ABSTRACT
Common bean consumption is associated with the prevention of chronic diseases and these effects are partially attributed to their polyphenol content. In this study, the bioaccessibility and anti-inflammatory activity of polyphenols from six Mexican cultivars of beans (Phaseolus vulgaris) varying in their seed colour, was evaluated. In all cultivars, digestion of starch and protein released significantly more polyphenols compared to soaking, cooking and methanol extraction. Pink cultivar ‘Marcela’ showed the highest total and bioaccessible polyphenol content (>50 mg GAE per gram dry bean). Black cultivar ‘Negro Jamapa’ showed a moderate anti-inflammatory effect, reducing mRNA levels of IL6, IL1β and iNOS by up to 20%. In contrast, cultivars ‘Marcela’ and yellow ‘Azufrado’ induced mRNA expression of IL1β, IL6, and HO-1, thus inducing both pro- and anti-oxidation pathways. Results indicate that enzymatic digestion promotes the bioaccessibility of common bean polyphenols and bean cultivars can elicit both anti- and pro-inflammatory responses in macrophages.
RESUMEN
El consumo de frijol común está asociado con la prevención de enfermedades crónicas y estos efectos se atribuyen en parte a su contenido de polifenoles. En este estudio se evaluó la bioaccesibilidad y actividad antiinflamatoria de polifenoles de seis cultivares mexicanos de frijol (Phaseolus vulgaris) que varían en el color de las semillas. En todos los cultivares, la digestión de almidón y proteína liberó significativamente más polifenoles en comparación con el remojo, la cocción y la extracción con metanol. El cultivar rosado ‘Marcela’ mostró el mayor contenido de polifenoles totales y bioaccesibles (> 50 mg de GAE por gramo de frijol seco). El cultivar ‘Negro Jamapa’ mostró un efecto antiinflamatorio moderado, reduciendo los niveles de ARNm de IL6, IL1β e iNOS hasta en un 20%. En contraste, los cultivares ‘Marcela’ y amarillo ‘Azufrado’ indujeron la expresión de ARNm de IL1β, IL6 y HO-1, induciendo así vias pro- y anti-oxidativas. Los resultados indican que la digestión enzimática promueve la bioaccesibilidad de los polifenoles del frijol común y los cultivares de frijol pueden provocar respuestas tanto antiinflamatorias como proinflamatorias en macrófagos.
1. Introduction
The common bean (Phaseolus vulgaris L.) is native to the Andean and Mesoamerican regions of the American continent (Chávez-Servia, Citation2016). Archaeological and genetic evidence have led to conclude that Mexico is the centre of origin, diversification and domestication of common beans (Bitocchi et al., Citation2012; Duranti, Citation2006). South-central Mexico is considered as the world’s biodiversity hotspot of common beans since this region contains the largest genetic diversity of this legume (Hernández-López et al., Citation2013). Thus, beans grown in Mexico possess diverse physical and chemical properties. Most of the beans grown in Mexico (including wild-type varieties) remain uncharacterised.
Common beans are a source of energy and protein for regions with a low animal food consumption, such as in Latin America and Africa (Duranti, Citation2006). Besides protein, common beans are a source of complex carbohydrates (starch and fibre), vitamins, minerals and polyphenols (Suárez-Martínez et al., Citation2016). Intake of legumes, including common beans, has been associated with the prevention of diseases such as cancer, obesity and diabetes as well as other conditions related to the metabolic syndrome (Leterme, Citation2002; Maphosa & Jideani, Citation2017). Polyphenols, including anthocyanins and flavonoids, are thought to play an important role in disease prevention (Chávez-Servia, Citation2016). Legume polyphenols have been shown to have antioxidant (Singh et al., Citation2017), antimutagenic (Barman et al., Citation2018), apoptosis‐related (Aparicio-Fernández et al., Citation2008), antiproliferative (Dong et al., Citation2007) and anti-inflammatory effects (García-Lafuente et al., Citation2014; Oomah et al., Citation2010; Zhu et al., Citation2018) both in vitro and in vivo. Polyphenols have been shown to attenuate inflammatory response by inhibiting the transcription factor nuclear factor-kappa B (NF-kB) signalling pathway (Choy et al., Citation2019; Rahman et al., Citation2006; Serreli & Deiana, Citation2020). Mexican beans show high diversity in seed coat colour which is due to the presence of a range of polyphenols such as flavonol glycosides, condensed tannins and anthocyanins (Chávez-Mendoza & Sánchez, Citation2017). Despite some knowledge about the composition of bean polyphenols, less is known about their bioaccessibility and putative bioactivities.
Although bean polyphenols have been associated with positive health benefits, these compounds may be lost upon domestic processing. Dried common beans (P. vulgaris L.) are hard seeds when uncooked and cannot be consumed raw, but these become sufficiently soft to eat after extensive soaking and cooking (Bender, Citation1983; Chu et al., Citation2020). Soaking helps accelerate the cooking process of legumes (del Socorro López-Cortez, Citation2016) and solubilises anti-nutritional factors. It has previously been reported that soaking and cooking of beans reduces the polyphenol content significantly (Mba et al., Citation2019; Ranilla et al., Citation2009). Dietary polyphenols may be found free, polymerized or associated (esterified or glycosylated) with proteins, lipids and carbohydrates (including dietary fibre) (Jakobek, Citation2015). When digestive enzymes hydrolyse these food macronutrients, the polyphenols associated to them may be liberated, thereby enhancing polyphenol bioaccessibility. We have previously demonstrated that, in Borlotti beans, starch digestion in particular releases higher amounts of polyphenols in comparison to methanol/water-based solvent extraction (Perez-Hernandez et al., Citation2020). We hypothesize that a similar behaviour would be observed in these Mexican cultivars
Therefore, the aim of this study was to evaluate the effect of domestic processing and in vitro digestion on the release of polyphenols from six Mexican common bean (P. vulgaris) cultivars which vary in their seed coat colour; and to assess the effect of cooked bean polyphenol extracts on the inflammatory response in macrophages, a cellular model of inflammation.
2. Materials and methods
A general scheme of the methodology used to conduct the present study is shown in . Six Mexican varieties of P. vulgaris beans were analysed: Negro Jamapa, Azufrado Higuera, Pinto Saltillo, Marcela (also known as Flor de Junio), Azufrado and Peruano Bola. Beans were locally produced in Villa Hidalgo, Nayarit, Mexico (21°44ʹ36” N; 105°13ʹ51” W). They were obtained as dried seeds from a local farmer during the winter 2013 growing season. The six bean samples were soaked, cooked and subjected to in vitro enzymatic digestion. The total polyphenol content (TPC) released during soaking, cooking and in vitro digestion, as well as those remaining in the undigested residue (dietary fibre) were determined. TPC and antioxidant activity were determined in methanolic extracts of ground raw and freeze-dried cooked beans. Finally, to evaluate the anti-inflammatory properties of Mexican beans, methanolic extracts of cooked freeze-dried Negro Jamapa, Marcela and Azufrado beans were applied to RAW 264.7 macrophages and mRNA levels of inflammatory targets determined.
