ABSTRACT
Biogenic amine (BA) is mostly produced through the decarboxylation of amino acids, the formation of BA (histamine, putrescine, cadaverine, and tyramine) was closely associated with microorganism. This study investigated the effects of tea polyphenol (TP), anise essential oil (AEO), cinnamon essential oil (CEO), and ginger essential oil (GEO) on the accumulation of four BAs and bacterial community in fermented (pork) sausage. Meanwhile, the pH and the residual nitrite content (added as preservative) were evaluated. The results revealed that the accumulation of nitrite and BA were significantly inhibited by TP and plant essential oils (PEO). Pediococcus and Staphylococcus were the dominant bacteria in all fermented sausage. The H2S-producing bacteria, Lactobacillus, Pseudomonas, Serratia, and Clostridium sensu stricto 1 was suppressed by TP and PEO. These results indicated that TP and PEO could improve the safety of fermented sausage by inhibiting spoilage bacterial growth and BA formation, and AEO showed the optimal inhibitory effect.
RESUMEN
Las aminas biógenas (BA), por ejemplo, histamina, putrescina, cadaverina y tiramina. se producen principalmente por la descarboxilación de aminoácidos y su formación está estrechamente asociada con microorganismos. Este estudio investigó los efectos del polifenol del té (TP), el aceite esencial de anís (AEO), el aceite esencial de canela (CEO) y el aceite esencial de jengibre (GEO) en la acumulación de cuatro BA y de la comunidad bacteriana presente en la salchicha fermentada (de cerdo). Asimismo, se evaluaron el pH y el contenido de nitrito residual (añadido como conservante). Los resultados dan cuenta de que la acumulación de nitrito y BA fue inhibida de manera significativa por el TP y los aceites esenciales vegetales (PEO). El análisis permitió constatar que las bacterias dominantes en todos los embutidos fermentados fueron Pediococcus y Staphylococcus y que el TP y los PEO suprimieron las bacterias productoras de H2S, Lactobacillus, Pseudomonas, Serratia y Clostridium sensu stricto. Estos resultados indican que el TP y los PEO pueden mejorar la seguridad de los embutidos fermentados pues inhiben el crecimiento de bacterias de deterioro y la formación de BA, siendo el AEO el aceite que produjo el efecto inhibidor óptimo.
1. Introduction
Fermented sausage is a famous traditional meat product, and it has become increasingly popular in China due to its unique flavor and rich nutrition (Chen et al., Citation2016). It is made of fresh pork, back fat, spices, salt, sugar, and other auxiliary materials. Then, the mixture is inoculated with or without starter culture, stuffed into the casing, and fermented under certain conditions (Yu et al., Citation2017). Fermented sausage has high nutritional value, long shelf-life, and unique flavor and it is easy to store. However, there are some potential safety problems with traditional fermented sausage (natural fermentation), such as harmful microorganisms and accumulation of excessive (BA).
BA is a low molecular weight organic compound with biological activity, which is produced by the action of amino acid decarboxylases on free amino acids (Ruiz-Capillas et al., Citation2007). BA is a nitrogenous organic compound, and is considered a toxic compound in foods when the content of biogenic amine exceeds the limit requirement (Han et al., Citation2019). They are generally found in fermented foods with high content of protein and amino acids, such as fermented fish, yogurt, cheese, and fermented sausage. Eight BAs (tryptamine, 2-phenylethylamine, putrescine, cadaverine, histamine, tyramine, spermidine, and spermine) have been detected in 120 sausage samples collected from the local markets of Van by HPLC (Ekici & Omer, Citation2018). Appropriate intake of BA can modulate a variety of physiological functions, such as metabolic vitality enhancement and intestinal function regulation, but excessive ingestion of BA can cause headache, nausea, vomiting, and other adverse physiological responses (Hernandez-Orte et al., Citation2008). Many studies have indicated that the beneficial microorganisms or starter culture can decrease the accumulation of BA and inhibit the growth of harmful microorganisms (Niu et al., Citation2019). The accumulation of BA has been reported to be significantly reduced by inoculating Staphylococcus equorum S2M7, Staphylococcus xylosus CECT7057, Lactobacillus sakei CV3C2, Lactobacillus sakei CECT7056, and Yeast 2RB4 in fermented sausage (Dias et al., Citation2020). Xie et al. (Citation2015) also reported that inoculating mixed starter can effectively reduce the contents of tryptamine (by 100%), phenylethylamine (100%), putrescine (86%), cadaverine (63%), histamine (82%), and tyramine (43%) in fermented sausage.
Essential oil is complex mixtures of aromatic and volatile liquids frequently distilled from plant, and it has distinctive flavors, antioxidation, and antibacterial effects (Khan et al., Citation2019), therefore it has been widely used in traditional food production (Sojic et al., Citation2019). The application of the plant essential oils to fermented sausage as natural additives has been widely investigated. For example, plant extracts (cinnamon, clove, and ginger) were added to fermented sausage, resulting in the significantly reduced accumulation of BA (Lu et al., Citation2015). Mah et al. (Citation2009) found that addition of garlic to fermented fish led to a significant decrease in the contents of putrescine, cadaverine, histamine, tyramine, and spermidine (p < .05).
High throughput sequencing technologies can generate a tremendous amount of sequencing data, and detect the complex microbial communities. The sequenced reads of the amplicons were clustered into operational taxonomic units (OTUs) based on sequence similarity, and then the relative abundances and read counts were evaluated (Troll et al., Citation2020). Based on OTUs, the microbial diversity and species abundance were examined (Soon et al., Citation2013). This technology exhibits advantages such as easy availability, rapidness, and preciseness (Singer et al., Citation2020). It can also detect microorganisms with low survival rate and those which cannot be grown in culture medium.
This study is aimed to explore the inhibition of spoilage microorganisms and the control of BA by adding tea polyphenols and essential oils of cinnamon, ginger, and anise during fermented (pork) sausage production.
2. Materials and methods
2.1. Materials
Cinnamon essential oils (CEO), ginger essential oils (GEO), and anise essential oils (AEO) were purchased from Zhongjing Food Co., Ltd., China. Tea polyphenol (TP) was purchased from Zhejiang Orient Tea Development Co., Ltd., China. de Man Rogosa and Sharpe (MRS) agar, mannitol salt agar (MSA), and iron agar medium were purchased from Aoboxing Mcrobial Company (Beijing, China). The pork were obtained 24 h after slaughter from local markets in TaiGu, China. The starter culture mainly contained Staphylococcus xylosus, Staphylococcus carnosus, Pediococcus pentosaceus, and Pediococcus acidilactici (Danisco Co., Ltd., Kunshan, Jiangsu, China) with the total bacteria content of 12 lg CUF/g.
