ABSTRACT
We assessed the changes in nutritional and putrescent components during enzymolysis of Acetes chinensis. Oligopeptide and astaxanthin both reached a maximum of 46.78 ± 1.03% and 14.71 ± 0.32 mg/kg at 6 h, respectively. The total free amino acid changed from 6.37 ± 0.08 g/100 g to 14.21 ± 0.17 g/100 g at 8 h. However, the volatile base nitrogen reached 143.50 ± 6.21 mg/100 g at 10 h. The total biogenic amine was first decreased and then increased to 6,324.00 ± 10.56 mg/kg. Moreover, the acid and peroxide values reached 4.93 ± 0.17 mg/g and 5.56 ± 0.23 mg/kg at 10 h, respectively. Therefore, although Acetes chinensis hydrolysate was rich in nutrients, the putrescent components, including volatile base nitrogen, biogenic amine, acid and peroxide values, were increased during enzymolysis. Collectively, the enzymatic hydrolysis process of Acetes chinensis should be appropriately regulated to reduce the contents of putrescent components.
RESUMEN
El presente estudio se propuso evaluar los cambios en los componentes nutricionales y putrescentes de Acetes chinensis. durante su enzimólisis Este proceso permitió comprobar que el oligopéptido y la astaxantina alcanzaron un máximo de 46.78 ± 1.03% y 14.71 ± 0.32 mg/kg a las seis horas, respectivamente. Además, el aminoácido libre total pasó de 6.37 ± 0.08 g/100 g a 14.21 ± 0.17 g/100 g a las ocho horas. El nitrógeno base volátil alcanzó 143.50 ± 6.21 mg/100 g a las 10 horas. Asimismo, la amina biogénica total disminuyó primero y aumentó después hasta 6,324.00 ± 10.56 mg/kg, mientras los valores de ácido y peróxido alcanzaron 4.93 ± 0.17 mg/g y 5.56 ± 0.23 mg/kg a las 10 horas, respectivamente. Por lo tanto, aunque el hidrolizado de Acetes chinensis es rico en nutrientes, durante su enzimólisis se incrementaron los componentes putrescentes, incluyendo el nitrógeno base volátil, la amina biogénica y los valores de ácido y peróxido. Se concluye que, en conjunto, el proceso de hidrólisis enzimática de Acetes chinensis debería ser regulado adecuadamente para reducir el contenido de componentes putrescentes.
PALABRAS CLAVE:
1. Introduction
As a type of low-value marine micro-shrimp, Acetes chinensis has a slightly lateral flat body and a grayish red color, and it is widely distributed in Liaodong Peninsula, Jiangsu, Zhejiang, and Guangdong offshore of China (Kang et al., Citation2015). The peak fishing season for Acetes chinensis is in summer, and its annual catch in Zhejiang Province alone is over 250,000 tons (Lu et al., Citation2011). Acetes chinensis will be rapidly self-dissolved and decomposed after capture due to the high temperature of summer (H. T. Wang et al., Citation2013). Therefore, the freshness of Acetes chinensis is difficult to maintain.
Acetes chinensis is rich in proteins, fatty acids, minerals, and other high-value nutrients. However, most Acetes chinensis are processed into powder through rough processing and used in the aquatic feed industry because of the difficult preservation (Nguyen & Wang, Citation2019). Only a few fresh big Acetes chinensis are made into shrimp bran and shrimp paste, which are used in the food industry (Amir & Mahdi, Citation2019). In the production process of Acetes chinensis powder, a large number of odors will be produced, causing serious pollution to the surrounding environment. Therefore, the powder-processing enterprises of Acetes chinensis are required to merge and integrate to meet the environmental requirements before the resumption of production, leading to a great economic loss. Besides, the long time and high temperature of the cooking and drying process result in a greater loss of nutrients. Therefore, it is urgently necessary to develop new high-nutrition and environmental-friendly products of Acetes chinensis.
Hydrolysis of macromolecular proteins by protease can effectively release the active substances of small molecules with various physiological functions (D. Li et al., Citation2018). The enzymatic hydrolysis remains an effective way to increase the economic value added (EVA) of Acetes chinensis, which has a good application prospect in the field of functional food. He et al. (Citation2006) have hydrolyzed Acetes chinensis with self-made crude protease to obtain the enzymatic hydrolysate with antioxidant activity. Wang et al. (Citation2008, Citation2018) have fermented Acetes chinensis to prepare hydrolytic products that inhibit the activity of angiotensin I-converting enzyme. J. H. Liu et al. (Citation2016) have degraded Acetes chinensis using alkaline lipase to decrease the lipid oxidation during preservation. These reports have mainly focused on the enzymolysis techniques of Acetes chinensis, while only few studies have investigated the changes in physicochemical components in the enzymatic hydrolysis process. In the present study, we determined the main nutrition and putrefaction indexes of the enzymatic hydrolysis of Acetes chinensis, and the changes in nutritional and putrescent compositions in the enzymatic hydrolysis process were also assessed. Collectively, our findings provided a scientific basis for the high-value application of the enzymatic hydrolysis of Acetes chinensis in the food industry.
