ABSTRACT
Amaranth has been of interest for its nutritional quality and its multiple benefits; however, there is little research on its toxicological effect. This work studied antinutritional compounds, the cytotoxic and genotoxic effect in the Amaranthus protein extract (PEAh). The content of lectins, tannins, saponins, and trypsin inhibitors was determined. Biological studies were conducted to determine toxicity in male CD-1 mice by intraperitoneal administration. The genotoxic potential was determined by the micronucleus test. The bone marrow cytotoxicity was determined by polychromatic erythrocyte (PE) rate and the cytotoxic effect was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test. Our results showed considerably high concentrations of lectins and trypsin inhibitors, lower concentrations of tannins and saponins in PEAh. The PEAh was toxic to mice, presenting genotoxic and cytotoxic damage to bone marrow and hepatocytes.
RESUMEN
El amaranto ha sido de interés por su calidad nutricional y sus múltiples beneficios; sin embargo, hay poca investigación sobre su efecto toxicológico. Este trabajo estudió compuestos antinutricionales, el efecto citotóxico y genotóxico en el extracto de proteína de Amaranthus hypochondriacus (PEAh). Se determinó el contenido de lectinas, taninos, saponinas e inhibidores de tripsina. Se realizaron estudios biológicos para determinar la toxicidad en ratones machos CD-1 mediante administración intraperitoneal. El potencial genotóxico se determinó mediante la prueba de micronúcleos. La citotoxicidad de la médula ósea se determinó mediante la tasa de eritrocitos policromáticos (PE) y el efecto citotóxico se evaluó mediante la prueba MTT. Los resultados mostraron concentraciones considerablemente altas de lectinas e inhibidores de tripsina, concentraciones más bajas de taninos y saponinas en PEAh. El PEAh fue tóxico para los ratones, presentando daño genotóxico y citotóxico en la médula ósea y los hepatocitos.
1. Introduction
Many people have argued that natural medicines are much safer than synthetic drugs (Valerio & Gonzales, Citation2005). This has caused exceptional growth in human exposure to natural products, as phytotherapeutic agents, phytopharmaceutical, and nutraceutical products. As a result, there has been a resurgence of scientific interest in the biological effects of natural products to guarantee the biological effect attributed to them and the possible toxicological risk to which consumers may be exposed. There is no regulatory system ensuring the safety and activity of natural products in most countries, and such products have not been sufficiently investigated analytically or toxicologically (Valerio & Gonzales, Citation2005). Currently, it has been shown that many plants used in traditional and folk medicines are potentially toxic, mutagenic, and carcinogenic (Déciga-Campos et al., Citation2007; Ferreira-Machado et al., Citation2004; Fonseca et al., Citation2000; Grobelnik Mlakar et al., Citation2009; Matthews et al., Citation2003; Teschke et al., Citation2009; Valerio & Gonzales, Citation2005).
One such plant of recently increased importance is the amaranth. It is an ancient plant belonging to the family of Amaranthaceae, which is believed to have originated from Central and South America (Gamel et al., Citation2006). Amaranthus hypochondriacus is a species considered native to Mexico (Sauer, Citation1950, Citation1967). This and other species of amaranth have been used in a wide variety of foods, such as cereals, candies, sauces, and porridges. Amaranth flours are used in the preparation of bread, tortillas, and desserts. The chemical and cosmetic industries have also taken advantage of the plant to make oils and dyes (Grobelnik Mlakar et al., Citation2009).
Amaranth cultivation dates back 5,000 to 7,000 years. The Mayans were probably the first to cultivate amaranth. In recent times, the amaranth crop reappeared, not only in Mexico and Central America but also in the Andean territories of South America, as well as in some Asian, European, and African countries (Escudero et al., Citation2004). The species of this plant are receiving significant attention in developing countries to fight against protein malnutrition because it has proteins of good quality, a favorable composition of amino acids and fiber, and high digestibility coefficients of nutrients. Also, the seeds of amaranth can undergo different treatments to increase their nutritional proprieties (Escudero et al., Citation2004; Písaříková et al., Citation2011).
Different studies have characterized the seeds of amaranth, showing its antioxidant activity (Lehmann et al., Citation1994), hypocholesterolemic properties (Klimczak et al., Citation2002), and its potent anticarcinogenic effects (Barrio & Añón, Citation2010). Unfortunately, controversial data exist about the balance in its beneficial and/or toxic potential. Some evidence shows that amaranth also contains compounds that reduce its nutritional quality by reducing the absorption and/or nutrients utilization (Correa et al., Citation1986), adversely affecting health. These include lectins (Rinderle et al., Citation1990), saponins, tannins, and trypsin inhibitors (Ivleva et al., Citation2000). They have been related to various alterations in the gastrointestinal tract, producing a poor absorption of nutrients and, in the long term, possible malnutrition. Some studies have shown that lectins can produce toxic effects in various biological models, both in vivo and in vitro (Gonzalez de Mejia et al., Citation2005). In the same way, it has been observed that trypsin inhibitors decrease the digestibility of animal proteins (Palliyeguru et al., Citation2011). Saponins and tannins have been shown to diminish the growth of laboratory animals due to their ability to trap metal ions relevant to the enzymatic activity of the digestion of various nutrients (Griffiths & Moseley, Citation1980; Laurena et al., Citation1984; Valerio & Gonzales, Citation2005). All these factors contribute to the analysis of the plant to find its beneficial and harmful properties; therefore, the proposed aim was to evaluate the content of antinutritional compounds, the cytotoxic and genotoxic effect of the proteins extracted from the seeds of Amaranthus hypochondriacus.
2. Materials and methods
2.1. Animals
Thirty-five male mice strain CD-1 with a mean weight of 13 g were used in the micronucleus assay. They were obtained from the Institute of Health Sciences-UAEH (Pachuca, Hidalgo, Mexico), and maintained under standard conditions in metallic cages at a temperature of 23 ± 2°C and 50 ± 10% humidity, with feed (TEKLAD Global 2018S, Harlan, Mexico City) and water ad libitum, in a 12 h light–dark period. The adjustment time period for the animals was one week before the treatments. Male Fisher rats weighing 180–200 g were employed to obtain hepatocytes. The animals were obtained from the Production Unit of Experimental Laboratory Animals (UPEAL-Cinvestav, Mexico City, Mexico). These were fed ad libitum rodent chow and tap water, and before liver perfusion, were fasted for 18 h.
2.2. Human erythrocytes
Peripheral blood erythrocytes Type O were obtained from one voluntary healthy male donor. All the procedures were realized by the Official Mexican Standard NOM-007-SSA3-2011 and NOM-253-SSA1-2012. The subject provided written informed consent, and the protocol was approved by the Ethics and Research Committee of the Autonomous University of Hidalgo State with the number CEEI-000011-2019.
2.3. Plants seeds
Amaranthus hypochondriacus seeds (amaranth) used in the experiment were obtained directly from farmers in the town of Progreso Hidalgo, Mexico 20°14′53″N 99°11′23″O. The seeds were cleaned. The less damaged ones were selected, and these were stored at 5°C until use.