2.2. Fermented sausage preparation
Fermented sausage was made according to the previously reported method with slight modification (Dias et al., Citation2020). Fermented sausage was prepared at the Meat Laboratory Center of Shanxi Agricultural University of China. The lean pork was minced through mincer (8 mm plate, Shaoguan Xintongli Foodstuff Machinery Co., Ltd., China, Model: MM-12) and the fat were cut into about 5 mm cubes. Fermented sausage formulation mainly included the lean pork and back fat at the ratio of 8:2 with the addition of 5% sugar, 2% salt, 0.015% nitrite, 0.2% compound phosphate 0.05% sodium isoascorbate. Subsequently, TP, GEO, CEO, or AEO were individually added into the sausage mixture. All the treatments were divided into five groups: a control batch without TP and plant essential oils (PEO) (batch PREC), a batch with additional 0.3% GEO (batch PRE1), a batch with additional 0.3% AEO (batch PRE2), a batch with additional 0.3% CEO (batch PRE3), and a batch with additional 0.3% TP (batch PRE4). Afterwards, the starter cultures were inoculated into the mixture with the final bacteria concentration of 107 CFU/g (based on the weight of meat). After a thorough mixing, the mixture was stuffed into natural pork casing (Far-Eastern Casing Company, Hebei, China) with a diameter of approximately 4 cm. The final sausage had a length of 8 ± 1 cm and a weight of 90 ± 10 g. All the sausages were fermented in constant temperature and humidity incubator (Yuejin medical apparatus factory, Shanghai, China, Model: LRHS-150) at 30°C and 90% humidity until pH dropped to 5.1, and then ripened in constant temperature and humidity incubator at 15 ± 1°C and 70–75% humidity until water activity dropped to 0.81. It took 10 days for ripening.
2.3. Determination of pH
The pH value was determined using an electronic pH meter (Meter Toledo, Columbus, USA). The 5 g sausage sample was homogenized with 20 mL deionized water by high-speed shearing (Fluko Machinery Manufacturing Co., Ltd., Shanghai, China, Model: FA25) (Wang, Zhou, et al., Citation2018).
2.4. Microbial counting
Using aseptic techniques, 10 g sample of fermented sausage was homogenized in 90 mL sterile physiological saline (0.85%) and shaken for 30 min. The suspension was serially diluted (1:9) with sterile physiological saline 0.85% to obtain a series of diluents (1, 10−1, 10−2, 10−3, 10−4, 10−5, 10−6, 10−7). One milliliter appropriate dilution sample was spread onto the agar plates (Wang, Zhang, et al., Citation2018)(21). The lactic acid bacteria (LAB), Staphylococcus, and H2S-producing bacteria were cultivated on MRS, MSA and iron agar medium, respectively, at 30°C for 48 h. After cultivation, the bacteria on selection plates with colony number ranging from 30 to 300 cfu/g were counted. The counting results were converted into the number of colonies per unit sample and expressed as lg (cfu/g). All the media were purchased from Aoboxing Mcrobial Company (Beijing, China).
2.5. Nitrite determination
Nitrite content was determined according to the previously reported method (Paika & Lee, Citation2014). The 5 g sample of fermented sausage was minced using meat grinder and placed into conical flask, and 12.5 mL saturated borax solution was added, then 150 mL distilled water (70°C) was added. The mixture was subjected to boiling water bath for 15 min, and then cooled to the room temperature. Five mL of K4Fe(CN)6 · 3H2O solution (106 g/L) was added and the mixture was shaken, and then 5 ml of Zn (CH3COO)2 · 2H2O solution (220 g/L) was added. Subsequently, the mixture was added with distilled water until the final volume reached 200 mL. The mixture was filtered with a filter paper (20 µm pore-size, Whatman, Mosu Scientific Equipment Co., China), and then the filtrate was collected. Two millilitres of p-aminobenzenesulfonic acid (4 g/L) was added to the filtrate of fermented sausage (40 mL). The mixture was stood for 5 min, then 1 mL of N-ethylenediamine Standard Solution (2 g/L) was added to the mixture. The mixture was stood for 15 min at room temperature. Absorbance was measured at 538 nm using a spectrophotometer (Shanghai Jinghua Technology Instrument Co., Ltd, China, Model: 723). A series of concentrations of standard nitrate was used to build standard curve.
2.6. BA determination
Standard solutions (0, 2.5, 5, 10, 20, and 40 mg/mL) of histamine, putrescine, cadaverine, and tyramine (Sigma-USA) were prepared used 0.4 M perchloric acid, and stored at 4°C. BA content was determined according to a previously reported method with slight modification (Mozuriene et al., Citation2016). The 5 g sample was homogenized in 20 mL 0.4 M perchloric acid for 4 min using Homogenizer (Ultra-Turrax FA25, FLUKO Company, Shanghai, China). Then, the mixture was centrifuged at 4°C, 2500 × g for 10 min using Refrigerated Centrifuge (Thermo Fisher company, USA). The supernatant was collected with 50 mL perchloric acid (0.4 M). One mL aqueous extract of fermented sausage was mixed with 200 μL Sodium hydroxide (2 M) to prepare the alkaline solution. The 300 μL of saturated sodium bicarbonate solution was added for buffering, and then 2 mL of dansyl chloride (10 mg/mL) were added. Reaction was conducted for 30 min at 40°C in the dark, and then 100 μL ammonia was added to stop the reaction. Finally, the mixture was adjusted to 5 mL with acetonitrile and centrifuged for 3 min at 4°C, 2500 × g. The final mixture was used for HPLC analysis.
BA was analyzed using high-performance liquid chromatograph (Agilent, USA) with C18 column (5 um, 4.6 mm × 150 mm, Agilent, USA) at 254 nm. The elution was performed with acetonitrile (solvent A) and water (solvent B). The gradient elution was performed as followed, 0–5 min, 65% A; 5–20 min, 70% A; 20–25 min, 100% A; and 25–30 min, 65% A. The flow rate was 1 mL/min.
2.7. DNA extraction, PCR amplification, and high-throughput sequencing
The bacterial genomic DNA of fermented sausage was extracted using the CTAB. The 1000 μL CTAB lysate (Nobleryder, China) was injected into 2.0 mL centrifuge tube. The adequate amount of sample was added, and then the 20 μL Lysozyme RT401 (TIANGEN, China) was added to fully lyse the bacteria in 70°C water bath pot. After 2 h water bath, 950 μL supernatant was harvested by centrifugation (12000 rpm, 10 min), and then equal volume of phenol-chloroform-isoamyl alcohol mixture (25:24:1) was added, then the mixture was centrifugated at 12000 rpm for 10 min. The supernatant was harvested, and an equal volume of chloroform: isoamyl alcohol (24:1) mixture was added. The mixture was centrifugated at 12000 rpm for 10 min. The supernatant was transferred to 1.5 mL centrifuge tube. Three quarters of the volume (supernatant) of isopropanol was added to precipitate DNA at −20°C, and the mixture solution was centrifuged at 2000 rpm for 10 min, the supernatant was removed, and the remaining precipitate was washed twice with 1 mL 75% ethanol, and blow dried in super workbench. The 51 μL ddH2O was added to dissolve the DNA sample, and then 1 μL RNase A (TIANGEN, China) was added to digest RNA. Finally, the mixture was placed at 37°C for 15 min.