2. Material and methods
2.1. Materials
Acetes chinensis was provided by Zhejiang Eiifne Marine Biological Products Co., Ltd. (Taizhou, China). Alcalase2.4 L, neutrase, protamex, and flavourzyme were supplied by Novozymes (China) Biotech (Tianjin, China). Astaxanthin standard was purchased from Aladdin Industrial Co., Ltd. (Shanghai, China). Fatty acid and biogenic amine standards were obtained from Sigma Chemical (St. Louis, USA). Amino acid mixture standard was provided by Wako Pure Chemical (Osaka, Japan). Other reagents were of analytical grade. Animal-related experimental procedures were approved by the ethical committee of Nantong College of Science and Technology. All protocols were made following relevant guidelines.
2.2. Enzymolysis of Acetes chinensis
The raw materials of Acetes chinensis were mixed with distilled water at a ratio of 1:0.5 (w/w), followed by homogenization using a colloid mill (Huiyou Machinery Co., Ltd., Langfang, China). The homogenate was divided into four parts and maintained in a water bath at 55°C, and the pH of these four parts was then adjusted to 8.5, 7.0, 7.0, and 7.0, respectively. Subsequently, alcalase2.4 L, neutrase, protamex, and flavourzyme were respectively added at a ratio of 0.2:100 (w/w), followed by agitation at 180 r/min for 10 h. The enzymatic hydrolysates were collected every 2 h and immediately stored at −80°C before subsequent physical and chemical analyses.
2.3. Proximate component and amino nitrogen assay
Proximate components, such as protein, lipid, ash, and moisture, were determined according to the corresponding methods from the Association of Official Analytical Chemists (Gaithersburg, Citation2003). The content of amino nitrogen was determined as previously described by Chen et al. (Citation2019).
2.4. Nutritional component assay
The conetent of amino acid was determined by a previously established approach (Yang et al., Citation2007) using an automatic amino acid analyzer (Hitachi, Tokyo, Japan). Oligopeptide content was determined using the method of Cai et al. (Citation2014), which was calculated as the total content of trichloroacetic acid-soluble proteins minus the free amino acid. The content of astaxanthin was analyzed according to the method of Ranga Rao et al. (Citation2013) using high-performance liquid chromatography (HPLC, Shimadzu, Kyoto, Japan) equipped with a C18 column. The content of fatty acid was analyzed according to the method of Xie et al. (Citation2018) using a gas chromatographic system (Agilent, Santa Clara, USA) equipped with a capillary column.
2.5. Putrescent component assay
Volatile base nitrogen was determined using the method of Mirzapour-Kouhdasht and Moosavi-Nasab (Citation2019). Biogenic amines were measured based on the method of Sang et al. (Citation2020) using an HPLC (Agilent, Santa Clara, USA) equipped with a C18 column. Acid and peroxide values were determined based on the method of D. Y. Li et al. (Citation2019).
2.6. Statistical analysis
The experiments were performed in triplicate, and the data were expressed as mean ± SD. Statistical analysis was conducted using SPSS Statistics 13.0, and significant differences (p < .05) were indicated with different lower-case letters. The results were calculated based on a dry basis.
3. Results
3.1. The proximate compositions of Acetes chinensis raw materials
shows that Acetes chinensis was rich in proximate components, such as protein, lipid, and ash. The protein content reached 12.21 ± 0.19%, which was up to 65.47 ± 2.62% when converted to the dry base. The contents of lipid and ash reached 9.91 ± 0.62% and 23.86 ± 1.76% when converted to the dry base, respectively. Besides, the moisture content of Acetes chinensis was as high as 81.31 ± 0.90%. Compositional analysis of amino acid showed that the total amino acid content of Acetes chinensis was 54.08 ± 0.19 g/100 g dry basis, consisting of 21.67 ± 0.34 g/100 g dry basis of essential amino acids (Lys, Phe, Met, Thr, Ile, Leu, and Val) and 32.32 ± 0.43 g/100 g dry basis of non-essential amino acids (Asp, Ser, Glu, Gly, Ala, Cys, Tyr, His, Arg, and Pro) ().
Table 1. Basic chemical constituents of Acetes chinensis.
