 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this research was to evaluate the polyphenolic compounds, the antioxidant activity, and the individual identification of the different types of phenolic compounds in dried extracts of Opuntia atropes. O. atropes extract was dehydrated in a drying oven at 50°C/24 h and by nano-spray drying at 105°C. Drying by nano-spray did not affect the content of phenolic compounds or the antioxidant activity of the O. atropes extract, presenting values of 11.80 ± 0.61 mg of gallic acid equivalent/g of phenolic acids, 22.26 ± 1.02 mg quercetin equivalent/g of flavonoids and inhibition percentage of the radical DPPH• (2,2-. diphenil-1-picrilhidrazil) and ABTS•+ (2,2ʹAzinobis(3-ethylbenzothiazoline-6 sulfonic acid)) of 17.92 ± 0.31 and 54.08 ± 0.48, respectively. In the powdered extracts of O. atropes, glycosides of kaempferol and isorhamnetin were mainly identified by ultra high-performance liquid chromatography coupled to mass spectrometry. Mediorresinol lignan was identified for the first time in this Opuntia species.
RESUMEN
El objetivo de esta investigación fue evaluar los compuestos polifenólicos, la actividad antioxidante y la identificación individual de los diferentes tipos de compuestos fenólicos en extractos secos de Opuntia atropes. El extracto de O. atropes se deshidrató en un horno de secado a 50°C/24 h y mediante secado por nano-pulverización a 105°C. El secado por nano-pulverización no afectó el contenido de compuestos fenólicos ni la actividad antioxidante del extracto de O. atropes, presentando valores de 11.80 ± 0.61 mg EAG (equivalentes de ácido gálico)/g de ácidos fenólicos, 22.26 ± 1.02 mg EQ (equivalentes de quercetina)/g de flavonoides y porcentaje de inhibición del radical DPPH• (2,2-difenil-1-picrilhidrazilo) y ABTS• + (ácido 2, 2ʹ Azinobis-3-etil- benzotiazolin-6 sulfónico) de 17.92 ± 0.31 y 54.08 ± 0.48, respectivamente. En los extractos en polvo de O. atropes, se identificaron principalmente los glucósidos de kaempferol e isoramnetina mediante cromatografía líquida de ultra alta resolución acoplada a espectrometría de masas. El lignano de mediorresinol se identificó por primera vez en esta especie de Opuntia.
1. Introduction
The consumption of the different species of Opuntia spp. (Cactaceae family) is highly related to a lower risk of chronic diseases associated with oxidative stress, such as cancer, diabetes, and cardiovascular diseases (Antunes-Ricardo et al., Citation2015; Keller et al., Citation2015; Rodríguez-Rodríguez et al., Citation2015;). These biological effects have related to the anti-inflammatory and antioxidant properties of the polyphenols present in Opuntia spp. (Luca et al., Citation2020; Myint et al., Citation2020). Opuntia Mill., is the most important genus of these Cactaceae and includes many species and varieties. Opuntia ficus-indica (L.) Mill. is the specie most studied worldwide. However, there are other species that are consumed, such as Opuntia atropes Rose, and of which there are few reports (López-Gutiérrez et al., Citation2015; Tranquilino-Rodríguez et al., Citation2020; Valadares et al., Citation2020). Recent studies have shown that cladodes of Opuntia spp. are good candidates for the development of new healthy foods, due to their high content of phenolic-type compounds, such as flavonoids (Serra et al., Citation2013). These physiologically active compounds are present mainly as glycosides and it has been shown that the sugar residues in flavonoids, their number and type, affect their biological activity and are related to differences in bioavailability (Antunes-Ricardo et al., Citation2015). In general, polyphenolic compounds and other antioxidant compounds have become more important economically and its increasingly used in the nutraceutical and cosmetic industries (Marqués et al., Citation2013). In the food sector, a wide range of products enriched with polyphenolic compounds have begun to be developed for the prevention of chronic diseases, which have been very well received by the consumer (Gómez & Martinez, Citation2018; Spina et al., Citation2019). To achieve separation processes, as well as the identification and characterization of bioactive compounds, plant materials must first undergo an extraction process (Gligor et al., Citation2019). Various extraction techniques such as maceration have been used to obtain antioxidants and other bioactive compounds from different parts of the plant (Benattia & Arrar, Citation2018). After extraction, it is recommended to carry out a drying process to increase the stability of the compounds and preserve their shelf life in storage for a longer time (Akbarian et al., Citation2013). The traditional drying process can damage quality by causing oxidative damage, browning, change in taste and solubility. During hot air drying, polyphenols can have enzymatic and non-enzymatic degradation (Abhay et al., Citation2016; Salazar et al., Citation2018). This is favored when the drying temperature of polyphenolic compounds exceeds 50°C (Aruwa et al., Citation2019; De Torres et al., Citation2010). Therefore, to maintain the quality of the polyphenols, the drying process must be carried out under a controlled temperature environment, preferably low, or applying vacuum (Wang et al., Citation2009). Spray drying is a simple, fast, reproducible and scalable drying technology that allows mild temperature conditions, suitable for heat-sensitive bioactive compounds and maintains the quality of nutrients, colors and flavors (Fazaeli et al., Citation2012). The powders produced are of high quality and generally have low moisture content and water activity, so they are resistant to microbiological and oxidative degradation, resulting in high storage stability (Islam et al., Citation2016; Shishir et al., Citation2018). The objective of this research was to evaluate two methods of drying in an extract of O. atropes, which allow the preservation of polyphenolic compounds with antioxidant activity, as well as the individual identification of the different types of phenolic compounds present in the extract.
2. Materials and methods
The cladodes of O. atropes were obtained from Ziracuaretiro, Michoacán, Mexico, and were identified by the Herbarium of the Faculty of Biology of the Universidad Michoacana de San Nicolás de Hidalgo.
2.1. Dehydration and characterization of the extract of O. atropes
The young cladodes of O. atropes (3 months) of 80 ± 40 g were disinfected with 1% sodium hypochlorite solution/15 min, then washed with distilled water, spines removed, cut into 1 × 1 cm pieces, and dehydrated at 50°C for 48 h in a oven (Felisa® FE-292, Mexico). The dehydrated samples were triturated in a blender (Oster®, 450,010,000 model, 400-watt power) and sieved to obtain nopal flour with a particle size of less than 260 μm. The nopal flours were stored in frozen at −5°C until their analysis.
