ABSTRACT
A liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) analytical method using a modified QuEChERS sample preparation for the analysis of insecticide residues in honey was developed and validated. The use of the Plackett Burman design in the sample preparation step proved to be effective in optimizing recovery and reducing the matrix effect. For quantification purposes, extract-matched analytical curves were constructed showing linearity (R2) higher than 0.99. The precision (RSD%) of the method was lower than 20% and the accuracy was between 74% and 104%. Lower limits of quantification than the maximum residue limits established by the European Commission and the Brazilian legislative framework were obtained. Insecticide residues were found in 37.3% of 51 real honey samples analyzed, with imidacloprid, clothianidin and dimethoate being the most frequently detected insecticides. The co-occurrence of insecticide residues in samples was frequent. Monitoring insecticide residues in honey is needed to avoid consumer exposure at unacceptable levels.
Graphical Abstract
RESUMEN
Se desarrolló y validó un método analítico por cromatografía líquida-espectrometría de masas en tándem (UHPLC-MS/MS) para el análisis de residuos de insecticidas en la miel, utilizando un preparo de muestras QuEChERS modificado. La utilización del diseño de Plackett Burman en la etapa de preparación de la muestra demostró ser eficaz para optimizar las tasas de recuperación y reducir el efecto matriz. Para propósitos de cuantificación se construyeron curvas analíticas preparadas en el extracto, con linealidad (R2) superior a 0,99. La precisión (RSD%) del método fue inferior al 20% y la exactitud estuvo entre el 74% y el 104%. Se obtuvieron límites de cuantificación inferiores a los límites máximos de residuos establecidos por la Comisión Europea y el marco legislativo brasileño. Se encontraron residuos de insecticidas en el 37,3% de las 51 muestras reales de miel analizadas, siendo imidacloprid, clotianidina y dimetoato los insecticidas detectados con mayor frecuencia. La co-ocurrencia de residuos de insecticidas en las muestras fue frecuente. Es necesario monitorear los residuos de insecticidas en la miel para evitar la exposición del consumidor a niveles inaceptables.
1. Introduction
Honey is complex food and has great diversity in terms of its chemical composition (more than 200 known substances). It consists mainly of carbohydrates (fructose, glucose and sucrose), water and other substances such as proteins, organic acids, vitamins, minerals, pigments and solid particles (Bogdanov, Citation2006). The use of honey has grown and has been adopted into consumption habits due to its high nutritional value, palatable flavor, and medicinal properties. Several authors have reported the presence in the honey of pesticide residues from different classes, mainly neonicotinoid insecticides, organophosphates and pyrethroids (Al Naggar et al., Citation2015; Codling et al., Citation2016; Orso et al., Citation2015).
The presence of insecticide residues induces adverse effects in bees (Jeschke & Nauen, Citation2005). Therefore, to protect human and environmental health, the European Union has set maximum residue limits (MRLs) for the presence of insecticides in honey (European Data Base, Citation2019). In Brazil, the Ministry of Agriculture, Livestock and Supply (MAPA) established reference limits for the residues of some pesticides in honey for inspection purposes (MAPA, Citation2019), and the National Health Surveillance Agency (ANVISA) of the Ministry of Health (ANVISA, Citation2019) established MRLs for amitraz and coumaphos.
The QuEChERS sample preparation approach has been the most used method for the simultaneous extraction and extract cleanup for the analysis of insecticide residues in honey (Codling et al., Citation2016; P. A. Tette et al., Citation2016a), and the Plackett Burman design is an alternative for optimization of the sample preparation step with a small number of experiments to assess the effects of the chosen variables under study and establish their levels of influence (Rodrigues & Iemma, Citation2014).
Methods for quantification of pesticide residues in honey report the use of gas chromatography with detectors such as electron capture (GC-ECD) (Orso et al., Citation2014), nitrogen-phosphorus (GC-NPD) (Farajzadeh et al., Citation2014) and mass spectrometry (GC-MS) (Bargańska et al., Citation2014). Also, liquid chromatography (LC) with ultraviolet detection (LC-UV) (Jovanov et al., Citation2015) has been reported. In particular, LC coupled with tandem mass spectrometry (LC-MS/MS) has been used in multiresidue methods and shows promising performance in the quantitative analysis of several classes of pesticides with different physicochemical characteristics in matrices such as honey (P. A. S. Tette et al., Citation2016b).