Tabla 1. Componentes químicos básicos de Acetes chinensis.
Table 2. Amino acid composition of Acetes chinensis.
Tabla 2. Composición de aminoácidos de Acetes chinensis.
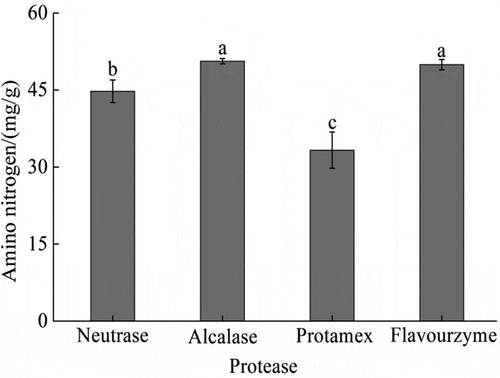
3.2. Enzymolysis effect of the four commercial proteases on Acetes chinensis
The optimal protease was selected based on the amino nitrogen content in the enzymatic hydrolysis solution of Acetes chinensis. The amino nitrogen contents enzymatically hydrolyzed with alcalase2.4 L and flavourzyme were the highest, which were up to 50 mg/g, followed by neutrase and protamex (). Therefore, the enzymatic hydrolysis effect of the four commercial proteases on Acetes chinensis was ranked as follows: alcalase2.4 L = flavourzyme > neutrase > protamex.
Figure 1. Enzymolysis effect of different commercial proteases on Acetes chinensis. There is a significant difference between different lowercase letters (P < .05).
Figura 1. Efecto de la enzimólisis de diferentes proteasas comerciales en Acetes chinensis. Existe una diferencia significativa entre las distintas letras minúsculas (P < .05)
3.3. Nutritional changes during the enzymolysis process of Acetes chinensis
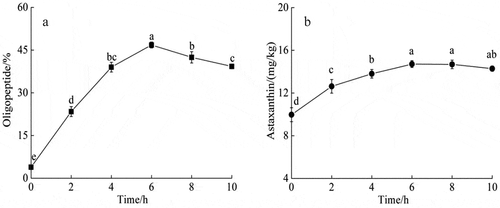
3.3.1. Changes in oligopeptide and astaxanthin
shows the changes of oligopeptide and astaxanthin contents during the enzymatic hydrolysis of Acetes chinensis. With the extension of hydrolysis time, Acetes chinensis protein was continuously enzymolyzed to produce oligopeptide, which reached the maximum (46.78 ± 1.03%) at 6 h (). Subsequently, the oligopeptide was further enzymolyzed to generate free amino acids, leading to a gradually decreasing trend of the oligopeptide content (). The astaxanthin was gradually released from Acetes chinensis with the extension of enzymatic hydrolysis time, and its content remained increasing and reached 14.71 ± 0.32 μg/g at 6 h ().
Figure 2. Changes in oligopeptide and astaxanthin during Acetes chinensis enzymolysis. (a) Change of oligopeptide content during the enzymatic hydrolysis of Acetes chinensis; (b) change of astaxanthin content during the enzymatic hydrolysis of Acetes chinensis. There is a significant difference between different lowercase letters (P < .05).
Figura 2. Cambios en el oligopéptido y la astaxantina durante la enzimólisis de Acetes chinensis. (a) Cambio en el contenido de oligopéptidos durante la hidrólisis enzimática de Acetes chinensis; (b) cambio en el contenido de astaxantina durante la hidrólisis enzimática de Acetes chinensis. Existe una diferencia significativa entre las distintas letras minúsculas (P < .05)
3.3.2. Change in free amino acid
reveals that the total amount of free amino acids was increased from 6.37 ± 0.08 g/100 g at the beginning of enzymatic hydrolysis to 14.21 ± 0.17 g/100 g at 8 h. Both the sweet (Asp, Glu, Thr, Ser, Gly, Pro, and Ala) and bitter amino acids (His, Leu, Ile, Val, Arg, Tyr, Met, and Phe) presented an increasing trend, which were increased to 5.40 ± 0.04 g/100 g and 7.60 ± 0.10 g/100 g, respectively.
Table 3. Changes in free amino acids during Acetes chinensis enzymolysis.
Tabla 3. Cambios en los aminoácidos libres durante la enzimólisis de Acetes chinensis.