The V3-V4 region of 16S rRNA was amplified by PCR (T100, Bio-rad, USA) using primers 341 F (5ʹ-CCTAYGGGRBGCASCAG-3ʹ) and 806 R (5ʹ-GGACTACNNGGGTATCTAAT-3ʹ). PCR was carried out in a system with a total volume of 50 μL containing 10 μL gDNA, 25 μL PCR Premix, 2 μL primers, 13 μL ddH2O. The PCR amplification was conducted as follows: predenaturation at 95°C for 5 min, 34 cycles of 94°C for 1 min, 57°C for 45 S, and 72°C for 1 min, and final extension at 72°C for 10 min, 16°C for 5 min. The PCR products (10 μL) were analyzed by agarose gel electrophoresis (Beijing Liuyi Biotechnology Co., Ltd., China, Model: DDY6C) to determine the size and number of the PCR amplicons. The Ion Plus Fragment Library Kit 48 rxns (Thermo Scientific, USA) was used for library construction. The resultant samples were sequenced with high-throughput sequencer (LonS5TMXL lon 530 Chip, Thermofisher, USA).
The raw sequence data were analyzed using the Quantitative Insights Into Microbial Ecology. The sequences were spliced and quality-controlled using FLASH (version 0.36) and Trimomatic (version 0.36). The sequences were clustered according to 97% similarity with Usearch, and single sequences and inclusions were removed during the clustering process. Alpha diversity (ACE, chao, Simpson and Shannon index) was evaluated based on OUTs using Mothur v 1.30.0. The heatmap analysis was performed using R packages (v3.2.0).
2.8. Statistical analysis
All the experiments in this study were conducted in triplicates. Data were analyzed using a Mixed model (SAS version 9.1; SAS Institute, Cary, NC). The data are expressed as mean ± SD. Statistical analyses were performed using the SPSS 14.0 (SPSS Inc., Chicago, IL, USA). p value < .05 was considered as statistically significant. Origin 8 was used to plot results.
3. Results and discussion
3.1. pH
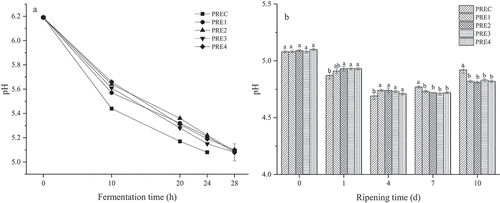
As shown in ), the pH in all the groups was significantly decreased during the fermentation. The reason for this might lie in that the LAB from the starter culture acted on carbohydrates such as endogenous or added sugars to decompose them into lactic acid, acetic acid, and/or other organic acids, resulting in a decrease in pH, and low pH can effectively inhibit the growth of miscellaneous bacteria. Similar results were observed in the study by Hu et al. (Citation2020). The pH value in control group was continuously decreased during the fermentation until it dropped to 5.08 at 24 h when fermentation ended. However, four treatment groups (PRE1, PRE2, PRE3, and PRE4) reached the end of fermentation at 28 h with their fermentation time longer than that of control group (24 h), which might be due to the fact that TP, AEO, GEO, and CEO inhibited the growth of LAB, slowing down the drop in pH. As shown in ), the pH during the ripening process dropped slowly. This might be attributed to the fact that the ripening temperature (15°C) was significantly lower than the fermentation temperature (30°C), resulting in slow growth of some microorganisms and inhibition of acid production capacity. Until the 7th day, the pH value began to rise, which might be mainly due to the water evaporation during the ripening process, the use of nitrogen-containing compounds as energy by LAB, and the generation of alkaline buffer substances such as biogenic amines after protein decomposition by protease (Ruiz-Capillas et al., Citation2007). The pH in control group was significantly higher than that in the treatment groups at end of ripening process (P < .05), which might be explained by the possibility that TP might inhibit the growth of amine-producing bacteria (Zhang et al., Citation2017).
Figure 1. Effect of plant extracts on pH during the fermentation (a) and ripening (b).
Figura 1. Efecto de extractos vegetales sobre el pH durante la fermentación (a) y la maduración (b)
3.1.1. Nitrite analysis
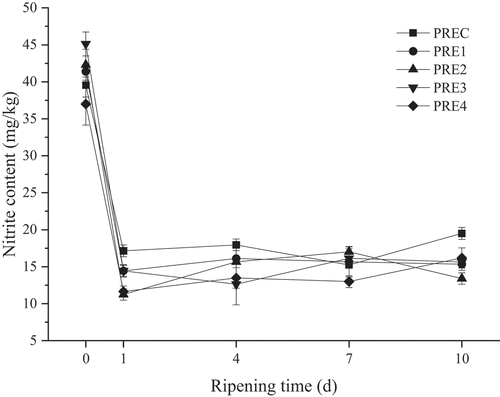
Nitrite has antibacterial and coloring effects in the production process of meat products. However, nitrite can promote the formation of N-nitrosamines, which poses a potential risk for meat product processing (Herrmann et al., Citation2015). The change of nitrite content is shown in . The nitrite contents in five groups ranged from 37.00 mg/kg to 45.12 mg/kg at the beginning of ripening. After one-day ripening, the residual nitrite contents in five groups were decreased rapidly to the range of 11.24 mg/kg to 17.14 mg/kg. Similar results were observed in the study by Wang et al. (Citation2013). At the end of ripening, the content of nitrite in treatment groups were less than 16.50 mg/kg, which were lower than that in the control group (19.50 mg/kg). The content of nitrite among the fermented sausage maximum residue limit of 30 mg/kg according to the National Food Safety Standard ‘GB/T 2760–2014ʹ. Our data indicated that the addition of TP, AEO, GEO, and CEO can reduce the nitrite content. This might be due to the strong reducing power of TP, AEO, GEO, and CEO as natural antioxidants, and they acted as reducing agents and played a role in effectively converting nitrite to nitrous acid. Nitrous acid could further generate nitric oxide to combine with myoglobin, thereby reducing residual nitrite level (Viuda-Martos et al., Citation2009). Plant extracts have antioxidation, thus they have an elimination effect on nitrite (Lucia et al., Citation2005). One previous study has revealed that the addition of 1% citrus dietary fiber and 0.02% plant essential oil results in the decrease by 56% in the nitrite content (Viuda-Martos et al., Citation2010). Another study has found that the addition of tea polyphenols and grape seed extracts to fermented sausage leads to the significant reduction in the content of nitrite (p < .05) (Vogel et al., Citation2005).