2.4. Protein extraction of Amaranthus hypochondriacus seeds
Proteins were obtained from the Amaranthus hypochondriacus seeds flour by shaking for 16 h at 4°C in 10 mM phosphate-buffered saline (PBS) solution. The mixture was centrifuged at 12,000 g for 60 min to yield the protein extract of A. hypochondriacus (PEAh). The PEAh was dialyzed overnight against deionized water at 4°C, then lyophilized and stored at −20°C. The protein concentration of Amaranthus hypochondriacus extract was determined by the Lowry method of using bovine serum albumin as standard (Waterborg & Matthews, Citation1984).
2.5. Antinutritional factors in PEAh
2.5.1. Lectin activity
The lectin was identified by hemagglutination, and the tests were carried out in microtiter 96-well (U-shaped) plates. The lectin was diluted serially (2-fold), adjusting the sample volume in each well to 50 µL with PBS. Each diluted sample was added to 50 µL of the 2% suspension of trypsinized human erythrocytes type O; these were obtained by incubation globular packages with trypsin (0.6 mg/mL), in PBS and incubated for one hour at 37°C in constant shaking. The trypsinized human erythrocytes exposed to protein sample diluted were incubated for 1 h at room temperature and then observed for positive agglutination. The titer was defined as the reciprocal of the highest dilution showing detectable agglutination (Jaffé et al., Citation1972).
2.5.2. Determination of tannins
Tannins were determined according to the modified vanillin-HCl method of Price et al. (Citation1978) using (+)-catechin (Sigma Chemical Co, St Louis, MO, USA) as standard. Tannin content was expressed as mg of catechin (CE) per gram of sample (mg CE/g) (Price et al., Citation1978).
2.5.3. Determination of Trypsin Inhibitors (TI)
Trypsin inhibitory activity was measured as the residual trypsin activity, using BAPNA (N-benzoyl-DL-arginine p-nitroanilide) as substrate (Martinez & Moyano, Citation2003). Trypsin inhibitory unit (TIU) was defined as the amount of inhibitor that inhibits 1 μg of pure trypsin. Total trypsin inhibitory activity was expressed as TIU per mg of lyophilized sample (TIU/mg). The reported results are the means from three separate measurements.
2.5.4. Determination of saponin
Saponins were extracted by the Soxhlet method using methanol-water mixture (85:15 v/v) for 2 h, and rotary evaporated to dryness and resuspended in saline solution (0.9%). The tests were carried out by the serial dilution method proposed by Girón (Citation1992), using type O human erythrocytes. Results were expressed as hemolytic units per milligram. The reported results are the means from three separate measurements.
2.6. Evaluation of the toxic effect of PEAh
Hepatocytes were isolated from male fisher rats (180–200 g) by the collagenase perfusion method (Mendoza-Figueroa et al., Citation1979). The cell was collected in a petri dish in Dulbecco’s modified Eagle’s medium (DMEM) F12 with 7% bovine serum, 5 μg insulin/mL, 100 U penicillin G/mL, and 100 μg streptomycin/mL. The cultures were maintained at 37°C in 5% CO2 in air (Sanyo CO2 Incubator US-Canada, Sanyo Scientific).
2.7. Cell viability assay
The hepatocytes were cultured for 24 h in DMEM medium supplemented with fetal bovine serum and antibiotics in a 4% CO2 and humid atmosphere at 37°C. The cell viability was measured by the tetrazolium dye colorimetric test, using (3-(4, 5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) (Hansen et al., Citation1989). The hepatocytes were cultured for 24 h in DMEM medium supplemented with fetal bovine serum and antibiotics in a 4% CO2 and humid atmosphere at 37°C. The cell viability was measured by the tetrazolium dye colorimetric test, using MTT (Hansen et al., Citation1989). Hepatocytes were treated with various concentrations of protein extract (0 to 5,550 μg/mL) under the same conditions previously described for 24 h. Then, MTT was added to the medium until getting a 5 mg/mL concentration and incubated for 3 h. At the end of that, the medium was removed, and 100 μL of dimethyl sulfoxide was added to each well to dissolve the formazan produced. The absorbance of each well was measured at 540 nm in a microplate reader (EPOCH, Biotek, Biotek Instruments Inc USA). The cell viability was expressed as a percentage of the value of the control culture.
2.8. Lethal median dose (LD50)
Lethal median dose was determinated by the Lorke method (Citation1983). Male mice CD-1 were randomly divided into seven lots and PEAh was administrated intraperitoneal via. The assay was made in two steps: in the first step, doses of 10, 100, and 1000 mg/kg of PEAh were administered intraperitoneally (i.p) and showed no lethality among the animals; then the effect of 1600, 2900, and 5000 mg/kg was tested, and mortality appeared among the treated mice.
2.9. Evaluation of the genotoxic capacity of PEAh by the micronucleus assay
After calculating DL50, we considered using doses of 50, 150, 250 mg/kg of PEAh to determine the genotoxic potential. The Committee of Ethics and biosecurity of the Institute of Sciences of the Health approved the protocol of micronucleus assay. Animals were organized into groups of five individuals each: a negative control group (saline solution 0.9%), a group positive treated with daunorubicin (DAU 3 mg/kg), and three groups treated with PEAh (50, 150, and 250 mg/kg) respectively. The PEAh were administered daily for a week, intraperitoneally, and according to the experimental groups mentioned. The DAU was injected in a single dose intraperitoneally. Rate of micronucleated polychromatic erythrocytes (MNPE) was determined before the experimental treatment and at 24, 48, 72, and 96 h after treatment. Two blood smears were made from the tail of each animal, fixed in methanol for 5 min, and stained for 20 min with a 4% Giemsa solution prepared in phosphate-buffered saline, at a pH of 6.8 (Madrigal-Santillán et al., Citation2007). One thousand erythrocytes per animal were scored to determine the rate of MNPE. To evaluate bone marrow cytotoxicity, we scored 1000 erythrocytes per animal and established the rate of polychromatic erythrocytes (PE) concerning the number of normochromic erythrocytes (PE/NE index).
2.10. Data analysis
The data obtained were analyzed with the statistical program GRAPHPAD INSTAT (San Diego, CA, USA) version 3.0, using ANOVA to determine if data were normally distributed within all groups. After that, a Tukey test (p < .05) was performed to establish the statistical differences between groups in each assay.
3. Results
3.1. Antinutritional compounds in A. hypochondriacus protein extract
According to the extraction method employed, the protein concentration in the extract was 2.1428 mg/mL, founding antinutritional compounds, including those of protein origin as lectin and trypsin inhibitors, as well as tannins and such as presented in .
Table 1. Tannins, lectins, saponins, and trypsin inhibitor’s activity content in A. hypochondriacus protein extract.
