 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The influence of Moringa oleifera leaves extract (MOLE) on the nutritional properties, thiobarbituric acid reactive substances, microbial composition and physicochemical characteristics of mutton patties during refrigerated storage was explored. The mutton patties were processed by incorporating different amounts (1, 2, 3 and 5%) of MOLE except for the control sample. The inclusion of MOLE significantly increased (p < .05) the protein (21.75%), ash (2.73%), total phenolic (41.96 mg GAE/g) and total flavonoids (20.93 mg CE/g) contents of mutton patties while moisture and fat contents decreased during storage. Lipid oxidation and microbial growth significantly increased while pH values of raw patties decreased. The lightness (L*), redness (a*), yellowness (b*) and chroma (C*) values significantly decreased; while hue angle (H°) value increased. The inclusion of MOLE exhibited cooking yield and moisture retention values of 66.68 and 67.32, respectively. The study results show that MOLE can be utilised as natural antioxidant and preservative of mutton patties.
RESUMEN
Este artículo se propuso examinar la influencia del extracto de hojas de Moringa oleifera (MOLE) en las propiedades nutricionales, las sustancias reactivas del ácido tiobarbitúrico, la composición microbiana y las características fisicoquímicas de los medallones de ovino durante su almacenamiento refrigerado. Los medallones de ovino se procesaron incorporándoles diferentes cantidades (1, 2, 3 y 5%) de MOLE, excepto en la muestra de control. La inclusión de MOLE aumentó significativamente (p < .05) sus contenidos de proteínas (21.75%), cenizas (2.73%), fenólicos totales (41.96 mg GAE/g) y flavonoides totales (20.93 mg CE/g). Sin embargo, durante el almacenamiento disminuyeron los contenidos de humedad y grasa. Por otra parte, se incrementaron significativamente la oxidación de lípidos y el crecimiento microbiano, mientras que se redujeron los valores de pH de los medallones crudos. Los valores de luminosidad (L*), rojez (a*), amarillez (b*) y croma (C*) disminuyeron significativamente, en tanto que el valor del ángulo de tonalidad (H°) aumentó. La incorporación de MOLE a los medallones registró valores de rendimiento de cocción y retención de humedad de 66.68 y 67.32, respectivamente. En conclusión, los resultados del estudio muestran que el MOLE puede utilizarse como antioxidante natural y conservante de los medallones de ovino.
1. Introduction
In recent years, the utilisation of plant extracts as natural antioxidants and preservatives is receiving substantial attention because of their ability to improve the shelf life of food and health of consumers (Falowo et al., Citation2016). Natural antioxidants extracted from plants rich in bioactive compounds such as polyphenols and flavonoids have been used to prevent lipid oxidation and improve quality characteristics of different meat products (Mancini et al., Citation2015; Zhang et al., Citation2017). As possible preservative agents, plant extracts can have high amounts of bioactive compounds that inhibit and disintegrate the cytoplasmic membrane as well as cell wall of pathogenic and spoilage microorganisms (Kim et al., Citation2013; Radha Krishnan et al., Citation2014). Phenolic compounds have antioxidant properties as well as antimicrobial activity and they are found in all parts of fruits, vegetables and seeds (Sayas-Barberá et al., Citation2020). Synthetic antioxidants such as butylated hydroxytoluene, butylated hydroxyanisol, etc. are added during meat processing to prevent lipid oxidation but they have recently come under scrutiny because of their possible toxicological effects (Choi et al., Citation2019; Ribeiro et al., Citation2019).
At present, processed meat products constitute a high percentage of the muscle foods consumed globally since they are convenient and reasonably inexpensive when compared with whole fresh meat cuts (De Oliveira et al., Citation2012; Soladoye et al., Citation2015). However, most of the processed meat products are prone to lipid and pigment oxidation during the manufacture or storage. This is due to mincing and thermal treatment disrupting the coherence of muscle membranes as well as exposing lipid membranes to metal ions which promotes pro-oxidants to interact with unsaturated fatty acids resulting in the initiation of free radicals and generation of oxidative reaction (Asghar et al., Citation1988; Sharma & Yadav, Citation2020). Lipid oxidation influences alteration of muscle fats and proteins and, thereby, compromising physicochemical, nutritional properties and sensory attributes of meat and meat products by producing rancid flavour and oxidised compounds that are harmful to the health of consumers (Falowo et al., Citation2014).
New research on the utilisation of plant extracts as non-synthetic preservatives have demonstrated that they can improve the storage life of meat and meat products by decreasing the growth of spoilage and pathogenic microorganisms (Kobus-Cisowska et al., Citation2014; Kozłowska et al., Citation2015). In addition, the use of plant extracts as preservatives also prevent lipid oxidation that might result in nutritional and economic losses to the meat processing industry (Falowo et al., Citation2014; Sánchez-Ortega et al., Citation2014).
Moringa oleifera leaves extract is associated with various functional properties such as antioxidant, anticancer, antiulcerogenic, antihypertensive, antispasmodic and antiasthmatic qualities (Abdull Razis et al., Citation2014). Therefore, the utilisation of natural antioxidants such as Moringa oleifera leaves extract in processed meat products such as patties might be useful because of the escalating consumer concerns on the application of synthetic antioxidants in processed food products. Moringa oleifera is the most extensively cultivated species among the thirteen species of Moringa trees in the family Moringaceae (Khawaja et al., Citation2010). The leaves can be consumed fresh, cooked or dried and can be stored for longer periods without compromising their nutritional value (Mendieta-Araica et al., Citation2011). Moringa oleifera leaves extract (MOLE) possesses antioxidant and biological activities that are ascribed to the availability of phenolic compounds such as flavonoids and other phenolic acids (Al-Owaisi et al., Citation2014) and its leaves are being evaluated as possible natural preservative for different processed meat products (Al-Juhaimi et al., Citation2016; Dillard & German, Citation2000; Falowo et al., Citation2016). Moringa oleifera contains many essential nutrients such as vitamins, minerals, proteins, dietary fibre, carotenoids, and fatty acids (Fahey, Citation2005).