The extracts of polyphenolic compounds of O. atropes were obtained according to the conditions described in a previous study by this same team (Tranquilino-Rodríguez et al., Citation2020), with minor modifications. Briefly, the extraction method was carried out as follows, 10 g of nopal flour were placed in 100 mL of 70% ethanol in a 250 ml Erlenmeyer flask, which was placed at 50°C in a shaking water bath SW22 (Julabo®, Seelbach, Germany) at 100 rpm for 120 min. The extract obtained was centrifuged at 3,087 g/10 min and vacuum filtered with Whatman filter paper No. 2, 4 and 5 until obtaining a particle size ≤2.5 µm. The ethanol was then removed in a rotary evaporator (Science Med® RE 100-Pro) at a temperature of 40°C, coupled to a vacuum system, obtaining an aqueous extract, that was vacuum filtered with Whatman paper No. 5 to obtain a particle size ≤ 2.5 microns. The extract of O. atropes adjusted to 1% of total solids, was dehydrated by two methods, drying in an oven at 50°C and drying by nano-spray at 105°C. For drying in the oven (Felisa® FE-292, Mexico), 20 mL of the O. atropes extract were placed in a glass Petri dish 100 × 20 mm, and placed in the oven at 50°C for 24 h and the dehydrated extract was collected with a stainless-steel spatula. Nano-spray drying was carried out in a Nano Spray Dryer Unit B-90 (Büchi® Labortechnik AG, Flawil, Switzerland). The process parameters were, inlet temperature of 105°C, outlet temperature of 49°C, air flow of 110 L/min, vacuum pressure of 33 mbar, aspersion of 70%, spray mesh size of 7 µm and feeding speed of 36 mL/h (5 h of process). The sample was collected with a silicone spatula. The powdered extracts (EOSE and EOSNA) were stored in an amber bottle and in a desiccator at 4°C until their analysis. The quantification of phenolic acids, flavonoids and antioxidant activity by capture of the radical DPPH• and ABTS•+ this was carried out both in the O. atropes extract before drying (EO), in the O. atropes extract dried in an oven at 50°C (EOSE) and, in the O. atropes extract dried by nano-spray (EOSNA). In the extracts obtained in powder (EOSE and EOSNA), it was determined; yield (%), humidity (%), particle size (nm), colour (luminosity, chroma, °hue) and polyphenolic compounds by UPLC-Q/TOF-MS2.
2.1.1. Determination of total phenolic acids or phenols
The phenolic acids were assayed using the Folin–Ciocalteu method, as described by Makkar et al. (Citation1993), with minor modifications suggested by Treviño-Gómez et al. (Citation2017). An aliquot of 250 μL of the extract was added to 250 μL of Folin–Ciocalteu reagent (2 N) and 250 μL of 20% Na2CO3, stirred and incubated at 40°C for 30 mins. Distilled water (2 mL) was added, and the mixture was stirred. Absorbance was read at 750 nm in a VELAB® (Germany) spectrophotometer. Gallic acid was used as the standard. The results were expressed as gallic acid equivalents per gram of dry sample (mg GAE/g).
2.1.2. Determination of total flavonoids
The total flavonoids were assessed following the method proposed by Liu et al. (Citation2002). A 150-μL aliquot of each sample and 150 μL of 5% NaNO2 were mixed. Then, 150 μL of 10% AlCl3 and 1 mL NaOH 0.1 M were added and stirred. The absorbance was measured at 510 nm (VELAB® spectrophotometer). Quercetin was used as the standard. The results were expressed as quercetin equivalents per gram of dry sample (mg QE/g).
2.1.3. DPPH• antioxidant assay
The scavenging of DPPH• radicals was determined, as detailed by Randhir and Shetty (Citation2007), with the modifications proposed by Treviño-Gómez et al. (Citation2017). A 50-μL aliquot of each sample was mixed with 2.95 mL of 60 μM methanol–DPPH• solution, to obtain a volume of 3 mL. The samples were stirred for 10 s and maintained for 30 min in darkness. Gallic acid was used as the standard. Absorbance was read at 517 nm (VELAB® spectrophotometer), and the results were expressed as gallic acid equivalents per gram of sample according to the calibration curve prepared with the same standard and the % inhibition of the DPPH• radical according to the following formula:
AR = Absorbance of the reference standard. AS = Absorbance of the sample.
2.1.4. ABTS•+ antioxidant assay
The ABTS•+ scavenging activity was evaluated according to Re et al. (Citation1999) method. A stock solution containing 2:1 v/v of 7 mM ABTS•+ and 2.45 mM potassium persulfate solution was left to stand at room temperature for 12 to 16 h, and then adjusted with ethanol until an absorbance of 0.70 ± 0.02 nm at 734 nm was reached. Afterward, 15 μL of each sample was mixed with 1,485 μL of the prepared ABTS•+ solution. After 15 mins, the absorbance was measured at 734 nm (VELAB® spectrophotometer), and the results were expressed as gallic acid equivalents per gram of sample according to the calibration curve prepared with the same standard and the % inhibition of the ABTS•+ radical according to the following formula:
AR = Absorbance of the reference standard. AS = Absorbance of the sample.
2.1.5. Determination of yield
The percentage yield of the O. atropes dehydrated extracts were determined gravimetrically (Gereniu et al., Citation2017).
1Total solids content of the extract was determined gravimetrically by oven-drying (105°C) until a constant weight (4 h) was reached.
2.1.6. Determination of moisture
A porcelain capsule at constant weight was used for each sample, 3 g of extract were weighed into each capsule and placed in a drying oven (Felisa®) 105°C/4 h. Subsequently, they were placed in a desiccator for 40 min and weighed to constant weight (AACC, Citation2000). The moisture percentage was calculated based on the following formula:
weight of the capsule and the wet sample in grams.
= weight of the capsule and dry sample in grams. S = weight of the wet sample in grams.
2.1.7. Determination of particle size
A solution of 1 mg of extract in 1 mL of distilled water was prepared for each treatment. The measuring was evaluated in a nanoparticle analyzer (Horiba® SZ-100, Ltd., Japan). The particle size was expressed in nm.