Tabla 1. Contenido de taninos, lectinas, saponinas e inhibidores de tripsina en el extracto proteico de A. hipochondriacus
3.2. Hepatotoxicity of the antinutritional compounds of A. hypochondriacus protein extract
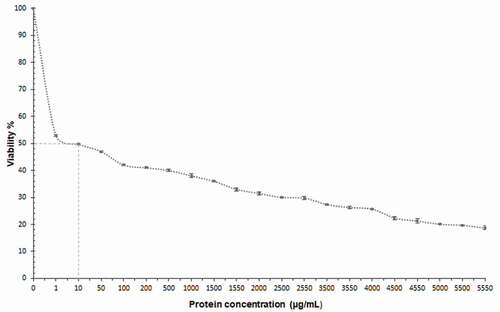
shows the cytotoxic effect of PEAh on primary cultured rat hepatocytes after 24 h of exposure. PEAh presented a dose depending cytotoxic effect. As can be seen, the hepatocyte cell viability decreases by 47.44% at the protein concentration of 1 μg/mL, and at 10 μg/mL the viability reaches 50%, obtaining the ID50. In contrast, at the maximum concentration, the viability decreased to 18%.
Figure 1. Cytotoxic effect of Amaranth protein extract on primary rat hepatocytes culture.
Figura 1. Efecto citotóxico del extracto de proteína de amaranto en cultivo primario de hepatocitos de rata
Acute toxicity assay of Amaranth proteins on mice presented a lethal dose (LD50) of 1254.9 mg/Kg of bodyweight, which classified the extract as moderately toxic. These results made it possible to establish the experimental conditions for the study of genotoxicity and cytotoxicity in mice not to cause death during the tests.
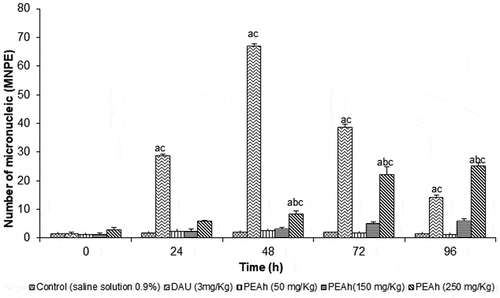
The results on the frequency of MNPE in the studied groups are shown in , observing that the animals belonging to the control group had no micronucleus increase in the four days of the experiment, having an average value of 1.0 MN/1000 PE. The mice treated with DAU (3 mg/kg) manifested a significant increase (p < .05) after 24 h of treatment with the most severe genotoxic damage. At 48 h, the micronucleus reached more than 60 MN/1000 PE. For the animals administered with the PEAh, there was no significant statistical difference (p < .05) regarding the control, at concentrations of 50 and 150 mg/kg during the test. It was observed an increase of MNPE rate at the doses of 150 and 250 mg/Kg. The greatest damage to genetic material was at the dose of 250 mg/kg during the 72 and 96 hours of treatment, obtaining a mean value of 24 MN/1000 PE; suggesting that the PEAh is a dose-dependent genotoxic agent.
Figure 2. Frequency of micronucleated polychromatic erythrocytes (MNPE) in mice treated with the protein extract of Amaranthus hypochondriacus seeds (PEAh) and daunorubicin (DAU).
Figura 2. Frecuencia de eritrocitos policromáticos micronucleados (MNPE) en ratones tratados con el extracto proteico de semillas de Amaranthus hypochondriacus (PEAh) y daunorrubicina (DAU)
3.3. Bone marrow cytotoxicity of the Antinutritional compounds of A. hypochondriacus protein extract
Results of the relation between PE and the number of normochromatic erythrocytes (PE/NE index) are shown in . This is an indicator of bone marrow cytotoxicity. At the beginning of the experiment, the PE/NE index was similar in all the groups. In the successive hours of treatment, the control group showed no significant variation in the index. The mice administered with DAU showed a significant increase in the rate of PE/NE at 96 h of treatment, equivalent to 72% concerning the control group level. In relation to the animals treated with the PEAhs, the PE/NE index increased with time. However, the concentration of 250 mg/kg reached a maximum at 48 h, followed by a fast decrease, which one statistically significant after 24 h of treatment in all concentrations analyzed. Based on these results, it can be inferred that the protein extract obtained from amaranth is cytotoxic to the bone marrow because of the increase in erythropoiesis.
Table 2. Relationship between the numbers of polychromatic erythrocytes and the number of normochromatic erythrocytes (PE/NE index).
Tabla 2. Relación entre el número de eritrocitos policromáticos y el número de eritrocitos normocromáticos (índice PE/NE)
4. Discussion
Amaranth grain has the potential to contribute to improving the nutrition of populations, especially in developing countries, because it has nutritional and functional properties (Muyonga et al., Citation2008). The presence of some compounds has been reported, such as phenols, fatty acids, tocopherols, carotenoids and others that contribute to health, reducing the risk of oxidative stress related to chronic diseases (Jo et al., Citation2015; López et al., Citation2013; Tang & Tsao, Citation2017). However, several researchers have demonstrated that amaranth seeds may contain antinutritional compounds, which could affect their nutritional value and limit their use. It has been observed that the content of compounds, such as trypsin inhibitors, phytates, saponins, tannins, and lectins, is related to the genus (Flavin & Pennington, Citation1982).
In this study, we analyzed the antinutritional compounds in the protein extract and determined the genotoxic and cytotoxic effects of PEAh, because this is the principal species cultivated and consumed in Mexico. Likewise, we chose to study the protein extract because of the significant interest for this grain due to its good quality proteins and being gluten-free (Alvarez-Jubete et al., Citation2010).
Contrary to its high nutritional quality, amaranth seeds contain antinutritional compounds, such as tannins, lectins, saponins, and trypsin inhibitors, which affect this plant’s nutritional value and consumers’ health (Flavin & Pennington, Citation1982). The concentration of antinutritional compounds depends on the species of Amaranthus, the methods employed for extraction and quantification, and the differences in time and place where amaranth was cultivated (Correa et al., Citation1986). This may be because the authors employed different extraction and quantification methods, and on the other hand, there are differences in terms of place and time of seed cultivation.
Globulins and albumins are the major seed storage proteins of food crops, including cereals. The main proteins present in amaranth seeds correspond to the group of globulins, glutelins, and albumins (Janssen et al., Citation2017; Venskutonis & Kraujalis, Citation2013) being the globulins 11S the main storage proteins (Chen & Paredes-Lopez, Citation1997; A. Quiroga et al., Citation2009; Silva-Sánchez et al., Citation2008).
In addition to the function of storage proteins in seed, the globulins have a secondary activity such as protease inhibitory activity (Rassam & Laing, Citation2006) and lectin-like activity (Soares et al., Citation2007). Sathe and Deshpande (Citation2003) reported that lectins are found in albumin and globulin fractions and that globulin in cereals can hemagglutinate rabbit red blood cells, and this lectin activity resides in the a-subunit of the globulin (Langston-Unkefer & Gade, Citation1984).
As in other research, the present work demonstrates that the hemagglutinating activity of lectins in PAEh has high activity. The lectins in amaranth seeds have been previously reported in A. hypochondryacus, A. leucocarpus, and A. caudatus, which are recognized as homodimeric proteins (Rinderle et al., Citation1989; Ozeki et al., Citation1996; Zenteno et al., Citation1992; Zenteno & Ochoa, Citation1988).