Moringa oleifera is a rich source of polyphenolic compounds, protein and antioxidants with high activity. Therefore, it is necessary to examine the potential of MOLE as a food enriching agent to substitute synthetic preservatives. Few studies have been conducted on the utilisation of MOLE in meat and meat products but none on mutton patties during refrigerated storage. The objective of this study was to explore the impact of MOLE on the nutritional characteristics and shelf life of mutton patties.
2. Materials and methods
2.1. Plant materials and reagents
Fresh Moringa oleifera leaves were purchased from a local farm in Thohoyandou, South Africa. All reagents used were of analytical grade. The reagents Folin-Ciocalteu’s, gallic acid, catechin, and thiobarbituric acid were purchased from Merck (Pty, Ltd, Sandton, South Africa).
2.2. Preparation of Moringa oleifera leaves extract
Moringa oleifera leaves were separated from the stalks and shaken to remove dirt and impurities then soaked in clean tap water and washed. The washed leaves were rolled out on the tray to air-dry for 20 min to drain out water. Moringa oleifera leaves were dried using an oven dryer (Prolab Model OTE 80. USA) at 50°C for 3 h. The dried leaves were milled using a Retsch miller (ultra-centrifugal mill ZM 200) and the ring sieves produced the final fine powder (40 µm). The milled powder was sieved using a sieve of 40 µm and the powder was kept in closed plastic (polyethylene bag) and stored in a cool dry place to avoid changes in physical and chemical properties. To obtain the extract, 100 mL boiled distilled water was added to 25 g of dried powder and allowed to rest for 5 min. The temperature of water for extraction and during extraction was 65 and 60°C. The extract was obtained by filtration.
2.3. Preparation of mutton patties
Five kilograms of boneless mutton meat was obtained from a local butchery. Fresh mutton meat was cut into strips and the meat strips were ground using a freddyl Hirsch mincer. Basic patties formulation was used (90% mutton meat, 9% water and 1% salt). The mixture was divided into five batches and MOLE was added at different levels: control (0%), 1%, 2%, 3% and 5% (w/w), respectively. All batches were mixed again for 5 min and patties were formed using a conventional burger forming machine (70–75 g/patty, 1.5 cm thickness and 10 cm diameter). The patties samples were wrapped with polyethylene plastic boxes for 15 days at 4 ± 1°C. The prepared samples were taken on days 0, 5, 10, 15 for analyses.
2.4. Proximate composition and polyphenolic compounds analyses
The Association of Official Analytical Chemists (AOAC) methods (Citation2007) were used to determine the moisture, protein, ash and fat contents of mutton patties. The AOAC method 945.32 was used to determine the moisture content with oven drying at 105°C for 3 h. Crude protein was determined using the Kjeldahl digestion method following AOAC method 978.02, and 6.25 × N factor was used. Muffle furnace was used to determine the ash content according to the official method 923.03. The fat content of samples was determined by solvent extraction method using petroleum ether (AOAC method 920.39). The polyphenolic compounds were extracted from one gram of sample by mixing it with 5 mL of the solvent extraction solution (acetone/distilled water/acetic acid; 70:29.5:0.5v/v/v) and the mixture was stirred for 1 min using vortex equipment. An ultrasonic bath (Treviglio, Italy) was used to sonicate the mixture for 10 min. The mixture was centrifuged at 492 g-force for 10 min and filtered through cellulose paper. Supernatant was then collected and stored at 2°C for total flavonoids and total phenolic contents analysis.
The total flavonoid content was determined using a colorimetric method with few modifications (Ordonez et al., Citation2006). About 2 g of each sample was weighed and transferred into beaker and 20 mL of methanolic acid (10%) was added and sonicated for 10 min in an ultrasonic bath and then centrifuged at 3075 g-force for 10 min and then filtered. An aliquot of 0.5 mL of 2% AlCl3 ethanol solution was added to 0.5 mL of the filtrate. The samples were incubated for 1 h at room temperature and the absorbance was measured at 506 nm using UV-visible spectrophotometer microplate reader. Results were expressed as mg catechin equivalent (CE)/g.
The total phenolic content was determined through the Folin–Ciocalteu reagent assay following the method described by Mahmoud et al. (Citation2017). Briefly, 2.4 mL of distilled water and 0.15 mL of 0.25 N Folin–Ciocalteu reagent were added to 0.15 mL of sample diluent (1 g patties extract/100 mL distilled water), vortexed, and reacted for 3 min. Afterwards, 0.3 mL of 1 N sodium carbonate was added, samples were left to stand in the dark for 1 h in a dark area with occasional shaking. After incubation, the absorbance of samples was measured at 725 nm using a UV–visible spectrophotometer microplate reader (Zenyth 200rt Biochrom, Cambridge, UK). Results were expressed as mg gallic acid equivalent (GAE)/g.
2.5. Determination of lipid oxidation
The degree of lipid oxidation in control and treated mutton patties was evaluated by the 2-thiobarbituric acid method (Rosmini et al., Citation1996). To prepare the sample extracts, 5 g of patties was mixed with 20 ml of distilled water followed by filtration. About 1.0 ml filtrate was deposited in a test tube, and 100 µl of 10% butylated hydroxytoluene and 4 ml of 20% thiobarbituric acid were added. The mixture was heated in a boiling water bath for 10 min after being vortexed. Afterwards, the mixture was cooled, centrifuged for 15 min and filtered. The absorbance of the samples was measured at 532 nm using UV spectrophotometer microplate reader (Zenyth 200rt Biochrom, Cambridge, UK). A standard curve of malonaldehyde (MDA) was used to calculate TBARS values and expressed as mg MDA/kg sample.
2.6. Microbiological analyses
Determination of microbial load, coliform bacteria, yeast and mould was carried out using the pour plate count following the method of Lorenzo et al. (Citation2015). About 10 g of raw mutton patties samples were weighed and homogenised with 90 mL of buffered peptone water (BPW) using a stomacher (260 oscillation per min) for 2.5 min. Subsequent serial sixfold dilutions were prepared in BPW up to a dilution. Afterwards, 1 mL of homogenised samples was inoculated into a sterile plate count agar using pour plate. The total plate count was enumerated on plate count agar (PCA, Merck, Sandton, South Africa), and Violet Red Bile agar (Merck, Sandton, South Africa) was used for total coliform count following incubation at 37°C for 48 h. Yeast and mould were determined on potato dextrose agar supplemented with antibiotics after incubation at 25°C for 120 h. The values were expressed as the log10 of colony forming units/g of sample (log10 CFU/g).