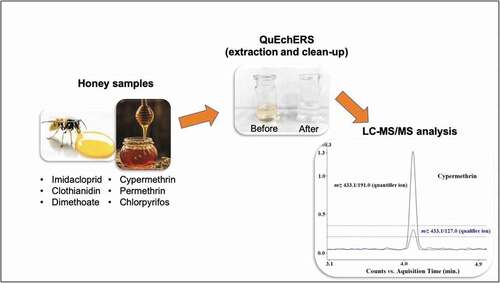
Thus, this work reports a method based on LC-MS/MS with a QuEChERS sample preparation approach optimized through the Placket Burman design given the physical-chemical difference between pesticides and the complexity of the matrix. The method was validated and applied to real samples for the analysis of insecticide (imidacloprid [IMI], clothianidin [CLO], dimethoate [DIM], chlorpyrifos [CPY], cypermethrin [CYP] and permethrin [PER]) residues in honey. Samples from different origins (Brazil, Mexico, Paraguay, and some European countries) were analyzed to evaluate the robustness and reliability of the method.
2. Materials and methods
2.1. Reagents and analytical standards
All analytical insecticide standards (imidacloprid, clothianidin, chlorpyrifos, permethrin, dimethoate, cypermethrin and the internal standard (IS) imidacloprid d-4) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and had purities greater than 99%. Acetonitrile and methanol of HPLC grade were purchased from J.T. Baker (Phillipsburg, NJ, USA). Acetone, ammonium formate, formic acid, anhydrous magnesium sulfate and sodium chloride provided by Sigma–Aldrich (St. Louis, MO, USA) were of analytical grade. Primary and secondary amine (PSA), silica-bonded C18 and graphitized carbon black (GCB) sorbents were supplied by Supelco (Bellefonte, PA, USA). PVDF (polyvinylidene fluoride) syringe filters (0.22 μm) were purchased from Analitica (São Paulo, SP, Brazil). Ultrapure deionized water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA).
2.2. Standard solutions
Individual stock standard solutions of all analytes (including the internal standard imidacloprid d-4) were prepared in acetonitrile at a concentration of 200 μg mL−1, except for clothianidin stock solution, which was prepared in acetone. From the stock solutions, intermediate solutions were prepared in acetonitrile at two concentrations (10 and 100 μg mL−1). For the preparation of analytical curves [in solvent (acetonitrile) and extract (extract-matched)], proper volumes of the intermediate standard solutions were added to acetonitrile and extracts to prepare 7-point analytical curves of each insecticide standard (equivalent to 10, 25, 50, 75, 100, 150 and 250 μg kg−1 in the blank honey matrix).
2.3. Instrumentation and chromatographic conditions
Analyses were carried out using an Agilent UHPLC 1290 system coupled with a 6460 triple quadrupole tandem mass spectrometer (Agilent Technologies, CA, USA). Chromatographic separation was achieved on an Agilent Zorbax Eclipse plus RRHD C18 column (2.1 mm × 50 mm, 1.8 μm) (Agilent Technologies, CA, USA). The mobile phase consisted of (A) 20 mmol L−1 ammonium formate at pH 3.0 and (B) methanol containing 0.1% formic acid. A gradient elution program was used, starting with 15% B increasing linearly until reaching 95% over 2.2 min, remaining constant for 2.5 min and then returning to 15%, with a total run time of 5.0 min. A post-run time of 3.0 min was necessary to re-equilibrate the column at the initial conditions. The flow rate was 0.6 mL min−1, the column temperature was 45°C, and the injection volume was 2 μL.
The MS/MS system was equipped with an electrospray ionization (ESI) source and operated in positive ionization mode. The ionization source conditions were optimized employing the Source Optimizer tool and were as follows: gas temperature, 300°C; gas flow, 10 L min−1; nebulizer, 25 psi; sheath gas flow 10 L min−1; sheath gas temperature, 300°C; capillary, 4.5 kV; and nozzle, 0 kV. These parameters were established by directly injecting individual standard solutions (1 μg mL−1) prepared in acetonitrile into the ESI source. The Optimizer tool was also used to provide the most abundant product ions (quantifier and qualifier ions) and the corresponding collision energies (CE) in selected reaction monitoring (SRM) mode (). The identification criteria were based on the Guidance document SANTE/12682/2019 for LC-MS/MS analysis with acquisition of two transitions ions (qualifier and quantifier ions), retention time (tolerance of ±0.2 min) and ion ratio compliance with a tolerance of ± 30% between the ions (SANTE, Citation2019).