3.1.2. BA analysis
Cadaverine, putrescine, tyramine, and histamine are the four main BAs (Lu et al., Citation2010). According to the Food and Drug Administration (FDA), the content of BA maximum residue limit of 1000 mg/kg in food. Excessive intake of BA can pose certain risks to consumers. The contents of BA have been associated with microbial growth (Suzzia & Gardinib, Citation2003). In this study, the contents of these four BAs were determined at the end of ripening ().
Figure 3. Accumulation of four biogenic amines at the end of ripening. Different letters within a column represent significant difference (p < .05).
Figura 3. Acumulación de cuatro aminas biógenas al final de la maduración. Las distintas letras dentro de una columna indican una diferencia significativa (p < .05)
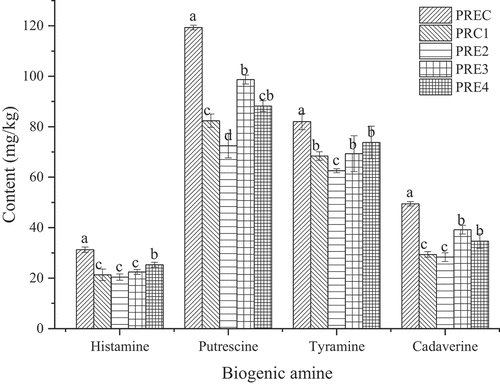
Histamine is one of the BA of the most toxic and the greatest hazard to consumers health, which can cause many adverse symptoms after excessive intake, such as headache (Zhang et al., Citation2017). Our data indicated that histamine content was 21.31 mg/kg, 20.42 mg/kg, 22.46 mg/kg, and 25.34 mg/kg at the end of ripening in the treatment groups PRE1, PRE2, PRE3, and PRE4, respectively, which was significantly (p < .05) lower than that in the control group PREC (31.29) mg/kg. Therefore, it could be concluded that TP, AEO, GEO, and CEO can inhibit the formation of histamine. Our results were similar to Cai et al. (Citation2015) who reported that the histamine content was reduced by 86.29% (clove oil), 90.52% (cumin oil), and 93.45% (spearmint oil), by adding essential oils to red drum (Sciaenops ocellatus) fillets. Similarly, Ozogul et al. (Citation2011) found that the addition of the extracts of rosemary and sage tea significantly inhibited the formation of histamine in sardine muscle. The maximum residue limit of 50 mg/kg according to the FDA. In our study, the content of histamine in fermented sausage is less than 50 mg/kg, which is in compliance with FDA standards, especially the treatment groups.
Tyramine is formed from tyrosine through the action of decarboxylase. In this study, tyramine content was 68.41 mg/kg (PRE1), 62.57 mg/kg (PRE2), 69.31 mg/kg (PRE3), and 73.76 mg/kg (PRE4) at the end of ripening, which exhibited a reduction by 16.64%, 23.76%, 15.55%, and 10.12%, respectively, compared with control group PREC (82.07 mg/kg). Thus, it could be concluded TP, AEO, GEO, and CEO could inhibit the formation of Tyramine. Likewise, Sun et al. (Citation2018) found that the addition of spice extracts (cinnamon, clove, and anise) could inhibit the tyramine formation.
Putrescine can enhance the toxicity of histamine and tyramine (Renes et al., Citation2014). Compared with the other three BAs, putrescine was the highest content in fermented sausage. The content of putrescine in four treatment groups was significantly lower than that in the control group PREC. Especially in the PRE2 treatment group, the content of putrescine was decreased by 39.31%, compare with that in the PREC control group. Our result was in line with one previous study finding that the addition of grape seed extract to Chinese traditional smoke-cured bacon could inhibit accumulation of putrescine (Wang, Zhang, et al., Citation2018).
Cadaverine is formed from lysine through the action of decarboxylase producted by microorganisms, and it can be used as an indicator of food hygiene (Li et al., Citation2014). It is a common BA in fermented sausage. In this study, the cadaverine content was 49.42 mg/kg, 29.39 mg/kg, 28.33 mg/kg, 39.20 mg/kg, 34.68 mg/kg at the end of ripening in the groups PREC, PRE1, PRE2, PRE3, and PRE4, respectively. The content of cadaverine in control group was significantly higher than that in 4 treatment groups (p < .05), indicating that cadaverine content was reduced by adding TP, AEO, GEO, and CEO. Our results were consistent with Mah et al. (Citation2009) findings that the addition of ginger and red pepper extracts significantly reduced cadaverine content in fermented anchovy. In this study, the maximal inhibition effect on cadaverine formation was observed in PRE2 treatment group with an addition of 0.3% AEO to fermented sausage.
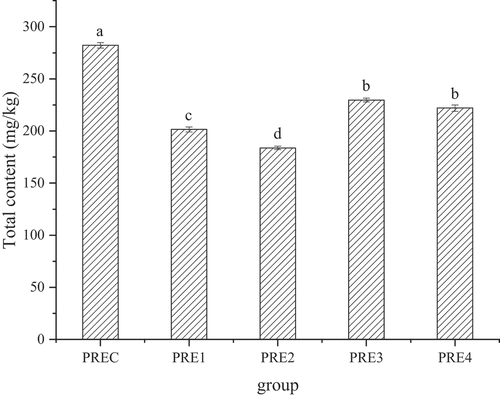
The total content of four BAs was different in five groups (). The total content of BA in control group (282.10 mg/kg) was significantly higher than that in all the 4 treatment groups (PRE1(201.48 mg/kg), PRE2(183.74 mg/kg), PRE3(229.68 mg/kg), PRE4 (221.99 mg/kg)) (p < .05). Therefore, adding TP, AEO, GEO and CEO to fermented sausage could significantly reduce the total content of BA. BA was formed by decarboxylation of free amino acids (Ruiz-Capillas et al., Citation2007) . The formation of BA closely associated with microorganisms (Durlu-Özkaya et al., Citation2001). Lactobacillus, Pseudomonas and Shewanella can secrete high-activity amino acid decarboxylase to produce BA during the storage of meat (Vogel et al., Citation2005). Similarly, the Enterobacteriaceae could promote the formation of tyramine, putrescine, cadaverine, and histamine (Durlu-Özkaya et al., Citation2001). Previous study has showed that the biogenic amine formation was inhibited by the antibacterial activity of plant extracts. For example, tea polyphenol suppressed the BA formation by inhibiting the growth of BA-producing bacteria (Fan et al., Citation2015). Lu et al. (Citation2015) found that plant extracts (cinnamon, clove, ginger, and fennel) effectively inhibited the growth of BA-producing bacteria, thus reducing biogenic amine content in fermented sausage. Mah et al. (Citation2009) demonstrated that the content of putrescine, cadaverine, histamine, tyramine and spermidine were reduced by up to 11.2%, 18.4%, 11.7%, 30.9% and 17.4%, respectively, compared to control in fermented anchovy.