2.7. Determination of physiochemical properties
The pH of raw mutton patties was measured by mixing 10 g of sample with distilled water (50 mL) using a stomacher (Pb international, L7). The pH of the patties was measured using a pH meter (model: 2.0, Crison instrument, S.A, Barcelona, Spain).
Colour measurements(L*, a* and b*values) were performed using Spectrophotometer Lovibond (model no: LC 100, RM 200, Beijing, China) with a D65 light source, 8° observer, Diffuse/O mode, 8 mm aperture of the instrument for illumination and 8 mm for measurement. Hue and Chroma were calculated as H = Arc tan(a*/b*) and Chroma = (a*2 + b*2)1/2. The black and white plates were used to calibrate the spectrophotometer and zero calibration followed. The vacuum packaged mutton patties were opened at each time and the surface colour was measured at 0, 5, 10 and 15 days after 30 min of blooming at 4°C. Three different locations of mutton patties were scanned during measurement to obtain average L*, a* and b* values.
2.8. Determination of cooking properties
Three patties from each treatment were cooked in an electric oven at 163°C to a core of 71°C (American Meat Science Association, Citation2015) and then cooled to room temperature (25 ± 2°C). The difference in weight of raw and cooked patties was used to calculate cooking yield, fat and moisture retention:
2.9. Statistical analysis
The analyses were performed in triplicate and the mean data ± SD (standard deviation) were reported. The statistical package for the social sciences (SPSS) software (version 23.0, IBM SPSS, Armonk, NY, United States) was used to analyse the data and two-way analysis of variance (ANOVA) was used with fixed effects of treatment, storage days and their interaction where control and treated samples (T1, T2, T3 and T4) and storage days (0, 5, 10, 15) were used as factors. Differences between means were considered statistically significant at p < .05.
3. Results and discussion
3.1. Proximate composition
shows the proximate composition of raw mutton patties added with MOLE. There was a significant decrease (p < .05) in moisture content of patties added with MOLE throughout the storage period. The decrease in the moisture content of patties might be due the presence of MOLE which contributed to low binding in the matrix of the meat (Aleson-Carbonell et al., Citation2005). Moreover, increase in solid contents of the patties also contributed to low moisture content during storage. Similar results were also reported by Al-Juhaimi et al. (Citation2016) and Elhadi et al. (Citation2017) where Moringa oleifera seed and leaves powder were added resulting in a decrease of the moisture content of beef and chicken patties.
Table 1. Proximate composition of raw mutton patties added with Moringa oleifera leaves extract during storage days.
Tabla 1. Composición proximal de los medallones de ovino crudos con extracto de hojas de Moringa oleifera añadido durante los días de almacenamiento
There was a significant increase in protein content of treated mutton patties with the inclusion of MOLE. The protein content significantly increased (p < .05) throughout the storage period, ranging between 18.42 (control) and 21.45% (T1). At day 15, treated patties had higher protein content when compared with control sample. Moringa oleifera leaves contain protein content ranging from 18.92 to 26.16% (Sánchez-Machado et al., Citation2010). Therefore, the inclusion of MOLE increased the protein content of mutton patties.
There was a significant increase in ash content of treated patties throughout the storage days. The increase in ash content in treated mutton patties throughout the storage period might be due to high mineral content such as calcium, potassium, zinc and magnesium in MOLE. The high values of protein and ash contents of treated mutton patties might be associated with MOLE being a rich source of these constituents. Elhadi et al. (Citation2017) reported similar results where the inclusion of Moringa oleifera leaves powder in chicken patties improved their protein and ash contents.
The fat content of patties treated with MOLE significantly decreased (p < .05) during storage. By day 15, the fat content had decreased from 11.46 (control) to 9.97% (T4). The decrease in fat content of treated samples was anticipated due to the inclusion of MOLE which is rich in dietary fibre and natural antioxidants.
3.2. Polyphenolic compounds
depicts the results of polyphenolic compounds of raw mutton patties incorporated with MOLE. The total flavonoid content (TFC) of treated mutton patties was significantly higher (p < .05) than control sample during storage. This is because MOLE has high amounts of flavonoids and therefore the addition of MOLE increased the TFC of mutton patties. Das et al. (Citation2012) reported a MOLE flavonoids content of 22.4 mg/g in terms of catechin equivalent. Sreelatha and Padma (Citation2009) reported that mature MOLE is a rich source of flavonoids. Therefore, the phenolic and flavonoids contents of MOLE demonstrate that they can be used to enhance the bioactive components of meat products. The antioxidant activities of plant extracts generally depend on the amount of phenolic acids such as gallic, feluric and caffeic as well as flavonoids (catechin, myricetin and quercetin) (Sayas-Barberá et al., Citation2020). Different studies have demonstrated that flavonoids are the chief contributor for plant’s antioxidant activity (Chun et al., Citation2007; Merken & Beecher, Citation2000).
Table 2. Effect of Moringa oleifera leaves extract on polyphenolic compounds of mutton patties during storage days.
Tabla 2. Efecto del extracto de hojas de Moringa oleifera en los compuestos polifenólicos de los medallones de ovino durante los días de almacenamiento
The total phenolic content (TPC) in treated mutton patties was higher (p < .05) with the inclusion of MOLE compared with the control during 15 days of storage. A small quantity of polyphenols and flavonoids observed in control patties might be due to the inclusion of phenolic compounds in animal diets in order to improve the antioxidant capacity of the produced meat (Kalogianni et al., Citation2020). The increase in TPC level in treated mutton patties is attributed to high amounts of phenolic compounds in MOLE. Iqbal and Bhanger (Citation2006) reported that MOLE contains between 88.2 and 127.9 mg/g of total phenolics of ethonolic extracts. The higher quantity of phenolic content suggests that treated mutton patties are nutritionally enriched by the inclusion of MOLE. Therefore, MOLE was useful in increasing the TPC of mutton patties and hence enhances the antioxidant activity than the control sample. The results are in line with reports of Das et al. (Citation2012) and Devatkal et al. (Citation2011) where the inclusion of MOLE, kinnow and pomegranate by-product extracts improved the phenolic content of goat meat patties and raw chicken patties during cold storage.