Table 1. Selected reaction monitoring (SRM) settings for the targeted insecticides using electrospray ionization (ESI) source in positive ionization mode.
Tabla 1. Configuraciones del monitoreo de reacciones seleccionadas (SRM) para los insecticidas investigados usando una fuente de ionización por electropulverización (ESI) en modo de ionización positiva
2.4. Samples
Blank samples of honey employed for optimization and validation purposes were obtained from Apiário Cambará, Rio Grande do Sul, Brazil.
A total of 51 honey samples were purchased at the retail market or supplied by producers and honey cooperatives in Brazil. Also, samples from other Latin American countries [Paraguay (n = 1) and Mexico (n = 1)] and Europe [France (n = 1), Spain (n = 1), Germany (n = 1), Portugal (n = 1), Greece (n = 1), Cyprus (n = 2) and Italy (n = 3)] were analyzed. All samples were collected between 2017 and 2019, stored at 10°C and analyzed within their listed shelf life.
2.5. Optimization of the sample preparation step
Sample preparation was based on the QuEChERS approach (Anastassiades et al., Citation2003), and optimized through the Plackett-Burman (PB) design (Rodrigues & Iemma, Citation2014). Eight sample preparation variables were studied at two levels, low (-1) and high (+1): the amount of salts used in the extraction step (NaCl and MgSO4), extraction time, amount of sorbents (C18, GCB, PSA) used in the dispersive solid-phase extraction (d-SPE) and extraction time (Table S1, supplemental material) The effect of each variable on insecticide recoveries was estimated using Statistica 7.0 software, adopting a significance level of 10% (p ≤ .1).
2.6. Sample preparation
Sample preparation was carried out using the in-house optimized QuEChERS. Ten grams of a homogenized honey sample was weighed into a 50 mL PTFE centrifuge tube and 10 mL of water was added, followed by vortexing for 5 min. Then, 10 mL of acetonitrile was added, and the samples were vortexed again for 5 min. To induce phase separation and force the insecticides into the acetonitrile phase, 4 g of MgSO4 and 1.5 g of NaCl were added into the tubes and the mixture was vortexed for 5 min followed by centrifugation at 3,000 g for 5 min. A 5 mL aliquot of the supernatant was transferred to another PTFE centrifuge tube containing 750 mg of MgSO4, 250 mg of PSA and 125 mg of C18 and was vortexed for 2 min and centrifuged at 3,000 g for 5 min. The cleaned extracts were filtered through a Millex HV filter unit (0.22 μm pore size, Millipore) directly into an LC vial and then injected into the UHPLC-MS/MS system.
2.7. In-house validation
The performance characteristics of the analytical method were established according to the European Commission (EU) guidance document for pesticide residue analysis in food and feed (SANTE, Citation2019). The parameters evaluated were selectivity, matrix effect, linearity, precision, accuracy, limit of quantification (LOQ) and robustness.
The selectivity was determined based on the LC-MS/MS analysis of analyte-free samples against spiked samples to provide the ability of the method to discriminate the targeted insecticides from interfering matrix compounds in SRM mode. Linearity was assessed through 7-point (10– 250 μg kg−1) analytical curves prepared in solvent (acetonitrile) and blank honey matrix extract (extract-matched). The matrix effects (ME) were estimated according to Sapozhnikova and Lehotay (Citation2013).
Accuracy and precision (intraday and interday) were assessed through recovery of additions of known amounts of the analytical insecticide standards to the blank honey matrix at two concentration levels, namely, a low level (the LOQ obtained for each target insecticide) and high level (100 μg kg−1). Accuracy, expressed as recovery (%), was obtained by comparison between the real and measured concentrations from five individual replicates at each concentration level. Precision, expressed as relative standard deviation (RSD%), was evaluated under repeatability conditions (intraday precision) from five individual replicates at each spiked concentration level analyzed on the same day and under within-laboratory reproducibility (interday precision), which was evaluated from a total of ten individual replicates at each spiked concentration level analyzed on two different days.
The LOQ was stated as the lowest analyte concentration in the matrix that could be quantified with acceptable accuracy (between 70% and 120%) and precision (RSD values ≤20%) with a signal to noise ratio of 10:1 (SANTE, Citation2019).
The robustness was evaluated by applying a 23 factorial design, considering small variations inherent in the laboratory routine, such as analytical column batch (# B13211 and # B12238), oven temperature (40°C and 50°C) and flow rate of the mobile phase (0.5 mL min−1 and 0.7 mL min−1). All data were analyzed using Statistica 7.0 software and adopting a significance level of 5% (p ≤ .05). The applicability of the method was verified through the analysis of real samples from different origin.
