ABSTRACT
The objective of this study was to evaluate the application of hot water treatment to tomato fruits (Solanum lycopersicum cv. TA234) genetically modified with silencing of the TomloxB gene. Unmodified and genetically modified tomato fruits were immersed in water at 40°C for 10, 20 and 30 seconds. Subsequently, fruits were stored at 25°C for 18 days. Physiology was assessed and electrolyte leakage, lipoxygenase and polygalacturonase activities were monitored. Treatment applied to the genetically modified tomatoes at 40°C for 30 seconds, resulted in slower ripening, decreased metabolic activity and maintained the attributes in optimal conditions for longer time, increased the postharvest life of the genetically modified tomatoes until 18 days. Lipoxygenase and polygalacturonase activities were partially inhibited.
El objetivo de este estudio fue evaluar la aplicación del tratamiento hidrotérmico a frutos de tomate (Solanum lycopersicum cv. TA234) modificados genéticamente con el silenciamiento del gen TomloxB. Los frutos de tomate no modificados y modificados genéticamente se sumergieron en agua a 40°C durante 10, 20 y 30 segundos. Posteriormente, se almacenaron a 25°C durante 18 días. Se evaluaron la fisiología, la fuga de electrolitos y las actividades de lipoxigenasa y poligalacturonasa. El tratamiento aplicado a los tomates modificados genéticamente a 40°C durante 30 segundos provocó un desarrollo lento de la maduración, disminuyó la actividad metabólica y mantuvo los atributos en condiciones óptimas durante más tiempo, aumentando la vida poscosecha de los tomates modificados genéticamente hasta 18 días. Las actividades de la lipoxigenasa y la poligalacturonasa se inhibieron parcialmente.
1. Introduction
Genetic engineering has been a revolutionary tool within agricultural biotechnology, specifically transgenic technology has been a means for the rapid development of improved crop plants and the stacking of multiple favorable traits (Klümper & Qaim, Citation2014; Kumar et al., Citation2020; Rodríguez et al., Citation1999). The increase in post-harvest life or delay in fruit ripening has also been explored with excellent results in apple, pear, kiwi, papaya, melon, pineapple and tomato (Kumar et al., Citation2020; Lobato et al., Citation2021). Therefore, the use of genetically modified (GM) crops has reduced the amount of soil erosion, provided more protection to plants, reduced the use of agricultural equipment and fuel consumption, and caused less greenhouse gas emissions in the area where the transgenic crops are grown. By modifying the chemical composition of foodstuffs it has fought malnutrition, improved food processing and marketing, and reduced waste. These are some of the benefits of using GM crops (Baghbani-Arani et al., Citation2021; Lobato et al., Citation2021).
In this sense, one of the alternatives to increase the postharvest life of tomato is the silencing of the TomloxB gene. This modification produced a delay in the onset of senescence, and improved its attributes such as color and texture, maintaining the complete integrity of the fruit, desirable by the consumer (León-García et al., Citation2017). On the other hand, postharvest treatments have been extensively studied to preserve the physicochemical attributes of fruits in optimal conditions and to keep them attractive to the consumer. In wild-type tomato, hot water treatment (HWT) has been shown to delay ripening, and control fungal growth (Fallik, Citation2004; McDonald et al., Citation1999; Pinheiro et al., Citation2015; Polenta et al., Citation2006).
In HWT, fruits are immersed in water at 40–60°C for times ranging from seconds to hours, a means by which heat is efficiently transferred to the interior of the fruit. Such process induces the fusion of epicuticular wax platelets, covering the cracks in the fruit epidermis, which prevents phytopathogens or insect attack, or the production of lignin at the site affected by abiotic conditions (Fallik, Citation2004; Mahajan et al., Citation2014).
The limitations of this treatment can be complemented with genetic modifications to extend post-harvest life. This is due to the synergism that is created in the mechanism of action of both and since there are few studies related to the behavior of genetically modified tomatoes after the application of a post-harvest treatment. The objective of this study was to evaluate the application of hot water treatment to tomato fruits (Solanum lycopersicum cv. TA234) genetically modified with an antisense TomloxB gene insertion, which effects a decrease in lipoxygenase activity. Physiology was assessed, and electrolyte leakage, lipoxygenase and polygalacturonase activities were monitored. Results are discussed.
2. Materials and methods
2.1. Plant material
In a previous work, León-García et al. (Citation2017) reported the production of transgenic tomato plants via Rhizobium radiobacter (prior Agrobacterium tumefaciens) with tomato lipoxygenase B (TomloxB) antisense constructs. Rhizobium radiobacter strain LBA4404 harboring the binary vector pCAMBIA 2301 which carried the CaMV 35S promoter. The Neomycin phosphotransferase (NPTII) and β-Glucuronidase (UIDA) genes were used as transformation marker and reporter genes, respectively. The plasmid contains the 2745 bp long TomloxB sequence (GenBank accession number U09025) in antisense direction. In the present work, the genetically modified (GM) lines 1 I, 6H and 5.1C which belong to the F1 line were employed, as well as unmodified or wild fruits (WT) of the same TA234 variety.