Figure 1. Native Mexican common beans (P. vulgaris) analysed and diagram of the methodology performed in this study.
Figura 1. Frijoles comunes mexicanos (P. vulgaris) analizados y diagrama de la metodología llevada a cabo en el presente estudio
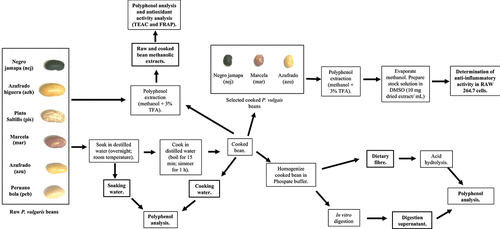
2.1. Domestic processing (soaking and cooking) of beans
P. vulgaris beans were prepared according to Perez-Hernandez et al. (Citation2020). Briefly, beans were soaked overnight (16 h) in deionized water (1:5, w:v), drained and boiled for 10 min in water (1:10, w:v) followed by simmering for 1 h. Aliquots of soaking and cooking water were kept at −20°C until further TPC analysis. Cooked beans were also preserved at −20°C until being subjected to in vitro enzymatic starch and protein digestion.
2.2. Determination of total polyphenol content (TPC) by the Folin-Ciocalteu assay
TPC of the different fractions was determined by the Folin-Ciocalteu assay as described by Singleton et al. (Citation1999) adapted to smaller volumes. Briefly, 50 µL of sample (cooking water, soaking water, digestion supernatant or fibre acid hydrolysis supernatant) was mixed with 50 µL of Folin-Ciocalteu reagent (1 N), 150 µL of Na2CO3 (20%, w:v) and 750 µL of water. The mixture was incubated for 30 min in the dark and the absorbance measured at 765 nm with a double beam spectrophotometer. To calculate TPC values of the samples, a TPC calibration curve was constructed using gallic acid as a polyphenol standard (range 10–250 µg/mL). Results were expressed as µg gallic acid equivalents (GAE)/g dry weight.
2.3. In vitro digestion of starch and protein
Digestions were performed as described by Perez-Hernandez et al. (Citation2020). Briefly, 1 g of cooked P. vulgaris bean was homogenized in 40 mL of sodium maleate buffer (50 mM with 2 mM CaCl2, pH 6.0) and digested with 250 U/mL of porcine pancreatic α-amylase (Sigma Aldrich, Gillingham, UK) for 16 h at 37°C. Samples were later digested with 30 U/mL protease (subtilisin A from Bacillus licheniformis, Megazyme International, Bray, Ireland) for 30 min at 60°C, pH 6.0, followed by addition of 80 U/mL amyloglucosidase (from Aspergillus niger, Megazyme International, Bray, Ireland) for 30 min at 60°C, pH 4.5. Afterwards, samples were boiled for 5 min to stop further enzymatic activity. The undigested residue (dietary fibre) was mixed with aqueous ethanol (80% v/v) and precipitated by centrifugation (9000 x g, 20 min). The supernatant was analysed for sugar and polyphenol content. Aliquots of the supernatants and fibre residues were kept at −20°C until further analysis.
2.4. Total starch extraction
Starch extraction was performed as described by Bustos et al. (Citation2004) with a few modifications. Briefly, 5 g of cooked beans were homogenized in 250 mL 50 mM Tris buffer containing 10 mM EDTA and 0.5 g/L sodium metabisulfite, pH 7.5 at 4°C and filtered through four layers of cheesecloth. The filtrate was centrifuged at 20,000 x g (4°C) for 15 min. The pellet was washed three times with Tris buffer and twice with acetone. The final pellet was dried at room temperature and weighed to calculate the starch yield.
2.5. Recovery of dietary fibre and acid hydrolysis
Dietary fibre was determined by weighing the remainder of the residue obtained after starch and protein digestion. Fibre was separated from the digestion supernatants and processed as described by (Aldwairji et al., Citation2014). Digestion supernatants and fibre residue were separated by filtration using 3 layers of miracloth filter. The fibre residue was washed with ethanol and acetone and then dried in an oven at 103°C until constant weight was achieved.
To release fibre-associated polyphenols, the fibre residue was hydrolysed with 1 N HCl (100°C, 1 h), the sample subsequently centrifuged (20,000 x g and 4°C for 15 min) and the supernatant stored at −20°C for further polyphenol analysis.
2.6. Determination of the antioxidant activity
In order to evaluate the effect of cooking on the antioxidant capacity of polyphenols, the antioxidant capacity of methanolic extracts of raw and cooked P. vulgaris beans was measured. Polyphenol extraction from ground raw and ground freeze-dried cooked P. vulgaris beans was performed by mixing 0.5 g of milled sample with 10 mL acidified methanol (97% with 3% Trifluoroacetic acid (TFA)). Samples were vortexed for 1 h, centrifuged (9000 x g) and the supernatant collected and kept at −20°C until further analysis. The methods used for assessing the antioxidant activity of the extracts were the Trolox equivalent antioxidant capacity (TEAC) assay and the ferric reducing antioxidant power (FRAP) assay.
The TEAC assay was performed as described by (Han et al., Citation2021). First, ABTS radical stock solution was prepared by mixing a 14 mM ABTS stock solution with a 4.9 mM potassium peroxodisulfate solution. The stock solution was left at room temperature for 12–24 h. Afterwards, an 2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) working solution was diluted with water until an absorbance value of 0.700 was achieved when measuring the absorbance at a wavelength of 734 nm. Later, 10 µL of the methanolic extract were mixed with 300 µL of ABTS radical working solution in a 96 well plate. Samples were incubated with the ABTS working solution for 6 min at room temperature and the absorbance was read at 734 nm using a Tecan spark 10 M plate reader. A calibration curve was constructed using trolox ranging from 0 to 750 µM. Results were expressed as µg trolox equivalent (TE)/g dry bean. Each bean extract was tested by triplicate.