3.3. Changes in thiobarbituric acid reactive substances (TBARS)
The values of TBARS of the mutton patties during storage are shown in . There was a significant difference in both treated and non-treated mutton patties. The TBARS values of mutton patties, irrespective of treatments, increased significantly (p < .05) from day 0 to day 15 for control and treated samples, respectively. The increase in TBARS value of all samples throughout the storage period might be attributed to lipid oxidation and generation of volatile metabolites in the presence of oxygen during aerobic storage (Domínguez et al., Citation2018; Prabakaran et al., Citation2018). Lipid in meat and meat products is easily oxidised, and the resulting hydroperoxides produce secondary lipid oxidation products such as aldehydes and ketones, which cause off-flavour (Cuong & Chin, Citation2016).
Figure 1. Effect of Moringa oleifera leaves extract on the TBARS on the mutton patties. Control (100% mutton patties), T1 (1%), T2 (2%), T3 (3%) and T4 (5%), Moringa oleifera leaves extract. Error bars are standard error of mean values (n = 3). Different letters show significant difference within same storage day at p ≤ .05.
Figura 1. Efecto del extracto de hojas de Moringa oleifera en las TBARS de los medallones de ovino. Control (100% medallones de ovino), T1 (1%), T2 (2%), T3 (3%) y T4 (5%), extracto de hojas de Moringa oleifera. Las barras de error representan el error estándar de los valores medios (n = 3). Las distintas letras indican diferencias significativas en el mismo día de almacenamiento a p ≤ .05
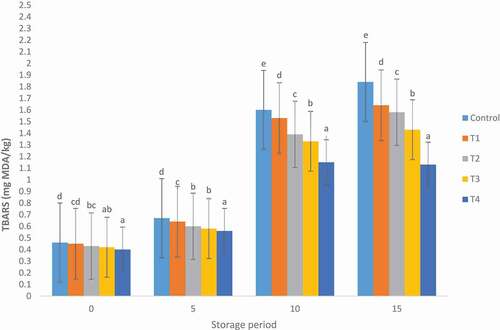
The gradual decrease of TBARS values in treated mutton patties could be attributed to the high amounts of phenolic compounds such as naphthoquinones, phenolic acids, flavonoids and other biochemical compounds in MOLE which might be the cause of the substantial antioxidant activity in mutton patties. Polyphenolic compounds reduce lipid oxidation through the donation of electrons as well as reacting with free radicals, thereby converting them to more stable products and terminating reactions of free chain (Das et al., Citation2012). Radha Krishnan et al. (Citation2014) found that antioxidant activity of polyphenolic compounds of plant extracts are related to the hydroxyl group connected to the aromatic ring which donate hydrogen atoms with electrons and neutralise free radicals. These results are similar to studies that have reported a positive relationship between polyphenolic content or antioxidant activity of plant extracts and decrease in lipid oxidation in meat and meat products (Devatkal et al., Citation2011; Jayathilakan et al., Citation2007). Various studies have demonstrated that lipid oxidation is delayed by the inclusion of plant powders or flours and extracts in meat and meat products such as MOLE in goat meat and pork patties (Das et al., Citation2012; Hayes et al., Citation2011).
3.4. Microbiological analysis
The impact of MOLE and storage time on total bacteria, coliform, yeast and moulds values were significant (p < .05). As shown in , at day 0 of storage, all treated samples remained below 3 log10 cfu/g with the control having 3.55 (total plate count), 3.77 (total coliform) and 4.44 (yeast/mould) log10 cfu/g, but these values increased throughout the storage period. The variation in microbial counts in day 0 might be due to the cell wall or outward membrane structure of microorganisms which restrict the penetration of the plant extract (Nisa et al., Citation2013). It was observed that, at day 15 control samples had the highest total bacterial count of 6.63 log cfu/g while treated sample (T4) had the lowest value of 4.30 log10 cfu/g. This shows that inclusion of MOLE significantly (p ≤ 0.05) reduced total bacterial count in patties. This is because Moringa oleifera leaves contain different types of bioactive compounds including flavonoids, saponins, tannins and other phenolic acids that possess antimicrobial activities (Fahey, Citation2005). Therefore, these components could be attributed to low bacterial count in treated mutton patties. In addition, the inclusion of MOLE decreases the total bacterial count during the cold storage of different meat products (Falowo et al., Citation2016; Najeeb et al., Citation2015).
Table 3. Effect of Moringa oleifera leaves extract on the microbiological analysis in of raw mutton patties during 15 days of storage.
Tabla 3. Efecto del extracto de hojas de Moringa oleifera en el análisis microbiológico de los medallones de ovino crudos durante 15 días de almacenamiento
The highest coliform count at day 0 was noticed in control sample (3.77 log cfu/g) compared to treated samples and the value increased by day 15 to 6.20 cfu/g. There was a significant increase (p < .05) in number of coliforms in all treated samples across the treatment during the period of 15 days. However, the presence of coliform in both treated and untreated samples might be due to dirty equipment and utensils used or poor hygienic handling and cross contamination during processing. Similar results of decrease in coliform count during cold storage of chicken and beef patties with the incorporation of moringa seed powder and MOLE have been reported (Al-Juhaimi et al., Citation2016; Elhadi et al., Citation2017).
Yeast/moulds count among days showed significant difference (p ˂ 0.05) in the control and the treated samples. However, treated mutton patties showed a significant increase throughout storage days. At day 15, treated samples values ranged from 5.90 (T1) to 3. 22 log cfu/g (T4), with the control samples having the highest value of 6.25 cfu/g. Bukar et al. (Citation2010) proved the ability of Moringa oleifera leaves to act as preservative against the growth spoilage microorganisms such as Escherichia coli, yeast and mould and pathogenic microorganisms such as Staphylococcus aureus, and Enterobacter aerogenes. The lower count of total bacteria, total coliform, yeast and mould in treated mutton patties compared with the control during storage could be attributed to the antimicrobial action of MOLE. On the basis of these results, it can be concluded that antimicrobial activity of MOLE is associated with phenolic compounds which are likely to be responsible for antibacterial activity.
