ABSTRACT
A novel, rapid, and reliable ultra-high performance supercritical fluid chromatography (UHPSFC) method was developed to correctly separate and quantitatively determine Sudan dyes in chilli products. The effect of different separation parameters on the retention characteristics of Sudan dyes was investigated. The results showed that diode array detection of Sudan I–II was accomplished at 480 nm, and that of Sudan III–IV at 500 nm. In the analysis of Sudan dyes, the whole analysis time was less than 10 minutes, the method had a limit of detection and limit of quantification of 0.25 μg/mL and 0.50 μg/mL, respectively. All calibration curves had good linearity (r2 > 0.9989) within the tested concentration ranges. The method had intra- and inter-day precision of less than 3.5% and 4.1%, respectively, with a recovery between 96.58% and 104.50%, and a relative standard deviation of less than 4.4%. The method was successfully applied to analyze chilli product sample.
Resumen
El presente estudio se propuso desarrollar un método novedoso, rápido y fiable de cromatografía de fluidos supercríticos de alto rendimiento (UHPSFC) para separar correctamente y determinar cuantitativamente los colorantes Sudán en productos de chile. Para ello, se investigó el efecto de diferentes parámetros de separación en las características de retención de los colorantes Sudán. Los resultados permitieron constatar que la detección por matriz de diodos de Sudán I-II se logró a 480 nm, mientras que la de Sudán III-IV ocurrió a 500 nm. Asimismo, al analizar los colorantes de Sudán, la totalidad del tiempo de análisis fue inferior a 10 minutos, el método tuvo un límite de detección y un límite de cuantificación de 0.25 μg/mL y 0.50 μg/mL, respectivamente. Todas las curvas de calibración mostraron buena linealidad (r2 > 0.9989) dentro de los rangos de concentración probados. Además, el método tuvo una precisión intra e interdiaria inferior a 3.5 y a 4.1%, respectivamente, registrando una recuperación de entre 96.58 y 104.50%, y una desviación estándar relativa inferior a 4.4%. En conclusión, el método se aplicó con éxito para analizar muestras de productos de chile.
1. Introduction
Sudan dyes are synthetic industrial dyes that are widely used as a colorant in oil, wax, gasoline, shoes, and floor polishes (Teng & Zhou, Citation2017). Sudan dyes are carcinogenic, genotoxic, sensitive, and mutagenic (Li et al., Citation2017; Xu et al., Citation2010), and are toxic to human organs such as the liver and kidney. The International Agency for Research on Cancer (IARC) has analyzed and evaluated the carcinogenic effect of Sudan Red and classified it as a category 3 carcinogen (Zuckerman, Citation1995). Thus, food materials that contain Sudan Red with amounts that exceed the restricted value must be withdrawn from the market (Rebane et al., Citation2010; The Comission of the European Communities, Citation2003). Nonetheless, because Sudan Red is cheap and provides a bright (not easily faded) color to food, which increases consumer appeal, illegal addition of the dyes persists. In 2003, France announced that pepper imported from India and Pakistan contained Sudan I and in 2005, chili sauce and Kentucky Fried Chicken (KFC) in China were found to contain Sudan I (European Commission). In the same year, the European Union (EU) announced that certain dyes should not be present in foodstuffs at any level, while the level for analytical detection methods was set at a maximum of 0.5–1.0 mg/kg for food control. Since then, Sudan dyes other than Sudan I have been detected in a variety of foods apart from chili powders including sausages, bubble noodles, cooked meat, pies, chilli pepper, and seasoning sauces. As a result, European Commission guidelines directives 2003/460/EC, 2004/92/EC, and 2005/402 extended legislation to include other types of Sudan dye and other food products. Nonetheless, the finding of Sudan dyes in food samples at levels of 1 and 2 mg/kg indicates that their illegal addition persists. Therefore, an accurate, rapid, and high-throughput extraction method is needed to determine their presence in foodstuffs.
Recent commonly used methods include magnetic metal–organic frameworks (Shi et al., Citation2018; J. Zhang et al., Citation2013), liquid–liquid extraction (Long et al., Citation2011; Yu et al., Citation2015), cloud point extraction (Liu et al., Citation2007), liquid–liquid microextraction (Hu et al., Citation2016; Sun et al., Citation2011), and solid-phase extraction (Deng et al., Citation2021; Z. Zhang et al., Citation2012). Some of these are slow, for example, detection of Sudan I–IV may take up 6–71 minutes using methods that rely on LC analysis, DAD and/or UV detectors, and MS detection (a more selective and sensitive detection) (Du & Sun, Citation2007; Fukuji et al., Citation2012; López-Jiménez et al., Citation2010; Qi et al., Citation2011; Zhao et al., Citation2012), and have the disadvantage of solvent consumption. The use of appropriate pretreatment method plays a vital role in the separation and analysis of Sudan dyes due to the low concentration of added Sudan I–IV and the complex food matrix. For instance, ultra-high performance supercritical fluid chromatography (UHPSFC) is a green separation and faster alternative to LC that has been used to detect Sudan dyes (Jia et al., Citation2020; Lesellier & West, Citation2015). This technique has many advantages over other chromatographic techniques such as low viscosity and high diffusivity of the supercritical fluid. Moreover, compared with earlier SFC, the technique uses a column with sub-micron particles (2 μm) that can further enhance mass transfer and improve the effectiveness of the separation (Grand-Guillaume Perrenoud et al., Citation2014; Lesellier et al., Citation2014). The use of SFC for the analysis of Sudan dyes has been described previously. Dolak (Dolak et al., Citation2007) and Lefler (Lefler & Chen, Citation2008) employed SFC to separate and quantify Sudan dyes and required an analysis time of 8 and 3 min, respectively. Khalikova (Khalikova et al., Citation2015) compared the use of UHPSFC and UPLC to analyze 11 illegal dyes and completed the former test within 5 minutes. Although the UHPSFC technology has been applied to detect illegal addition of dye, parameters that affect Sudan I–IV detection have not been studied in detail.