Figure 4. The total accumulation of four total biogenic amines at the end of ripening. Different letters within a column represent significant differences (p < .05).
Figura 4. Acumulación total de cuatro aminas biógenas al final de la maduración. Las distintas letras dentro de una columna indican una diferencia significativa (p < .05)
3.1.3. Microbial counting
The change of microbial number in fermented sausage during the ripening process are shown in . The numbers of LAB and Staphylococcus were 8.61 log10 CFU g−1 and 6.99 log10 CFU g−1, respectively, in PREC at the end of ripening. The growth of aerobic bacteria was rapid during the early stage of ripening, which might be due to high water activity and appropriate pH. The number of Staphylococcus was significantly lower than that of LAB in all five groups, which might be attributed to the fact that the lactic acid and bacteriocins produced by LAB were unfavorable for Staphylococcus proliferation, or the fact that there were less Staphylococcus than LAB in starter culture. The growth of LAB accelerated the decrease in pH to ensure the safe production of sausage. The numbers of LAB and Staphylococcus in control group were higher than those in the treatment groups at the end of ripening.
Table 1. The microbial contents (log10 cfu g−1) (mean±standard deviation) during ripening of fermented sausage. Different letters within a column mean significant difference significantly (p < .05).
Tabla 1. Contenido microbiano (log10 cfu g−1) (media±desviación estándar) durante la maduración de embutidos fermentados. Las distintas letras dentro de una columna indican una diferencia significativa (p < .05)
H2S-producing bacteria such as Enterobacteriaceae and Pseudomonas are common spoilage microorganisms in meat. Enterobacteriaceae and Pseudomonas can secrete a high-activity lysine and ornithine decarboxylase, and they are main bacteria to produce BA. The number of H2S-producing bacteria decreased from 3.61 log10 CFU g−1 to 2.62 log10 CFU g−1 in PREC control group, and it was higher than that in the treatment groups at the end of ripening, indicating that TP, GEO, CEO, and AEO had an inhibitory effect on H2S-producing bacteria. This might be due to the fact that the plant extract could enter the cell wall, and cell membrane to destroy the structure of the cell membrane, increase the permeability of the membrane, leading to the leakage of cell contents, eventually destroying bacterial structure (Gonelimali et al., Citation2018). Our results were consistent with many previous study findings that coptis chinensis extracts and laurel essential oil significantly decreased the Enterobacteriaceae number in fermented sausage (p < .05) (Botsaris et al., Citation2015), and that the addition of plant extracts (cinnamon, clove, and ginger) significantly reduced (p < .05) the numbers of Enterobacteriaceae and Pseudomonas in the fermented sausage (Lu et al., Citation2015).
3.1.4. Bacterial diversity
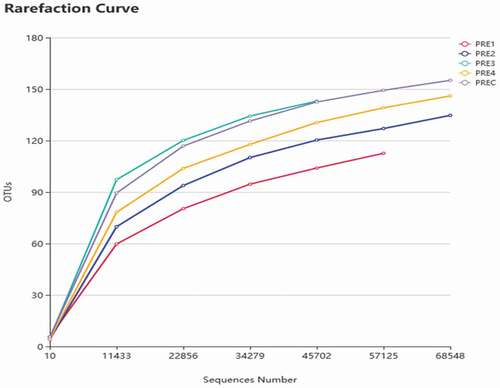
As shown in , rarefaction curves of all samples reached a plateau at approximately 11433 sequences, indicating that the sequencing depth was sufficient to capture an acceptable number of observed bacterial species, thus the sequencing data were reasonable.
Figure 5. Rarefaction curve for the bacterial community of fermented sausage samples.
Figura 5. Curva de rarefacción de la comunidad bacteriana de las muestras de salchichas fermentadas
Diversity indices (Simpson and Shannon) and community abundance indices (Ace and Chao1) were calculated to evaluate the alpha-diversity of bacterial communities. shows the abundance and diversity of bacterial communities in five groups. Some obvious differences were observed. Among all the groups, PRE3 treatment group exhibited the highest ACE, Chao1 and Shannon indices and the lowest Simpson index, indicating that the bacterial abundance diversity was the highest in PRE3. The bacterial abundance and diversity in PRE1, PRE2, and PRE4 treatment groups were lower than those in PREC control group. Therefore, the abundance and diversity of bacteria were changed by adding TP, AEO, GEO, and CEO in fermented sausage. Zhang et al.’s (Citation2017) study has shown that the abundance and diversity of bacteria were changed by adding 0.3% rose polyphenols to fermented sausage. Lu et al. (Citation2015) reported that plant extracts (cinnamon, clove, ginger, and fennel) effectively inhibited the growth of spoilage bacteria, such as Pseudomonas and Enterobacter.
Table 2. Diversity indices for the 16S rDNA sequences of five fermented sausages in the end of ripening. Different letters within a column mean significant difference (p < .05).