The FRAP method used in this study was adapted from Benzie and Strain (Citation1996). Initially, a FRAP reagent solution was prepared by mixing 300 mM acetate buffer, 10 mM (TPTZ) solution, and 20 mM ferric chloride hexahydrate solution (v:v:v = 10:1:1). Later, 10 µL of the extract was mixed with 300 µL of the FRAP reagent in the 96 well plate. The absorbance was read at 593 nm after a 15 min incubation at 37°C. A trolox calibration curve was constructed with concentrations that ranged from 0–1000 µM. Results were expressed as µg trolox equivalent (TE)/g dry bean. Each bean extract was tested by triplicate.
2.7. Cell culture conditions and preparation of bean extracts
RAW 264.7 murine macrophages were obtained from the European Collection of Authenticated Cell Cultures (ECACC). Macrophages were cultured in Dulbecco’s modified Eagle’s medium (DMEM), high glucose (4.5 g/l), supplemented with 10% (v/v) foetal bovine serum, and 1% antibiotics mix (Penicillin-Streptomycin by Gibco, Waltham, Massachusetts, USA). Initially, a stock solution of freeze-dried Negro Jamapa, Marcela and Azufrado was prepared. First, cooked freeze-dried methanolic extracts were prepared as mentioned previously. Methanol was later evaporated using a Genevac EZ-2 Series equipment (Ipswich, UK). Next, 100 mg of dried extract were weighted and dissolved in 1 mL of 100% dimethyl sulfoxide (DMSO) to obtain 100 mg of dried bean extract /mL stock solutions. The stock solutions were later diluted in DMEM to obtain the desired testing concentrations (10–200 mg of sample/mL, equivalent to 0.20–2 mM). This range of concentrations was used in similar studies such as Chen et al. (Citation2017) and thought to reflect physiological conditions in human serum.
2.8. Determination of bean extract cytotoxicity in RAW 264.7 murine macrophages
Cytotoxicity was determined by the neutral red assay according to Boesch-Saadatmandi et al. (Citation2011). Briefly, cells were incubated (37°C and 5% CO2; 24 h) with bean extracts (50–100 μg/mL). Afterwards, the medium was changed to DMEM with 60 µg/mL neutral red and incubated for 2 h. The medium was later removed, and the dye extracted from cells using a mixture of ethanol, deionized water and glacial acid (50:49:1). The absorbance was read at 540 nm and the viability calculated in percentage of control cells. Solvent controls were included (0.1% dimethyl sulfoxide (DMSO).
2.9. Determination of anti-inflammatory activity in RAW 264.7 murine macrophages
The anti-inflammatory activity of extracts from three selected cooked P. vulgaris beans (Negro Jamapa, Marcela and Azufrado) was performed as described by Perez-Hernandez et al. (Citation2020). First, cells were incubated with bean extracts (10, 50, 100 μg/mL) for 1 h and later stimulated with 100 ng/mL of lipopolysaccharide (LPS) for 6 h. Sulforaphane (5 µM) was used as positive inhibition control. For RNA isolation, macrophages were washed with phosphate-buffered saline (PBS) and lysed with Trisure (Bioline, UK). Experiments were performed in three independent passages in duplicate. RNA was then isolated according to Perez-Hernandez et al. (Citation2020) and quantified using Nanoquant plate.
The cDNA synthesis was performed using iScript cDNA synthesis kit (Biorad, UK). Afterwards, real time qPCR was performed with the SensiMix SYBR High-ROX Kit on the StepOne real time cycler (Thermo Fisher Scientific). The primers used were for the following target genes: IL-6, IL1β, iNOS and HO-1. The housekeeper gene used was beta-actin (ACBT). Forward (5ʹ-3ʹ) and reverse (3ʹ-5ʹ) sequences of the target genes are listed in Table S1 (supplementary material). Each sample was run in duplicate. The ΔΔC T method was used for calculations. The results were expressed as the ratio of target gene to housekeeper gene mRNA.
2.10. Statistical analysis
One-factor ANOVA and the Tukey test were applied to determine statistical differences in compositional factors between bean varieties. Anti-inflammatory activity data was analyzed with one-way ANOVA followed by Dunnett’s t-test to compare treatments with control group. Significance was accepted when p < .05. All statistical analyses were performed using SPSS 22.0 statistical package.
3. Results
3.1. Bioaccessibility of polyphenols from Mexican beans
TPC values measured in soaking and cooking water suggested domestic processing led to minor losses of polyphenols from the beans. Negro Jamapa bean lost the highest amounts of TPC upon domestic processing (2.5 mg GAE/ mg of dry sample TPC loss) compared to other varieties (). TPC released after digestion of cooked beans with pancreatic α-amylase, amyloglucosidase and protease was consistently higher than any of the other fractions analysed (soaking water, cooking water, raw and cooked bean methanolic extracts and dietary fibre hydrolysis) in all cultivars. TPC values of digestion supernatants (polyphenols released upon enzymatic digestion) ranged from 28.2 ± 1.9 to 38.0 ± 1.0 mg GAE/g dry sample (mean: 33.2 ± 4.0 mg GAE/g dry sample). The highest TPC release upon in vitro digestion was observed for the Azufrado bean, whereas Negro Jamapa presented the lowest TPC value for the same process. We observed that there were small but significant variations in the starch content and digestibility amongst the various cultivars (), and there was an inverse correlation between the amount of digestible starch and polyphenol release (supplementary Figure S2).
Table 1. Starch content (total, digestible and resistant) and fibre content of cooked Mexican common beans (P. vulgaris).
Tabla 1. Contenido de almidón (total, digerible y resistente) y contenido de fibra de frijoles comunes mexicanos (P. vulgaris) cocidos
Figure 2. Total polyphenol content (TPC) of cooked common beans (P. vulgaris) released after domestic processing (soaking and cooking), enzymatic digestion and acid hydrolysis of dietary fibre. Values show mean ± standard deviation (n = 3). Different letters indicate significant differences between values of the sum of the three fractions.
Figura 2. Contenido de polifenoles totales (CPT) extraídos de frijoles comunes cocinados (P. vulgaris) después del procesamiento doméstico (remojo y cocinado), digestión enzimática e hidrólisis ácida de la fibra. Los valores muestran promedio ± desviación estándar (n = 3). Las diferentes letras indican diferencias significativas entre muestras
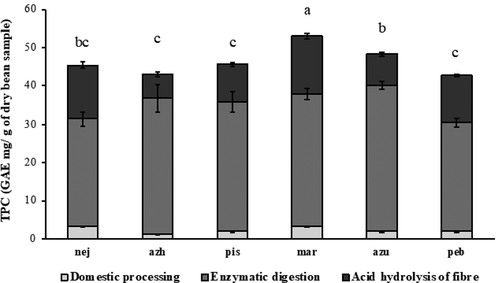
Acid hydrolysis of the remaining dietary fibre resulted in an additional release of polyphenols. TPC values present in the dietary fibre of P. vulgaris ranged from 6.5 ± 0.6 to 15.1 ± 0.7 mg GAE/g dry sample (). The highest TPC release upon this process was observed for the Marcela and Negro Jamapa bean cultivars. Azufrado and Azufrado Higuera beans (both possessing a light seed coat colour) presented the lowest TPC values released upon acid hydrolysis of the dietary fibre. Values of the sum of TPC values released upon domestic processing (soaking and cooking), in vitro digestion and acid hydrolysis of the dietary fibre, ranged from 36.5–52.3 mg GAE/g dry sample. The highest polyphenol release after all the processes tested was observed for Marcela bean (52.3 mg GAE/g dry sample), followed by Negro Jamapa (44.9 mg GAE/g dry sample), Azufrado Higuera (42.9 mg GAE/g dry sample), Azufrado (42.5 mg GAE/g dry sample), Peruano Bola (42.5 mg GAE/g dry sample) and Pinto Saltillo (36.5 mg GAE/g dry sample).