The lectins are widely distributed in plant tissues and are particularly abundant in seeds, reaching up to 10% of total proteins. However, they have also been located in smaller quantities in leaf stems, roots, and barks (Sharon & Lis, Citation2004). These proteins have been reported to be cytotoxic in several cell lines such as SW480, C33-A, Hela, 3T3 (Barzideh et al., Citation2014; Valadez-Vega et al., Citation2011; Valadez-Vega et al. Citation2011), which was observed in the present work since the protein extract was rich in lectins. This result allows attributing the observed cytotoxic effect to this type of protein. Since lectins can bind to cell membrane glycoconjugates in the digestive tract, they affect the transport of nutrients; this is why there has been increased interest in their antitumor effect in recent years, which prevents cell interactions causing cell damage (Quiroga et al., Citation2015).
Lectins, even in very low concentrations, can cause death to different laboratory animals (Reynoso-Camacho et al., Citation2003; Benson et al., Citation2011). The antinutritional compounds reported in this work, especially lectins, were found in high concentrations and may be responsible for the death of mice. It is well known that this type of protein can cause the death of laboratory animals by damaging several organs, promoting malabsorption of nutrients, inhibiting protein synthesis, and increasing the development of bacteria, among others (Endo et al., Citation1991; Pusztai & Bardocz, Citation1996).
Regarding the trypsin inhibitor level found in the PEAh, this was superior to in A. cruentus protein extract (Escudero et al., Citation2004). At the same time, the trypsin level was lower than reported in amaranth flours. Trypsin inhibitors in amaranth seeds have been reported to range from 938 to 2100 TIU/g for the black seeds, and from 300 to 5150 TIU/g units for the yellow seeds. These differences may be due to the species and the analytical methods employed (Correa et al., Citation1986; Valdes-Rodriguez et al., Citation1993). It has been demonstrated that this type of protease inhibitors present cytotoxic effects in some malignant and normal cell lines, such as MCF-7, HeLa, A549, HEK (Lanza et al., Citation2004; Patriota et al., Citation2020; Shamsi et al., Citation2017) and can also cause hypertrophy and hyperplasia of the pancreas, and lead to adenomas and carcinomas of the exocrine pancreas in rats (Hathcock, Citation1991). This supports the results obtained, since trypsin inhibitors were found in the amaranth extract, which may be contributing to the cytotoxic and toxicological effects in animals.
The concentration of tannins reported in the present work is in accordance with Akin-Idowu et al. (Citation2017) and Venskutonis and Kraujalis (Citation2013) results, who studied different species of Amaranthus, but are lower than that reported for amaranth grains. These differences relate to the fact that the compounds were determined in a protein extract in the present work, and in the other studies, it was from the seeds. However, it is known that tannins are preferably found in the seed coat than in perisperm, and their concentration depends on the color of the seed, being higher in colorful and dark seeds (Akin-Idowu et al., Citation2017; Osuntogun & Oke, Citation1983).
The hemolytic activity of saponins in PEAh was previously analyzed. The activity of this compound was lower in PEAh than in other amaranth species, such as A. hypochondriacus, A.cruentus, and A. hybridus, which differ considerably in their biological activity (Kohda et al., Citation1991; Oleszek et al., Citation1999).
The genotoxic assay showed that at a low concentration of PEAh, the quantity of MNPE did not show a significant statistical difference (p < .05) for the negative control. This result suggested that at these concentrations, the PEAh does not present a genotoxic effect. However, at the highest concentration, the proteins extract exerted genotoxic activity in the in vivo test system, inducing the production of MNPE. The cytotoxic effect observed in the in vitro and in vivo assays showed that the protein extract exerted a dose-dependent effect, causing damage to both the hepatocytes and the bone marrow.
The genotoxic damage found for PEAh is supported by the findings of other studies, since it has been reported that protein extract and pure lectins in plants present genotoxic effect, demonstrated by micronucleus, ames, and comet assays (Faheina-Martins et al., Citation2011; Patriota et al., Citation2020; Rolim et al., Citation2011).
The findings of this work suggest that the protein extract contains compounds responsible for the observed biological damage. Compounds such as lectins and trypsin inhibitors are globular proteins with anti-physiological effects and were extracted with the method used to obtain the PEAh. The concentration at which some of them were found may have been high enough to produce the observed cytotoxic and genotoxic damage. Other types of anti-physiological compounds, such as tannins and saponins, were found. Although their concentration were low in comparison to other reports, they could also collaborate in the biological damage observed. (Akubugwo et al., Citation2007; Venskutonis & Kraujalis, Citation2013)
Several papers have been published showing the cytotoxic effect of lectins and extracts rich in lectins on normal and malignant cells (Reynoso-Camacho et al., Citation2003; Gonzalez de Mejia et al., Citation2005; Valadez-Vega et al., Citation2011; Valadez-Vega et al. Citation2011). Likewise, trypsin inhibitors, tannins, and saponins, from different plant sources, have also been shown to have cytotoxic activity in several cell lines (Akhtar et al., Citation2017; Buyukleyla et al., Citation2012; Jaramillo-Carmona, Guillén-Bejarano et al., Citation2018; Jaramillo-García, Trindade et al., Citation2018; Mbaveng et al., Citation2018; Shamsi et al., Citation2017; Zhu et al., Citation2018). Saponins, trypsin inhibitors, and tannins from different sources have been reported as genotoxic, inducing the formation of micronuclei and chromosomal aberrations (Akhtar et al., Citation2017; Blaszkowska et al., Citation2009; Buyukleyla et al., Citation2012; Dauer et al., Citation2003; Demma et al., Citation2013).
It can be inferred that the antinutritional compounds found are related to the toxicological effect since, as mentioned above, lectins, trypsin inhibitors, tannins and saponins have shown cytotoxic and genotoxic effects by themselves. Since all these compounds were found in PEAh, it can be deduced that all of them contribute to the cytotoxic and genotoxic effects observed. Although lectins and trypsin inhibitors may be the principal responsible for the cyto- and genotoxic effect found, they were found in higher concentrations; however, tannins and saponins could also contribute to the adverse effect.
5. Conclusions
Our results showed considerably high concentrations of lectins and trypsin inhibitors, lower concentrations of tannins and saponins in PEAh. The PEAh was toxic to mice, presenting genotoxic and cytotoxic damage to bone marrow and hepatocytes. In this study, high concentrations of lectins and low concentrations of tannins, saponins and trypsin inhibitors were found in PEAH, which have been reported to have toxicological effects in cell lines and in laboratory animals, even causing death.
PEAh showed a dose-dependent cytotoxic effect on hepatocytes, determining the ID50 at 10 μg/mL, as well, a time- and dose-dependent genotoxic effect of PEAh.
The Bone marrow cytotoxicity of PEAhs, corresponded whit the PE/NE index, increased with time. The major effect was observed at 96 h at the concentrations of 50 and 150 mg/kg, while at 250 mg/kg the greatest effect was found at 48 h.
The effects observed in cytotoxicity and genotoxicity studies, caused by PEAh, can be attributed to the presence of the antinutritional compounds found in the protein extract.