2.1.8. Determination of colour
Determination of color. A manual colorimeter (BYK® Gardner, USA) was used, with an illuminant and standard observer D65/10 ° at T = 24°C. The samples (EOSE and EOSNA) were placed in a 4 cm container of diameter. The thickness of each sample was 1 cm and the parameters of luminosity, chroma or saturation and the °hue were determined.
2.1.9. Identification of polyphenolic compounds by UPLC-Q/TOF-MS2
For the identification of polyphenolic compounds of the O. atropes extract obtained both by drying in an oven (EOSE) and by drying by nano-spray (EOSNA), a solution was prepared at 500 ppm with HPLC grade water for each sample and filtered on paper Whatman with 0.2 µm pore and was placed in the 2 mL glass chromatography vials.
For the evaluation of the polyphenolic profile, an ultra-high-resolution liquid chromatograph was used (ACQUITY® UPLC I-Class, Singapore). The qualitative identification of the polyphenols was performed with a BEH PHENYL analytical column (2.1 mm x 100 mm, 1.7 μm, WATERS, UK) operated at 40°C. Gradient separation was performed for each sample using a mobile phase of solvent A: water with 0.1% (v/v) formic acid and solvent B: 100% acetonitrile, with a constant flow rate of 0.3 mL per min. The samples were injected (3 μL) with an automatic sampler at a scan time of 10 min, starting with the 90% A and 10% B gradient program, followed by 87% of A and 13% of B at 0.5 min, 85% of A and 15% of B at 2.0 min, 83% of A and 17% of B at 3.50 min, 80% of A and 20% of B at 5.0 min, and finally 10% of A and 90% of B at 8.50 min. The UPLC system was coupled to a Q-TOF orthogonal accelerated Q-TOF mass spectrometer (XEVO® G2-XS Q-TOF 4 K, UK) equipped with an electrospray ionization source. The PDA detector was used to record the chromatograms. The detection of the mass spectra was carried out in the negative ion mode in an m/z mass range of 50–1200 Da, using a capillary voltage of −3.5 and +4.0 kV, dry gas temperature of 210°C, 8.0 L gas flow per min, 2.0 bar nebulizer pressure, and 1 Hz spectrum speed. Automatic MS/MS experiments were performed using a 15–35 V ramp collision energy with argon as the collision gas and adjusting the scan time every second. Each polyphenolic compound was identified according to its characteristic aglycone fragment ions, through the interpretation of its fragmentation patterns and with the interpretation of the mass spectra that were obtained, these data were compared with databases such as Phenol-Explorer and MassBank and with the information reported by other research (Astello et al., Citation2015; Guevara-Figueroa et al., Citation2010; Melgar et al., Citation2017; Mena et al., Citation2018; Santos-Zea et al., Citation2011).
2.2. Statistic analysis
Results obtained from the triplicates were reported as a mean ± standard deviation (SD). Data were subjected to Student’s t tests or one-way analysis of variance (ANOVA) followed by Tukey’s test using JMP6 software (SAS, Institute, Cary, NC, USA). P values < .05 were regarded as significant. Chromatograms obtained by UPLC -ESI-Q/TOF-MS2 were plotted using OriginPro 2016 software.
3. Results and discussion
3.1. Dehydration and characterization of the extract of O. atropes
No significant difference was found in the content of phenolic acids (P > .05) in the extracts, being 11.13 mg GAE/g for the O. atropes extract before drying and 10.62 mg GAE/g for EOSE and of 11.80 mg GAE/g for EOSNA (). The flavonoid content of the O. atropes extract before drying was 24.05 QE/g and there was no significant difference (P > .05) with EOSNA, which was 22.26 QE/g; however, in EOSE there was a difference significant (P < .05) and flavonoids of 19.22 QE/g were quantified, so there was a decrease of 20.08% with respect to the O. atropes extract before drying. For the antioxidant activity by capturing the radical DPPH•, no significant difference was found in the % inhibition of the O. atropes extract before drying, which was 18.32%, compared to the two drying treatments, which was around 17% (). For the antioxidant activity as a function of the ABTS•+ radical, there was no statistically significant difference between the O. atropes extract before drying (53.49%) compared with the EOSNA (54.08%), however, was found less inhibition in EOSE (49.30%) (), which indicated that the oven drying treatment decreased 7.8% of the antioxidant activity for this radical.
Table 1. Dehydration of Opuntia atropes extract.
Tabla 1. Deshidratación del extracto de Opuntia atropes.
The O. atropes extract dehydrated in an oven (EOSE), was obtained in the form of dark amber crystals, and its recovery was difficult, due to the presence of high hygroscopicity and adherence to the glass petri dish, and therefore, presented a low yield, 49.70% (). The extract of O. atropes dried by nano-spray (EOSNA), was obtained in the form of a fine beige color powder, and presented low hygroscopicity, so that its recovery was higher, obtaining a yield of 74.29%, which is classified as a high yield for the use of this technology (Arpagaus et al., Citation2017).
Table 2. Characterization of dehydrated Opuntia atropes extracts.
Tabla 2. Caracterización de extractos de Opuntia atropes deshidratados
The moisture in the obtained extracts was determined. The EOSE extract presented 9.67 ± 0.86% and the EOSNA extract presented 4.38 ± 0.42% (). Moisture is a critical factor in dehydrated products since it determines their shelf life based on the deterioration reactions that take place in the product. As mentioned, the EOSE extract presented high hygroscopicity, which increased the moisture content of the extract, this moisture could favor contact with free radicals, thus causing their deterioration (Silva et al., Citation2013). On the other hand, in the EOSNA extract, the stability of the bioactive compounds and antioxidant activity could be related to the lower moisture and hygroscopicity.