The aim of this work was to develop a high-throughput UHPSFC-based method coupled with DAD (UHPSFC/DAD) for simultaneous determination of Sudan I–IV in chilli products. The effects of the column stationary phase, organic modifier, back pressure, and column temperature on the retention characteristics of Sudan I–IV were investigated. Finally, the method was applied to quantify Sudan I–IV in real chilli product samples.
2. Material and methods
2.1. Chemicals, reagents, and samples
The standard Sudan dyes I, II, III, and IV (>98.5%; ) were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Food-grade carbon dioxide (purity >99.993%), which was stored in 50 kg steel cylinders without a dip tube, was purchased from Hui Neng. LC-grade isopropanol, acetonitrile, ethanol, and methanol were purchased from Merck (Darmstadt, Germany). All other chemicals were of analytical grade.
2.2. Chilli product samples
The following 13 commercial samples were studied: five chilli products and three chilli oils purchased from local markets, and five chilli oil samples submitted by the government department. Chilli sauces were stored in the dark at 4°C. The samples were homogenized before use.
2.3. Standard preparation and calibration
To prepare the stock solutions, standard Sudan dyes I, II, III, and IV were accurately weighed and then dissolved in hexane/isopropanol (1:1, v/v), each to a concentration of 200 μg/mL. Each stock solution was then diluted with hexane/isopropanol (1:1, v/v) to eight different concentrations: 200, 100, 50, 10, 1.0, 0.5, and 0.25 μg/mL. Approximately 1.5 mL of each diluted standard was transferred to a 2 mL amber vial and closed with a crimp cap. The standards were stored at −20℃ before analysis.
2.4. Preparation of samples for UHPSFC
Each sample of chilli powder, chilli sauce or chilli oil was thoroughly homogenized. Two grams of each sample was then homogenized and subjected to ultrasonic extraction with 10.0 mL of hexane/isopropanol (1:1, v/v) at room temperature for 2.0 min. Each solution (except for chilli oil) was then centrifuged at 11200 RCF for 5.0 min. The supernatant was filtered through a 0.22 μm membrane filter (Agilent, USA) and 2.0 μL of the filtrate injected into a UHPSFC for analysis.
2.5. UHPSFC analysis
The target compounds were analyzed using an ultra-performance convergence chromatography (UPC2) system (Waters, USA) equipped with a binary solvent delivery pump, an autosampler, a convergence manager with a back pressure regulator (BPR), and a column oven, coupled to a DAD controlled by Empower 3 software. The separation was carried out on an Acquity UPC2 HSS SB C18 (150 × 2.1 mm, 1.7 μm) column. The gradient elution (eluent A: CO2; eluent B: IPA) was set as follows: 1.0% B (initial); 1–20% B at 0–4.0 min; and 1% B for 0.5 min (to re-equilibrate the column). Other operating conditions were as follows: back pressure, 12.41 MPa; flow rate, 1.6 mL/min; injection volume, 2.0 μL; column temperature 40℃; sample temperature, 20℃; and detection, scan mode 200–550 nm.
2.6. Method validation
To ensure that the method could reliably determine and quantify four Sudan dyes in chilli product samples, the investigation of methodology is very necessary. The selectivity of the method was determined using spiked and un-spiked (blank) samples to ensure that the target compounds were not contaminated. The precision and accuracy of the method were determined using chilli samples spiked with standard Sudan dyes at a low, medium or high concentration before pretreatment. The intra-day precision was determined on the same day using six replicate samples. The inter-day precision was determined for three consecutive days using six replicate samples. The calibration curves were prepared using stock solutions of Sudan dyes diluted to appropriate concentrations. The limit of quantification (LOQ) and the limit of detection (LOD) are the concentrations at which the signal-to-noise ratio (S/N) is 10 and 3, respectively. Extraction recovery was evaluated using Sudan dyes at low, medium, or high concentration by comparing the ratio of peak area of spiked Sudan dyes before and after extraction. Each experiment was performed in triplicate.