Future research is required to explain the mechanisms of action of these proteins; in addition to, purify the main antinutritional compounds found in the extract as well as evaluate toxicological effects in vitro studies with other cell lines.
Ethics declarations
All animal procedures were realized in accordance with the Official Mexican Standard NOM-062-ZOO-1999 and were approved with number CIECUAL/009/2018 by the Ethics Committee on Animal Care and Use of Autonomous University of Hidalgo State.
Acknowledgments
The authors would like to acknowledge the Autonomous University of Hidalgo for technical services during this study
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Akhtar, M. F., Sharif, A., Saleem, M., Saleem, A., Akhtar, B., Raza, M., Ijaz, H., Shabbir, M., Ali, S., Sharif, A., Nasim, M. B., & Peerzada, S. (2017). Genotoxic and cytotoxic potential of Alternanthera bettzickiana, an important ethno-medicinal plant. Cellular and Molecular Biology (Noisy-le-grand, France), 63(8), 109–114. https://doi.org/https://doi.org/10.14715/cmb/2017.63.8.23
- Akin-Idowu, P. E., Ademoyegun, O., Olagunju, Y., Aduloju, A. O., Akin-Idowu, P. E., Ademoyegun, O. T., Olagunju, Y. O., & Adebo, U. G. (2017). Phytochemical content and antioxidant activity of five grain amaranth species breeding for disease resistance tomato varieties view project control of bug of grain amaranthus view project phytochemical content and antioxidant activity of five grain amaran. American Journal of Food Science and Technology, 5(6), 249–255. https://doi.org/https://doi.org/10.12691/ajfst-5-6-5
- Akubugwo, I. E., Obasi, N. A., Chinyere, G. C., & Ugbogu, A. E. (2007). Nutritional and chemical value of Amaranthus hybridus l. leaves from Afikpo, Nigeria. African Journal of Biotechnology, 6(24), 2833–2839. https://doi.org/https://doi.org/10.5897/AJB2007.000-2452
- Alvarez-Jubete, L., Arendt, E. K., & Gallagher, E. (2010). Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends in Food Science and Technology, 21 (2), 106–113. Elsevier. https://doi.org/https://doi.org/10.1016/j.tifs.2009.10.014
- Barrio, D. A., & Añón, M. C. (2010). Potential antitumor properties of a protein isolate obtained from the seeds of Amaranthus mantegazzianus. European Journal of Nutrition, 49(2), 2. https://doi.org/https://doi.org/10.1007/s00394-009-0051-9
- Barzideh, Z., Latiff, A. A., Gan, C.-Y., Benjakul, S., & Karim, A. A. (2014). Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). International Journal of Food Science & Technology, 49(6), 1490–1499. https://doi.org/https://doi.org/10.1111/ijfs.12464
- Benson, J. M., Gomez, A. P., Wolf, M. L., Tibbetts, B. M., & March, T. H. (2011). The acute toxicity, tissue distribution, and histopathology of inhaled ricin in Sprague Dawley rats and BALB/c mice. Inhalation Toxicology, 23(5), 5. https://doi.org/https://doi.org/10.3109/08958378.2011.565490
- Blaszkowska, J., Bratkowska, W., Lopaczynska, D., & Ferenc, T. (2009). Cytogenetic study of Ascaris trypsin inhibitor in cultured human lymphocytes with metabolic activation. Journal of Genetics, 88(1), 69–75. https://doi.org/https://doi.org/10.1007/s12041-009-0009-y
- Buyukleyla, M., Azirak, S., Rencuzogullari, E., Kocaman, A. Y., Ila, H. B., Topaktas, M., & Darici, C. (2012). The genotoxic and antigenotoxic effects of tannic acid in human lymphocytes. Drug and Chemical Toxicology, 35(1), 11–19. https://doi.org/https://doi.org/10.3109/01480545.2011.564181
- Chen, S., & Paredes-Lopez, O. (1997). Isolation and characterization of the 11S globulin from amaranth seeds. Journal of Food Biochemistry, 21(6), 53–65. https://doi.org/https://doi.org/10.1111/j.1745-4514.1997.tb00224.x
- Correa, A. D., Jokl, L., & Carlsson, R. (1986). Chemical constituents, in vitro protein digestibility, and presence of antinutritional substances in amaranth grains. Archivos Latinoamericanos de Nutricion, 36(2), 319–26. https://www.alanrevista.org/ediciones/1986/2/art-11/
- Dauer, A., Hensel, A., Lhoste, E., Knasmüller, S., & Mersch-Sundermann, V. (2003). Genotoxic and antigenotoxic effects of catechin and tannins from the bark of Hamamelis virginiana L. in metabolically competent, human hepatoma cells (Hep G2) using single cell gel electrophoresis. Phytochemistry, 63(2), 199–207. https://doi.org/https://doi.org/10.1016/S0031-9422(03)00104-3
- Déciga-Campos, M., Rivero-Cruz, I., Arriaga-Alba, M., Castañeda-Corral, G., Angeles-López, G. E., Navarrete, A., & Mata, R. (2007). Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. Journal of Ethnopharmacology, 110(2), 334–342. https://doi.org/https://doi.org/10.1016/j.jep.2006.10.001
- Demma, J., El-Seedi, H., Engidawork, E., Aboye, T. L., Göransson, U., & Hellman, B. (2013). An in vitro study on the DNA damaging effects of phytochemicals partially isolated from an extract of glinus lotoides. Phytotherapy Research, 27(4), 507–514. https://doi.org/https://doi.org/10.1002/ptr.4744
- Endo, Y., Glück, A., & Wool, I. G. (1991). Ribosomal RNA identity elements for ricin A-chain recognition and catalysis. Journal of Molecular Biology, 221(1), 193–207. https://doi.org/https://doi.org/10.1016/0022-2836(91)80214-F
- Escudero, N. L., De Arellano, M. L., Luco, J. M., Giménez, M. S., & Mucciarelli, S. I. (2004). Comparison of the chemical composition and nutritional value of Amaranthus cruentus flour and its protein concentrate. Plant Foods for Human Nutrition, 59(1), 15–21. https://doi.org/https://doi.org/10.1007/s11130-004-0033-3
- Faheina-Martins, G. V., da Silveira, A. L., Ramos, M. V., Marques-Santos, L. F., & Araujo, D. A. M. (2011). Influence of fetal bovine serum on cytotoxic and genotoxic effects of lectins in MCF-7 cells. Journal of Biochemical and Molecular Toxicology, 25(5), 5. https://doi.org/https://doi.org/10.1002/jbt.20388
- Ferreira-Machado, S. C., Rodrigues, M. P., Nunes, A. P. M., Dantas, F. J. S., De Mattos, J. C. P., Silva, C. R., Moura, E. G., Bezerra, R. J. A. C., & Caldeira-de-Araujo, A. (2004). Genotoxic potentiality of aqueous extract prepared from Chrysobalanus icaco L. leaves. Toxicology Letters, 151(3), 481–487. https://doi.org/https://doi.org/10.1016/j.toxlet.2004.03.014
- Flavin, D. F., & Pennington, J. A. T. (1982). Food composition and nutrition tables 1981–1982. Journal of AOAC International, 65(6), 1529. https://doi.org/https://doi.org/10.1093/jaoac/65.6.1529
- Fonseca, C. A., Otto, S. S., Paumgartten, F. J., & Leitão, A. C. (2000). Nontoxic, mutagenic, and clastogenic activities of Mate-Chimarrão (Ilex paraguariensis). Journal of Environmental Pathology, Toxicology and Oncology: Official Organ of the International Society for Environmental Toxicology and Cancer, 19(4), 333–346. https://pubmed.ncbi.nlm.nih.gov/11213015/
- Gamel, T. H., Linssen, J. P., Mesallam, A. S., Damir, A. A., & Shekib, L. A. (2006). Effect of seed treatments on the chemical composition of two amaranth species: Oil, sugars, fibres, minerals and vitamins. Journal of the Science of Food and Agriculture, 86(1), 82–89. https://doi.org/https://doi.org/10.1002/jsfa.2318
- Girón, M. (1992). Determinación semi cuantitativa de saponinas en muestras vegetales aprovechando su capacidad hemolítica. Tesis Facultad de Química UNAM.