3.3.3. Change in fatty acid
shows the change of fatty acid during the enzymatic hydrolysis of Acetes chinensis. With the extension of enzymatic hydrolysis time, the compositions of saturated fatty acid (C4:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C22:0, C24:0), unsaturated fatty acid (C14:1, C16:1, C18:1n9t, C18:1n9c, C18:2n6c, C18:3n6, C20:1, C18:3n3, C20:2, C20:3n6, C22:1n9, C20:4n6, C22:2, C20:5n3, C24:1, C22:6n3) and omega-3polyunsaturated fatty acid (C18:3n3, C20:5n3, C22:6n3) were all not significantly changed, which were about 40%, 60%, and 39%, respectively.
Table 4. Change in fatty acid during Acetes chinensis enzymolysis.
Tabla 4. Cambios en los ácidos grasos durante la enzimólisis de Acetes chinensis.
3.4. Changes in putrescent components during the enzymolysis process of Acetes chinensis
3.4.1. Changes in protein putrescent components
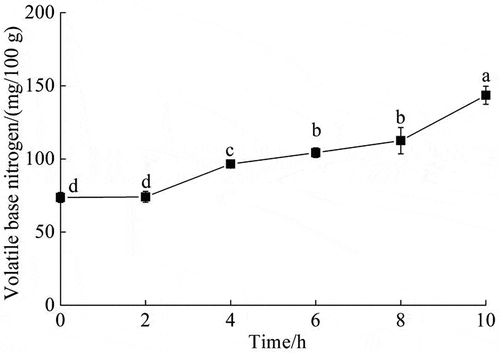
With the extension of enzymatic hydrolysis time, the volatile base nitrogen of protein putrescent components was gradually increased and reached 143.5 ± 6.21 mg/100 g at 10 h (). On the other hand, the total amount of biogenic amines was decreased from 5,803.12 ± 4.38 mg/kg to 5,205.65 ± 6.57 mg/kg at 2 h, and then it was increased to 6,324.00 ± 10.56 mg/kg at 8 h (). The contents of putrescine, tryptamine, and histamine were increased from 960.54 ± 3.66 mg/kg to 1177.02 ± 3.77 mg/kg, from 1,127.04 ± 2.96 mg/kg to 1,828.89 ± 4.71 mg/kg, and from 294.49 ± 1.35 mg/kg to 415.45 ± 4.92 mg/kg at 8 h, respectively (). The content of cadaverine was decreased from 1,788.81 ± 1.86 mg/kg to 1,425.27 ± 3.61 mg/kg at 2 h, and then it was increased to 1,919.56 ± 5.32 mg/kg at 8 h (). The contents of tyramine and phenylethylamine were decreased from 1,159.18 ± 3.21 mg/kg to 773.03 ± 2.74 mg/kg and from 473.07 ± 2.79 mg/kg to 210.06 ± 1.78 mg/kg at 8 h, respectively (). Besides, spermidine, spermine, and octopamine were not detected.
Table 5. Change in bioamine during Acetes chinensis enzymolysis.
Tabla 5. Cambio en la bioamina durante la enzimólisis de Acetes chinensis.
Figure 3. Change in volatile base nitrogen during Acetes chinensis enzymolysis. There is a significant difference between different lowercase letters (P < .05).
Figura 3. Cambio en el nitrógeno base volátil durante la enzimólisis de Acetes chinensis. Existe una diferencia significativa entre las distintas letras minúsculas (P < .05)
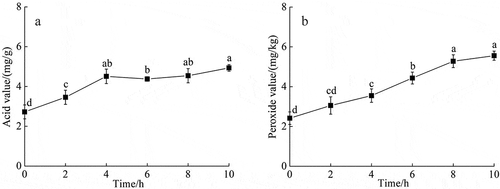
3.4.2. Changes in lipid putrescent components
depicts the changes of lipid putrescent components during the enzymatic hydrolysis of Acetes chinensis. The acid and peroxide values of oil oxidation index were both gradually increased, reaching 4.93 ± 0.17 mg/g and 5.56 ± 0.23 mg/kg at 10 h, respectively.