3. Results and discussion
3.1. Selection of stationary phase
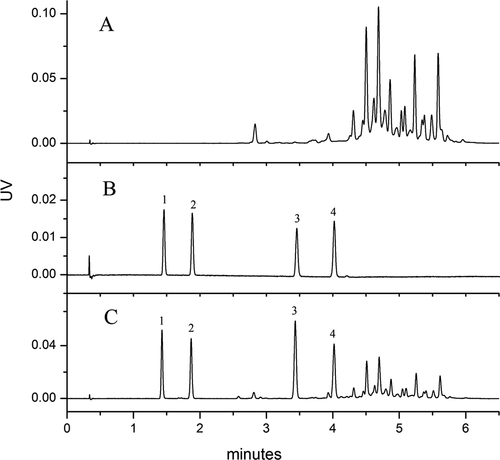
The complex composition of chilli products renders separation by UHPSFC challenging. To select the most suitable column for separation, four columns containing different stationary phases were tested. The four stationary phases were divided into three groups: (i) Bare hybrid phase (BEH) and bare hybrid phase modified with 2-ethylpyridine (BEH 2-EP) that can form different polar interactions with solutes, such as dipole–dipole and dipole-induced dipole interactions; (ii) The second type of stationary phase that can form additional retention interactions, such as π–π, H-bonding, and dipole–dipole interactions, due to the charged hybrid phase modified with a fluoro-phenyl group (CSH FL); and (iii) the stationary phases that comprise HSS C18, an inorganic silica phase modified with a C18 group, this stationary-phase optimization is used for method development under low pH conditions and provides complementary selectivity, especially for alkaline compounds. (Grand-Guillaume Perrenoud et al., Citation2012; Lesellier, Citation2012; Wang et al., Citation2017). In this experiment, Sudan I–IV were the target compounds, and the separations were performed under the conditions described in 2.4, except for the following conditions: gradient elution, 1–20% IPA (a co-solvent); flow rate, 1.6 mL/min; temperature, 40℃; and pressure, 12.41 MPa ().
Figure 2. Separation of Sudan I–IV on four different stationary phases.
Figura 2. Separación de Sudán I - IV en cuatro fases estacionarias diferentes
Separation of Sudan I–IV by BEH and BEH 2-EP columns had substantial peak tailing, likely because the dyes, which are azo compounds, had weak interaction with these stationary phases ()). The peaks were also distorted, possibly caused by secondary interactions between the azo compound and Si-OH or 2-pyridine of the stationary phase, or may be related to inappropriate dilution solvent. According to the literature, BEH and BEH 2-EP are suitable for separation of polar compounds (Grand-Guillaume Perrenoud et al., Citation2012), and thus have weak interactions with Sudan dyes. These results indicate that BEH and BEH 2-EP are not suitable for separation of Sudan dyes. In contrast, the separation of Sudan I–IV by CSH fluoro-phenyl stationary phase reached baseline separation, although peak tailing was observed ()). This observation is similar to that of a previous study by Khalikova (Khalikova et al., Citation2015). Among all four stationary phases tested, the HSS C18 SB phase could best separate Sudan I–IV ()); peak of the target compounds reached a baseline separation within 5.0 min. Therefore, although its peak shape and baseline were not symmetrical, the HSS C18 SB column was selected for further optimization.
3.2. Optimization of organic modifiers
Supercritical fluid carbon dioxide is highly nonpolar and therefore has weak elution ability for polar compounds such as glycosides and organic acid compounds. In other words, the elution strength of supercritical fluid CO2 is weak for polar compounds (Shaaban & Górecki, Citation2013). Under the same chromatographic conditions (described in 2.4), Sudan I–IV were better separated when the four modifiers were present, and their retention times decreased with increasing polarity of the alcohol modifiers ()), similar to results previously reported (Khalikova et al., Citation2015; Wang et al., Citation2017). Although ACN is a non-hydrogen bond-donating modifier, it had little effect on the separation and selectivity of Sudan I–IV ()), also reported previously by Khalikova (Khalikova et al., Citation2015).
Figure 3. Effect of (A) acetonitrile, (B) ethanol, (C) methanol, and (D) isopropanol on the separation of Sudan I–IV mixture.
Figura 3. Efecto de (A) acetonitrilo, (B) etanol, (C) metanol y (D) isopropanol en la separación de la mezcla Sudán I - IV
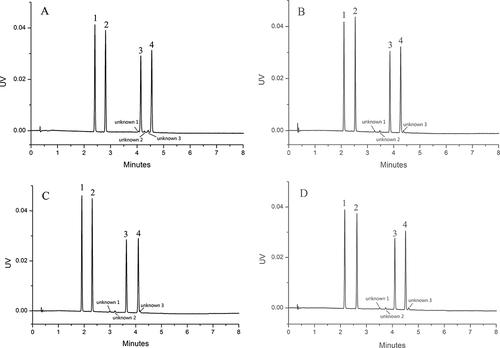
In addition, because the Sudan standard solvent contains three unknown impurities, we temporarily named the three unknown impurities as unknown 1, 2, 3, respectively. Using ACN as a modifier, unknown three spiked with Sudan III could not reach baseline separation. Using alcohol as modifiers, the resolution of unknown 3 and Sudan IV decreased with increasing polarity of alcohol. Although unknown 3 and Sudan IV were well separated when both ethanol and isopropanol were used, IPA was the best modifier considering that it has good solubility in greasy samples.