The weight of the tomatoes ranged from 18 to 25 g, and a total of 162 fruits were analyzed. Tomatoes were grown and collected in the greenhouse of the postharvest handling laboratory of the Instituto Tecnológico de Veracruz. The tomatoes were selected at the break stage ensuring that they did not display physical damage [supplemental content 1]. Pretreatments were performed based on literature reports (Lurie & Klein, Citation1992; Lurie et al., Citation1998; McDonald et al., Citation1999; S. Wang et al., Citation2001), selecting the conditions reported by S. Wang et al. (Citation2001). WT and GM tomatoes, selected for treatment, were washed and disinfected with a 50 µL/L sodium hypochlorite solution. They were then immersed in a water bath (Fisher Scientific Isotemp Model 28 L-M, Pittsburgh, PA) at 40°C for 10, 20, or 30 seconds. Once the exposure time was completed, fruits were immersed into a tap water bath for 20 seconds, and left to rest on a grid for one hour to eliminate surface water; then fruits were stored in open containers at 25°C for 18 days at a relative humidity of 60–70%. Post-harvest life parameters were analyzed every 3 days. The experimental unit was 1 fruit.
2.2. External color
External color was measured employing the method of Kalantari et al. (Citation2015). Three measurements were performed, at three different points on the equatorial region of the fruit. The CIE-lab Hue angle (ºH) was calculated, using a Hunter Lab colorimeter model 4500L (Reston, Virginia, USA).
2.3. Firmness
Tomato pericarp firmness was determined by puncture at three equidistant points on the equatorial zone, using a fruit texture analyzer (Fruit Texture Analyser, model GS25, China) with an operating range of 50– 25,000 kg, using an 8-mm stainless-steel needle probe.
2.4. Weight loss
According to the method of Kalantari et al. (Citation2015), three fruits of each treatment were measured at the beginning of the experiment and every 3 days during the storage period. Weight loss was expressed as the percentage loss with respect to the initial total weight on an electronic balance (Sartorius model BL 2100, Germany).
2.5. CO2 and ethylene production rate
To quantify ethylene and CO2 production rates, the method proposed by De La Cruz et al. (Citation2010) was followed. Three previously weighed tomato fruits were placed in 300 mL glass jars. After 1 hour, a sample of 1 mL was withdrawn from the headspace with a precision analytical syringe (SUPELCO, Pressure-Lok, Baton Rouge, LA) and injected into a gas chromatograph (Agilent Technologies model 7820A, Waldbronn, Germany); the analysis was performed every 3 days. The operating conditions of the chromatograph were as follows: 100°C in the oven, 150°C in the injector and 250°C in the FID (flame ionization detector) and TCD (thermal conductivity detector). High-purity nitrogen was used as the carrier gas.
2.6. Lipoxygenase (LOX) activity
This analysis was based on the technique reported by Velázquez-López et al. (Citation2020). The extract was prepared from the samples in triplicate by macerating the tomato pulp. The extracted juice was placed in 1.5-mL Eppendorf tubes. Subsequently, it was centrifuged at 8765 ×g in an Eppendorf centrifuge model 5415R (Hamburg, Germany) for 5 minutes. The supernatant was recovered for a second centrifugation at 4036 ×g for 3 minutes. The supernatant was filtered through 0.45 µm pore diameter Millipore membrane discs. Linoleic acid was used as a substrate. The absorbance was then measured at 234 nm using a UV-Vis spectrophotometer (Agilent Technologies, model 8453, Waldbronn, Germany). Lipoxygenase activity was defined as the increase in absorbance of 0.001*mg protein−1*minute−1. Protein concentration was determined by the method of Bradford (Citation1976): 150 mL of Bradford’s reagent was prepared by mixing 15 mg of Coomassie Blue G250, 4.5 mL of reagent grade ethanol, 15 mL of phosphoric acid, gauged with Milli-Q water. For the albumin standard, 10 mg of bovine serum albumin were weighed and gauged to 10 mL with Milli-Q water. A calibration curve was made relating the concentration of the albumin with the absorbance of the sample, which was then measured at 595 nm with the UV-Vis spectrophotometer.