3.5. Changes in pH
The pH values of control and mutton patties treated with MOLE are shown in . The inclusion of MOLE increased the pH values of control and mutton patties treated with MOLE during storage. The high counts of microorganisms during storage of mutton patties might be responsible for variation in pH among samples, may be due to putrefaction of protein in the patties by these microorganisms (Al-Juhaimi et al., Citation2016). Moreover, Microbial metabolites might also be responsible for the increase in pH value of mutton patties during storage. The low pH of T3 and T4 samples during day 15 of storage might be due to the inhibitory effects of MOLE on protein and lipid oxidation as well as some antimicrobial effects of the plant extracts.
Figure 2. Effect of Moringa oleifera leaves extract on the pH of mutton patties. Values expressed as mean ± standard deviation. Different letters show significant difference within same storage day at p ≤ .05. Control (100% mutton patties), T1 (1%), T2 (2%), T3 (3%) and T4 (5%), Moringa oleifera leaves extract.
Figura 2. Efecto del extracto de hojas de Moringa oleifera en el pH de los medallones de ovino. Los valores representan la media ± desviación estándar. Las distintas letras indican diferencias significativas en el mismo día de almacenamiento a p ≤ .05. Control (100% medallones de ovino), T1 (1%), T2 (2%), T3 (3%) y T4 (5%), extracto de hojas de Moringa oleifera.
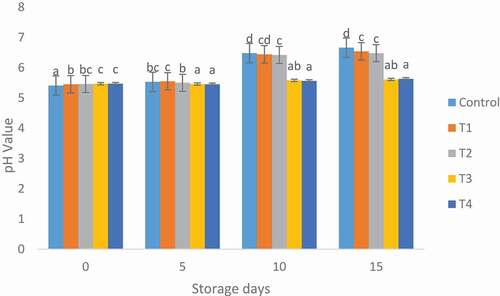
High pH values are related to the use of amino acids by bacteria, and the unpleasant production of ammonia and amines. On the depletion of stored glucose, bacteria use amino acids liberated during protein disintegration and ammonia multiplies as amino acid breakdown and the pH value increases (Gill, Citation1983). This results in the generation and accumulation of ammonia which give rise to pH value of mutton patties during storage (Radha Krishnan et al., Citation2014). Madane et al. (Citation2019) also observed an increase in pH of chicken nuggets added with Moringa oleifera flower extract during the storage.
3.6. Colour measurement
The colour measurement of mutton patties added with MOLE is shown in . There was a significant decrease in lightness (L*) values of treated samples with the concentration of MOLE compared to control. The decrease in the L* values (p < .05) of treated samples could be the result of lower moisture with the inclusion of MOLE, since moisture is associated with the lightness values (Pérez-Álvarez et al., Citation1999). Moreover, the inclusion of MOLE decreased the lightness of patties because MOLE contains a green pigment (chlorophyll) that affected the colour of the patties by diluting meat pigment, haemoglobin.
Table 4. Colour changes of raw mutton patties added with Moringa oleifera leaves extract during storage days.
Tabla 4. Cambios de color de los medallones de ovino crudos con extracto de hojas de Moringa oleifera añadido durante los días de almacenamiento
The treated samples had lower redness (a*) values than control sample which is due to the green colour of MOLE. The inclusion of the MOLE resulted in the greening (a*) of patties because of the green pigment in MO leaves, thus the redness decreased with the addition of MOLE. It was reported that high levels of antioxidant compound in Moringa oleifera leaves affect the colour of red meat because most antioxidants contain green pigments in high content, and the leaves have large amounts of green chlorophyll (Siddhuraju & Becker, Citation2003). However, Lynch and Faustman (Citation2000) indicated that the decrease in a* values is due to the interrelationship between lipid oxidation and meat colour oxidation. The yellowness (b*) values significantly decreased with the concentration of MOLE in treated patties compared to control. The decrease of yellowness in patties is due to natural antioxidants that MOLE contains.
There was a significant decrease in the C* (Chroma) values of treated patties samples with the concentration of MOLE. The H° (Hue angle) values of treated patties were significantly (p < .05) higher than the control sample. This shows that the addition of MOLE increased H° values. Luciano et al. (Citation2011) state that the lower values of a* and C* and higher H* values indicate meat discolouration due to their positive association with metmyoglobin quantity. Nkukwana et al. (Citation2014) reported similar results whereby the inclusion of Moringa oleifera leaf meal increased the H° values of chicken breast meat.
3.7. Cooking properties
Cooking properties such as cooking yield, fat and moisture retention are important quality attributes of meat products. The inclusion of MOLE significantly increased the cooking yield and moisture and fat retention as shown in . The increase in cooking yield of treated mutton patties could be due to the high ability of MOLE to retain moisture and fat in the meat matrix. In addition, higher cooking yield of treated patties might also be attributed to the presence of dietary fibres in MOLE since they bind water and fat (Cofrades et al., Citation2000). Therefore, the inclusion of MOLE contributed to the formation of a stronger structure of meat matrix in the patties (Essa & Elsebaie, Citation2018).
Table 5. Effect of Moringa oleifera leaves extract on the cooking properties of raw mutton patties during storage days.
Tabla 5. Efecto del extracto de hojas de Moringa oleifera en las propiedades de cocción de los medallones de ovino crudos durante los días de almacenamiento
The moisture retention of treated mutton patties was significantly higher (p < .05) than that of control sample These results are important since high water and fat retention influences properties such as texture and juiciness of the patties. Similar results of increase in moisture retention of patties with the inclusion of plant extracts were reported by Al-Juhaimi et al. (Citation2016) who used Moringa oleifera seed flour.
The fat retention of treated patties significantly increased (p < .05) when compared with the control sample. This result could be attributed to dietary fibre and starch in MOLE that form a gel network at high temperatures, resulting in greater entrapment of fat and water within the food matrix (Feiner, Citation2006). However, the fat retention decreased with the amount of MOLE and this could be due to reduction of fat content of patties. According to Besbes et al. (Citation2010), fat retention is associated with the ability of the meat matrix to bind fat. Therefore, high fat retention in treated patties samples might be due to the swelling of the starch, fibre and the fat absorbed by the fibre which interacted with the protein of the patties meat matrix which inhibited the migration of fat from the product (Alakali et al., Citation2010). Similar results were reported by Al-Juhaimi et al. (Citation2016) where Moringa oleifera seed flour increased the fat retention of beef patties during storage.