- Gonzalez de Mejia, E., Valadez-Vega, M. D. C., Reynoso-Camacho, R., & Loarca-Pina, G. (2005). Tannins, trypsin inhibitors and lectin cytotoxicity in tepary (Phaseolus acutifolius) and common (Phaseolus vulgaris) beans. Plant Foods for Human Nutrition, 60(3), 3. https://doi.org/https://doi.org/10.1007/s11130-005-6842-0
- Griffiths, D. W., & Moseley, G. (1980). The effect of diets containing field beans of high or low polyphenolic content on the activity of digestive enzymes in the intestines of rats. Journal of the Science of Food and Agriculture, 31(3), 255–259. https://doi.org/https://doi.org/10.1002/jsfa.2740310307
- Grobelnik Mlakar, S., Turinek, M., Jakop, M., Bavec, M., & Bavec, F. (2009). Nutrition value and use of grain amaranth: Potential future application and bread making. Agricultura, 2(6), 43–53. https://www.doc-developpement-durable.org/file/Culture/Culture-plantes-alimentaires/FICHES_PLANTES/amarante/Nutrition%20value%20and%20use%20of%20grain%20amaranth_potential%20future%20crop.pdf
- Hansen, M. B., Nielsen, S. E., & Berg, K. (1989). Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. Journal of Immunological Methods, 119(2), 203–210. https://doi.org/https://doi.org/10.1016/0022-1759(89)90397-9
- Hathcock, J. N. (1991). Residue trypsin inhibitor: Data needs for risk assessment. Advances in Experimental Medicine and Biology, 289, 273–279. https://doi.org/https://doi.org/10.1007/978-1-4899-2626-5_20
- Ivleva, E. V., Rudenskaya, Y. A., Zimacheva, A. V., & Mosolov, V. V. (2000). Trypsin inhibitor from amaranth (Amaranthys cruentus) leaves. Prikladnaya Biokhimiya I Mikrobiologiya, 36, 5. https://pubmed.ncbi.nlm.nih.gov/11042876/
- Jaffé, W. G., Brücher, O., & Palozzo, A. (1972). Detection of four types of specific phytohemagglutinins in different lines of beans (Phaseolus vulgaris). Zeitschrift fur Immunitatsforschung, Experimentelle und Klinische Immunologie, 142(5), 5. https://www.fundacionbengoa.org/wp-content/uploads/publicaciones/Detection-of-four-types-of-specifity-Phytohemagglutinins-in-different-lines-of-beans-Phaseolus-vulgaris.pdf
- Janssen, F., Pauly, A., Rombouts, I., Jansens, K. J. A., Deleu, L. J., & Delcour, J. A. (2017). Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): A food science and technology perspective. Comprehensive Reviews in Food Science and Food Safety, 16(1), 39–58. https://doi.org/https://doi.org/10.1111/1541-4337.12240
- Jaramillo-Carmona, S., Guillén-Bejarano, R., Jiménez-Araujo, A., Rodríguez-Arcos, R., & López, S. (2018). In vitro toxicity of asparagus saponins in distinct multidrug-resistant colon cancer cells. Chemistry & Biodiversity, 15(11), e1800282. https://doi.org/https://doi.org/10.1002/cbdv.201800282
- Jaramillo-García, V., Trindade, C., Lima, E., Guecheva, T. N., Villela, I., Martinez-Lopez, W., Corrêa, D. S., Ferraz, A. D. B. F., Moura, S., Sosa, M. Q., Da Silva, J., & Henriques, J. A. P. (2018). Chemical characterization and cytotoxic, genotoxic, and mutagenic properties of Baccharis trinervis (Lam, Persoon) from Colombia and Brazil. Journal of Ethnopharmacology, 213(1), 210–220. https://doi.org/https://doi.org/10.1016/j.jep.2017.10.027
- Jo, H. J., Chung, K. H., Yoon, J. A., Lee, K. J., Song, B. C., & An, J. H. (2015). Radical scavenging activities of tannin extracted from amaranth (Amaranthus caudatus l.). Journal of Microbiology and Biotechnology, 25(6), 795–802. https://doi.org/https://doi.org/10.4014/jmb.1409.09088
- Klimczak, I., Małecka, M., & Pachołek, B. (2002). Antioxidant activity of ethanolic extracts of amaranth seeds. Nahrung - Food, 46(3), 3. https://doi.org/https://doi.org/10.1002/1521-3803(20020501)46:3<184::AID-FOOD184><184::AID-FOOD184>3.0.CO;2-H
- Kohda, H., Tanaka, S., Yamaoka, Y., & Ohhara, Y. (1991). Saponins from Amaranthus hypochondriacus. Chemical & Pharmaceutical Bulletin, 39(10), 2609–2612. https://doi.org/https://doi.org/10.1248/cpb.39.2609
- Langston-Unkefer, P. J., & Gade, W. (1984). A seed storage protein with possible self-affinity through lectin-like binding. Plant Physiology, 74(3), 675–680. https://doi.org/https://doi.org/10.1104/pp.74.3.675
- Lanza, A., Tava, A., Catalano, M., Ragona, L., Singuaroli, I., Robustelli Della Cuna, F. S., & Robustelli Della Cuna, G. (2004). Effects of the Medicago scutellata Trypsin Inhibitor (MsTI) on cisplatin-induced cytotoxicity in human breast and cervical cancer cells. Anticancer Research, 24(1). https://www.researchgate.net/publication/5390399_Effects_of_the_Medicago_scutellata_Trypsin_Inhibitor_MsTI_on_Cisplatin-induced_Cytotoxicity_in_Human_Breast_and_Cervical_Cancer_Cells
- Laurena, A. C., Van Den, T., & Mendoza, E. M. T. (1984). Effects of condensed tannins on the in vitro protein digestibility of cowpea [Vigna unguiculata (L.) Walp.]. Journal of Agricultural and Food Chemistry, 32(5), 1045–1048. https://doi.org/https://doi.org/10.1021/jf00125a025
- Lehmann, J. W., Putnam, D. H., & Qureshi, A. A. (1994). Vitamin E isomers in grain amaranths (Amaranthus spp.). Lipids, 29(3), 177–181. https://doi.org/https://doi.org/10.1007/BF02536726
- López, V. R. L., Razzeto, G. S., Escudero, N. L., & Gimenez, M. S. (2013). Biochemical and molecular study of the influence of Amaranthus hypochondriacus flour on serum and liver lipids in rats treated with ethanol. Plant Foods for Human Nutrition, 68(4), 396–402. https://doi.org/https://doi.org/10.1007/s11130-013-0388-3
- Lorke, D. (1983). A new approach to practical acute toxicity testing. Archives of Toxicology, 54(4), 275–287. https://doi.org/https://doi.org/10.1007/BF01234480