Figure 4. Changes in acid and peroxide value during Acetes chinensis enzymolysis. (a) Change in acid value during the enzymatic hydrolysis of Acetes chinensis; (b) change in peroxide value during the enzymatic hydrolysis of Acetes chinensis. There is a significant difference between different lowercase letters (P < .05).Figura 4. Cambios en el valor de ácido y peróxido durante la enzimólisis de Acetes chinensis. (a) Cambio en el valor del ácido durante la hidrólisis enzimática de Acetes chinensis; (b) Cambio en el valor del peróxido durante la hidrólisis enzimática de Acetes chinensis. Existe una diferencia significativa entre las distintas letras minúsculas (P < .05)
4. Discussion
In the present study, we aimed to assess the changes in nutritional and putrescent components in the enzymatic hydrolysis process of Acetes chinensis to provide a scientific basis for the high-value application of the enzymatic hydrolysis of Acetes chinensis in the food industry. Knowledge of protein content is crucial for the utilization of Acetes chinensis raw materials. The protein content of the Acetes chinensis was about 66% on a dry basis (), which was close to that of raw materials from other marine shrimp proteins, such as Antarctic krill (CitationTharaka et al., Citation2020). High protein content in raw material can provide sufficient protein substrates for enzymatic hydrolysis. Besides, the ratio of essential amino acids and non-essential amino acids to total amino acids was 0.40 ± 0.01 and 0.67 ± 0.02, respectively (), showing an ideal protein model according to the United Nations Food and Agriculture Organization and the World Health Organization. Therefore, Acetes chinensis might be a perfect resource for enzymatic hydrolysis due to its high protein content and well-balanced amino acid composition. To select the appropriate protease, four main commercial proteases (alcalase2.4 L, neutrase, protamex, and flavourzyme) were employed to hydrolyze Acetes chinensis. The contents of amino nitrogen enzymatically hydrolyzed with alcalase2.4 L and flavourzyme were the highest (). Then Acetes chinensis was hydrolyzed by alcalase2.4 L due to the consideration of market price, and the variations of main nutritional and putrescent compositions during the enzymatic hydrolysis were analyzed.
Oligopeptide, composed of 2 ~ 10 amino acid residues with a molecular weight of less than 1,000 Da, is a class of small molecular nutrients with various physiological activities (Qin et al., Citation2020). With the extension of enzymatic hydrolysis time, oligopeptide was continuously produced from the Acetes chinensis protein (Chatterjee et al., Citation2020), reaching the maximum of 46.78 ± 1.03%, and then it was gradually decreased due to the further production of free amino acids (Fujiya & Tavares, Citation2015) (). Astaxanthin, a type of bright red carotenoid, has many physiological functions, such as anti-inflammatory (Farruggia et al., Citation2018) and immunity-improving effects (Jiang et al., Citation2020). The astaxanthin was gradually released from the astaxanthin-protein complex (Armenta-Lopez et al., Citation2002) of Acetes chinensis with the extension of enzymatic hydrolysis time, which remained increasing and reached 14.71 ± 0.32 μg/g (). Enzymatic hydrolysis can destroy the protein structure and continuously free astaxanthin from the astaxanthin-protein complex (Y. X. Liu et al., Citation2020), thus increasing the release of astaxanthin. Free amino acids are extremely important non-volatile flavor substances for shrimp products (Zzaman et al., Citation2017), rendering Acetes chinensis unique flavor and taste. The total amount of free amino acids presented a steadily increasing trend, and the ratio of sweet amino acids to bitter amino acids in the enzymatic hydrolysates of Acetes chinensis was maintained at 0.7 (). The continuously increased total free amino acids and the balanced relationship between sweet amino acids and bitter amino acids together constitute the flavor basis (El-Adawy et al., Citation2001) of Acetes chinensis enzymolytic products. Furthermore, the variations of fatty acid composition during the enzymatic hydrolysis of Acetes chinensis were not significantly changed (). It is worth mentioning that the omega-3 polyunsaturated fatty acid is an important functional fatty acid associated with decreased risk for chronic diseases, such as inflammation, arrhythmia, hyperlipidemia, and atherosclerosis (Yates et al., Citation2009). The composition of omega-3 polyunsaturated fatty acid (C18:3n3, C20:5n3, C22:6n3) in Acetes chinensis hydrolysate was about 39% (), and its content was on a par with Antarctic krill (Gigliotti et al., Citation2011). Therefore, Acetes chinensis hydrolysate represented a perfect source of nutritional value for its rich nutrients. However, analyzing the nutritional composition alone of Acetes chinensis hydrolysate only has limited effect, and more attention should be paid to the variation of putrescent components during enzymolysis.
Volatile base nitrogen and biogenic amines, two typical protein putrefaction products, are positively correlated with the degree of decomposition of aquatic products (Heerthana & Preetha, Citation2019), which can reflect the freshness of aquatic products, such as fish and shrimp. With the extension of enzymatic hydrolysis time, the volatile base nitrogen of protein putrescent components was gradually increased, reaching 143.5 ± 6.21 mg/100 g at 10 h (). On the other hand, the total amount of biogenic amines was decreased from 5,803.12 ± 4.38 mg/kg to 5,205.65 ± 6.57 mg/kg first, and then it was increased to 6,324.00 ± 10.56 mg/kg (). Excessive amounts of volatile base nitrogen produce ammonia odor (Du et al., Citation2011), and excessive intake of biogenic amines can cause adverse reactions, such as headache and nausea (Moret et al., Citation1992). Therefore, more attention should be paid to the regulation of volatile base nitrogen and biogenic amines during the enzymatic hydrolysis of Acetes chinensis. Moreover, acid and peroxide, two typical oil rancidity products (Muttagi et al., Citation2014), were both gradually increased, which reached 4.93 ± 0.17 mg/g and 5.56 ± 0.23 mg/kg at 10 h, respectively, showing a decreasing trend of the oil freshness during the hydrolysis process of Acetes chinensis.