3.3. Optimization of initial gradient
Elution of target compounds in UHPSFC can be achieved by isocratic and gradient elution. The gradient elution in UHPSFC separation was achieved by varying the ratio of organic modifiers that can affect the solvent strength of supercritical fluid. Moreover, it does not vary in a linear fashion when you change pressure or temperature, and sometimes not even in a monotonous fashion. In a word, changing solvent proportion mostly affects solvent strength, way more than changing density (Berger, Citation2017; Berger, Citation2018). As described in 3.4, change of initial gradient greatly affected retention of the target compound; therefore, in UHPSFC separation, especially for the separation of compounds with similar structure, it is necessary to perform gradient screening. In this study, the initial gradient elution from HSS C18 SB column using 1–5% to 20% IPA was examined (). As depicted in , with increasing initial gradient (increasing IPA concentration), the retention time of Sudan I–IV decreased by 32.60%, 27.77%, 12.45%, and 9.00%, respectively; their Tf was 0.90 to 1.45, and Rs was higher than 2.80. The retention of Sudan I–IV caused by the change in the initial gradient elution of the mobile phase was similar to that caused by temperature and back pressure, and the influence on earlier eluting compounds was higher than that of later eluting compounds. These data show that it is convenient to set up a wide elution window for the initial gradient elution of mixtures that contain compounds with different physicochemical properties. Thus, considering the efficiency of sample separation and limitation of system pressure, the initial gradient elution was set at 5% to 20%.
Figure 4. Resolution, retention time and tailing factor of Sudan I–IV separated using different initial gradient (1–5% to 20%), temperature (30–70°C), and back pressure (1700–2100 psi), and IPA as an organic modifier.
Figura 4. Resolución, tiempo de retención y factor de cola de Sudán I-IV separados utilizando diferentes gradientes iniciales (1–5% a 20%), temperatura (30–70 °C), presión de retorno (1700–2100 psi), e IPA como modificador orgánico
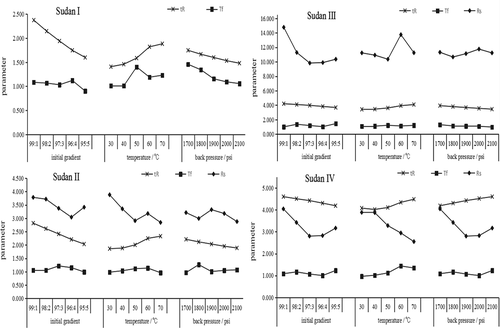
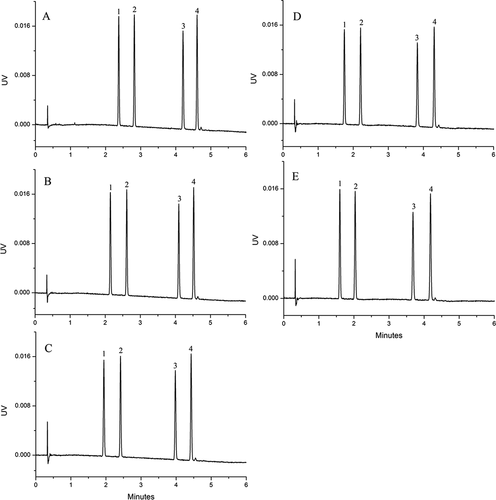
Figure 5. Influence of different initial gradients on separation of Sudan I–IV.