- Madrigal-Santillán, E., Álvarez-González, I., Márquez-Márquez, R., Velázquez-Guadarrama, N., & Madrigal-Bujaidar, E. (2007). Inhibitory effect of mannan on the toxicity produced in mice fed aflatoxin B1 contaminated corn. Archives of Environmental Contamination and Toxicology, 53(3), 466–472. https://doi.org/https://doi.org/10.1007/s00244-006-0074-7
- Martinez, T. F., & Moyano, F. J. (2003). Determination of trypsin inhibitor activity of soy products: A collaborative analysis of an improved procedure. Journal of the Science of Food and Agriculture, 83(11), 5. https://doi.org/https://doi.org/10.1016/S0031-9422(00)88169-8
- Matthews, S. C., Camacho, A., Lawson, K., & Dimsdale, J. E. (2003). Use of herbal medications among 200 psychiatric outpatients: Prevalence, patterns of use, and potential dangers. General Hospital Psychiatry, 25(1), 24–26. https://doi.org/https://doi.org/10.1016/S0163-8343(02)00237-2
- Mbaveng, A. T., Ndontsa, B. L., Kuete, V., Nguekeu, Y. M. M., Çelik, İ., Mbouangouere, R., Tane, P., & Efferth, T. (2018). A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine, 43(1), 78–85. https://doi.org/https://doi.org/10.1016/j.phymed.2018.03.035
- Mendoza-Figueroa, T., López-Revilla, R., & Villa-Trevino, S. (1979). Dose-dependent dna ruptures induced by the procarcinogen dimethylnitrosamine on primary rat liver cultures. Cancer Research, 39(8), 3254–32527. https://cancerres.aacrjournals.org/content/39/8/3254.long
- Muyonga, J. H., Nabakabya, D., Nakimbugwe, D. N., & Masinde, D. (2008). Efforts to promote amaranth production and consumption in Uganda to fight malnutrition. In Robertson, G. L. & Lupien, J. R. (Eds.), Using food and technology to improve nutrition and promote national development, August 2015 (pp. 1–10). International Union of Food Science & Technology (IUFoST). http://www.iufost.org/iufostftp/IUFoST_Case%20Studies-1.pdf
- Oleszek, W., Junkuszew, M., & Stochmal, A. (1999). Determination and toxicity of saponins from Amaranthus cruentus seeds. Journal of Agricultural and Food Chemistry, 47(9), 3685–3687. https://doi.org/https://doi.org/10.1021/jf990182k
- Osuntogun, A. B., & Oke, O. L. (1983). A note on the nutritive value of amaranth seeds. Food Chemistry, 12(4), 287–289. https://doi.org/https://doi.org/10.1016/0308-8146(83)90017-1
- Ozeki, M., Kamemura, K., Moriyama, K., Itoh, Y., Furuichi, Y., Umekawa, H., & Takahashi, T. (1996). Purification and characterization of a lectin from Amaranthus hypochondriacus var. Mexico seeds. Bioscience, Biotechnology and Biochemistry, 60(12), 2048–2051. https://doi.org/https://doi.org/10.1271/bbb.60.2048
- Palliyeguru, M. W. C. D., Rose, S. P., & Mackenzie, A. M. (2011). Effect of trypsin inhibitor activity in soya bean on growth performance, protein digestibility and incidence of sub-clinical necrotic enteritis in broiler chicken flocks. British Poultry Science, 52(3), 3. https://doi.org/https://doi.org/10.1080/00071668.2011.577054
- Patriota, L. L. D. S., Ramos, D. D. B. M., dos Santos, A. C. L. A., Silva, Y. A., Gama E Silva, M., Torres, D. J. L., Procópio, T. F., de Oliveira, A. M., Coelho, L. C. B. B., Pontual, E. V., Da Silva, D. C. N., Paiva, P. M. G., De Lorena, V. M. B., Mendes, R. L., & Napoleão, T. H. (2020). Antitumor activity of Moringa oleifera (drumstick tree) Flower Trypsin Inhibitor (MoFTI) in sarcoma 180-bearing mice. Food and Chemical Toxicology, 145(1), 111691. https://doi.org/https://doi.org/10.1016/j.fct.2020.111691
- Písaříková, B., Zralý, Z., Kráčmar, S., Trčková, M., & Herzig, I. (2011). Nutritional value of amaranth (genus Amaranthus L.) grain in diets for broiler chickens. Czech Journal of Animal Science, 50(No. 12), 568–573. https://doi.org/https://doi.org/10.17221/4263-CJAS
- Price, M. L., Van, S. S., & Butler, L. G. (1978). A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. Journal of Agricultural and Food Chemistry, 26(5), 5. https://doi.org/https://doi.org/10.1021/jf60219a031
- Pusztai, A., & Bardocz, S. (1996). Biological effects of plant lectins on the gastrointestinal tract: Metabolic consequences and applications. Trends in Glycoscience and Glycotechnology, 8(41), 41. https://doi.org/https://doi.org/10.4052/tigg.8.149
- Quiroga, A., Martínez, E. N., Rogniaux, H., Geairon, A., & Añón, M. C. (2009). Globulin-p and 11S-globulin from amaranthus hypochondriacus: Are two isoforms of the 11S-globulin. Protein Journal, 28(9–10), 457–467. https://doi.org/https://doi.org/10.1007/s10930-009-9214-z
- Quiroga, A. V., Barrio, D. A., & Añón, M. C. (2015). Amaranth lectin presents potential antitumor properties. LWT - Food Science and Technology, 60(1), 478–485. https://doi.org/https://doi.org/10.1016/j.lwt.2014.07.035
- Rassam, M., & Laing, W. A. (2006). The interaction of the 11S globulin-like protein of kiwifruit seeds with pepsin. Plant Science, 171(6), 663–669. https://doi.org/https://doi.org/10.1016/j.plantsci.2006.06.014
- Reynoso-Camacho, R., González de Mejía, E., & Loarca-Piña, G. (2003). Purification and acute toxicity of a lectin extracted from tepary bean (Phaseolus acutifolius). Food and Chemical Toxicology, 41(1), 21–27. https://doi.org/https://doi.org/10.1016/S0278-6915(02)00215-6
- Rinderle, S. J., Goldstein, I. J., Matta, K. L., & Ratcliffe, R. M. (1989). Isolation and characterization of amaranthin, a lectin present in the seeds of Amaranthus caudatus, that recognizes the T- (or Cryptic T)-antigen. Journal of Biological Chemistry, 264(27), 16123–16131. https://doi.org/https://doi.org/10.1016/S0021-9258(18)71595-0