The color parameters of the extracts are shown in . The parameters were evaluated; luminosity, this parameter was evaluated in a range from 0 to 100, where 100 was white and 0 was black. The saturation or chroma was also determined and the purity or how vivid the color is indicated, so that dull colors can be differentiated near center and become more vivid as they move away from center and finally the °hue was quantified, which is the visual appreciation of color, the angle found and the tone this represented was: 90 ° = yellow (HunterLab, Citation2012). For EOSE, luminosity values of 26.83 ± 1.99, chroma of 16.37 ± 0.53 and °hue of 75.30 ± 0.92 were obtained, so this extract presented a bright dark yellow color, whereas EOSNA presented luminosity values of 66.54 ± 1.24, chroma of 11.33 ± 1.14 and °hue of 88.35 ± 2.42, so this extract presented a light and opaque yellow color (). The color in antioxidant extracts is an important quality parameter. Brown colors are associated with oxidation processes. This characteristic could be verified, because the EOSE extract presented a darker color, and therefore, lower polyphenolic and antioxidant content compared to EOSNA.
Figure 1. Dehydrated extracts (a) EOSE and (b) EOSNA.
Figura 1. Extractos deshidratados (a) EOSE y (b) EOSNA

The size of the particles was evaluated in the two dehydrated samples (). It was obtained that the extract of O. atropes obtained by EOSE was in the micrometric scale >1 µm (1307.8 ± 81.18 nm), while the particles obtained by EOSNA were of nanometric size <1 µm (105.4 ± 25.40 nm) as reported by Fang and Bhandari (Citation2012), Ezhilarasi et al. (Citation2013), and Wui (Citation2015). With nano-spray drying technology (Buchi B-90), it is possible to obtain ultrafine powders with a size smaller than 500 nm, compared to the aggregation in the form of flakes obtained by other methods such as oven drying or lyophilization (Chopde et al., Citation2020). The main advantage of nano-spray drying is the production of nano-scale particles, which improves the bioavailability of bioactive components, due to a higher surface–volume ratio, stability, and a higher rate of penetration into cells (Arpagaus et al., Citation2018).
Spray dryers can dry a product very quickly compared to other drying methods and are suitable for heat-sensitive products such as polyphenols, as the exposure time to high temperatures is short (Sandoval-Peraza et al., Citation2016). Spray drying has been shown to have higher retention of phenolic compounds and flavonoids compared to lyophilization in papaya pulp (Gomes et al., Citation2018). A previous study showed that spray drying did not affect the polyphenolic and antioxidant content of a black rice (Oryza sativa L., var. Artemide) extract (Papillo et al., Citation2018). It has also been shown that freeze-drying and oven drying at 45°C do not affect the polyphenolic and antioxidant content of the pulp and peel of the fruit of O. ficus-indica (Aruwa et al., Citation2019). And that lyophilization is a more efficient method to preserve polyphenolic compounds with antioxidant activity, in Opuntia spp. cladode flour, compared to spray drying or tunnel drying (Martínez-Soto et al., Citation2016). However, until now the impact of nano-spray drying or oven on the polyphenolic extract of cladodes of O. atropes had not been reported.
Based on these results, it was established that drying by nano-spray at 105°C did not affect the content of polyphenolic compounds (phenolic acids and flavonoids) or the antioxidant activity (DPPH• and ABTS•+) of the O. atropes extract, in addition the nano-spray drying at 105°C presented some advantages over drying in an oven at 50°C, which are related to the yield, moisture, color, particle size and the content of flavonoids and antioxidant activity in ABTS•+, however, if nano-spray drying is not available, drying in an oven at 50°C/24 h could be a feasible method for the dehydration of O. atropes extract. The decrease in the amount of some polyphenolic compounds such as flavonoids and the antioxidant activity of the extract should be considered, lower quality characteristics and that the drying time is longer.
Lemos et al. (Citation2016) reported 1.2 mg GAE/g of phenolic acids or phenols and 2.26 mg QE/g of flavonoids in flour from O. atropes and Guevara-Figueroa et al. (Citation2010) reported concentrations of up to 5.2 mg GAE/g for phenolic acids and 9.7 mg QE/g for flavonoids also in O. atropes flour. The content of both phenolic acids and flavonoids in this study were higher than those reported by Lemos et al. (Citation2016) and Guevara-Figueroa et al. (Citation2010). These variations in the polyphenol content are related to the agroecological conditions of the region where the cladodes were collected, variety, cladode maturity, extraction method and compound quantification (Koolen et al., Citation2013).
The tests with radical’s DPPH• and ABTS•+ are used because they show an idea of the structure and its relationship in the antioxidant activity of the compounds contained in the samples (Bernini et al., Citation2018), this antioxidant characterization provided us valuable information on the chemical reactivity of the compounds present in the samples and that effectively present the ability to eliminate free radicals by electron transfer. It has been reported that the values of the total antioxidant activity in extracts of Opuntia spp. its correlated with the content of flavonoids mainly (Fernández-López et al., Citation2010). The extracts obtained in this study presented a significant number of polyphenolic compounds, and their antioxidant activity was also shown, so cladodes of O. atropes can be attractive foods for the food industry as functional ingredients, to prevent or reduce the risk of chronic degenerative diseases.
3.2. Identification of polyphenolic compounds by UPLC-Q/TOF-MS2
show the polyphenolic profiles of the oven-dried O. atropes extract (EOSE) and the extract dried by nano-spray (EOSNA) respectively, obtained by UPLC-Q/TOF-MS2. It is important to mention that this is a tentative chemical characterization of the polyphenolic compounds present in the O. atropes extracts. show the retention times and the 8 peaks found for both EOSE and EOSNA respectively, in both cases they correspond mainly to polyphenolic compounds of the flavonoid type, from the group of flavonols, which are kaempferol glycosides and isorhamnetin, see , as can be seen, the two extracts presented very similar chromatograms, and the peaks corresponded to the same polyphenolic compounds. Therefore, the dehydrated extracts of O. tropes (EOSE and EOSNA) conserved similar proportions of these bioactive compounds.
Table 3. Polyphenolic profile from EOSE obtained by UPLC-Q/TOF-MS2.
Tabla 3. Perfil polifenólico de EOSE obtenido por UPLC-Q/TOF-MS2
Table 4. Polyphenolic profile from EOSNA obtained by UPLC-Q/TOF-MS2.
Tabla 4. Perfil polifenólico de EOSNA obtenido por UPLC-Q/TOF-MS2
Figure 2. Peak chromatogram of the polyphenolic compounds obtained by UPLC-Q/TOF-MS2 for EOSE. For the assignment of each peak see Table 3.