Figura 5. Influencia de diferentes gradientes iniciales en la separación de Sudán I–IV
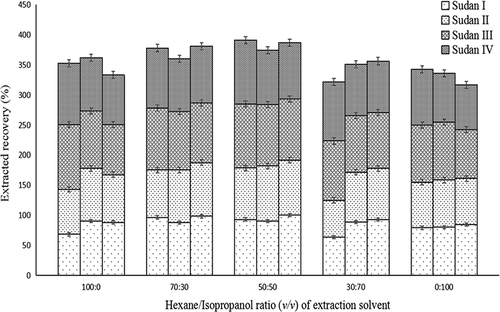
3.4. Optimization of extraction solvent
Extraction solvents can directly affect the accuracy of analysis or extraction. In this study, standard solutions of Sudan I–IV at low, medium, and high concentrations (or 0.5, 2.0, and 10.0 mg/L, respectively) were added to chilli powder. Sudan I–IV were then extracted at room temperature by ultrasonic extraction using 100% hexane, 30% (v/v) IPA, 50% IPA, 70% IPA, or 100% IPA as the extraction solvent, and the extraction efficiencies compared. The results depicted in show that the extraction solvent 50% IPA was the most efficient with recovery rates of 85.4% to 106.4%. The solvent 100% hexane achieved extraction rates of 68.4% to 107.4%. The recovery rates of 30% IPA, 70% IPA 100% IPA were 78.6–103.2%, 61.4–98.6%, and 74.4–96.5%, respectively. The average extraction rate of Sudan I–IV at concentrations of 0.5, 2.0 and 10.0 mg/L using 50% IPA increased by 9.9%, 3.4%, and 13.9%, respectively, compared with that using 100% hexane. The extraction rate of Sudan I–IV at concentrations of 0.5, 2.0, and 10.0 mg/L using 100% IPA decreased by 13.3%, 10.4%, and 18.3%, respectively, compared with that using 50% IPA. This may have been due to high polarity of IPA at concentrations greater than 50% that could increase the extraction efficiency of impurities in the sample but reduce the extraction yield of Sudan I–IV. Based on these results, 50% (v/v) IPA was used in subsequent Sudan I–IV extraction.
3.5. Method validation
3.5.1. Selectivity
The UHPSFC chromatograms of blank sample and blank sample spiked with Sudan I–IV are shown in . The retention time of Sudan I–IV was 1.445, 1.882, 3.440, and 4.024 min, respectively, and their peaks were not observed in the blank sample.
3.5.2. System suitability
The system suitability test was carried out under the chromatographic conditions described in 2.4 using repeated injection of mixed standard solutions of Sudan I–IV. The following parameters were examined: retention time (Tr) and peak area (A), tailing factor (Tf), resolution (Rs), separation factor (α), and retention factor (k). According to the results (), the RSD values for Tr were lower than 0.04% and those for Pa were lower than 0.09%; Tf values ranged from 0.984 to 1.297. These results are better than those reported by Khalikova (Khalikova et al., Citation2015), possibly due to the use of different modifiers and stationary phases. Additionally, all target compounds had an Rs higher than 2.5. This is the first validated UHPSFC method that can separate standard Sudan I–IV within only 4.5 min.
Table 1. Results from system suitability test (c = 5.00 mg/kg).
Tabla 1. Resultados de la prueba de idoneidad del sistema (c = 5.00 mg/kg)
3.5.3. Linear range and sensitivity
The sensitivity of the method in separating standard Sudan I–IV and different real samples (hot pot, chilli oil, and chilli powder) was determined using LOD and LOQ. Direct extraction with hexane/isopropanol (1:1, v/v) can lead to a complex matrix, which causes inaccuracy; thus, matrix calibration curves were prepared using the matrix at six different concentrations (the actual sample matrix was free of the target compounds). The linearity of the proposed method was studied at the range of 0.125–50.00 mg/kg (depending on different target compounds and sample matrix) in standard solutions and spiked real samples. The results showed that the correlation coefficient in all calibration curves exceeded 0.9990, indicating that the curves had good linearity at the investigated concentration range. Calibration curves, as well as LOQs and LODs, determined from the signal-to-noise ratio are summarized in . LOD of the method in detecting Sudan I and Sudan II in hot pot, chilli oil and chilli powder was 0.20, 0.25, 0.25, and 0.25 mg/kg, respectively, whereas those in detecting Sudan III were 0.125, 0.25, 0.25, and 0.20 mg/kg, respectively. Those for detecting Sudan IV were 0.25, 0.25, 0.50, and 0.25 mg/kg, respectively. This result indicates that the method is more sensitive to the standard solution than to real samples, likely due to different SN ratios caused by different compositions (matrix) of the samples. Similar results were observed by Khalikova (Khalikova et al., Citation2015). Although the sensitivity of this method is lower than that of MS detector, pretreatment with concentration, and/or purification (Fukuji et al., Citation2012; Shi et al., Citation2018), it is worth mentioning that the LOD to all target compounds fulfilled the requirements of the EU (Zuckerman, Citation1995). Most importantly, the method is simple with good reproducibility as well as requiring less operation time (less than 18 min, including sample preparation and UHPSFC analysis).
Table 2. The results of method linearity and sensitivity of developed method (n = 5).
Tabla 2. Resultados de la linealidad y la sensibilidad del método desarrollado (n=5)
3.5.4. Accuracy and precision
Accuracy and intra-day and inter-day precision of the method in the analysis of Sudan I–IV at low, medium and high concentrations (0.5, 2.0, and 10 mg/kg, respectively) are summarized in . In the analysis of Sudan I–IV, the method had intra-day and inter-day precision, presented as RSD, of 0.91–4.11%.
Table 3. Inter-day and intra-day precision of the Sudan I–IV.