3. Results and discussion
3.1. Chromatographic conditions
The studied compounds belong to three different chemical classes of insecticides, including neonicotinoids, pyrethroids and organophosphates, with partition coefficients (log P) ranging from 0.57 (IMI) to 6.6 (CYP). Therefore, to achieve suitable simultaneous analyses of the insecticides in terms of selectivity and peak shape, different compositions and proportions of the mobile phase, such as water-methanol, water-acetonitrile or water-acetonitrile-methanol, as well as an aqueous ammonium formate solution at different concentrations (5, 10 or 20 mmol L−1) and pH (3.0 or 6.0) were tested using a reverse-phase C18 column.
The ionization of pyrethroids is favored with methanol, and this insecticide class ionized more efficiently with ammonium formate at a concentration of 20 mmol L−1, increasing ammonium adduct ion formation [M + NH4]+. A pH of 3.0 provided enhanced ionization of the neonicotinoids and the acidification of methanol with 0.1% formic acid provided analytical signals with optimal shape with ESI in positive ionization mode, as indicated by Lehotay and Mastovska (Citation2005).
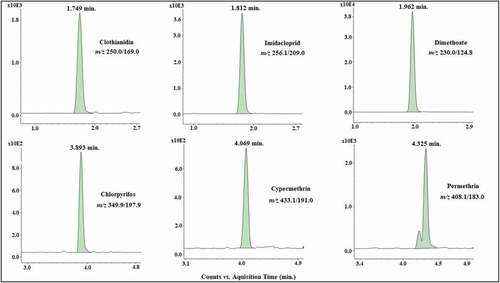
Neonicotinoids are extremely polar compounds (log P 0.57 and 0.7 for IMI and CLO, respectively) and show poor interaction with the stationary phase used; thus, the elution gradient was optimized to improve the separation efficiency and avoid the elution of these compounds in the column dead volume. In addition, the permethrin isomers could be visualized in the chromatogram, and both were considered for quantification purposes. Thus, the chosen mobile phase was a 20 mmol L−1 aqueous ammonium formate solution at pH 3.0 (A) and methanol with 0.1% formic acid (B) with gradient elution. shows SRM chromatograms of a standard solution in the honey extract at a level of 100 μg kg−1.
Figure 1. Representative of selected reaction monitoring (SRM) chromatograms of spiked honey extracts with clothianidin, imidacloprid, dimethoate, chlorpyrifos, cypermethrin and permethrin (100 μg kg−1).
Figura 1. Cromatogramas del monitoreo de reacciones seleccionadas (SRM) característicos de extractos de miel enriquecidos con clotianidina, imidacloprid, dimetoato, clorpirifos, cipermetrina y permetrina (100 μg kg−1)
3.2. Optimization of the sample preparation step
Honey is a complex matrix that requires a sample preparation with efficient cleanup step to eliminate possible LC-MS/MS interferences (Bogdanov, Citation2006; Rissato et al., Citation2007). The QuEChERS procedure has been widely used for honey analyses and several modifications to the original method have been reported due to the physicochemical characteristics of the compounds investigated (Al Naggar et al., Citation2015; Codling et al., Citation2016; P. A. Tette et al., Citation2016a). In the present study, the target insecticides cover a wide range of polarities with log P values between 0.57 (IMI) and 6.6 (CYP). Thus, a Plackett-Burman screening design was used to evaluate sample preparation variables that could affect the insecticide extraction as well as to minimize the amount of chemicals employed in the sample preparation step.
The experimental design was conducted in according to Rodrigues and Iemma (Citation2014) and resulted in recoveries from 36% to 138% (Table S1, supplemental material). The effect (%) was calculated from the recoveries obtained ()
Table 2. Main effects of evaluated parameters on the recovery (%) of insecticides estimated according the Plackett-Burman design.
Tabla 2. Principales efectos de los parámetros evaluados sobre la recuperación (%) de insecticidas, estimados según el diseño de Plackett-Burman
It was verified that the amount of NaCl and MgSO4 salts used to induce phase separation as well as the amount of PSA and C18 sorbents used in the cleanup step significantly influenced (p ≤ .1) the insecticide recoveries.