4. Conclusions
The inclusion of MOLE improves the polyphenolic compounds, protein and ash contents of mutton patties during storage. Moreover, the inclusion of MOLE is also effective in inhibiting lipid oxidation and microbial growth extending the shelf life of mutton patties. The other benefits of adding MOLE in mutton patties is that it increases the cooking properties. This is likely due to the polyphenolic compounds, antimicrobial and antioxidant properties of MOLE. Our results show that MOLE can be utilised as a natural antioxidant to preserve mutton meat products. It would be beneficial to carry out consumer acceptance studies to test the results of the current work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdull Razis, A. F., Ibrahim, M. D., & Kntayya, S. B. (2014). Health benefits of Moringa oleifera. Asian Pacific Journal of Cancer Prevention, 15(20), 8571–8576. https://doi.org/https://doi.org/10.7314/APJCP.2014.15.20.8571
- Alakali, J. S., Irtwange, S. V., & Mzer, M. T. (2010). Quality evaluation of beef patties formulated with bambara groundnut (Vigna subterranean L.) seed flour. Meat Science, 85(2), 215–223. https://doi.org/https://doi.org/10.1016/j.meatsci.2009.12.027
- Aleson-Carbonell, L., Fernández-López, J., Pérez-Álvarez, J. A., & Kuri, V. (2005). Characteristics of beef burger as influenced by various types of lemon albedo. Innovative Food Science & Emerging Technologies, 6(2), 247–255. https://doi.org/https://doi.org/10.1016/j.ifset.2005.01.002
- Al-Juhaimi, F., Ghafoor, K., Hawashin, M. D., Alsawmahi, O. N., & Babiker, E. E. (2016). Effects of different levels of Moringa (Moringa oleifera) seed flour on quality attributes of beef burgers. CyTA – Journal of Food, 14(1), 1–9. https://doi.org/https://doi.org/10.1080/19476337.2015.1034784
- Al-Owaisi, M., Al-Hadiwi, N., & Khan, S. A. (2014). GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrine (Forssk.) fiori leaves. Asian Pacific Journal of Tropical Biomedicine, 4(12), 964–970. https://doi.org/https://doi.org/10.12980/APJTB.4.201414B295
- American Meat Science Association. (2015). Research guidelines for cookery, sensory evaluation, and instrumental tenderness measurements of meat. AMSA.
- Asghar, A., Gray, J. I., Buckley, D. J., Pearson, A. M., & Booren, A. M. (1988). Perspectives on warmed-over flavour. Food Technology, 42(6), 102–108.
- Associaton of Official Analytical Chemists. (2007). Official methods of analysis of AOAC international (18th ed.). Association of Official Analytical Chemists AOAC.
- Besbes, S., Ghorbel, R., Salah, R. B., Masmoudi, M., Jedidi, F., Attia, H., & Blecker, C. (2010). Date fibre concentrate: Chemical composition, functional properties and effect on quality characteristics of beef burgers. Journal of Food and Drug Analysis, 18(1), 8–14. https://doi.org/https://doi.org/10.38212/2224-6614.2220
- Bukar, A., Uba, A., & Oyeyi, T. I. (2010). Antimicrobial profile of Moringa oleifera lam extracts against some food – borne microorganisms. Bayero Journal of Pure and Applied Sciences, 3(1), 43 – 48.
- Choi, J. H., Kim, N., Kim, G. W., & Choi, H. Y. (2019). Effect of cacao nip extracts on quality characteristics of pork patties during cold storage period. Food Science of Animal Resources, 39(6), 918–933. https://doi.org/https://doi.org/10.5851/kosfa.2019.e77
- Chun, O. K., Chung, S. J., & Song, W. O. (2007). Estimated dietary flavonoid intake and major food sources of U.S. adults. Journal of Nutrition, 137(5), 1244–1252. https://doi.org/https://doi.org/10.1093/jn/137.5.1244
- Cofrades, S., Guerra, M. A., Carballo, J., Fernandez-Martin, F., & Jiménez-Colmenero, F. (2000). Plasma protein and soy fibre content effect on bologna sausage properties as influenced by fat level. Journal of Food Science, 65(2), 281–287. https://doi.org/https://doi.org/10.1111/j.1365-2621.2000.tb15994.x
- Cuong, T. V., & Chin, K. B. (2016). Effects of annatto (Bixa orellana L.) seeds powder on physicochemical properties, antioxidant and antimicrobial activities of pork patties during refrigerated storage. Korean Journal for Food Science of Animal Resources, 36(4), 476–486. https://doi.org/https://doi.org/10.5851/kosfa.2016.36.4.476
- Das, A. K., Rajkumar, V., Verma, A. K., & Swarup, D. (2012). Moringa oleiferia leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. International Journal of Food Science and Technology, 47(3), 585–591. https://doi.org/https://doi.org/10.1111/j.1365-2621.2011.02881.x
- De Oliveira, T. L. C., De Carvalhob, S. M., Soaresa, R. D., Andradeb, M. A., Cardosob, M. D., Ramos, E. M., & Piccolia, R. H. (2012). Antioxidant effects of Satureja montana L. essential oil on TBARS and colour of mortadella-type sausages formulated with different levels of sodium nitrite. LWT - Food Science and Technology, 45(2), 204–212. https://doi.org/https://doi.org/10.1016/j.lwt.2011.09.006
- Devatkal, S. K., Narsaiah, K., & Borah, A. (2011). The effect of salt, extract of kinnow and pomegranate fruit by-products on colour and oxidative stability of raw chicken patties during refrigerated storage. Journal of Food Science and Technology, 48(4), 472–477. https://doi.org/https://doi.org/10.1007/s13197-011-0256-9
- Dillard, C. J., & German, J. B. (2000). Phytochemicals: Nutraceuticals and human health: A review. Journal of the Science of Food and Agriculture, 80(12), 1744–1756. https://doi.org/https://doi.org/10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W
- Domínguez, R., Barba, F. J., Gómez, B., Putnik, P., Bursa´c Kovaˇcevi´c, D., Pateiro, M., Santos, E. M., & Lorenzo, J. M. (2018). Active packaging films with natural antioxidants to be used in meat industry: A review. Food Research International, 113, 93–101. https://doi.org/https://doi.org/10.1016/j.foodres.2018.06.073
- Elhadi, D. A. E., Elgasim, A. E., & Mohamed Ahmed, I. A. (2017). Microbial and oxidation characteristics of refrigerated chicken patty incorporated with moringa (Moringa oleifera) leaf powder. CyTA – Journal of Food, 15(2), 234–240. https://doi.org/https://doi.org/10.1080/19476337.2016.1242157
- Essa, R. Y., & Elsebaie, E. (2018). Effect of using date pits powder as a fat replacer and anti-oxidative agent on beef burger quality. Journal of Food Dairy Science, Mansoura University, 9(2), 91–96. https://doi.org/https://doi.org/10.21608/jfds.2018.35225
- Fahey, J. W. (2005). Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees for Life Journal, 1, 5. http://www.TFLJournal.org/article.php/2005/201124931586
- Falowo, A. B., Fayemi, P. O., & Muchenje, V. (2014). Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Research International, 64, 171–181. https://doi.org/https://doi.org/10.1016/j.foodres.2014.06.022
- Falowo, A. B., Muchenje, V., Hugo, C. J., & Charimba, G. (2016). In vitro antimicrobial activities of Bidens pilosa and Moringa oleifera leaf extracts and their effects on ground beef quality during cold storage. CyTA – Journal of Food, 14(4), 541–546. https://doi.org/https://doi.org/10.1080/19476337.2016.1162847
- Feiner, G. (2006). Meat products handbook: Practical science and technology (1st ed ed.). Woodhead Publishing.