Tabla 3. Precisión interdiaria e intradiaria del Sudán I – IV
3.5.5. Spiked recovery
The recovery rate of the target compounds in spiked samples was high; nonetheless, it contradicted that of spiked real samples. Three types of chilli products (chilli powder, hot pot, and chilli oil) were spiked with Sudan dyes I–IV at three different concentrations (high, medium, and low) and then incubated for 72 h; the dyes were then extracted and analyzed. The results are shown in . The recovery rates at all three concentrations of Sudan I and Sudan II were 76.60–101.28% and 74.40–102.90%, respectively, and those of Sudan III and Sudan IV were 76.57–106.20% and 75.91–103.40%, respectively.
Table 4. Recoveries for the assay of four target compounds in different samples (n = 3).
Tabla 4. Recuperaciones para el ensayo de cuatro compuestos objetivo en diferentes muestras (n=3)
3.5.6. Analysis of Sudan I–IV in chilli products
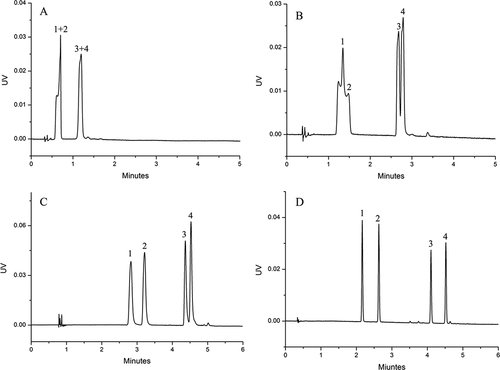
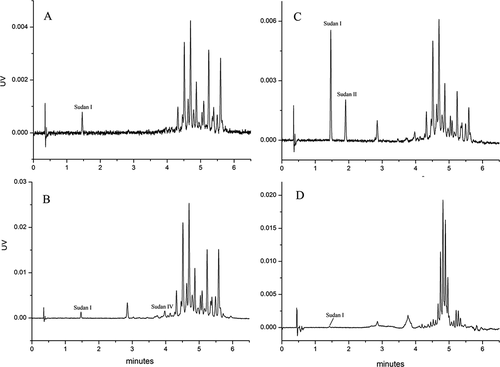
The typical chromatograms of standard Sudan I–IV solutions and chilli product samples are shown in , respectively. The chilli product samples including chili powder, chili sauce, chilli oil and hot pot were obtained from local supermarkets and the government department. The identities of the target compounds were confirmed by their retention times and UV spectra. Whereas none of the samples purchased from local supermarkets contained Sudan I–IV, some samples submitted by the government department contained Sudan dyes. Some samples contained Sudan I, others contained Sudan II and/or Sudan IV (). Furthermore, only a few impurities were observed to interfere with the target compounds, according to the chromatogram, indicating that the method had high selectivity. Taken together, the method developed in this study could effectively separate and detect Sudan I–IV in different chilli product samples, including those with lower Sudan concentration. Thus, the developed method may be suitable for the analysis of Sudan I–IV in various other food matrices.
Figure 8. UHPSFC-UV chromatograms of Sudan I–IV in different chilli products.
Figura 8. Cromatogramas UHPSFC-UV de Sudán I–IV en diferentes productos de chile
4. Conclusions
In this study, a simultaneous determination of Sudan I–IV in chilli products based on UHPSFC-DAD was established, and did not require the use of chlorinated solvents or acetonitrile (a supercritical fluid property). Nor did it require solid-phase extraction (SPE) or polyethylene glycol (GPC) to purify the sample. Since samples can be extracted and analyzed directly, this novel method is very user-friendly for analysts. This method has the advantages of high yield, high separation efficiency, short analysis time, and high sensitivity. It is an ideal method to separate fat soluble/non-polar compounds such as fat pigments, fat vitamins, and flavonoids, and has potential application in food control analysis or when screening for banned additives. More importantly, the results of this study that analyzed three different pepper products show that the method is scientific and reliable.
Disclosure statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Berger, T. A. (2017). Preliminary kinetic evaluation of an immobilized polysaccharide sub-2 μm column using a low dispersion supercritical fluid chromatograph. Journal of Chromatography. A, 1510(8), 82–88. https://doi.org/https://doi.org/10.1016/j.chroma.2017.06.021
- Berger, T. A. (2018). Effect of density on kinetic performance in supercritical fluid chromatography with methanol modified carbon dioxide. Journal of Chromatography. A, 1564(24), 188–198. https://doi.org/https://doi.org/10.1016/j.chroma.2018.06.021
- Deng, Y. Y., Jia, Q. C., Shi, X., Wan, J., Zhou, C., Wang, Q., & Wang, Q. (2021). Characterization of key odorants in peeled and unpeeled flaxseed powders using solvent-assisted flavor evaporation and odor activity value calculation. LWT - Food Science and Technology, 138(9), 110724. https://doi.org/https://doi.org/10.1016/j.lwt.2020.110724
- Dolak, L. A., Cole, J., Lefler, J. L., & Jennifer, L. (2007). Resolution and identification of Sudan dyes I-IV via supercritical fluid chromatography. LC GC Europe, 13(2), 22–23.