3.2. Antioxidant activity of Mexican beans (laboratory assays)
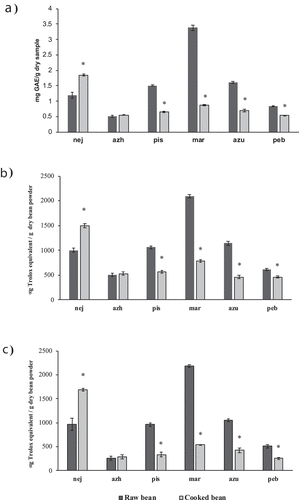
TPC values of most raw P. vulgaris methanolic extracts were significantly higher than cooked bean extracts (), indicating a TPC reduction upon cooking. Raw Marcela beans presented the highest TPC values (3.4 ± 0.1 mg GAE/g dry sample) of all the P. vulgaris cultivars tested; however, this sample also showed the highest TPC loss upon cooking (74% TPC loss). Surprisingly, TPC values of Negro Jamapa beans increased by 55% upon cooking; methanolic raw bean extracts of this sample presented a TPC value of 1.2 ± 0.1 mg GAE/g dry sample, whereas the cooked bean extract showed a TPC value of 1.8 ± 0.0 mg GAE/g dry sample. The antioxidant activity as measured with TEAC and FRAP assays of most of P. vulgaris beans decreased after cooking (). This behaviour was not observed in the Negro Jamapa bean cultivar. A two-fold increase of the antioxidant activity of Negro Jamapa beans was observed after cooking. The antioxidant activity of Azufrado Higuera bean was maintained upon cooking. TPC values positive correlated with TEAC and FRAP values (r2 = 0.85 and r2 = 0.65, respectively), a positive correlation (r2 = 0.86) between the TEAC and FRAP results was also observed (Figure S1, supplementary data).
Figure 3. Total polyphenol content (TPC) values (A) and antioxidant activity of raw and cooked common beans (P. vulgaris) determined by the TEAC (B) and FRAP (C) assays. TPC results are expressed as mg GAE/g dry sample. TEAC and FRAP values are expressed as mg Trolox equivalent/g dry sample. Samples: nej: Negro Jamapa; azh: Azufrado Higuera; pis: Pinto Saltillo; mar: Marcela; azu: Azufrado; peb: Peruano Bola. Values show mean ± standard deviation (n = 3). * indicates significant differences between raw and cooked sample (p < .05).
Figura 3. Valores de contenido de polifenoles totales (CPT) (A) y actividad antioxidante de frijoles comunes crudos y cocidos (P. vulgaris) determinada por los métodos TEAC (B) y FRAP (C). Los resultados de CPT han sido expresados como mg GAE / g muestra seca. Los valores de TEAC y FRAP han sido expresados como mg equivalentes Trolox / g muestra seca. Muestras: nej: Negro Jamapa; azh: Azufrado Higuera; pis: Pinto Saltillo; mar: Marcela; azu: Azufrado; peb: Peruano Bola. Los valores muestran promedio ± desviación estándar (n = 3). * indica diferencias significativas entre muestras crudas y cocidas (p < .05)
3.3. Effect of bean extracts on gene expression of inflammatory target genes in macrophages
Bean extracts from Negro Jamapa, Marcela (flor de junio) and Azufrado had no effect on RAW 264.7 cell viability in comparison to control cells (macrophages with no extract added, data not shown). The effect of these bean extracts on mRNA levels of inflammatory target genes IL6, IL1-β, iNOS and HO-1 are shown in . Negro Jamapa bean extract decreased the expression of IL6 by 18% and 20% when incubating with bean extract at concentrations of 50 and 100 µg/mL, respectively. Marcela bean extract showed no suppressive or stimulant effect of this target gene. Interestingly, Azufrado bean extract increased IL6 mRNA levels by 50% when incubating with 50 µg/mL; no decrease or increase was observed with the other concentrations tested (10 and 100 µg/mL).
Figure 4. Effect of Negro Jamapa, Marcela and Azufrado bean extracts on mRNA inflammatory target genes IL6 (A), IL1-β (B), iNOS (C) and HO-1 (D). Macrophages were incubated with increasing concentrations of cooked bean extracts (10–100 μg/mL) and stimulated with LPS (100 ng/mL). Controls used were unstimulated medium control (DMEM control), LPS stimulated control and sulforaphane (5 μM). *Indicates statistically different values between samples and LPS control (Dunnett’s test) at p < .05.
Figura 4. Efecto de los extractos de frijoles Negro Jamapa, Marcela y Azufrado en la producción de ARNm de los genes diana IL6 (A), IL1-β (B), iNOS (C) and HO-1 (D). Los macrófagos fueron incubados con diferentes concentraciones de extractos de frijol cocido (10–100 μg/mL) y estimulados con LPS (100 ng/mL). Los controles utilizados fueron: control con medio de cultivo no estimulado (control DMEM), control estimulado con LPS y control estimulado con sulforano (5 μM). * indica diferencias significativas entre las muestras y el control estimulado con LPS (p < .05)
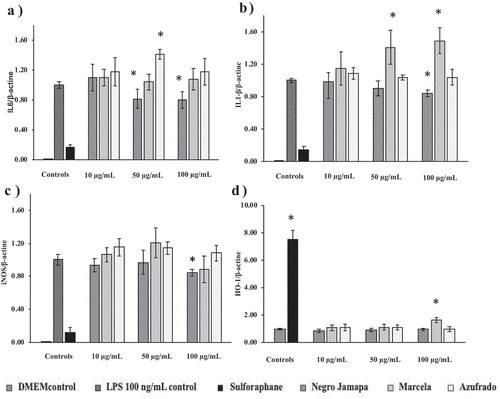
Negro Jamapa extract (100 µg/mL) decreased mRNA levels of IL1β by 16%. Marcela bean increased the levels of this inflammatory target gene by 30% and 35% at 50 and 100 µg/mL, respectively. No significant increase or decrease of this marker was observed with any of the Azufrado bean concentrations tested.