2.7. Polygalacturonase (PG) activity
The method of Miller (Citation1959) was used. To 500 µL of crude extract 250 µL of 0.2 M sodium acetate were added, the samples were incubated at 37°C for 5 minutes, then 250 µL of 1% polygalacturonic acid were added and incubated for one hour at 37°C; then 500 µL of DNS were added and incubated at 65°C for 10 minutes. To stop the reaction, the samples were cooled rapidly in ice water. For the determination of polygalacturonase activity, the absorbance was measured at 540 nm using the UV-Vis spectrophotometer. The unit of enzyme activity was defined as the release of 1 µmol galacturonic acid*mg protein−1*minute−1.
2.8. Electrolyte leakage (EL)
Tomato cells’ membrane damage was determined employing the methodology of Li et al. (Citation2016) with some modifications. Two mesocarp discs (1–2 mm thick and 5 mm in diameter) from each fruit, taken from the equatorial region were immersed in 30 mL of distilled water for 4 h. The initial conductivity was determined with a benchtop conductivity meter (Thermo Scientific Orion 1114000-WA, 3star, Madrid, Spain). The final conductivity was also measured while each sample was rinsed for 4 h after being boiled for 15 min. The relative leakage rate was defined as the relative conductivity (initial conductivity/final conductivity)*100%.
2.9. Experimental design and statistical analysis
For the analysis of HWT for 10, 20 and 30 seconds, a completely randomized design was used. Results were analyzed using a one-way ANOVA; comparison between means was performed by Tukey’s rank test (P ≤ .05) using the statistical package Minitab version 18. Three replicates were considered in each determination.
3. Results and Discussion
Silencing the TomloxB gene promoted significant changes in the ripening of transformed tomatoes, being the increase in postharvest life the most important (León-García et al., Citation2017). In contrast to other mutants like Nr and rin tomato GM fruits, in which polygalacturonase enzyme activation was greatly reduced in Nr mutants and in trace levels in rin fruits, leading to deficiencies in carotenoid synthesis, lycopene production, firmness and an inability to ripen normally (Griffiths et al., Citation1999; Tucker et al., Citation1980). Fruits silenced with the TomloxB gene showed a delay in the senescence stage, allowing carotenoid synthesis and ethylene production, although ethylene production was reduced (León-García et al., Citation2017; Velázquez-López et al., Citation2020). When normal ethylene production was affected, the enzymes involved in ripening were also affected, but not to the extent of preventing complete ripening as in the case of the Nr and rin mutants. In our case, the hot water treatment combined very well with the transgenic fruit. The fruit did not lose its ripening capacity and retained its sensory properties for a longer period.
Hot water treatment for 30 seconds extended postharvest life in the GM tomatoes to 18 days, while 20 seconds did it for 15 days, and 10 seconds for 12 days, compared to the WT tomato that lasted 9 days.
3.1. External color
WT tomatoes subjected to HWT showed no significant difference (P ≤ .05) between treatments. Only tomatoes subjected for 30 seconds, showed significantly greater °hue angle (P ≤ .05) compared to control tomatoes [supplemental content 2]. However, GM tomatoes with HWT for 30 seconds showed greater significant difference in °hue angle (P ≤ .05) compared to untreated GM tomatoes during the first 6 days of storage (), observing that the longer the immersion time, the lesser the change in coloration from green to red. These results may be attributed to that, in the early stages of ripening of the modified tomato, the genes encoding for TomLoxB accumulated slowly resulting in lower lipoxygenase activity, in contrast to WT tomatoes where the highest activity of this enzyme is present in the early stages of ripening (Sheng et al., Citation2000). The delay in the appearance of the carotenoid pigments responsible for developing the reddish coloration in ripening fruit, resumed their synthesis when placed at 25°C after treatment. This effect has been observed in tomatoes subjected to heat treatment [supplemental content 2] (Cheng et al., Citation1988; Klein & Lurie, Citation1991; Zhang et al., Citation2018) and the delay in color change, caused by hot water treatment, has been reported in tomatoes treated at 42°C for 30 and 60 minutes (Polenta et al., Citation2006) and at 40°C for 30 minutes (Pinheiro et al., Citation2015). From day 9, no significant differences were observed between the two groups, maintaining this behavior until the conclusion of storage, registering values close to 40 ºHue, a value that indicates that the fruits were in the mature state (Pathare et al., Citation2013).