- Du, Z., & Sun, S. (2007). Determination of Sudan red I-IV in duck egg yolk using ultra performance liquid chromatography-tandem mass spectrometry. Chinese Journal of Applied and Environmental Biology, 25(5), 705–710. https://doi.org/https://doi.org/10.1016/S1872-2059(07)60023-6
- Fukuji, T. S., Castro-puyana, M., Tavares, M. F. M., & Cifuentes, A. (2012). Sensitive and fast determination of Sudan dyes in chilli powder by partial-filling micellar electrokinetic chromatography-tandem mass spectrometry. Electrophoresis, 33(4), 705–712. https://doi.org/https://doi.org/10.1002/elps.201100272
- Grand-Guillaume Perrenoud, A., Veuthey, J. L., & Guillarme, D. (2012). Comparison of ultra-high performance supercritical fluid chromatography and ultra-high performance liquid chromatography for the analysis of pharmaceutical compounds. Journal of Chromatography. A, 1266(11), 158–167. https://doi.org/https://doi.org/10.1016/j.chroma.2012.10.005
- Grand-Guillaume Perrenoud, A., Veuthey, J. L., & Guillarme, D. (2014). The use of columns packed with sub-2-µm particles in supercritical fluid chromatography. Trac-Trends in Analytical Chemistry, 63(11), 44–54. https://doi.org/https://doi.org/10.1016/j.trac.2014.06.023
- Hu, M. Z., Wu, L. J., Song, Y., Li, Z. C., Ma, Q., Zhang, H. Q., & Wang, Z. M. (2016). Determination of Sudan dyes in juice samples via solidification of ionic liquid in microwave-assisted liquid-liquid microextraction followed by high-performance liquid chromatography. Food Analytical Methods. 9(7), 2124–2132. https://doi.org/https://doi.org/10.1007/s12161-015-0389-y
- Jia, X., Wang, L., Zheng, C., Yang, Y., Wang, X., Hui, J., & Zhou, Q. (2020). Key odorant differences in fragrant Brassica napus and Brassica juncea oils revealed by gas chromatography–olfactometry, odor activity values, and aroma recombination. Journal of Agricultural and Food Chemistry, 68(50), 14950–14960. https://doi.org/https://doi.org/10.1021/acs.jafc.0c05944
- Khalikova, M. A., Šatínský, D., Solich, P., & Nováková, L. (2015). Development and validation of ultra-high performance supercritical fluid chromatography method for determination of illegal dyes and comparison to ultra-high performance liquid chromatography method. Analytica chimica acta, 874(5), 84–96. https://doi.org/https://doi.org/10.1016/j.aca.2015.03.003
- Lefler, J. L., & Chen, R. (2008). A feasibility study of using supercritical fluid chromatography (SFC)-UV-ELSD for food and beverage analyses. Lc Gc North America, 26(3), 42–43. https://doi.org/https://doi.org/10.1556/JPC.21.2008.3.15
- Lesellier, E. (2012). Extension of the carotenoid test to superficially porous C18 bonded phases, aromatic ligand types and new classical C18 bonded phases[J]. Journal of Chromatography. A, 1266(1), 34–42. https://doi.org/https://doi.org/10.1016/j.chroma.2012.09.068
- Lesellier, E., Latos, A., & Lopes, D. O. (2014). Ultra high performance/low pressure supercritical fluid chromatography with superficially porous particles for triglycerides separation. Journal of Chromatography. A, 1327(1), 141–148. https://doi.org/https://doi.org/10.1016/j.chroma.2013.12.046
- Lesellier, E., & West, C. (2015). The many faces of packed column supercritical fluid chromatography - A critical review. Journal of Chromatography. A, 1382(1), 2–46. https://doi.org/https://doi.org/10.1016/j.chroma.2014.12.083
- Li, T., Hao, M. L., Pan, J., Zong, W. S., & Liu, R. T. (2017). Comparison of the toxicity of the dyes Sudan II and Sudan IV to catalase. Journal of Biochemical and Molecular Toxicology, 31(10), 234–242. https://doi.org/https://doi.org/10.1002/jbt.21943
- Liu, W., Zhao, W. J., Chen, J. B., & Yang, M. M. (2007). A cloud point extraction approach using Ttriton X-100 for the separation and preconcentration of Sudan dyes in chilli powder. Analytica chimica acta, 605(1), 41–45. https://doi.org/https://doi.org/10.1016/j.aca.2007.10.034
- Long, C. Y., Mai, Z. B., Yang, X. F., Zhu, B. H., Xu, X. M., Huang, X. D., & Zou, X. Y. (2011). A new liquid-liquid extraction method for determination of 6 azo-dyes in chilli products by high-performance liquid chromatography. Food Chemistry, 126(3), 1324–1329. https://doi.org/https://doi.org/10.1016/j.foodchem.2010.11.089