The variation in the amount of MgSO4 from 2 g to 6 g resulted in a significantly negative effect (p ≤ .1) on the recovery of neonicotinoid (imidacloprid and clothianidin) and organophosphate (dimethoate and chlorpyrifos) insecticides, whereas an increase in the amount of NaCl from 0.5 to 1.5 g resulted in a significantly positive effect (p ≤ .1) on the recovery of pyrethroids (permethrin and cypermethrin). Therefore, 4 g of MgSO4 (central point condition) and a high amount of NaCl (1.5 g) were chosen as optimal conditions for the extraction step. In addition, variations in the time of vortex agitation from 5 to 25 min resulted in no significant effects (p > .1) on the recoveries; thus, the time was maintained at 5 min.
Regarding the d-SPE step, variations in the amount of C18 (from 125 to 375 mg), GCB (from 0 to 75 mg) and PSA (from 125 to 375 mg) sorbents were evaluated. C18 has been used in d-SPE to remove lipids, and GCB is extremely efficient in removing pigments such as chlorophyll and carotenoids, but it has a significant effect on the recovery of some structurally planar pesticides. PSA is an anion-exchange sorbent used mainly to remove polar coextracted matrix components, including sugars, organic acids and some pigments, such as anthocyanidins (Lehotay & Mastovska, Citation2005). The increase in the amounts of C18 and GCB resulted in a significantly negative effect (p ≤ .1) on permethrin recovery; thus, the minimal amount of both sorbents was adopted for the cleanup step.
An increase in the amount of PSA resulted in a significantly positive effect (p ≤ .1) on the recoveries of imidacloprid, clothianidin and dimethoate, all compounds with relatively low log P values (0.57, 0.7 and 0.78, respectively). On the other hand, negative effects were observed for compounds with relatively high log P values, such as permethrin (6.5) and cypermethrin (6.6). Thus, the amount of PSA was fixed at the central point (250 mg).
In addition to cleanup sorbents, the amount of MgSO4 used in the d-SPE step and the time of vortex agitation were also evaluated. Variation in the amount of MgSO4 from 250 to 750 mg resulted in positive effects on the recovery of all compounds; thus, the maximum amount studied was adopted on the d-SPE step. Since the increment in the agitation time from 2 to 8 min produced negative effects on the recovery of cypermethrin and clothianidin, this variable was fixed at the minimum level studied.
Therefore, based on the Plackett-Burman screening design, the optimal sample preparation conditions were 4 g of MgSO4 and 1.5 g of NaCl in the liquid-liquid partitioning step, under 5 min of vortex agitation, followed by 750 mg of MgSO4, 250 mg of PSA and 125 mg of C18 in the d-SPE step and under 2 min of vortex agitation. Under these conditions, clean honey extracts were obtained, and the insecticide recoveries ranged from 77% to 88%, which met the criteria established by SANTE 12682/2019 (SANTE, Citation2019) (Figure S1, supplemental material).
3.3. LC–MS/MS method validation
No analytical signals were observed at the insecticide retention times when comparing the SRM chromatograms obtained from the blank matrix with those obtained from the spiked honey sample, demonstrating the selectivity of the method (Figure S2, supplemental material).
Linearity was evaluated and the presence of outliers was checked by the Grubbs test, and the homoscedasticity of the regression residuals was evaluated by the Cochran test. A weighted (1/x) linear least-squares regression (WLS) was used, and the coefficients of determination (R2) obtained were ≥0.99 for all analytes. The distribution of residues at each point was less than ± 20% in the range studied ().
Table 3. Maximum residue limits (MRL) and validation data of the analytical method for the determination of insecticide residues in honey.
Tabla 3. Valores de límites máximos de residuo (LMR) y datos de validación del método analítico para la determinación de residuos de insecticidas en la miel
Matrix effects (%) were estimated with a signal increment observed for all analytes except for dimethoate, which showed signal suppression. The results demonstrated the effectiveness of the cleanup step on the removal of coextracted matrix compounds that are associated with the suppression or enhancement of analytical signals (Sapozhnikova & Lehotay, Citation2013).
An LOQ of 10 μg kg−1 was obtained for all target insecticides, except for cypermethrin which showed a LOQ of 50 μg kg−1. These LOQ values were suitable for the monitoring of the targeted insecticides at the MRL values established by the EU (European Data Base, Citation2019) and the reference limits established by the Brazilian Ministry of Agriculture, Livestock and Food Supply (MAPA, Citation2019) for pesticide residues in honey.
For all insecticides, the average recovery values ranged from 84% to 104% at the low concentration level and were between 74% and 85% at the high concentration level ().