Based on the above-mentioned findings, Acetes chinensis hydrolysate was rich in nutrients, such as oligopeptide, astaxanthin, and amino acides. Hence, it could serve as a valuable source of food with potential bioactive functions. Meanwhile, the putrescent compositions, such as volatile base nitrogen, biogenic amine, acid value, and peroxide value, were increased during enzymolysis of Acetes chinensis. Therefore the enzymatic hydrolysis process of Acetes chinensis should be appropriately regulated to reduce the contents of undesirable components as much as possible.
Author contributions
Conceived and designed the experiments: Feng Lv and Jiang Sun. Performed the experiments: Feng Lv, Jiang Sun, Linghua Wang, and Chenhui Zhong. Analyzed the data: Shuiqing Wu and Haiyan Wu. Wrote the paper: Feng Lv and Jiang Sun. All authors have read and approved the final manuscript.
Data sharing statement
All data generated or analyzed during this study are available from the corresponding author Feng Lv upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amir, N., & Mahdi, C. (2019). Quality and food safety of shrimp paste produced in district of takalar, province of south sulawesi. International Journal of ChemTech Research, 12(1), 100–104. https://doi.org/https://doi.org/10.20902/IJCTR.2019.120111
- Armenta-Lopez, R., Guerrero, L. I., & Huerta, S. (2002). Astaxanthin extraction from shrimp waste by lactic fermentation and enzymatic hydrolysis of the carotenoprotein complex. Journal of Food Science, 67(3), 1002–1006. https://doi.org/https://doi.org/10.1111/j.1365-2621.2002.tb09443.x
- Cai, M. Y., Gu, R. Z., Li, C. Y., Ma, Y., Dong, Z., Liu, W. Y., Jin, Z. T., Lu, J., & Yi, W. X. (2014). Pilotscale production of soybean oligopeptides and antioxidant and antihypertensive effects in vitro and in vivo. Journal of Food Science and Technology, 51(9), 1866–1874. https://doi.org/https://doi.org/10.1007/s13197-012-0701-4
- Chatterjee, R., Dey, T. K., Roychoudhury, A., Paul, D., & Dhar, P. (2020). Enzymatically excised oligopeptides from bellamya bengalensis shows potent antioxidative and anti-hypertensive activity. Journal of Food Science and Technology -mysore, 57(7), 2586–2601. https://doi.org/https://doi.org/10.1007/s13197-020-04295-8
- Chen, L., Wang, J. L., Ni, H., & Zhu, M. J. (2019). Disruption of Phaffia rhodozyma cells and preparation of microencapsulated astaxanthin with high water solubility. Food Science and Biotechnology, 28(1), 111–120. https://doi.org/http://dx.doi.org/10.1007/s10068-018-0443-9
- Du, X., Plotto, A., Baldwin, E., & Rouseff, R. (2011). Evaluation of volatiles from two subtropical strawberry cultivars using gc-olfactometry, gc-ms odor activity values, and sensory analysis. Journal of Agricultural and Food Chemistry, 14(23), 12569–12577. https://doi.org/https://doi.org/10.1021/jf2030924
- El-Adawy, T. A., Rahma, E. H., El-Bedawey, A. A., & Gafar, A. F. (2001). Nutritional potential and functional properties of sweet and bitter lupin seed protein isolates. Food Chemistry, 74(4), 455–462. https://doi.org/https://doi.org/10.1016/S0308-8146(01)00163-7
- Farruggia, C., Kim, M. B., Bae, M., Lee, Y., Pham, T. X., Yang, Y., Han, M. J., Park, Y. K., & Lee, J. Y. (2018). Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manner. Journal of Nutritional Biochemistry, 62, 202–209. https://doi.org/https://doi.org/10.1016/j.jnutbio.2018.09.005
- Fujiya, N. M., & Tavares, M. F. M. (2015). Analysis of underivatized amino acids in protein hydrolysates for cosmetic application by capillary electrophoresis. Journal of Separation Science, 26(6–7), 562–568. https://doi.org/https://doi.org/10.1002/jssc.200390077
- Gaithersburg, M. D. (2003). OfficialMethodsofAnalysisofAOACInternational,seventeenthed. Association of the Official Analytical Chemists (AOAC) International.