Negro Jamapa bean extract (100 µg/mL) decreased the mRNA levels of iNOS target gene by 16%; no significant changes were observed with lower Negro Jamapa bean extract concentrations. There were no significant differences in the mRNA levels of this target gene at any of the concentrations tested of Marcela and Azufrado bean extracts.
Unlike the rest of the target genes tested, the increase in HO-1 production is beneficial for the oxidative balance of the cells. For this target gene, a stimulating effect was observed only with the Marcela bean extract at the highest concentration tested (100 μg/mL). In this case, the increase in the production of HO-1 mRNA levels was of 66%.
4. Discussion
For most of the samples tested, domestic processing decreased TPC values. Polyphenol decrease upon cooking is due to the dissolution of polyphenols during soaking and cooking, as well as to the degradation during thermal processing and chemical transformation of these molecules (Bubelová et al., Citation2018). In this study, TPC values of Negro Jamapa beans increased upon cooking and were maintained for Azufrado Higuera beans. Lafarga et al. (Citation2019) and Siah et al. (Citation2014) observed a similar increase of TPC values in methanolic extracts of cooked legumes. Chen et al. (Citation2015) found that thermal processing stimulated the release of bound polyphenols (flavonols and hydroxycinnamic acids). The increase of TPC values after legume cooking could be due to an increase in the yield of polyphenols extraction enhanced by the disruption of the legume cells during thermal processing (Ferracane et al., Citation2008; Lafarga et al., Citation2019). Differences regarding the effect of thermal processing on TPC may be particular for every bean variety (Yang et al., Citation2018). The Folin–Ciocalteu method used in this study is based on electron transfer reactions between the Folin–Ciocalteu reagent and reacting molecules; it has been reported that other compounds, such as sugars and amino acids might react with the Folin-Ciocalteu reagent (Muñoz-Bernal et al., Citation2017). However, LC-MS analysis of the extracts identified a variety of polyphenols in the soaking, cooking and digestion supernatants (Supplementary Figure S3 and Table S2), so we can attribute the TPC values to the presence of polyphenols, though we cannot rule out the presence of other reactive compounds.
In all varieties, much higher amounts of polyphenols were released upon in vitro digestion in comparison to domestic processing and methanolic extraction. Previous studies have also shown that enzymatic hydrolysis of legumes, including beans, results in higher polyphenol release in comparison to solvent extraction (Lafarga et al., Citation2019; Perez-Hernandez et al., Citation2020; Sangsukiam & Duangmal, Citation2017). Polyphenols bound to polysaccharides and proteins require hydrolysis by digestive enzymes or intestinal microorganisms before being absorbed (Wang et al., Citation2014). Previous studies have shown that a higher amount of polyphenols are released during the intestinal stage of digestion in comparison to the gastric phase in cooked lentils (Zhang et al., Citation2017) and cranberry beans (P. vulgaris) (Chen et al., Citation2015) .
In the present study, digestible starch content and polyphenols released upon in vitro digestion were inversely correlated (r2 = 0.71) suggesting an important relationship between polyphenols and starch digestion in P. vulgaris beans (Figure S2). Previously, Perez-Hernandez et al. (Citation2020) found that starch enzymatic hydrolysis increased polyphenol bioaccessibility. Our results add to the increasing evidence that digestion is essential for polyphenol bioaccessibility and that polyphenols may modulate starch bioaccessibility. Starch in legumes is particularly resistant to digestion in comparison to other starchy sources such as cooked potatoes and cereals. Further research is needed to understand the relationship between polyphenols and starches in legumes.
Cárdenas-Castro et al. (Citation2020) determined the release of polyphenols upon in vitro gastro-intestinal digestion of Negro Jamapa and Azufrado beans obtained from the same region as the ones used in the present study (Nayarit, Mexico). In contrast to the present study, the polyphenol yields released upon in vitro digestion were lower than those released with solvent extraction. Cárdenas-Castro et al. (Citation2020) found that the main polyphenol released upon in vitro digestion were tentatively identified as chlorogenic acid followed by kaempferol 3-O-glucoside and quercetin 3-O-glucoside. Similar to our results, Cárdenas-Castro et al. (Citation2020) found that greater amounts of polyphenols could be released from Azufrado than Negro Jamapa beans during digestion. We also identified the profile of polyphenols in our samples using LC-MS analysis (supplementary Figure S3 and Table S2). We identified a wide range of polyphenols in the extracts, and confirmed that complex mixtures are extracted from beans. This complexity complicates the interpretation of the bioactivity evaluation.
The values of the fibre or digestion residue obtained in this study were similar as those reported by Aldwairji et al. (Citation2014). Acid hydrolysis of the digestion residue resulted in further polyphenol release indicating that bean polyphenols are strongly bound to indigestible components. These are likely to be cell walls polysaccharides such pectin which may bind polyphenols through O- or C-glycosylation (Acosta-Estrada et al., Citation2014). In addition, cell wall polysaccharides may interact with polyphenols by hydrogen bonding, van der Waals or hydrophobic interactions (Jakobek, Citation2015). During the simulated digestion, the different pH and ionic strength of compartments could disrupt these bonds, with a consequent increase of polyphenols released and their bioaccessibility (Missaoui et al., Citation2020). The biological fate and effects of fibre-bound polyphenols are poorly understood.
Yang et al. (Citation2020) evaluated the TPC and the antioxidant activity (by the DPPH, ABTS and FRAP assays) of 10 P. vulgaris raw bean cultivars. TPC values of raw beans ranged among 2 to 4.23 mg GAE/g dw. These values are higher than the values obtained in the present study (0.50 to 2.28 mg GAE/g dw). However, the TPC values presented in our study are higher than those presented by Ombra et al. (Citation2016) (0.14 to 1.25 mg GAE/g dw) for P. vulgaris bean cultivars. Hence, these differences in TPC values could be due to genetic variations among the bean cultivars analysed, environmental and growing conditions or extraction methods. Although TPC values obtained by (Yang et al., Citation2020) were higher than those presented in our study, TPC values were highly correlated with ABTS and FRAP values in both studies. The positive correlation of TEAC and FRAP values indicates that the antioxidant activity of the beans is likely to be associated with polyphenols present in the legumes (Figure S1).