For intraday precision, the highest RSD value (16%) was observed for cypermethrin at the LOQ level (50 µg kg−1), whereas within interday precision, an RSD value of 15% was achieved for chlorpyrifos and clothianidin at the LOQ level (10 µg kg−1) ()
The robustness of the proposed method was demonstrated based on the same analytical variations inherent to the pre-established conditions when analyzing honey samples from different origin. No significant influences (p ≤ .05) on insecticide peak area were observed (Table S2 and Figure S3, supplemental material).
3.4. Occurrence of insecticide residues in honey
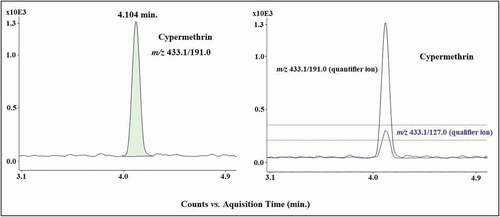
Fifty-one honey samples were analyzed for the presence of the target insecticides using the validated LC-MS/MS method, including samples from European and Latin American countries. Overall, 62.7% of the samples analyzed did not show detectable residues. However, residues of the targeted insecticide were detected in 35.3% of the samples at levels lower than the LOQ. The MRLs established by the EU (European Data Base, Citation2019), as well as the reference limits established by MAPA (Citation2019) were exceeded in 2% of the samples. Regarding the samples from Brazil (n = 39), residues were found in 15 samples (38.5%), all with levels below the LOQ. Among the samples from Europe (n = 10) and other Latin American countries (n = 2), three samples (25%) presented residues below the LOQ, whereas one sample (8.3%) exceeded the MRL (). This noncompliant honey sample was from Spain and contained cypermethrin residues at 102 µg kg−1 ().
Figure 2. Distribution of insecticide residues in real honey samples acquired at the retail market.
Figura 2. Distribución de residuos de insecticidas en muestras reales de miel adquiridas en el mercado al por menor
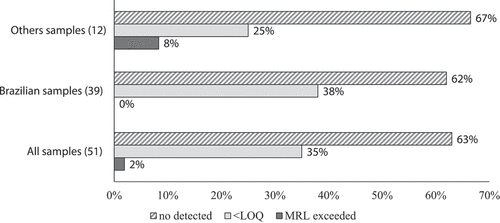
Figure 3. Selected reaction monitoring (SRM) chromatogram obtained from a honey sample from Spain contaminated with cypermethrin at 102 µg kg−1.
Figura 3. Cromatograma del monitoreo de reacciones seleccionadas (SRM) obtenido de una muestra de miel de España contaminada con cipermetrina a 102 µg kg−1
In all samples the most common insecticides detected (in at least five samples) were imidacloprid, clothianidin, dimethoate and cypermethrin. The frequency of detection for imidacloprid (27.5%, 13 Brazilian and 1 Spanish sample), clothianidin (11.8%, 4 Brazilian, 1 Italian and 1 Spanish samples), dimethoate (31,4%, 14 Brazilian, 1 French and 1 Spanish sample) and cypermethrin (9.8%, 2 Brazilian, 1 Italian, 1 Greek and 1 Spanish sample) were relatively high. Since 2013, imidacloprid and clothianidin have use restrictions in the European Union to protect pollinating insects (European Food Safety Authority [EFSA], Citation2018).
In monitoring studies conducted in Brazil, the presence of chlorpyrifos and dimethoate at levels below the MRL established by the EU and the reference limits adopted in Brazil (MAPA, Citation2019) was reported in honey samples from the State of Rio Grande do Sul (Orso et al., Citation2015). Tomasini et al. (Citation2012) did not found residues of imidacloprid nor thiametoxan in samples from the south of Brazil.
Regarding other monitoring studies, neonicotinoid insecticides such as imidacloprid, thiametoxican, thiacloprid and some metabolites have been reported in honey from Canada (Codling et al., Citation2016), Serbia (Jovanov et al., Citation2015), Spain (Sánchéz-Hernández et al., Citation2016) and Poland (Gaweł et al., Citation2019).
In the most recent annual report, the European Food Safety Authority (EFSA) reported the results of the EU-coordinated control program on food analyzed in 2018. Among the honey and other bee products analyzed (762 samples), 78.9% of samples did not show pesticide residues, whereas 1.2% of samples contained residues above the MRL. The substances that exceeded the MRL value established by the EU were the insecticide acetamiprid, the herbicide glyphosate, the fungicide boscalid and the antibiotic dimoxystrobin (EFSA, Citation2020).