- Gigliotti, J. C., Davenport, M. P., Beamer, S. K., Tou, J. C., & Jaczynski, J. (2011). Extraction and characterisation of lipids from antarctic krill (euphausia superba). Food Chemistry, 125(3), 1028–1036. https://doi.org/https://doi.org/10.1016/j.foodchem.2010.10.013
- He, H. L., Chen, X. L., Sun, C. Y., Zhang, Y. Z., & Gao, P. J. (2006). Preparation and functional evaluation of oligopeptide-enriched hydrolysate from shrimp (Acetes chinensis) treated with crude protease from Bacillus sp. SM98011. Bioresource Technology, 97(3), 385–390. https://doi.org/https://doi.org/10.1016/j.biortech.2005.03.016
- Heerthana, V. R., & Preetha, R. (2019). Biosensors: A potential tool for quality assurance and food safety pertaining to biogenic amines/volatile amines formation in aquaculture systems/products. Aquaculture, 11(1), 220–233. https://doi.org/https://doi.org/10.1111/raq.12236
- Jiang, X. D., Zu, L., Wang, Z. Y., Cheng, Y. X., Yang, Y. H., & Wu, X. G. (2020). Micro-algal astaxanthin could improve the antioxidant capability, immunity and ammonia resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish and Shellfish Immunology, 102(8), 499–510. https://doi.org/https://doi.org/10.1016/j.fsi.2020.05.021
- Kang, J. H., Noh, E. S., Park, J. Y., An, C. M., & Kim, J. K. (2015). Rapid origin determination of the Northern Mauxia Shrimp (Acetes chinensis) based on allele specific polymerase chain reaction of partial mitochondrial 16S rRNA gene. Asian-Australasian Journal of Animal Sciences, 28(4), 568–572. https://doi.org/https://doi.org/10.5713/ajas.14.0613
- Li, D., Li, L., Xu, T., Wang, T. X., Ren, J. W., Liu, X. R., & Li, Y. (2018). Effect of low molecular weight oligopeptides isolated from sea cucumber on diabetic wound healing in db/db mice. Marine Drugs, 16(1), 1–16. https://doi.org/https://doi.org/10.3390/md16010016
- Li, D. Y., Xie, H. K., Liu, Z. Y., Li, A., Li, J. X., Liu, B., Liu, X. Y., & Zhou, D. Y. (2019). Shelf life prediction and changes in lipid profiles of dried shrimp (Penaeus vannamei) during accelerated storage. Food Chemistry, 297(1), 1–9. https://doi.org/https://doi.org/10.1016/j.foodchem.2019.124951
- Liu, J. H., Zhao, P. C., Liu, L., Zhang, J. Y., Liu, S. L., & Zhang, Z. F. (2016). Decrease of lipid oxidation for dried shrimp (Acetes chinensis) preservation using alkaline lipase hydrolysis technology. Journal of Aquatic Food Product Technology, 25(2), 169–176. https://doi.org/https://doi.org/10.1080/10498850.2013.839017
- Liu, Y. X., Huang, L., Li, D. H., Wang, Y. B., Chen, Z. H., Zou, C., Liu, W. Q., Ma, Y., Cao, M. J., & Liu, G. M. (2020). Re-assembled oleic acid-protein complexes as nano-vehicles for astaxanthin: Multispectral analysis and molecular docking. Food Hydrocolloids, 103, 105689. https://doi.org/https://doi.org/10.1016/j.foodhyd.2020.105689
- Lu, F., Zhang, J. Y., Liu, S. L., Wang, Y., & Ding, Y. T. (2011). Chemical, microbiological and sensory changes of dried Acetes chinensis during accelerated storage. Food Chemistry, 127(1), 159–168. https://doi.org/https://doi.org/10.1016/j.foodchem.2010.12.120
- Mirzapour-Kouhdasht, A., & Moosavi-Nasab, M. (2019). Shelf-life extension of whole shrimp using an active coating containing fish skin gelatin hydrolysates produced by a natural protease. Food Science & Nutrition, 8(1), 214–223. https://doi.org/https://doi.org/10.1002/fsn3.1293
- Moret, S., Bortolomeazzi, R., & Lercker, G. (1992). Improvementof extraction procedure for biogenic amines in foods and their high-performance liquid chromatographic determination. Journal of Chromatography A, 591(1–2), 175–180. https://doi.org/https://doi.org/10.1016/0021-9673(92)80235-M
- Muttagi, G. C., Joshi, N., Shadakshari, Y. G., & Chandru, R. (2014). Storage stability of value added products from sunflower kernels. Journal of Food Science and Technology, 51(9), 1806–1816. https://doi.org/https://doi.org/10.1007/s13197-014-1261-6