Tabla 2. Índices de diversidad para las secuencias de ADNr 16S de cinco salchichas fermentadas al final de su maduración. Las distintas letras dentro de una columna indican una diferencia significativa (p < .05)
3.1.5. Bacterial relative abundance
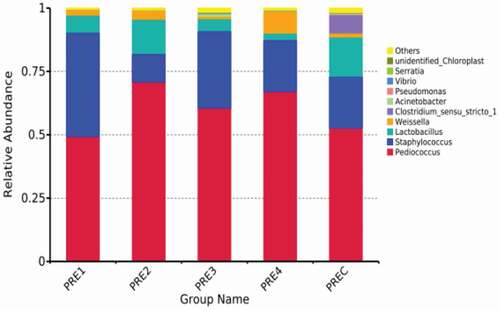
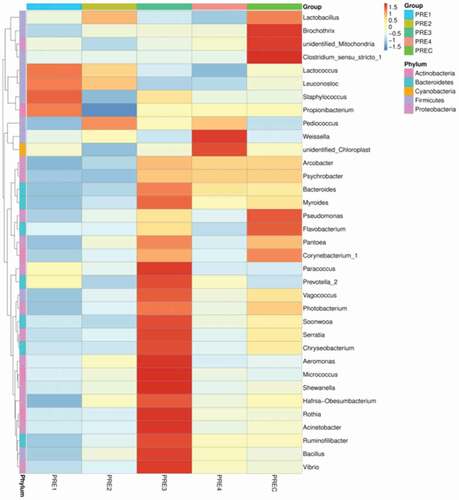
The bacterial relative abundance in fermented sausage at the genus level is shown in . Colored bars indicated the percentage of the relative abundance of different bacterial species in all the species. Heatmap analysis of five groups of fermented sausage showed the similarities and differences in the bacterial community composition (). Pediococcus and Staphylococcus were the dominant bacteria in the five groups. However, there were differences in Pediococcus and Staphylococcus abundance among the five groups. The total abundance of Pediococcus and Staphylococcus in treatment groups higher than that in control group. Pediococcus and Staphylococcus were mainly originated from the starter cultures, and they were carefully selected for controlling the formation of biogenic amines. Lactobacillus, Pseudomonas, Serratia, and Clostridium sensu stricto 1 are also found in which may be originated from raw from raw meat or environment. Pseudomonas is gram-negative psychrophilic bacterium, and one of the main bacteria to cause spoilage for food that rich in protein and fat under warm conditions (Olanya et al., Citation2014). Serratia and Clostridium sensu stricto 1 are common spoilage bacteria in meat products (Akhtar et al., Citation2009). The relative abundance of Lactobacillus in PRE1, PRE2, PRE3, PRE4, and PREC was (6.08 ± 0.02)%, (13.48 ± 0.09)%, (4.79 ± 0.02)%, (2.64 ± 0.01)%, and (16.02 ± 0.17)%, respectively. The relative abundance of Lactobacillus was highest in PREC group. The relative abundance of Pseudomonas in PREC group (0.38%) was significantly higher than that in PRE1 (0.04%), PRE2 (0.12%), PRE3 (0%), and PRE4 (0.12%) groups. The relative abundance of Serratia in PRE4 group (0.03%) and PRE1 (0.02%) group was significantly lower than that in the PREC group (0.08%), and the Serratia was not detected in PRE2 and PRE3 groups. Clostridium sensu stricto 1 was detected only in RREC control group with the relative abundance of 6.93%. Thus, it could be concluded that the growth of Lactobacillus, Pseudomonas, Serratia, and Clostridium sensu stricto 1 could be inhibited by adding TP, AEO, GEO, and CEO to fermented sausage. Previous studies have found that Lactobacillus, Pseudomonas, Serratia, and Clostridium sensu_stricto_1 are common biogenic amine-producing bacteria (De las Rivas et al., Citation2006). Hence, the addition of TP, AEO, GEO, and CEO could inhibit the growth of spoilage microbial, thus improving safety of fermented sausage.
3.1.6. Correlation analysis of bacterial relative abundance and BA content
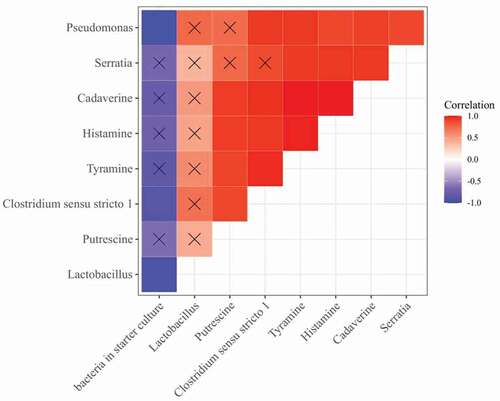
reveals the correlation between the bacterial relative abundance and the content of four BAs. As shown in , there were positive correlations between the relative abundance of Lactobacillus, Pseudomonas, Serratia, Clostridium sensu_stricto_1, and histamine, tyramine, putrescine, cadaverine, respectively. Especially, the correlation coefficient between the relative abundance of Pseudomonas and histamine, tyramine, cadaverine were ranged from 0.89 to 0.94, and the correlation are significant (p < .05), the correlation coefficient between the relative abundance of Serratia and histamine, tyramine, cadaverine were 0.94, 0.93, 0.94, respectively, which were significant (p < .05), the correlation coefficient between the relative abundance of Clostridium sensu_stricto_1 and histamine, tyramine, cadaverine were 0.94, 0.96, 0.97, respectively, which were significant (p < .05), indicating that Lactobacillus, Pseudomonas, Serratia, and Clostridium sensu_stricto_1 could promote the accumulation of BA. The relative abundance of bacteria in starter culture (bacteria of inoculation in fermented sausage) has negative correlations with the BA, which indicated that bacteria in starter culture could inhibit the accumulation of BA. This results similar to Lu et al. (Citation2015) who reported that plant extracts (cinnamon, clove, ginger, and fennel) could prevent the accumulation of BA by inhibiting the growth of Pseudomonas and Enterobacter.
4. Conclusions
In this study, the effect of TP, AEO, CEO, and GEO on BA content and bacterial abundance and diversity in fermented sausage were investigated. The results indicated that the total BAs were respectively reduced by 28.58%, 34.87%, 21.63%, and 21.65% by adding 0.3% GEO, 0.3% AEO, 0.3% CEO, 0.3% TP to fermented sausage. TP, AEO, CEO, and GEO significantly reduced the content of residual nitrite, and the lowest residual nitrite (13.40 mg/kg) was observed in AEO addition group. The addition of TP, AEO, CEO, and GEO inhibited the growth of spoilage bacteria, such as H2S-producing bacteria. Pediococcus and Staphylococcus were the dominant bacteria in all the fermented sausage groups at the end of ripening. The relative abundance of Lactobacillus, Pseudomonas, Serratia, and Clostridium sensu stricto 1 was reduced by adding TP, AEO, CEO, and GEO. These results indicate that TP and PEO (especially AEO) could be used to reduce the accumulation of BA and by inhibiting the growth of spoilage bacterial in fermented sausage, thereby improving the safety of fermented sausage.
Author contribution
Ji Wang, Xiaohong Li, and Yingchun Zhu contributed to the conception of the study; Ji Wang, Jingrong Hu, and Xiaohong Li performed the experiment; Ji Wang, Zhihui Yu, and Yingchun Zhu, contributed significantly to analysis and manuscript preparation; Ji Wang, Xin Zhang, and Jingrong Hu performed the data analyses and wrote the manuscript; Ji Wang, Xin Zhang, Zhihui Yu, and Yingchun Zhu helped perform the analysis with constructive discussions.