The results from the anti-inflammatory effect pathways in macrophages showed that Negro Jamapa had a moderate beneficial effect. The reduction in the gene expression of pro-inflammatory genes could be considered small in comparison to sulforaphane, which is a powerful anti-inflammatory molecule used as a positive control. It has previously been reported that catechin derivatives (epigallocatechin gallate, epicatechin gallate and theaflavin-3ʹ3-digalate) decreases the expression of IL6 cytokine and iNOS in cellular models (Y. Chen et al., Citation2003; Ganeshpurkar & Saluja, Citation2019). Ichikawa et al. (Citation2004) studied the effect of epicatechin gallate in myocardial injury after ischemia and reperfusion in rats. Their results indicate that this compound decreased the levels of IL6 cytokine, the activation of IKK and degradation of IκB-α (supressing the NFκB pathway). In contrast to Negro Jamapa, Marcela bean extract increased IL1β mRNA levels by more than 40%. This indicates that this extract, at the concentrations tested, stimulated some inflammation pathways. However, this same sample increased the mRNA levels of HO-1, which indicates a stimulation of the Nrf2 pathway. A similar effect was also observed with the Azufrado bean. HO-1 is a target gene for heme-oxygenase production. This enzyme is involved in the cleavage of the porphyrin ring of haeme into Fe2+, carbon monoxide and biliverdin, which is later transformed to bilirubin, an antioxidant. This decreases the oxidative status of the cell, inhibiting NF-κB (Wardyn et al., Citation2015). NF-κB creates an oxidative environment in the cell, which activates the antioxidant Nrf2 pathway in order to restore cell homeostasis (Yerra et al., Citation2013). No cytotoxic effects were found when incubating the cells with these extracts, so it is unlikely that any effects are severe enough to kill cells. We do not know what specific molecules or combinations of molecules are responsible for these effects. As seen in this study, different bean cultivars show different inflammatory responses and may possibly exert other biological effects differently. Hence, when evaluating the potential health benefits of common beans, it is important to test different cultivars and not generalize the behaviour observed for one specific bean cultivar.
5. Conclusion
The polyphenol content of six native Mexican common beans which vary in their seed colour has been evaluated for the first time. The results from this study confirmed that gastrointestinal digestion increases polyphenol release from the food matrix. The antioxidant activity of extracts was significantly associated with TPC, but did not always reflect the anti-inflammatory response in macrophages. Hence, future research is required to characterize the bioactive compounds and health benefits of different bean cultivars that comprise the highly diverse common bean species.
Supplemental Material
Download MS Word (2.2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability material
Data is available from the authors upon request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Acosta-Estrada, B. A., Gutiérrez-Uribe, J. A., & Serna-Saldívar, S. O. (2014). Bound phenolics in foods, a review. Food Chemistry, 152, 46–55. Elsevier Ltd. https://doi.org/https://doi.org/10.1016/j.foodchem.2013.11.093
- Aldwairji, M. A., Chu, J., Burley, V. J., & Orfila, C. (2014). Analysis of dietary fibre of boiled and canned legumes commonly consumed in the United Kingdom. Journal of Food Composition and Analysis, 36(1–2), 111–116. Elsevier Inc. https://doi.org/https://doi.org/10.1016/j.jfca.2014.06.010
- Aparicio-Fernández, X., Reynoso-Camacho, R., Castaño-Tostado, E., García-Gasca, T., González de Mejía, E., Guzmán-Maldonado, S. H., Elizondo, G., Yousef, G. G., Lila, M. A., & Loarca-Pina, G. (2008). Antiradical capacity and induction of apoptosis on HeLa cells by a Phaseolus vulgaris extract. Plant Foods for Human Nutrition, 63(1), 35–40. https://doi.org/https://doi.org/10.1007/s11130-007-0066-4
- Barman, A., Marak, C. M., Barman, R. M., & Sangma, C. S. (2018). Nutraceutical properties of legume seeds and their impact on human health. In Jimenez-Lopez, J. C., & Clemente, A. (Eds.), Legume seed nutraceutical research (pp. 0–22). IntechOpen. https://doi.org/https://doi.org/10.5772/intechopen.78799
- Bender, A. E. (1983). Haemagglutinins (lectins) in beans. Food Chemistry, 11(4), 309–320. https://doi.org/https://doi.org/10.1016/0308-8146(83)90077-8
- Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/https://doi.org/10.1006/abio.1996.0292
- Bitocchi, E., Nanni, L., Bellucci, E., Rossi, M., Giardini, A., Spagnoletti Zeuli, P., Logozzo, G., Stougaard, J., McClean, P., Attene, G., & Papa, R. (2012). Mesoamerican origin of the common bean (Phaseolus vulgaris L.) is revealed by sequence data. Proceedings of the National Academy of Sciences of the United States of America, 109(14), E788–E796. https://doi.org/https://doi.org/10.1073/pnas.1108973109.