- Gill, C. O. (1983). Meat spoilage and evaluation of the potential storage life of fresh meat. Journal of Food Protein, 46(5), 444–452. https://doi.org/https://doi.org/10.4315/0362-028X-46.5.444
- Hayes, J. E., Stepanyan, V., Allen, P., O’Grady, M. N., & Kerry, J. P. (2011). Evaluation of the effects of selected plant-derived nutraceuticals on the quality and shelf-life stability of raw and cooked pork sausages. LWT - Food Science and Technology, 44(1), 164–172. https://doi.org/https://doi.org/10.1016/j.lwt.2010.05.020
- Iqbal, S., & Bhanger, M. I. (2006). Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. Journal of Food Composition and Analysis, 19(6–7), 544–551. https://doi.org/https://doi.org/10.1016/j.jfca.2005.05.001
- Jayathilakan, K., Sharma, G. K., Radhakrishna, K., & Bawa, A. S. (2007). Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chemistry, 105(3), 908–916. https://doi.org/https://doi.org/10.1016/j.foodchem.2007.04.068
- Kalogianni, A. I., Lazou, T., Bossis, I., & Gelasakis, A. I. (2020). Natural phenolic compounds for the control of oxidation, bacterial spoilage, and foodborne pathogens in meat. Foods, 9(6), 794. https://doi.org/https://doi.org/10.3390/foods9060794
- Khawaja, T. M., Tahira, M., & Lkram, U. K. (2010). Moringa oleifera: A natural gift - A review. Journal of Pharmaceutical Sciences and Research, 2(11), 775–778.
- Kim, S., Cho, A. R., & Han, J. (2013). Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control, 29(1), 112–120. https://doi.org/https://doi.org/10.1016/j.foodcont.2012.05.060
- Kobus-Cisowska, J., Flaczyk, E., Rudzińska, M., & Kmiecik, D. (2014). Antioxidant properties of extracts from Ginkgo biloba leaves in meatballs. Meat Science, 97(2), 174–180. https://doi.org/https://doi.org/10.1016/j.meatsci.2014.01.011
- Kozłowska, M., Ścibisz, I., Zaręba, D., & Ziarno, M. (2015). Antioxidant properties and effect on lactic acid bacterial growth of spice extracts. CyTA – Journal of Food, 13(4), 573–577. https://doi.org/https://doi.org/10.1080/19476337.2015.1022228
- Lorenzo, J. M., Bermúdez, R., Domínguez, R., Guiotto, A., Franco, D., & Purriños, L. (2015). Physicochemical and microbial changes during the manufacturing process of dry-cured lacón salted with potassium, calcium and magnesium chloride as a partial replacement for sodium chloride. Food Control, 86(2), 763–769. https://doi.org/https://doi.org/10.1016/j.foodcont.2014.10.019
- Luciano, G., Vasta, V., Monahan, F. J., Lopez-Andres, A., Biondi, L., & Lanza, M. (2011). Antioxidant status, colour stability, myoglobin resistance to oxidation of longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chemistry, 124(3), 1036–1042. https://doi.org/https://doi.org/10.1016/j.foodchem.2010.07.070
- Lynch, M. P., & Faustman, C. (2000). Effect of aldehyde lipid oxidation products on myoglobin. Journal of Agricultural and Food Chemistry, 48(3), 600–604. https://doi.org/https://doi.org/10.1021/jf990732e
- Madane, P., Das, A. K., Pateiro, M., Nanda, P. K., Bandyopadhyay, S., Jagtap, P., Barba, F. J., Shewalkar, A., Banibrata Maity, B., & Lorenzo, J. M. (2019). Drumstick (Moringa oleifera) flower as an antioxidant dietary fibre in chicken meat nuggets. Foods, 8(8), 307. https://doi.org/https://doi.org/10.3390/foods8080307
- Mahmoud, M. H., Abou-Arab, A. A., & Abu-Salem, F. M. (2017). Quality characteristics of beef burger as influenced by different levels of orange peel powder. American Journal of Food Technology, 12(4), 262–270. https://doi.org/https://doi.org/10.3923/ajft.2017.262.270
- Mancini, S., Preziuso, G., Dal Alessandro, B., Roscini, V., Szendro, Z., Fratini, F., & Paci, G. (2015). Effect of turmeric powder (Curcuma longa L.) and ascorbic acid on physical characteristics and oxidative status of fresh and stored rabbit burgers. Meat Science, 110, 93–100. https://doi.org/https://doi.org/10.1016/j.meatsci.2015.07.005
- Mendieta-Araica, B., Spörndly, R., Reyes-Sánchez, N., & Spörndly, E. (2011). Moringa (Moringa oleifera) leaf meal as a source of protein in locally produced concentrates for dairy cows fed low protein diets in tropical areas. Livestock Science, 137(1–3), 10–17. https://doi.org/https://doi.org/10.1016/j.livsci.2010.09.021
- Merken, H. M., & Beecher, G. R. (2000). Measurement of food flavonoids by high-performance liquid chromatography: A review. Journal of Agricultural and Food Chemistry, 48(3), 577–599. https://doi.org/https://doi.org/10.1021/jf990872o