- López-Jiménez, F. J., Rubio, S., & Pérez-Bendito, D. (2010). Supramolecular solvent-based microextraction of Sudan dyes in chilli-containing foodstuffs prior to their liquid chromatography-photodiode array determination. Food Chemistry, 121(3), 763–769. https://doi.org/https://doi.org/10.1016/j.foodchem.2009.12.081
- Qi, P., Zeng, T., Wen, Z. J., Liang, X. Y., & Zhang, X. W. (2011). Interference-free simultaneous determination of Sudan dyes in chili foods using solid phase extraction coupled with HPLC-DAD. Food Chemistry, 125(4), 1462–1467. https://doi.org/https://doi.org/10.1016/j.foodchem.2010.10.059
- Rebane, R., Leito, I., Yurchenko, S., & Herodes, K. (2010). A review of analytical techniques for determination of Sudan I-IV dyes in food matrixes. Journal of Chromatogr A, 1217(17), 2747–2757. https://doi.org/https://doi.org/10.1016/j.chroma.2010.02.038
- Shaaban, H., & Górecki, T. (2013). Green ultra-fast high-performance liquid chromatographic method using a short narrow-bore column packed with fully porous sub-2 μm particles for the simultaneous determination of selected pharmaceuticals as surface water and wastewater pollutants. Journal of Separation Science, 36(2), 252–261. https://doi.org/https://doi.org/10.1002/jssc.201200335
- Shi, X. R., Chen, X. L., Hao, Y. L., Li, L., Xu, H. J., & Wang, M. M. (2018). Magnetic metal-organic frameworks for fast and efficient solid-phase extraction of six Sudan dyes in tomato sauce. Journal of Chromatography B, 1086(4), 146–152. https://doi.org/https://doi.org/10.1016/j.jchromb.2018.04.022
- Sun, S., Wang, Y., Yu, W. Z., Zhao, T. Q., Gao, S. Q., Kang, M. Q., Zhang, Y. P., Zhang, H. Q., & Yu, Y. (2011). Determination of Sudan dyes in red wine and fruit juice using ionic liquid-based liquid-liquid microextraction and high-performance liquid chromatography. Journal of Separation Science, 34(14), 1730–1737. https://doi.org/https://doi.org/10.1002/jssc.201100037
- Teng, Y., & Zhou, Q. X. (2017). Adsorption behavior of Sudan I-IV on a coastal soil and their forecasted biogeochemical cycles. Environmental Science and Pollution Research, 24(11), 10749–10758. https://doi.org/https://doi.org/10.1007/s11356-017-8723-0
- The Comission of the European Communities. (2003). Commission decision of 20 June 2003 on emergency measures regarding hot chilli and hot chilli products (2003/460/EC). Official Journal of the European Union, L154, 114–115.
- Wang, B., Liu, X. H., Zhou, W., Hong, Y., & Feng, S. L. (2017). Fast separation of flavonoids by supercritical fluid chromatography using a column packed with a sub-2 μm particle stationary phase. Journal of Separation Science, 40(6), 1410–1420. https://doi.org/https://doi.org/10.1002/jssc.201601021
- Xu, H. Y., Heinze, T. M., Paine, D. D., Cerniglia, C. E., & Chen, H. Z. (2010). Sudan azo dyes and para red degradation by prevalent bacteria of the human gastrointestinal tract. Anaerobe, 16(2), 114–119. https://doi.org/https://doi.org/10.1016/j.anaerobe.2009.06.007
- Yu, W., Liu, Z. L., Li, Q., Zhang, H. Q., & Yu, Y. (2015). Determination of Sudan I-IV in candy using ionic liquid/anionic surfactant aqueous two-phase extraction coupled with high-performance liquid chromatography. Food Chemistry, 173(4), 815–820. https://doi.org/https://doi.org/10.1016/j.foodchem.2014.10.091
- Zhang, J., Shao, J. B., Guo, P., & Huang, Y. M. (2013). A simple and fast Fe3O4 magnetic nanoparticles-based dispersion solid phase extraction of Sudan dyes from food and water samples coupled with high-performance liquid chromatography. Analytical Methods : Advancing Methods and Applications, 5(10), 2503–2510. https://doi.org/https://doi.org/10.1039/c3ay40242h
- Zhang, Z., Xu, S. F., Li, J. H., Xiong, H., Peng, H. L., & Chen, L. X. (2012). Selective solid-phase extraction of Sudan I in chilli sauce by single-hole hollow molecularly imprinted polymers. Journal of Agricultural and Food Chemistry, 60(1), 180–187. https://doi.org/https://doi.org/10.1021/jf2041609
- Zhao, S., Yin, J., Zhang, J., Ding, X. J., Wu, Y. N., & Shao, B. (2012). Determination of 23 dyes in chili powder and paste by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Food Anal Method, 5(5), 1018–1026. https://doi.org/https://doi.org/10.1007/s12161-011-9337-7
- Zuckerman, J. A. (1995). Monographs on the evaluation of carcinogenic risks to humans. Journal of Clinical Pathology, 48(7), 691. https://doi.org/https://doi.org/10.1136/jcp.48.7.691-a