Figura 2. Picos del cromatograma de compuestos polifenólicos obtenidos por UPLC-Q/TOF-MS2 para EOSE. Para la asignación de cada pico ver la Tabla 3
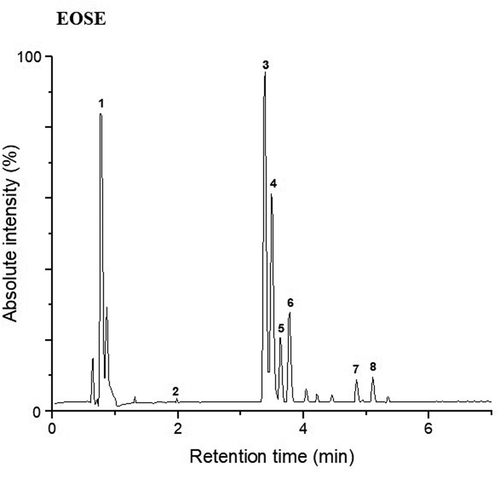
Figure 3. Peak chromatogram of the polyphenolic compounds obtained by UPLC-Q/TOF-MS2 for EOSNA. For the assignment of each peak see Table 4.
Figura 3. Picos del cromatograma de compuestos polifenólicos obtenidos por UPLC-Q/TOF-MS2 para EOSNA. Para la asignación de cada pico ver la Tabla 4
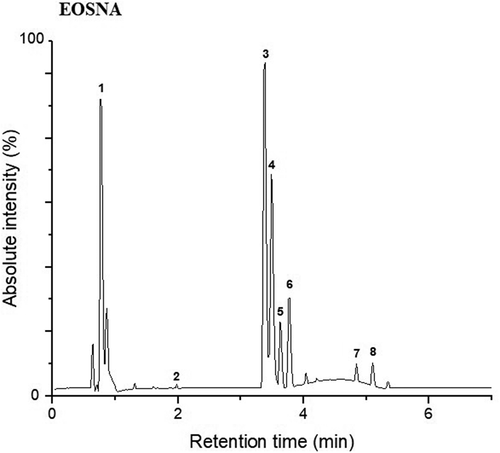
show that for the two dehydration treatments (EOSE and EOSNA), peaks 3 and 4 belong to kaempferol 3-O- (rhamnosyl-galactoside) −7-O-rhamnoside; however, these 2 compounds had different retention times; therefore, its designated as isomers, and in peak 7, kaempferol 3-O-galactoside 7-O-rhamnoside was detected. In peaks 5 and 6, two isomers of isoramnetin 3-O-rhamnoside-7-O- (rhamnosyl-hexoside) were present, and in peak 8 another isoramnetin glucoside was found, which was isoramnetin 3-O-glucoside 7 -O-rhamnoside. Peak 1 corresponded to medioresinol, which is a lignan, which has not been reported so far in the polyphenolic profile of the genus Opuntia, this compound was found in a similar proportion with respect to kaempferol glycosides (kaempferol 3-O- (rhamnosyl-galactoside) −7-O-rhamnoside), for this reason its identification in O. atropes is important. Lignans are attributed many physiological properties that positively influence human health (Durazzo, Citation2018). Its intake has been mainly related to its possible chemopreventive actions against cancer, due to its phytoestrogenic properties and in the prevention of cardiovascular diseases (Anandhi et al., Citation2018; Xiao et al., Citation2018). The presence of this lignan increases the number of bioactive compounds in the cladodes of O. atropes and, therefore, its interest in human health, either as an individual bioactive molecule or in conjunction with the other molecules present in the extract. Finally, a phenolic acid was detected in peak 2, which was p-coumaric acid 4-O-glucoside in a low proportion with respect to the other polyphenolic compounds. It is noteworthy to mention that the methodology used in this study (UPLC-Q/TOF-MS2), does not indicate the concentration of each polyphenolic compound present in the crude extract; however, it does provide us information about the proportion of the polyphenolic compounds, which are mainly flavonoid glycosides, and as can be seen in the chromatograms presented in , the proportion of kaempferol glycosides with respect to isorhamnetin glycosides is greater, and the lignan (mediorresinol) is detected in a very similar proportion to kaempferol glycosides.
The results found in this study coincide with what was previously reported by Park et al. (Citation2007) who indicated that both kaempferol and isorhamnetin are largely found as glycosides in flowers, fruits and cladodes of Opuntia spp. De Santiago et al. (Citation2018), reported flavonoids in cladodes of the species of O. ficus-indica as the predominant polyphenolic compounds, of which approximately 80% were derived from isorhamnetin, while those derived from quercetin and kaempferol were in lower concentration. In the research carried out by Santos-Zea et al. (Citation2011) identified the flavonoids present in different varieties of Opuntia cladodes such as isorhamnetin and kaempferol, mainly in their glycosidic forms in crude extracts. Flavanols such as kaempferol and isorhamnetin 3-O-glucosides, identified in this study have been associated with the antioxidant effect, an anti-inflammatory effect assigned to intestinal inflammation and cardioprotective effects (Kuti, Citation2004; Matias et al., Citation2014); therefore, the consumption of these extracts can provide important health benefits.
4. Conclusions
Nano-spray drying did not affect the polyphenol content or antioxidant activity of the O. atropes extract. Polyphenolic screening for O. atropes showed the majority presence of flavonols kaempferol and isorhamnetin in the form of glycosides and medioresinol, which is a lignan in this nopal species, was identified for the first time. The contribution in the identification of new bioactive molecules is relevant since it contributes to the existing relationship between the consumption of O. atropes with the potential benefits for human health. The extracts of O. atropes obtained in this work could be used as nutraceuticals and, therefore, have potential for application in the food and health industries.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
Eunice Tranquilino Rodriguez gratefully acknowledges the support of CONACyT for the PhD scholarship received, Scholar number 286273.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- AACC. (2000). American Association of Cereal Chemists. Approved methods 10th St. Paul, MN, USA: American Association of Cereal Chemists Methods 44-15A (Moisture).
- Abhay, S. M., Hii, C. L., Law, C. L., Suzannah, S., & Djaeni, M. (2016). Effect of hot-air drying temperature on the polyphenol content and the sensory properties of cocoa beans. International Food Research Journal, 23(4), 1479–1484 http://www.ifrj.upm.edu.my/23%20(04)%202016/(19).pdf.