Acknowledgments
The authors thank the novogene for the microbial community and diversity analysis. Great gratitude goes to linguistics Prof. Ping Liu from Huazhong Agriculture University, Wuhan, China for her work at English editing and language polishing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akhtar, S., Paredes-Sabja, D., Torres, J. A., Sarker, M. R. (2009). Strategy to inactivate Clostridium perfringens spores in meat products. Food Microbiology, 26(3), 272–277. https://doi.org/http://doi.org/10.1016/j.fm.2008.12.011
- Botsaris, G., Orphanides, A., Yiannakou, E., Gekas, V., & Goulas, V. (2015). Antioxidant and antimicrobial effects of Pistacia lentiscus L. extracts in pork sausages. Food Technology and Biotechnology, 53(4), 472–478. https://doi.org/https://doi.org/10.17113/ftb.53.04.15.4051
- Cai, L. Y., Cao, A. L., Li, Y. C., Song, Z., Leng, L., & Li, J. (2015). The effects of essential oil treatment on the biogenic amines inhibition and quality preservation of red drum (Sciaenops ocellatus) fillets. Food Control, 56, 1–8. https://doi.org/https://doi.org/10.1016/j.foodcont.2015.03.009
- Chen, X., Li, J., Zhou, T., Li, J., Yang, J., Chen, W., & Xiong, Y. L. (2016). Two efficient nitrite-reducing Lactobacillus strains isolated from traditional fermented pork (Nanx Wudl) as competitive starter cultures for Chinese fermented dry sausage. Meat Science, 121, 302–309. https://doi.org/https://doi.org/10.1016/j.meatsci.2016.06.007
- De las Rivas, B., Marcobal, Á., Carrascosa, A. V., & MUÑOZ, R. (2006). PCR detection of foodborne bacteria producing the biogenic amines histamine, tyramine, putrescine, and cadaverine. . Journal of Food Protection, 69(10), 2509–2514. https://doi.org/https://doi.org/10.4315/0362-028X-69.10.2509
- Dias, I., Laranjo, M., Potes, M. E., Agulheiro-Santos, A. C., Ricardo-Rodrigues, S., Fialho, A. R., Véstia, J., Fraqueza, M. J., Oliveira, M., & Elias, M. (2020). Autochthonous starter cultures are able to reduce biogenic amines in a traditional Portuguese smoked fermented sausage. Microorganisms, 8(5), 686. https://doi.org/https://doi.org/10.3390/microorganisms8050686
- Durlu-Özkaya, F., Ayhan, K., & Vural, N. U. (2001). Biogenic amines produced by Enterobacteriaceae isolated from meat products. Meat Science, 58(2), 163–166. https://doi.org/https://doi.org/10.1016/S0309-1740(00)00144-3
- Ekici, K., & Omer, A. K. (2018). The determination of some biogenic amines in Turkish fermented sausages consumed in Van. Toxicology Reports, 5, 639–643. https://doi.org/https://doi.org/10.1016/j.toxrep.2018.05.008
- Fan, W., Yi, Y., Zhang, Y., & Diao, P. (2015). Effect of an antioxidant from bamboo leaves combined with tea polyphenol on biogenic amine accumulation and lipid oxidation in pork sausages. Food Science and Biotechnology, 24(2), 421–426. https://doi.org/https://doi.org/10.1007/s10068-015-0055-6
- Gonelimali, F. D., Lin, J., Miao, W., Xuan, J., Charles, F., Chen, M., & Hatab, S. R. (2018). Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. . Frontiers in Microbiology, 9, 1639. https://doi.org/https://doi.org/10.3389/fmicb.2018.01639
- Han, S.-Y., Hao, -L.-L., Shi, X., Niu, J.-M., & Zhang, B. (2019). Development and application of a new QuEChERS method in UHPLC-QqQ-MS/MS to detect seven biogenic amines in Chinese Wines[J]. Foods, 8(11), 552–569. https://doi.org/https://doi.org/10.3390/foods8110552
- Hernandez-Orte, P., Lapena, A. C., Peña-Gallego, A., Astrain, J., Baron, C., Pardo, I., Polo, L., Ferrer, S., Cacho, J., & Ferreira, V. (2008). Biogenic amine determination in wine fermented in oak barrels: Factors affecting formation. Food Research International, 41(7), 7. https://doi.org/https://doi.org/10.1016/j.foodres.2008.05.002
- Herrmann, S. S., Granby, K., & Duedahl-Olesen, L. (2015). Formation and mitigation of N-nitrosamines in nitrite preserved cooked sausages. Food Chemistry, 174, 516–526. https://doi.org/https://doi.org/10.1016/j.foodchem.2014.11.101
- Hu, Y., Zhang, L., Zhang, H., Wang, Y., Chen, Q., & Kong, B. (2020). Physicochemical properties and flavour profile of fermented dry sausages with a reduction of sodium chloride. LWT - Food Science Technology, 124, 109061. https://doi.org/https://doi.org/10.1016/j.lwt.2020.109061
- Khan, A., Nadeem, M., Bhutto, M. A., Yu, F., Xie, X., El-Hamshary, H., El-Faham, A., Ibrahim, U. A., & Mo, X. (2019). Physico-chemical and biological evaluation of PLCL/SF nanofibers loaded with oregano essential oil. Pharmaceutics, 11(8), 386. https://doi.org/https://doi.org/10.3390/pharmaceutics11080386
- Li, N. Q., Chou, H., Yu, L. J., & Xu, Y. (2014). Cadaverine production by heterologous expression of Klebsiella oxytoca Lysine decarboxylase. . Biotechnology and Bioprocess Engineering, 19(6), 965–972. https://doi.org/https://doi.org/10.1007/s12257-014-0352-6
- Lu, S., Ji, H., Wang, Q., Li, B., Li, K., Xu, C., & Jiang, C. (2015). The effects of starter cultures and plant extracts on the biogenic amine accumulation in traditional Chinese smoked horsemeat sausages. Food Control, 50, 869–875. https://doi.org/https://doi.org/10.1016/j.foodcont.2014.08.015
- Lu, S., Xu, X., Zhou, G., Zhu, Z., Meng, Y., & Sun, Y. (2010). Effect of starter cultures on microbial ecosystem and biogenic amines in fermented sausage. Food Control, 21(4), 444–449. https://doi.org/https://doi.org/10.1016/j.foodcont.2009.07.008
- Lucia, P., Manini, P., Napolitano, A., & d’Ischia, M. (2005). The acid-promoted reaction of the green tea polyphenol epigallocatechin gallate with nitrite ions. . Chemical Research in Toxicology, 18(4), 722–729. https://doi.org/https://doi.org/10.1021/tx0496486
- Mah, J.-H., Kim, Y. J., & Wang, H.-J. (2009). Inhibitory effects of garlic and other spices on biogenic amine production in Myeolchi-jeot, Korean salted and fermented anchovy product. Food Control, 20(5), 449–454. https://doi.org/https://doi.org/10.1016/j.foodcont.2008.07.006
- Mozuriene, E., Bartkiene, E., Krungleviciute, V., Zadeike, D., Juodeikiene, G., Damasius, J., & Baltusnikiene, A. (2016). Effect of natural marinade based on lactic acid bacteria on pork meat quality parameters and biogenic amine contents. LWT - Food Science and Technology, 69, 319–326. https://doi.org/https://doi.org/10.1016/j.lwt.2016.01.061