- Nguyen, V. B., & Wang, S. L. (2019). Production of potent antidiabetic compounds from shrimp head powder via paenibacillus conversion. Process Biochemistry, 76, 18–24. https://doi.org/https://doi.org/10.1016/j.procbio.2018.11.004
- Qin, X. Y., Zhang, J. T., Li, G. M., Cai, M. Y., Lu, J., Gu, R. Z., & Liu, W. Y. (2020). Selenium-chelating corn oligopeptide as a potential antioxidant supplement: Investigation of the protein conformational changes and identification of the antioxidant fragment composition. International Journal of Food Engineering, 16(4), 629–656. https://doi.org/https://doi.org/10.1515/ijfe-2019-0166
- Ranga Rao, A., Baskaran, V., Sarada, R., & Ravishankar, G. A. (2013). In vivo bioavailability and antioxidant activity of carotenoids from microalgal biomass–A repeated dose study. Food Research International, 54(1), 711–717. https://doi.org/https://doi.org/10.1016/j.foodres.2013.07.067
- Sang, X., Li, K. X., Zhu, Y. L., Ma, X. X., Hao, H. S., Bi, J. B., Zhang, G. L., & Hou, H. M. (2020). The impact of microbial diversity on biogenic amines formation in grasshopper sub shrimp paste during the fermentation. Frontiers in Microbiology, 11, 1–13. https://doi.org/https://doi.org/10.3389/fmicb.2020.00782
- Tharaka, K., Benitez-Santana, T., Gunathilaka, B. E., Kim, M. G., Lee, C., Shin, J., & Lee, K. J. (2020). Evaluation of Antarctic krill (Euphausia superba) meal supplementation in diets for olive flounder (Paralichthys olivaceus). Aquaculture Research, 51(6), 2291–2302. https://doi.org/https://doi.org/10.1111/are.14573
- Wang, H. T., Wang, W., Chen, M., Yuan, N., Zhao, Y. H., & Mao, X. Z. (2013). Active peptide from Acetes chinensis with inhibitory activity on neuraminidase of influenza virus. Chemical Research in Chinese Universities, 34(11), 2540–2545. https://doi.org/https://doi.org/10.7503/cjcu20130334
- Wang, L. M., Sun, J., Ding, S. H., & Qi, B. (2018). Isolation and identification of novel antioxidant and antimicrobial oligopeptides from enzymatically hydrolyzed anchovy fish meal. Process Biochemistry, 74, 148–155. https://doi.org/https://doi.org/10.1016/j.procbio.2018.08.021
- Wang, Y. K., He, H. L., Chen, X. L., Sun, C. Y., Zhang, Y. Z., & Zhou, B. C. (2008). production of novel angiotensin I-converting enzyme inhibitory peptides by fermentation of marine shrimp Acetes chinensis with Lactobacillus fermentum SM 605. Applied Microbiology and Biotechnology, 79(5), 785–791. https://doi.org/https://doi.org/10.1007/s00253-008-1489-z
- Xie, D., Mu, H. Y., Tang, T. P., Wang, X. S., Wei, W., Jin, J., Wang, X. G., & Jin, Q. Z. (2018). Production of three types of krill oils from krill meal by a three-step solvent extraction procedure. Food Chemistry, 248, 279–286. https://doi.org/https://doi.org/10.1016/j.foodchem.2017.12.068
- Yang, Y., Tao, G., Liu, P., & Liu, J. (2007). Peptide with angiotensin I-converting enzyme inhibitory activity from hydrolyzed corn gluten meal. Journal of Agricultural and Food Chemistry, 55(19), 7891–7895. https://doi.org/https://doi.org/10.1021/jf0705670
- Yates, A., Norwig, J., Maroon, J. C., Bost, J., Bradley, J. P., Duca, M., Wecht, D. A., Grove, R., Iso, A., Cobb, I., Ross, N., & Borden, M. (2009). Evaluation of lipid profiles and the use of omega-3 essential fatty acid in professional football players. Sports Health: A Multidisciplinary Approach, 1(1), 21–30. https://doi.org/https://doi.org/10.1177/1941738108326978
- Zzaman, W., Bhat, R., Yang, T. A., & Easa, A. M. (2017). Influences of superheated steam roasting on changes in sugar, amino acid and flavour active components of cocoa bean (Theobroma cacao). Journal of the Science of Food and Agriculture, 97(13), 4429–4437. https://doi.org/https://doi.org/10.1002/jsfa.8302