- Rinderle, S. J., Goldstein, I. J., & Remsen, E. E. (1990). Physicochemical properties of amaranthin, the lectin from Amaranthus caudatus seeds. Biochemistry, 29(46), 46. https://doi.org/https://doi.org/10.1021/bi00498a019
- Rolim, L. A. D. M. M., Macêdo, M. F. S., Sisenando, H. A., Napoleão, T. H., Felzenszwalb, I., Aiub, C. A. F., Coelho, L. C. B. B., Medeiros, S. R. B., & Paiva, P. M. G. (2011). Genotoxicity evaluation of Moringa oleifera seed extract and lectin. Journal of Food Science, 76(2), 2. https://doi.org/https://doi.org/10.1111/j.1750-3841.2010.01990.x
- Sathe, S. K., & Deshpande, S. S. (2003). Beans. In Benjamin Caballero (Eds.), Encyclopedia of food sciences and nutrition (2nd ed., pp. 403–412). Academic Press. https://doi.org/https://doi.org/10.1016/b0-12-227055-x/00083-3
- Sauer, J. D. (1950). The grain amaranths: A survey of their history and classification. Annals of the Missouri Botanical Garden, 37(4), 561. https://doi.org/https://doi.org/10.2307/2394403
- Sauer, J. D. (1967). The grain amaranths and their relatives: A revised taxonomic and geographic survey. Annals of the Missouri Botanical Garden, 54(2), 103. https://doi.org/https://doi.org/10.2307/2394998
- Shamsi, T. N., Parveen, R., & Fatima, S. (2017). Trypsin inhibitors demonstrate antioxidant activities, inhibit A549 cell proliferation, and increase activities of reactive oxygen species scavenging enzymes. Indian Journal of Pharmacology, 49(2), 155–160. https://doi.org/https://doi.org/10.4103/ijp.IJP_553_16
- Sharon, N., & Lis, H. (2004). History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology, 14(11), 53R–62R. https://doi.org/https://doi.org/10.1093/glycob/cwh122
- Silva-Sánchez, C., Barba De La Rosa, A. P., León-Galván, M. F., De Lumen, B. O., De León-Rodríguez, A., & González de Mejía, E. (2008). Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. Journal of Agricultural and Food Chemistry, 56(4), 1233–1240. https://doi.org/https://doi.org/10.1021/jf072911z
- Soares, E. L., Freitas, C. D. T., Oliveira, J. S., Sousa, P. A. S., Sales, M. P., Barreto-Filho, J. D. M., Bandeira, G. P., & Ramos, M. V. (2007). Characterization and insecticidal properties of globulins and albumins from Luetzelburgia auriculata (Allemao) Ducke seeds towards Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Journal of Stored Products Research, 43(4), 459–467. https://doi.org/https://doi.org/10.1016/j.jspr.2006.12.007
- Tang, Y., & Tsao, R. (2017). Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Molecular Nutrition & Food Research, 61 (7), 1600767. Wiley-VCH Verlag. https://doi.org/https://doi.org/10.1002/mnfr.201600767
- Teschke, R., Genthner, A., & Wolff, A. (2009). Kava hepatotoxicity: Comparison of aqueous, ethanolic, acetonic kava extracts and kava-herbs mixtures. Journal of Ethnopharmacology, 123(3), 378–384. https://doi.org/https://doi.org/10.1016/j.jep.2009.03.038
- Valadez-Vega, C., Alvarez-Manilla, G., Riverón-Negrete, L., García-Carrancá, A., Morales-González, J. A., Zuñiga-Pérez, C., Madrigal-Santillán, E., Esquivel-Soto, J., Esquivel-Chirino, C., Villagómez-Ibarra, R., Bautista, M., & Morales-González, Á. (2011). Detection of cytotoxic activity of lectin on human colon adenocarcinoma (Sw480) and epithelial cervical carcinoma (C33-A). Molecules, 16(3), 2107–2118. https://doi.org/https://doi.org/10.3390/molecules16032107
- Valadez-Vega, C., Guzmán-Partida, A. M., Soto-Cordova, F. J., Álvarez-Manilla, G., Morales-González, J. A., Madrigal-Santillán, E., Villagómez-Ibarra, J. R., Zúniga-Pérez, C., Gutiérrez-Salinas, J., & Becerril-Flores, M. A. (2011). Purification, biochemical characterization, and bioactive properties of a lectin purified from the seeds of white tepary bean (Phaseolus acutifolius variety latifolius). Molecules, 16(3), 3. https://doi.org/https://doi.org/10.3390/molecules16032561
- Valdes-Rodriguez, S., Segura-Nieto, M., Chagolla-Lopez, A., Verver, Y., Vargas-Cortina, A., Martinez-Gallardo, N., & Blanco-Labra, A. (1993). Purification, characterization, and complete amino acid sequence of a trypsin inhibitor from amaranth (Amaranthus hypochondriacus) seeds. Plant Physiology, 103(4), 1407–1412. https://doi.org/https://doi.org/10.1104/pp.103.4.1407
- Valerio, L. G., & Gonzales, G. F. (2005). Toxicological aspects of the South American herbs cat’s claw (Uncaria tomentosa) and maca (Lepidium meyenii): A critical synopsis. Toxicological Reviews, 24(1), 11–35. https://doi.org/https://doi.org/10.2165/00139709-200524010-00002
- Venskutonis, P. R., & Kraujalis, P. (2013). Nutritional components of amaranth seeds and vegetables: A review on composition, properties, and uses. Comprehensive Reviews in Food Science and Food Safety, 12(4), 381–412. https://doi.org/https://doi.org/10.1111/1541-4337.12021
- Waterborg, J. H., & Matthews, H. R. (1984). The lowry method for protein quantitation. Methods in Molecular Biology (Clifton, NJ), 1, 1–3. https://doi.org/https://doi.org/10.1385/0-89603-062-8:1
- Zenteno, E., Lascurain, R., Montaño, L. F., Vazquez, L., Debray, H., & Montreuil, J. (1992). Specificity of Amaranthus leucocarpus lectin. Glycoconjugate Journal, 9(4), 204–208. https://doi.org/https://doi.org/10.1007/BF00731166
- Zenteno, E., & Ochoa, J. L. (1988). Purification of a lectin from Amaranthus leucocarpus by affinity chromatography. Phytochemistry, 27(2), 313–317. https://doi.org/https://doi.org/10.1016/0031-9422(88)83088-7
- Zhu, F. R., Li, Y. N., He, S. L., Chen, Q. S., & Xu, X. Y. (2018). Cytotoxic activities of total saponins from plena clematis on human tumor cell lines in vitro. Chinese Journal of Integrative Medicine, 24(10), 763–767. https://doi.org/https://doi.org/10.1007/s11655-018-2839-z