- Akbarian, M., Ghasemkhani, N., & Moayedi, F. (2013). Osmotic dehydration of fruits in food industrial: A review. International Journal of Biosciences, 3(12), 1–16. https://doi.org/http://dx.doi.org/10.12692/ijb/4.1.42-57
- Anandhi, S. H., Fata, J. E., & Kennelly, E. J. (2018). Phytoestrogens: The current state of research emphasizing breast pathophysiology. Phytotherapy Research, 32(9), 707–1719. https://doi.org/https://doi.org/10.1002/ptr.6115
- Antunes-Ricardo, M., Gutiérrez-Uribe, J. A., López-Pacheco, F., Alvarez, M. M., & Serna-Saldívar, S. O. (2015). In vivo anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica (L.) Mill cladodes. Industrial Crops and Products, 76, 803–808. https://doi.org/https://doi.org/10.1016/j.indcrop.2015.05.089
- Arpagaus, C., Collenberg, A., Rütti, D., Assadpour, E., & Jafari, S. M. (2018). Nano spray drying for encapsulation of pharmaceuticals. International Journal of Pharmaceutics, 546(1–2), 194–214. https://doi.org/https://doi.org/10.1016/j.ijpharm.2018.05.037
- Arpagaus, C., John, P., Collenberg, A., & Ruetti, D. (2017). Nanocapsules formation by nano spray drying. In S. M. Jafari (Ed.), Nanoencapsulation technologies for the food and nutraceutical industries (pp. 346–401). Elsevier Inc. https://doi.org/https://doi.org/10.1016/B978-0-12-809436-5.00010-0
- Aruwa, C. E., Amoo, S., & Kudanga, T. (2019). Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. Lwt, 111, 337–344. https://doi.org/https://doi.org/10.1016/j.lwt.2019.05.028
- Astello, M., Cervantes, I., Nair, V., Santos, M., Reyes, A., & Guéraud, F. (2015). Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. Journal of Food Composition Analysis, 43, 119–130. https://doi.org/https://doi.org/10.1016/j.jfca.2015.04.016
- Benattia, F. K., & Arrar, Z. (2018). Antioxidative and antiradical activities of bioactive compounds of extracts from Algerian prickly pear (Opuntia ficus-indica L.) fruits. Current Nutrition & Food Science, 14(3), 211–217. https://doi.org/https://doi.org/10.2174/1573401313666170609101639
- Bernini, R., Barontini, M., Cis, V., Carastro, I., Tofani, D., Chiodo, R. A., Lupattelli, P., & Incerpi, S. (2018). Synthesis and evaluation of the antioxidant activity of lipophilic phenethyl trifluoroacetate esters by in vitro ABTS, DPPH and in cell-culture DCF assays. Molecules, 23(1), 208. https://doi.org/https://doi.org/10.3390/molecules23010208
- Chopde, S., Datir, R., Deshmukh, G., Dhotre, A., & Patil, M. (2020). Nanoparticle formation by nanospray drying & its application in nanoencapsulation of food bioactive ingredients. Journal of Agriculture and Food Research, 2, 100085. https://doi.org/https://doi.org/10.1016/j.jafr.2020.100085
- De Santiago, E., Domínguez-Fernández, M., Cid, C., & De Peña, M.-P. (2018). Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia Ficus-Indica). Food Chemistry, 240, 1055–1062. https://doi.org/https://doi.org/10.1016/j.foodchem.2017.08.039
- De Torres, C., Díaz-Maroto, M. C., Hermosín-Gutiérrez, I., & Pérez-Coello, M. S. (2010). Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Analytica Chimica Acta, 660(1–2), 177–182. https://doi.org/https://doi.org/10.1016/j.aca.2009.10.005
- Durazzo, A. (2018). Lignans. In L. M. L. Nollet & J. A. Gutierrez-Uribe (Eds.), Phenolic compounds in food: Characterization and analysis (pp. 185–200). CRC Pres Inc. https://doi.org/https://doi.org/10.1201/9781315120157
- Ezhilarasi, P. N., Karthik, P., Chhanwal, N., & Anandharamakrishnan, C. (2013). Nanoencapsulation techniques for food bioactive components: A review. Food and Bioprocess Technology, 6(3), 628–647. https://doi.org/https://doi.org/10.1007/s11947-012-0944-0
- Fang, Z., & Bhandari, B. (2012). Encapsulation techniques for food ingredient systems. In B. Bhandari & Y. H. Roos (Eds.), Food Materials Science and Engineering (pp. 320–348). Blackwell Publishing Ltd.