- Boesch-Saadatmandi, C., Loboda, A., Wagner, A. E., Stachurska, A., Jozkowicz, A., Dulak, J., Döring, F., Wolffram, S., & Rimbach, G. (2011). Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. Journal of Nutritional Biochemistry, 22(3), 293–299. Elsevier Inc. https://doi.org/https://doi.org/10.1016/j.jnutbio.2010.02.008
- Bubelová, Z., Sumczynski, D., & Salek, R. N. (2018). Effect of cooking and germination on antioxidant activity, total polyphenols and flavonoids, fiber content, and digestibility of lentils (Lens culinaris L.). Journal of Food Processing and Preservation, 42(1), e13388. https://doi.org/https://doi.org/10.1111/jfpp.13388
- Bustos, R., Fahy, B., Hylton, C. M., Seale, R., Nebane, N. M., Edwards, A., Martin, C., & Smith, A. M. (2004). Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. Proceedings of the National Academy of Sciences of the United States of America, 101(7), 2215–2220. https://doi.org/https://doi.org/10.1073/pnas.0305920101
- Cárdenas-Castro, A. P., Pérez‐Jiménez, J., Bello‐Pérez, L. A., Tovar, J., & Sáyago‐Ayerdi, S. G. (2020). Bioaccessibility of phenolic compounds in common beans (Phaseolus vulgaris L.) after in vitro gastrointestinal digestion: A comparison of two cooking procedures. Cereal Chemistry, 97(3), 670–680. https://doi.org/https://doi.org/10.1002/cche.10283
- Chávez-Mendoza, C., & Sánchez, E. (2017). Bioactive compounds from mexican varieties of the common bean (Phaseolus vulgaris): Implications for health. Molecules, 22(8), 1360. https://doi.org/https://doi.org/10.3390/molecules22081360
- Chávez-Servia, J. L., Heredia-García, E., Mayek-Pérez, N., Aquino-Bolaños, E. N., Hernández-Delgado, S., Carrillo-Rodríguez, J. C., Gill-Langarica, H. R., & Vera-Guzmán, A. M. (2016). Diversity of common bean (Phaseolus vulgaris L.) landraces and the nutritional value of their grains. In A. Goyal, (Ed.), Grain Legumes (pp. 1–33). In-Tech. https://doi.org/https://doi.org/10.5772/61382
- Chen, P. X., Bozzo, G. G., Freixas-Coutin, J. A., Marcone, M. F., Pauls, P. K., Tang, Y., Zhang, B., Liu, R., & Tsao, R. (2015). Free and conjugated phenolic compounds and their antioxidant activities in regular and non-darkening cranberry bean (Phaseolus vulgaris L.) seed coats. Journal of Functional Foods, 18(B), 1047–1056. https://doi.org/https://doi.org/10.1016/j.jff.2014.10.032
- Chen, P. X., Zhang, H., Marcone, M. F., Pauls, K. P., Liu, R., Tang, Y., Zhang, B., Renaud, J. B., & Tsao, R. (2017). Anti-inflammatory effects of phenolic-rich cranberry bean (Phaseolus vulgaris L.) extracts and enhanced cellular antioxidant enzyme activities in Caco-2 cells. Journal of Functional Foods, 38(B), 675–685. https://doi.org/https://doi.org/10.1016/j.jff.2016.12.027
- Chen, Y., Dai, H., & Chang, H. (2003). Suppression of inducible nitric oxide production by indole and isothiocyanate derivatives from Brassica plants in stimulated macrophages Planta medica, 69(8), 696‐700. https://doi.org/https://doi.org/10.1055/s-2003-42790
- Choy, K. W., Murugan, D., Leong, X.-F., Abas, R., Alias, A., & Mustafa, M. R. (2019). Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Frontiers in Pharmacology, 10(OCT), 1–8. https://doi.org/https://doi.org/10.3389/fphar.2019.01295
- Chu, J., Ho, P., & Orfila, C. (2020). Growth region impacts cell wall properties and Hard-to-Cook Phenotype of Canned Navy Beans (Phaseolus vulgaris). Food and Bioprocess Technology, 13(5), 818–826. https://doi.org/https://doi.org/10.1007/s11947-020-02436-7
- Dong, M., He, X., & Rui, H. L. (2007). Phytochemicals of black bean seed coats: Isolation, structure elucidation, and their antiproliferative and antioxidative activities. Journal of Agricultural and Food Chemistry, 55(15), 6044–6051. https://doi.org/https://doi.org/10.1021/jf070706d
- Duranti, M. (2006). Grain legume proteins and nutraceutical properties. Fitoterapia, 77(2), 67–82. https://doi.org/https://doi.org/10.1016/j.fitote.2005.11.008
- Ferracane, R., Pellegrini, N., Visconti, A., Graziani, G., Chiavaro, E., Miglio, C., & Fogliano, V. (2008). Effects of different cooking methods on antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. Journal of Agricultural and Food Chemistry, 56(18), 8601–8608. https://doi.org/https://doi.org/10.1021/jf800408w
- Ganeshpurkar, A., & Saluja, A. (2019). In silico interaction of hesperidin with some immunomodulatory targets: A docking analysis. Indian Journal of Biochemistry & Biophysics, 56(1), 28–33. http://op.niscair.res.in/index.php/IJBB/article/view/27731/465477123
- García-Lafuente, A., Moro, C., Manchón, N., Gonzalo-Ruiz, A., Villares, A., Guillamón, E., Rostagno, M., & Mateo-Vivaracho, L. (2014). In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chemistry, 161, 216–223. https://doi.org/https://doi.org/10.1016/j.foodchem.2014.04.004
- Han, R., Hernández Álvarez, A. J., Maycock, J., Murray, B. S., & Boesch, C. (2021). Comparison of alcalase- and pepsin-treated oilseed protein hydrolysates – Experimental validation of predicted antioxidant, antihypertensive and antidiabetic properties. Current Research in Food Science, 4(March), 141–149. https://doi.org/https://doi.org/10.1016/j.crfs.2021.03.001
- Hernández-López, V. M., Vargas-Vázquez, M. L. P., Muruaga-Martínez, J. S., Hernández-Delgado, S., & Mayek-Pérez, N. (2013). Origin, domestication and diversification of common beans. advances and perspectives. Revista Fitotecnia Mexicana, 36(2), 95–104. https://doi.org/https://doi.org/10.35196/rfm.2013.2.95
- Ichikawa, D., Matsui, A., Imai, M., Sonoda, Y., & Kasahara, T. (2004). Effect of various catechins on the IL-12p40 production by murine peritoneal macrophages and a macrophage cell line, J774.1. Biological & Pharmaceutical Bulletin, 27(9), 1353–1358. https://doi.org/https://doi.org/10.1248/bpb.27.1353
- Jakobek, L. (2015). Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chemistry, 175, 556–567. Elsevier Ltd. https://doi.org/https://doi.org/10.1016/j.foodchem.2014.12.013
- Lafarga, T., Villaró, S., Bobo, G., Simó, J., & Aguiló‐Aguayo, I. (2019). Bioaccessibility and antioxidant activity of phenolic compounds in cooked pulses. International Journal of Food Science and Technology, 54(5), 1816–1823. https://doi.org/https://doi.org/10.1111/ijfs.14082
- Leterme, P. (2002). Recommendations by health organizations for pulse consumption. British Journal of Nutrition, 88(S3), 239–242. https://doi.org/https://doi.org/10.1079/bjn2002712