- Najeeb, A. P., Mandal, P. K., & Pal, U. K. (2015). Efficacy of leaves (drumstick, mint and curry leaves) powder as natural preservatives in restructured chicken block. Journal of Food Science and Technology, 52(5), 3129–3133. https://doi.org/https://doi.org/10.1007/s13197-014-1316-8
- Nisa, H., Kamili, A. N., Bandh, S. A., Amin, S., Lone, B. A., & Parray, J. A. (2013). Phytochemical screening, antimicrobial and antioxidant efficacy of different extracts of Rumex dentatus L. – A locally used medicinal herb of Kashmir Himalaya. Asian Pacific Journal of Tropical Disease, 3(6), 434–440. https://doi.org/https://doi.org/10.1016/S2222-1808(13)60097-3
- Nkukwana, T. T., Muchenje, V., Pieterse, E., Masika, P. J., Mabusela, T. P., Hoffman, L. C., & Dzama, K. (2014). Effect of Moringa oleifera leaf meal on growth performance, apparent digestibility, digestive organ size and carcass yield in broiler chickens. Livestock Science, 161(1), 139–146. https://doi.org/https://doi.org/10.1016/j.livsci.2014.01.001
- Ordonez, A., Gomez, J., Vattuone, M., & Lsla, M. (2006). Antioxidant activities of sechium edule (Jacq.) swartz extracts. Food Chemistry, 97(3), 452–458. https://doi.org/https://doi.org/10.1016/j.foodchem.2005.05.024
- Pérez-Álvarez, J. A., Sayas-Barberá, M. E., Fernández-López, J., & Aranda-Catalá, V. (1999). Physicochemical characteristics of Spanish-type dry-cured sausage. Food Research International, 32(9), 599–607. https://doi.org/https://doi.org/10.1016/S0963-9969(99)00104-0
- Prabakaran, M., Kim, S. H., Sasireka, A., Chandrasekaran, M., & Chung, I. M. (2018). Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera.. Food Bioscience, 26, 23–29. https://doi.org/https://doi.org/10.1016/j.fbio.2018.09.003
- Radha Krishnan, K., Babuskina, S., Azhagu Saravana Babu, P., Sasikala, M., Sabina, K., Archana, G., Sivarajan, M., & Sukumar, M. (2014). Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. International Journal of Food Microbiology, 171, 32–40. https://doi.org/https://doi.org/10.1016/j.ijfoodmicro.2013.11.011
- Ribeiro, J. S., Santos, M. J. M. C., Silva, L. K. R., Pereira, L. C. L., Santos, I. A., Da Silva Lannes, S. C., & Da Silva, M. V. (2019). Natural antioxidants used in meat products: A brief review. Meat Science, 148, 181–188. https://doi.org/https://doi.org/10.1016/j.meatsci.2018.10.016
- Rosmini, M. R., Perlo, F., Pérez-Alvarez, J. A., Pagán-Moreno, M. J., Gago-Gago, M. A., López-Santoveña, F., & Aranda-Catalá, V. (1996). TBA test by an extractive method applied to ‘paté’. Meat Science, 42(1), 103–110. https://doi.org/https://doi.org/10.1016/0309-1740(95)00010-0
- Sánchez-Machado, D. I., Núñez-Gastélum, J. A., Reyes-Moreno, C., Ramírez-Wong, B., & López-Cervantes, J. (2010). Nutritional quality of edible parts of Moringa oleifera. Food Analytical Methods, 3(3), 175–180. https://doi.org/https://doi.org/10.1007/s12161-009-9106-z
- Sánchez-Ortega, I., García-Almendárez, B. E., Santos-López, E. M., Amaro-Reyes, A., Barboza-Corona, J. E., & Regalado, C. (2014). Antimicrobial edible films and coatings for meat and meat products preservation. Scientific World Journal, 2014, 18. Article ID 248935. https://doi.org/https://doi.org/10.1155/2014/248935
- Sayas-Barberá, E., Martín-Sánchez, A. N., Cherif, S., Jamel Ben-Abda, J., & Pérez-Álvarez, J. A. (2020). Effect of date (Phoenix dactylifera L.) pits on the shelf life of beef burgers. Foods, 9(1), 102. https://doi.org/https://doi.org/10.3390/foods9010102
- Sharma, P., & Yadav, S. (2020). Effect of incorporation of pomegranate peel and bagasse powder and their extracts on quality characteristics of chicken meat patties. Food Science of Animal Resources, 40(3), 388–400. https://doi.org/https://doi.org/10.5851/kosfa.2020.e19
- Siddhuraju, P., & Becker, K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam). Journal of Agricultural and Food Chemistry, 51(8), 2144–2155. https://doi.org/https://doi.org/10.1021/jf020444+
- Soladoye, O. P., Juárez, M. L., Aalhus, J. L., Shand, P., & Estévez, M. (2015). Protein oxidation in processed meat: Mechanisms and potential implications on human health. Comprehensive Review in Food Science and Food Safety, 14(2), 106–122. https://doi.org/https://doi.org/10.1111/1541-4337.12127
- Sreelatha, S., & Padma, P. R. (2009). Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods for Human Nutrition, 64(4), 303–311. https://doi.org/https://doi.org/10.1007/s11130-009-0141-0
- Zhang, H., Peng, X., Li, X., Wu, J., & Guo, X. (2017). The application of clove extract protects Chinese-style sausages against oxidation and quality deterioration. Korean Journal for Food Science of Animal Resources, 37(1), 114–122. https://doi.org/https://doi.org/10.5851/kosfa.2017.37.1.114