- Fazaeli, M., Emam-Djomeh, Z., Kalbasi, A. A., & Omid, M. (2012). Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food and Bioproducts Processing, 90(4), 667–675. https://doi.org/https://doi.org/10.1016/j.fbp.2012.04.006
- Fernández-López, J. A., Almela, L., Obón, J. M., & Castellar, R. (2010). Determination of antioxidant constituents in cactus pear fruits. Plant Foods for Human Nutrition, 65(3), 253–259. https://doi.org/https://doi.org/10.1007/s11130-010-0189-x
- Gereniu, C. R. N., Saravana, P. S., Getachew, A. T., & Chun, B. S. (2017). Characteristics of functional materials recovered from Solomon Islands red seaweed (Kappaphycus alvarezii) using pressurized hot water extraction. Journal of Applied Phycology, 29(3), 1609–1621. https://doi.org/https://doi.org/10.1007/s10811-017-1052-3
- Gligor, O., Mocan, A., Moldovan, C., Locatelli, M., Crișan, G., & Ferreira, I. C. (2019). Enzyme-assisted extractions of polyphenols–A comprehensive review. Trends in Food Science & Technology, 88, 302–315. https://doi.org/https://doi.org/10.1016/j.tifs.2019.03.029
- Gomes, W. F., França, F. R. M., Denadai, M., Andrade, J. K. S., Da Silva Oliveira, E. M., De Brito, E. S., Rodrigues, S., & Narain, N. (2018). Effect of freeze-and spray-drying on physico-chemical characteristics, phenolic compounds and antioxidant activity of papaya pulp. Journal of Food Science and Technology, 55(6), 2095–2102. https://doi.org/https://doi.org/10.1007/s13197-018-3124-z
- Gómez, M., & Martinez, M. M. (2018). Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Critical Reviews in Food Science and Nutrition, 58(13), 2119–2135. https://doi.org/https://doi.org/10.1080/10408398.2017.1305946
- Guevara-Figueroa, T., Jiménez-Islas, H., Reyes-Escogido, M. L., Mortensen, A. G., Laursen, B. B., Lin, L.-W., De León-Rodríguez, A., Fomsgaard, I. S., & Barba De la Rosa, A. P. (2010). Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). Journal of Food Composition and Analysis, 23(6), 525–532. https://doi.org/https://doi.org/10.1016/j.jfca.2009.12.003
- HunterLab. 2012 Application note, AN 1005.00. Hunter L, a, b vs. CIE L*, a*, b* Measuring color using Hunter L, a, b versus CIE 1976 L*, a*, b* (Reston, VA.: Hunter Associates Laboratory Inc.) . . [On line]. http://www.hunterlab.com/an-1005b.pdf
- Islam, S. M. R., Taip, F. S., Aziz, N. A., Talib, R. A., & Sarker, M. S. H. (2016). Optimization of spray drying parameters for pink guava powder using RSM. Food Science and Biotechnology, 25(2), 1–8. https://doi.org/https://doi.org/10.1007/s10068-016-0064-0
- Keller, J., Camaré, C., Bernis, C., Astello-García, M., Barba de la Rosa, A. P., Rossignol, M., Santos, D. M. S., Salvayre, R., Negre-Salvayre, A., & Guéraud, F. (2015). Antiatherogenic and antitumoral properties of Opuntia cladodes: Inhibition of low density lipoprotein oxidation by vascular cells, and protection against the cytotoxicity of lipid oxidation product 4-hydroxynonenal in a colorectal cancer cellular model. Journal of Physiology and Biochemistry, 71(3), 577–587. https://doi.org/https://doi.org/10.1007/s13105-015-0408-x
- Koolen, H. H., Da Silva, F. M., Gozzo, F. C., de Souza, A. Q., & de Souza, A. D. (2013). Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC–ESI-MS/MS. Food Research International, 51(2), 467–473. https://doi.org/https://doi.org/10.1016/j.foodres.2013.01.039
- Kuti, J. O. (2004). Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chemistry, 85(4), 527–533. https://doi.org/https://doi.org/10.1016/S0308-8146(03)00184-5
- Lemos, A. F. A., Pereira, A. A., Alcantara, B. R. L., & Dos Santos, D. C. (2016). Study of the variability, correlation and importance of chemical and nutritional characteristics in cactus pear (Opuntia and Nopalea). African Journal of Agricultural Research, 11(31), 2882–2892. https://doi.org/https://doi.org/10.5897/AJAR2016.11025
- Liu, C.-L., Wang, J.-M., Chu, C.-Y., Cheng, M.-T., & Tseng, T.-H. (2002). In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food and Chemical Toxicology, 40(5), 635–641. https://doi.org/https://doi.org/10.1016/S0278-6915(02)00002-9
- López-Gutiérrez, D. M., Reyes-Agüero, J. A., Muñoz, A., Robles, J., & Cuevas, E. (2015). Morphological comparison between wild and cultivated populations of Opuntia atropes (Cactaceae) in Michoacán, Mexico. Revista Mexicana de Biodiversidad, 86(4), 1072–1077. https://doi.org/https://doi.org/10.1016/j.rmb.2015.08.006
- Luca, S. V., Macovei, I., Bujor, A., Miron, A., Skalicka-Woźniak, K., Aprotosoaie, A. C., & Trifan, A. (2020). Bioactivity of dietary polyphenols: The role of metabolites. Critical Reviews in Food Science and Nutrition, 60(4), 626–659. https://doi.org/https://doi.org/10.1080/10408398.2018.1546669
- Makkar, H. P. S., Blümmel, M., Borowy, N. K., & Becker, K. (1993). Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. Journal of the Science of Food and Agriculture, 61(2), 161–165. https://doi.org/https://doi.org/10.1002/jsfa.2740610205
- Marqués, J., Della, P. G., Reverchon, E., Renuncio, J. A. R., & Mainar, A. M. (2013). Supercritical antisolvent extraction of antioxidants from grape seeds after vinification. Journal of the Supercritical Fluids, 82, 238–243. https://doi.org/https://doi.org/10.1016/j.supflu.2013.07.005
- Martínez-Soto, G., Celis-Fabián, F., Hernández-Pérez, T., & Paredes-López, O. (2016). Effect of drying methods on the nutraceutical potential of cactus cladodes (Opuntia spp.). International Journal of Food and Nutritional Science, 2(6), 1. https://doi.org/https://doi.org/10.15436/2377-0619.15.023
- Matias, A., Nunes, S. L., Poejo, J., Mecha, E., Serra, A. T., Amorim, M. P. J., Bronze, M. R., & Duarte, C. M. M. (2014). Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food and Function, 5(12), 3269–3280. https://doi.org/https://doi.org/10.1039/C4FO00071D
- Melgar, B., Dias, M. I., Ciric, A., Sokovic, M., Garcia-Castello, E. M., Rodriguez-Lopez, A. D., Barros, L., & Ferreira, I. (2017). By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Industrial Crops and Products, 107, 353–359. https://doi.org/https://doi.org/10.1016/j.indcrop.2017.06.011
- Mena, P., Tassotti, M., Andreu, L., Nuncio-Jáuregui, N., Legua, P., Del Rio, D., & Hernández, F. (2018). Phytochemical characterization of different prickly pear (Opuntia ficus-indica (L.) mill.) cultivars and botanical parts: UHPLC-ESI-MSn metabolomics profiles and their chemometric analysis. Food Research International, 108, 301–308. https://doi.org/https://doi.org/10.1016/j.foodres.2018.03.062
- Myint, K. Z., Wu, K., Xia, Y., Fan, Y., Shen, J., Zhang, P., & Gu, J. (2020). Polyphenols from Stevia rebaudiana (Bertoni) leaves and their functional properties. Journal of Food Science, 85(2), 240–248. https://doi.org/https://doi.org/10.1111/1750-3841.15017
- Papillo, V. A., Locatelli, M., Travaglia, F., Bordiga, M., Garino, C., Arlorio, M., & Coïsson, J. D. (2018). Spray-dried polyphenolic extract from Italian black rice (Oryza sativa L., var. Artemide) as new ingredient for bakery products. Food Chemistry, 269, 603–609. https://doi.org/https://doi.org/10.1016/j.foodchem.2018.07.059
- Park, S. H., Kim, H., & Rhyu, D. Y. (2007). Flavonoids from the stems of Eastern pickly pear Opuntia humifusa, Cactaceae. Journal of Applied Biological Chemistry, 50(4), 254–258 https://www.koreascience.or.kr/article/JAKO200706717341579.pdf.