- Niu, T. J., Li, X., Guo, Y. J., & Ma, Y. (2019). Identification of a lactic acid bacteria to degrade biogenic amines in Chinese rice wine and its enzymatic mechanism. Foods, 8(8), 312. https://doi.org/https://doi.org/10.3390/foods8080312
- Olanya, O. M., Ukuku, D. O., & Niemira, B. A. (2014). Effects of temperatures and storage time on resting populations of Escherichia coli O157:H7 and Pseudomonas fluorescens in vitro. Food Control, 39, 128–134. https://doi.org/https://doi.org/10.1016/j.foodcont.2013.11.006
- Ozogul, F., Kenar, M., & Kuley, E. (2011). Effects of rosemary and sage tea extract on biogenic amines formation of sardine (Sardina pilchardus) fillets. International Journal of Food Science & Technology, 46(4), 761–766. https://doi.org/https://doi.org/10.1111/j.1365-2621.2011.02560.x
- Paika, H.-D., & Lee, J.-Y. (2014). Investigation of reduction and tolerance capability of lactic acid bacteria isolated from kimchi against nitrate and nitrite in fermented sausage condition. Meat Science, 97(4), 609–614. https://doi.org/https://doi.org/10.1016/j.meatsci.2014.03.013
- Renes, E., Diezhandino, I., Fernandez, D., Ferrazza, R. E., Tornadijo, M. E., & Fresno, J. M. (2014). Effect of autochthonous starter cultures on the biogenic amine content of ewe’s milk cheese throughout ripening. Food Microbiology, 44, 271–277. https://doi.org/https://doi.org/10.1016/j.fm.2014.06.001
- Ruiz-Capillas, C., Colmenero, F. J., Carrascosa, A. V., & Muñoz, R. (2007). Biogenic amine production in Spanish dry-cured “chorizo” sausage treated with high-pressure and kept in chilled storage. Meat Science, 77(3), 365–371. https://doi.org/https://doi.org/10.1016/j.meatsci.2007.03.027
- Singer, D., Duckert, C., Hedenec, P., Lara, E., Hiltbrunner, E., & Mitchell, E. A. D. (2020). High-throughput sequencing of litter and moss eDNA reveals a positive correlation between the diversity of Apicomplexa and their invertebrate hosts across alpine habitats. Soil Biology and Biochemistry, 147, 107837. https://doi.org/https://doi.org/10.1016/j.soilbio.2020.107837
- Sojic, B., Pavlic, B., Tomovic, V., Ikonić, P., Zeković, Z., Kocić-Tanackov, S., Đurović, S., Škaljac, S., Jokanović, M., & Ivić, M. (2019). Essential oil versus supercritical fluid extracts of winter savory (Satureja Montana L.) – Assessment of the oxidative, microbiological and sensory quality of fresh pork sausages. Food Chemistry, 287, 280–286. https://doi.org/https://doi.org/10.1016/j.foodchem.2018.12.137
- Soon, W. W., Hariharan, M., & Snyder, M. P. (2013). High‐throughput sequencing for biology and medicine. Molecular Systems Biology, 9(1), 640. https://doi.org/https://doi.org/10.1038/msb.2012.61
- Sun, Q. X., Zhao, X. X., Chen, H. S., Zhang, C., & Kong, B. (2018). Impact of spice extracts on the formation of biogenic amines and the physicochemical, microbiological and sensory quality of dry sausage. Food Control, 92, 190–200. https://doi.org/https://doi.org/10.1016/j.foodcont.2018.05.002
- Suzzia, G., & Gardinib, F. (2003). Biogenic amines in dry fermented sausages: A review. International Journal of Food Microbiology, 88(1), 41–54. https://doi.org/https://doi.org/10.1016/S0168-1605(03)00080-1
- Troll, M., Brandmaier, S., Reitmeier, S., Adam, J., Sharma, S., Sommer, A., Bind, M.-A., Neuhaus, K., Clavel, T., Adamski, J., Haller, D., Peters, A., & Grallert, H. (2020). Investigation of adiposity measures and Operational Taxonomic unit (OTU) data transformation procedures in stool samples from a German Cohort study using machine learning algorithms. Microorganisms, 8(4), 547. https://doi.org/https://doi.org/10.3390/microorganisms8040547
- Viuda-Martos, M., Ruiz-Navajas, Y., Fernández-López, J., & Pérez-Álvarez, J. A. (2009). Effect of adding citrus waste water, thyme and oregano essential oil on the chemical, physical and sensory characteristics of a Bologna sausage. . Innovative Food Science & Emerging Technologies, 10(4), 655–660. https://doi.org/https://doi.org/10.1016/j.ifset.2009.06.001
- Viuda-Martos, M., Ruiz-Navajas, Y., Fernández-López, J., & Pérez-Álvarez, J. A. (2010). Effect of added citrus fibre and spice essential oils on quality characteristics and shelf-life of mortadella. Meat Science, 85(3), 568–576. https://doi.org/https://doi.org/10.1016/j.meatsci.2010.03.007
- Vogel, B. F., Venkateswaran, K., Satomi, M., & Gram, L. (2005). Identification of Shewanella baltica as the most important H2 S-producing species during iced storage of Danish marine fish. Applied and Environmental Microbiology, 71(11), 6689–6697. https://doi.org/https://doi.org/10.1128/AEM.71.11.6689-6697.2005
- Wang, X., Zhou, P., Cheng, J., Chen, Z., & Liu, X. (2018). Use of straw mushrooms (Volvariella volvacea) for the enhancement of physicochemical, nutritional and sensory profiles of Cantonese sausages. Meat Science, 146, 18–25. https://doi.org/https://doi.org/10.1016/j.meatsci.2018.07.033
- Wang, X. H., Ren, H. Y., Liu, D. Y., Zhu, W. Y., & Wang, W. (2013). Effects of inoculating Lactobacillus sakei starter cultures on the microbiological quality and nitrite depletion of Chinese fermented sausages. Food Control, 32(2), 591–596. https://doi.org/https://doi.org/10.1016/j.foodcont.2013.01.050
- Wang, X. H., Zhang, Y. L., & Ren, H. Y. (2018). Effects of grape seed extract on lipid oxidation, biogenic amine formation and microbiological quality in Chinese traditional smoke-cured bacon during storage. Journal of Food Safety, 38(2), 2–8. https://doi.org/https://doi.org/10.1111/jfs.12426
- Xie, C., Wang, H. H., Nie, X. K., Chen, L., Deng, S. L., Xu, X. L. (2015). Reduction of biogenic amine concentration in fermented sausage by selected starter cultures. CyTA - Journal of Food, 13(4), 491–497. https://doi.org/http://doi.org/10.1080/19476337.2015.1005027
- Yu, -H.-H., Choi, J. H., Kang, K. M., & Hwang, H.-J. (2017). Potential of a lactic acid bacterial starter culture with gamma-aminobutyric acid (GABA) activity for production of fermented sausage. Food Science and Biotechnology, 26(5), 1333–1341. https://doi.org/https://doi.org/10.1007/s10068-017-0161-8
- Zhang, Q. Q., Jiang, M., Rui, X., Li, W., Chen, X. H., & Dong, M. S. (2017). Effect of rose polyphenols on oxidation, biogenic amines and microbial diversity in naturally dry fermented sausages. Food Control, 78, 324–330. https://doi.org/https://doi.org/10.1016/j.foodcont.2017.02.054