Considering the results found in this study, it is suggested to improve the management on the use of pesticides in general, mainly insecticides, in agricultural practice to avoid inappropriate residue levels in honey, protecting the human and environmental health and maintaining trade for this food commodity.
4. Conclusion
The LC-MS/MS analytical method developed and validated according to European Commission SANTE/12682/2019 was reliable and appropriate for the intended purpose of quantification of insecticide residues in honey. The optimization of the sample preparation step (extraction and cleanup) using an experimental design (Plackett Burman approach) enable higher analytes recovery and reduces the matrix effect, allowing obtaining lower LOQ values. The analysis of real honey samples from different origins proved the method’s robustness and applicability. Imidacloprid, clothianidin, dimethoate and cypermethrin were the insecticide more detected in the samples, being that in one sample cypermethrin MRL was exceed. These indicate there is a recurrent environmental contamination. Thus, it is recommended that regulatory agencies conduct surveillance programs to assess pesticide residues in honey to avoid unnecessary consumer exposure to these toxic compounds.
Supplemental Material
Download MS Word (3.7 MB)Acknowledgments
FGRR acknowledges the National Council for Scientific and Technological Development for the research scholarship (CNPq process number 306141/2017-5).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Al Naggar, Y., Codling, G., Vogt, A., Naiem, E., Mona, M., Seif, A., & Giesy, J. P. (2015). Organophosphorus insecticides in honey, pollen and bees (Apis mellifera L.) and their potential hazard to bee colonies in Egypt. Ecotoxicology and Environmental Safety, 114, 1–8. https://doi.org/https://doi.org/10.1016/j.ecoenv.2014.12.039
- Anastassiades, M., Lehotay, S. J., Stajnbaher, D., & Schenck, F. J. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of AOAC International, 86(2), 412–431. https://doi.org/https://doi.org/10.1093/jaoac/86.2.412
- ANVISA. (2019). Agencia Nacional de Vigilância Sanitária do Brasil - Instrução Normativa N° 51, de 19 de dezembro de 2019, Estabelece a lista de limites máximos de resíduos (LMR), ingestão diária aceitável (IDA) e dose de referência aguda (DRfA) para insumos farmacêuticos ativos (IFA) de medicamentos veterinários em alimentos de origem animal. http://www.in.gov.br/web/dou/-/instrucao-normativa-n-51-de-19-de-dezembro-de–2019–235414514
- Bargańska, B., Olkowska, E., Dymerski, T., & Namieśnik, J. (2014). Determination of pesticide residues in honey using the GC×GC-TOFMS Technique. Journal of Bioprocessing & Biotechnology, 4, 1–5. https://doi.org/https://doi.org/10.4172/2155–9821.1000182
- Bogdanov, S. (2006). Contaminants in bee products. Apidologie, 37(1), 1–8. https://doi.org/https://doi.org/10.1051/apido:2005043
- Codling, G., Al Naggar, Y., Giesy, J. P., & Robertson, A. J. (2016). Concentrations of neonicotinoid insecticides in honey, pollen and honey bees (Apis mellifera L) in central Saskatchewan, Canada. Chemosphere, 144, 2321–2328. https://doi.org/https://doi.org/10.1016/j.chemosphere.2015.10.135
- European Data Base. (2019). Maximum limits residue levels. https://ec.europa.eu/food/plant/pesticides/max_residue_levels_en
- European Food Safety Authority (EFSA). (2018). Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid considering the uses as seed treatments and granules. EFSA Journal, 16(2), 5178, 113. https://doi.org/https://doi.org/10.2903/j.efsa.2018.5178
- European Food Safety Authority (EFSA). (2020). The 2018 European Union report on pesticide residues in food. EFSA Journal, 18(4), 6057, 103. https://doi.org/https://doi.org/10.2903/j.efsa.2020.6057
- Farajzadeh, M. A., Mogaddam, M. R. A., & Ghorbanpour, H. (2014). Development of a new microextraction method based on elevated temperature dispersive liquid-liquid microextraction for determination of triazole pesticides residues in honey by gas chromatography-nitrogen phosphorus detection. Journal of Chromatography A, 1347, 8–16. https://doi.org/https://doi.org/10.1016/j.chroma.2014.04.067
- Gaweł, M., Kiljanek, T., Niewiadowska, A., Semeniuk, S., Goliszek, M., Burek, O., & Posyniak, A. (2019). Determination of neonicotinoids and 199 other pesticide residues in honey by liquid and gas chromatography coupled with tandem mass spectrometry. Food Chemistry, 282, 36–47. https://doi.org/https://doi.org/10.1016/j.foodchem.2019.01.003
- Jeschke, P., & Nauen, R. (2005). Neonicotinoid insecticides. In L. I. Gilbert, K. Iatrou, & S. S. Gill (Eds.), Comprehensive molecular insect science (pp. 53–105). Elsevier.