- Randhir, R., & Shetty, K. (2007). Mung beans processed by solid-state bioconversion improves phenolic content and functionality relevant for diabetes and ulcer management. Innovative Food Science & Emerging Technologies, 8(2), 197–204. https://doi.org/https://doi.org/10.1016/j.ifset.2006.10.003
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biological and Medicine, 26(9–10), 1231–1237. https://doi.org/https://doi.org/10.1016/S0891-5849(98)00315-3
- Rodríguez-Rodríguez, C., Torres, N., Gutiérrez-Uribe, J. A., Noriega, L. G., Torre-Villalvazo, I., Leal-Díaz, A. M., Antunes-Ricardo, M., Márquez-Mota, C., Ordaz, G., Chavez-Santoscoy, R. A., Serna-Saldívar, S. O., & Tovar, A. R. (2015). The effect of isorhamnetin glycosides extracted from Opuntia ficus-indica in a mouse model of diet induced obesity. Food & Function, 6(3), 805–815. https://doi.org/https://doi.org/10.1039/C4FO01092B
- Salazar, N. A., Alvarez, C., & Orrego, C. E. (2018). Optimization of freezing parameters for freeze-drying Mango (Mangifera indica L.) slices. Drying Technology, 36(2), 192–204. https://doi.org/https://doi.org/10.1080/07373937.2017.1315431
- Sandoval-Peraza, V. M., Cu-Cañetas, T., Peraza-Mercado, G., & Acereto-Escoffié, P. O. M. (2016). Introducción en los procesos de encapsulación de moléculas nutracéuticas. In M. E. Ramírez Ortiz (Ed.), Alimentos Funcionales de Hoy (pp. 181–218). OmniaScience. https://doi.org/https://doi.org/10.3926/oms.358
- Santos-Zea, L., Gutiérrez-Uribe, J. A., & Serna-Saldívar, S. O. (2011). Comparative analyses of total phenolics, antioxidant activity, and flavonol glycoside profile of cladode flours from different varieties of Opuntia spp. Journal of Agricultural and Food Chemistry, 59(13), 7054–7061. https://doi.org/https://doi.org/10.1021/jf200944y
- Serra, A. T., Poejo, J., Matias, A. A., Bronze, M. R., & Duarte, C. M. M. (2013). Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29). Food Research International, 54(1), 892–901. https://doi.org/https://doi.org/10.1016/j.foodres.2013.08.043
- Shishir, M. R. I., Xie, L., Sun, C., Zheng, X., & Chen, W. (2018). Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends in Food Science & Technology, 78, 34–60. https://doi.org/https://doi.org/10.1016/j.tifs.2018.05.018
- Silva, P. I., Stringheta, P. C., Teófilo, R. F., & De Oliveira, I. R. N. (2013). Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. Journal of Food Engineering, 117(4), 538–544. https://doi.org/https://doi.org/10.1016/j.jfoodeng.2012.08.039
- Spina, A., Brighina, S., Muccilli, S., Mazzaglia, A., Fabroni, S., Fallico, B., Rapisarda, P., & Arena, E. (2019). Wholegrain durum wheat bread fortified with citrus fibers: Evaluation of quality parameters during long storage. Frontiers in Nutrition, 6(13), 1–13. https://doi.org/https://doi.org/10.3389/fnut.2019.00013
- Tranquilino-Rodríguez, E., Martínez-Flores, H. E., Rodiles-López, J. O., & Martínez-Avila, G. C. G. (2020). Nanoencapsulation and identification of phenolic compounds by UPLC-Q/TOF-MS2 of an antioxidant extract from Opuntia atropes. Functional Foods and Health Disease, 10(12), 505–519. https://doi.org/https://doi.org/10.31989/ffhd.v10i12.763
- Treviño-Gómez, D. M., Sánchez-Alejo, E. J., Gontes-Pérez, I. C., Wong-Paz, J., Rojas, R., & Martínez-Avila, G. C. G. (2017). Antioxidant profile of different types of herbal infusions and teas commercially available in Mexico. American Scientific Research Journal for Engineering, Technology and Sciences, 31(1), 67–77 https://asrjetsjournal.org/index.php/American_Scientific_Journal/article/view/2861/1123.
- Valadares, P. D., De Pereira, A. A., Rodrigues, M. A. L., Teodoro, A. L., Dos Cordeiro, S. D., De Garcia, L. A. G., de Nunes, M. A., Do Bezerra, N. D., De Lima, V. R., & Barros, C. D. (2020). Forage nutritional differences within the genus Opuntia. Journal of Arid Environments, 181, 104243. https://doi.org/https://doi.org/10.1016/j.jaridenv.2020.104243
- Wang, S. R., Gu, Y., Liu, Q., Yao, Y., Guo, Z., Luo, Z., & Cen, K. (2009). Separation of bio-oil by molecular distillation. Fuel Processing Technology, 90(5), 738–745. https://doi.org/https://doi.org/10.1016/j.fuproc.2009.02.005
- Wui, W. T. (2015). Nanospray drying technology: Existing limitations and future challenges. Recent Patents on Drug Delivery & Formulation, 9(3), 185–186. https://doi.org/https://doi.org/10.2174/1872211309666150513111150
- Xiao, Y., Zhang, S., Tong, H., & Shi, S. (2018). Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytotherapy Research, 32(Suppl.1), 384–394. https://doi.org/https://doi.org/10.1002/ptr.5966
