmon fillets during refrigerated storage. Journal of Food Process Engineering, 40(1), e12270. https://doi.org/https://doi.org/10.1111/jfpe.12270
- Tsironi, T., Dermesonloouglou, E., Giannakourou, M., & Taoukis, P. (2009). Shelf life modelling of frozen shrimp at variable temperature conditions. LWT-Food Science and Technology, 42(2), 664–671. https://doi.org/https://doi.org/10.1016/j.lwt.2008.07.010
- Uçak, I. (2018). Determınatıon of the lıpıd oxıdatıon levelın fısh oıl enrıched wıth propolıs extract. GIDA, 43(3), 523–532. https://doi.org/https://doi.org/10.15237/gida.GD18031
- Ucak, I., Khalily, R., Carrillo, C., Tomasevic, I., & Barba, F. J. (2020). Potential of propolis extract as a natural antioxidant and antimicrobial in gelatin films applied to rainbow trout (Oncorhynchus mykiss) fillets. Foods, 9(11), 1584. https://doi.org/https://doi.org/10.3390/foods9111584
- Viji, P., Binsi, P. K., Visnuvinayagam, S., Bindu, J., Ravishankar, C. N., & Gopal, T. K. S. (2015). Efficacy of mint (Mentha arvensis) leaf and citrus (Citrus aurantium) peel extracts as natural preservatives for shelf life extension of chill stored Indian mackerel. Journal of Food Science and Technology, 52(10), 6278–6289. https://doi.org/https://doi.org/10.1007/s13197-015-1788-1
- Wu, J., Sun, X., Guo, X., Ji, M., Wang, J., Cheng, C., ..., Zhang, Q. (2018). Physicochemical, antioxidant, in Vitro release, and heat sealing properties of fish gelatin films incorporated with ?-cyclodextrin/curcumin complexes for apple juice. Preservation Food and Bioprocess Technology, 11(2), 447–461.https://doi.org/https://doi.org/10.1007/s11947-017-2021–1
- Yazgan, H., Burgut, A., Durmuş, M., & Koske, A. R. (2020). The impacts of water and ethanolic extracts of propolis on vacuum packaged sardine fillets inoculated with Morganella psychrotolerans during chilly storage. Journal of Food Safety, 40(2), e12767. https://doi.org/https://doi.org/10.1111/jfs.12767
- Yu, D., Xu, Y., Jiang, Q., & Xia, W. (2017). Effects of chitosan coating combined with essential oils on quality and antioxidant enzyme activities of grass carp (Ctenopharyngodon idellus) fillets stored at 4 °C. International Journal of Food Science and Technology, 52(2), 404–412. https://doi.org/https://doi.org/10.1111/ijfs.13295