- Jovanov, P., Guzsvány, V., Lazić, S., Franko, M., Sakač, M., Šarić, L., & Kos, J. (2015). Development of HPLC-DAD method for determination of neonicotinoids in honey. Journal of Food Composition and Analysis, 40, 106–113. https://doi.org/https://doi.org/10.1016/j.jfca.2014.12.021
- Lehotay, S. J., & Mastovska, K. (2005). Determination of pesticide residues. In S. Otles (Ed.), Methods of analysis of food components and additives (pp. 329–359). CRC press.
- MAPA. (2019). Brazilian Ministry of Agriculture, Livestock and Food Supply. Normative instruction 5 of April 23, 2019. National Plan for the Control of Residues and Contaminants in Animal Products - PNCRC of 2019. https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/PNCRC2019SamplingPlan.pdf/view
- Orso, D., Floriano, L., Ribeiro, L. C., Bandeira, N. M., Prestes, O. D., & Zanella, R. (2015). Simultaneous determination of multiclass pesticides and antibiotics in honey samples based on ultra-high performance liquid chromatography-tandem mass spectrometry. Food Analytical Methods, 9(6), 1638–1653. https://doi.org/https://doi.org/10.1007/s12161-015-0339-8
- Orso, D., Martins, M. L., Donato, F. F., Rizzetti, T. M., Kemmerich, M., Adaime, M. B., & Zanella, R. (2014). Multiresidue determination of pesticide residues in honey by modified QuEChERS method and gas chromatography with electron capture detection. Journal of the Brazilian Chemical Society, 25(8), 1355–1364. https://doi.org/https://doi.org/10.1007/s12161-015-0339-8
- Rissato, S. R., Galhiane, M. S., Almeida, M. V., Gerenutti, M., & Apon, M. B. (2007). Multiresidue determination of pesticides in honey samples by gas chromatography-mass spectrometry and application in environmental contamination. Food Chemistry, 101(4), 1719–1726. https://doi.org/https://doi.org/10.1016/j.foodchem.2005.10.034
- Rodrigues, M. L., & Iemma, A. F. (2014). Experimental design and process optimization. CRC Press.
- Sánchez-Hernández, L., Hernández-Domínguez, D., Martín, M. T., Nozal, M. J., Higes, M., & Yague, J. L. B. (2016). Residues of neonicotinoids and their metabolites in honey and pollen from sunflower and maize seed dressing crops. Journal of Chromatography A, 1428, 220–227. https://doi.org/https://doi.org/10.1016/j.chroma.2015.10.066
- SANTE. (2019). Safety of the food chain pesticides and biocides. Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed (SANTE/12682/2019). https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf
- Sapozhnikova, Y., & Lehotay, S. J. (2013). Multi-class, multi-residue analysis of pesticides, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers and novel flame retardants in fish using fast, low-pressure gas chromatography-tandem mass spectrometry. Analytica Chimica Acta, 758, 80–92. https://doi.org/https://doi.org/10.1016/j.aca.2012.10.034
- Tette, P. A. S., Oliveira, F. A., Pereira, E. N., Silva, G., Glória, M. B., & Fernandes, C. (2016a). Multiclass method for pesticides quantification in honey by means of modified QuEChERS and UHPLC-MS/MS. Food Chemistry, 211, 130–139. https://doi.org/https://doi.org/10.1016/j.foodchem.2016.05.036
- Tette, P. A. S., Guidi, L. R., Glória, M. B.de A., & Fernandes, C. (2016b). Pesticides in honey: A review on chromatographic analytical methods. Talanta, 149, 124–141. https://doi.org/https://doi.org/10.1016/j.talanta.2015.11.045
- Tomasini, D.,Sampaio, M. R. F., Caldas, S. S., Buffon, J. G. Duarte, F. A., & Primel, E. G . (2012). Simultaneous determination of pesticides and 5- hydroxymethylfurfural in honey by the modified QuEChERS method and liquid chromatography coupled to tandem mass spectrometry. Talanta, 99, 380–386. https://doi.org/https://doi.org/10.1016/j.talanta.2012.05.068