- López-Cortez, M. D. del S., Rosales-Martínez, P., Arellano-Cárdenas, S., & Cornejo-Mazón, M. et al. (2016). Antioxidants properties and effect of processing methods on bioactive compounds of legumes. In A. Goyal, (Ed.), Grain Legumes (pp. 103–126). In-Tech. https://doi.org/https://doi.org/10.5772/61382
- Maphosa, Y., & Jideani, V. A. (2017). The role of legumes in human nutrition. In Hueda, M. C. (Ed.),Functional food - Improve health through adequate food (pp. 103–121). IntechOpen. https://doi.org/https://doi.org/10.5772/intechopen.69127
- Mba, O. I., Kwofie, E. M., & Ngadi, M. (2019). Kinetic modelling of polyphenol degradation during common beans soaking and cooking. Heliyon, 5(5), e01613. https://doi.org/https://doi.org/10.1016/j.heliyon.2019.e01613
- Missaoui, M., D’antuono, I., D’imperio, M., Linsalata, V., Boukhchina, S., Logrieco, A. F., & Cardinali, A. (2020). Characterization of Micronutrients, Bioaccessibility and Antioxidant Activity of Prickly Pear Cladodes as Functional Ingredient. Molecules, 25(9), 2176. https://doi.org/https://doi.org/10.3390/molecules25092176
- Muñoz-Bernal, Ó. A., Torres-Aguirre, G. A., Núñez-Gastélum, J. A., De La Rosa, L. A., Rodrigo-García, J., Ayala-Zavala, J. F., & Álvarez-Parrilla, E. (2017). Nuevo Acercamiento a La Interacción Del Reactivo De Folin-Ciocalteu Con Azúcares Durante La Cuantificación De Polifenoles Totales. Tip, 20(2), 23–28. https://doi.org/https://doi.org/10.1016/j.recqb.2017.04.003
- Ombra, M. N., d’Acierno, A., Nazzaro, F., Riccardi, R., Spigno, P., Zaccardelli, M., Pane, C., Maione, M., & Fratianni, F. (2016). Phenolic Composition and Antioxidant and Antiproliferative Activities of the Extracts of Twelve Common Bean (Phaseolus vulgaris L.) Endemic Ecotypes of Southern Italy before and after Cooking. Oxidative Medicine and Cellular Longevity, 2016, 1–12. https://doi.org/https://doi.org/10.1155/2016/1398298
- Oomah, B. D., Corbé, A., & Balasubramanian, P. (2010). Antioxidant and anti-Inflammatory activities of bean (Phaseolus vulgaris L.) Hulls. Journal of Agricultural and Food Chemistry, 58(14), 8225–8230. https://doi.org/https://doi.org/10.1021/jf1011193
- Perez-Hernandez, L. M., Nugraheni, K., Benohoud, M., Sun, W., Hernández-Álvarez, A. J., Morgan , M. R., Boesch, C., & Orfila C., et al. (2020). Starch digestion enhances bioaccessibility of anti-inflammatory polyphenols from borlotti beans (Phaseolus vulgaris). Nutrients, 12(2), 295. PMID: 31978996; PMCID: PMC7070432. https://doi.org/https://doi.org/10.3390/nu12020295
- Rahman, I., Biswas, S. K., & Kirkham, P. A. (2006). Regulation of inflammation and redox signaling by dietary polyphenols. Biochemical Pharmacology, 72(11), 1439–1452. https://doi.org/https://doi.org/10.1016/j.bcp.2006.07.004
- Ranilla, L. G., Genovese, M. I., & Lajolo, F. M. (2009). Effect of different cooking conditions on phenolic compounds and antioxidant capacity of some selected Brazilian bean (Phaseolus vulgaris L.) cultivars. Journal of Agricultural and Food Chemistry, 57(13), 5734–5742. https://doi.org/https://doi.org/10.1021/jf900527v
- Sangsukiam, T., & Duangmal, K. (2017). A comparative study of physico-chemical properties and antioxidant activity of freeze-dried mung bean (Vigna radiata) and adzuki bean (Vigna angularis) sprout hydrolysate powders. International Journal of Food Science and Technology, 52(9), 1971–1982. https://doi.org/https://doi.org/10.1111/ijfs.13469
- Serreli, G., & Deiana, M. (2020). Extra virgin olive oil polyphenols: Modulation of cellular pathways related to oxidant species and inflammation in aging. Cells, 9(2), 478. https://doi.org/https://doi.org/10.3390/cells9020478
- Siah, S., Wood, J. A., Agboola, S., Konczak, I., & Blanchard, C. L. (2014). Effects of soaking, boiling and autoclaving on the phenolic contents and antioxidant activities of faba beans (vicia faba l.) differing in seed coat colours. Food Chemistry, 142, 461–468. Elsevier Ltd. https://doi.org/https://doi.org/10.1016/j.foodchem.2013.07.068
- Singh, B., Singh, J. P., Kaur, A., & Singh, N. (2017). Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Research International, 101(September), 1–16. Elsevier. https://doi.org/https://doi.org/10.1016/j.foodres.2017.09.026
- Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299(1999), 152–178. https://doi.org/https://doi.org/10.1016/S0076-6879(99)99017
- Suárez-Martínez, S. E., Ferriz-Martínez, R. A., Campos-Vega, R., Elton-Puente, J. E., de la Torre Carbot, K., & García-Gasca, T. (2016). Bean seeds: Leading nutraceutical source for human health. CYTA - Journal of Food, 14(1), 131–137. Taylor & Francis. https://doi.org/https://doi.org/10.1080/19476337.2015.1063548
- Wang, T., He, F., & Chen, G. (2014). Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. Journal of Functional Foods, 7(1), 101–111. Elsevier Ltd. https://doi.org/https://doi.org/10.1016/j.jff.2014.01.033
- Wardyn, J. D., Ponsford, A. H., & Sanderson, C. M. (2015). Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochemical Society Transactions, 43(4), 621–626. https://doi.org/https://doi.org/10.1042/BST20150014
- Yang, -Q.-Q., Farha, A. K., Cheng, L., Kim, G., Zhang, T., & Corke, H. (2020). Phenolic content and in vitro antioxidant activity in common beans (Phaseolus vulgaris L.) are not directly related to anti-proliferative activity. Food Bioscience, 36(May), 100662. Elsevier Ltd. https://doi.org/https://doi.org/10.1016/j.fbio.2020.100662
- Yang, -Q.-Q., Gan, R.-Y., Ge, -Y.-Y., Zhang, D., & Corke, H. (2018). Polyphenols in Common Beans (Phaseolus vulgaris L.): Chemistry, Analysis, and Factors Affecting Composition. Comprehensive Reviews in Food Science and Food Safety, 17(6), 1518–1539. https://doi.org/https://doi.org/10.1111/1541-4337.12391
- Yerra, V. G., Negi, G., Sharma, S. S., & Kumar, A. (2013). Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biology, 1(1), 394–397. Elsevier. https://doi.org/https://doi.org/10.1016/j.redox.2013.07.005
- Zhang, B., Deng, Z., Tang, Y., Chen, P. X., Liu, R., Dan Ramdath, D., Liu, Q., Hernandez, M., & Tsao, R. (2017). Bioaccessibility, in vitro antioxidant and anti-inflammatory activities of phenolics in cooked green lentil (Lens culinaris). Journal of Functional Foods, 32, 248–255. https://doi.org/https://doi.org/10.1016/j.jff.2017.03.004
- Zhu, F., Du, B., & Xu, B. (2018). Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Critical Reviews in Food Science and Nutrition, 58(8), 1260–1270. Taylor & Francis. https://doi.org/https://doi.org/10.1080/10408398.2016.1251390