Figure 1. Hue angle in wild-type (a) and genetically modified (b) tomatoes with hot water treatment at 40°C for 18 days. Control (![]()
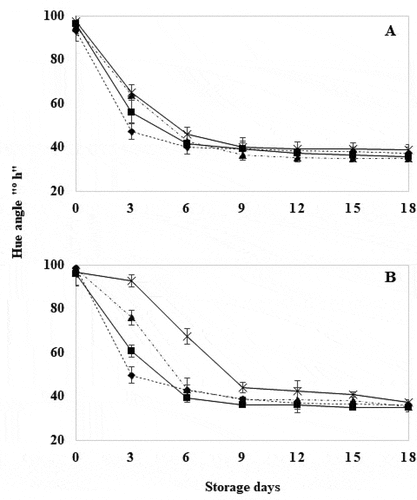
The results of the application of HWT suggest that short exposure times delayed the appearance of red color in treated tomatoes, as well as inhibit or delay ethylene production (Biggs et al., Citation1988; Cheng et al., Citation1988), together with the effect of silencing the TomLoxB gene, which showed lower ethylene production rates compared to WT tomatoes of this variety (Velázquez-López et al., Citation2020). Under stress conditions, free radicals catalyzed by lipoxygenase convert ACC to ethylene (Sheng et al., Citation2002), showing that the joint effect of both processes, reduced ethylene production. This is related to the biosynthesis of β-carotene, and added to the effect of heat that inhibits the mRNA responsible for carotenoid synthesis and chlorophyll degradation, which once removed from heat stress, recover their function (Zhang et al., Citation2018). With respect to lightness and chroma (data not shown) no significant differences (P ≤ .05) were found between treatments for both GM and WT tomatoes compared to untreated fruits, respectively, during storage time.
3.2. Firmness
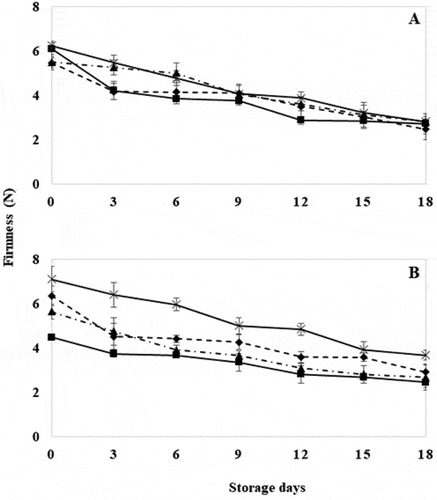
GM tomatoes with HWT for 30 seconds showed significantly higher firmness (P ≤ .05) between treatments until day 9 (), when a slow decline in firmness was observed. This is because properly delivered heat can induce membrane resistance and stabilization mechanisms, as well as inactivation of cell wall hydrolytic enzymes (Mama et al., Citation2016; Paull & Chen, Citation2000). However, WT tomatoes with HWT for 20 and 30 seconds showed a significantly lower decrease (P ≤ .05) between treatments up to day 6 (). These results agree with those reported by Polenta et al. (Citation2006) where a delay in fruit softening was observed after the application of heat treatment for short times. Regarding the behavior between GM and WT tomatoes subjected to HWT for 30 seconds, fruits with silenced TomLoxB gene together with the effect of hot water treatment showed a retardation of softening, thus extending one of the attributes of main importance for the consumer ().
Figure 2. Firmness in wild tomatoes (a) and genetically modified tomatoes (b) with hot water treatment at 40°C for 18 days. Control (![]()
3.3. Weight loss
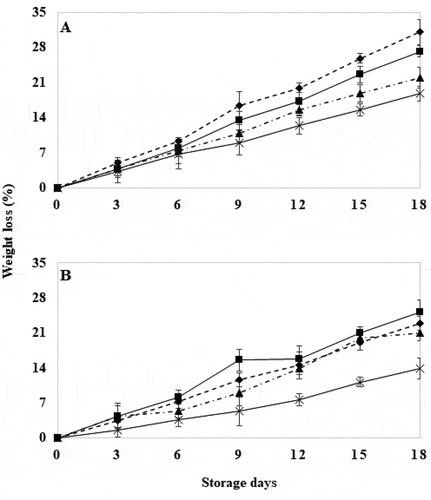
Wild-type tomatoes with HWT for 30 seconds showed significantly less weight loss (P ≤ .05) than the control and the rest of the treatments (). Also, GM tomatoes with HWT for 30 seconds showed significantly (P ≤ .05) less weight loss during storage compared to the other treatments (). This fact can be attributed to the decrease in fruit respiration and transpiration caused by HWT (Cheng et al., Citation1988; Kalantari et al., Citation2015), that delayed fruit softening by disrupting protein synthesis and enzyme inactivation, and prevented cell membrane degradation, which in turn shortened their postharvest life (Bouzayen et al., Citation2010; Paull & Chen, Citation2000). However, GM tomatoes with HWT for 10 seconds showed significantly (P ≤ .05) greater weight loss during storage compared to the other treatments (). This trend can be attributed to membrane permeability, where osmotic water leaked from the fruit as a consequence of the exposure time which acted as an accelerator of the enzymatic activity present in the cell wall. This is a parameter that decreases as the fruit reaches ripening (Mama et al., Citation2016; sGuevara & Ramos, Citation2014).
Figure 3. Weight loss in wild tomatoes (a) and genetically modified tomatoes (b) with hot water treatment at 40°C for 18 days. Control (![]()
3.4. CO2 production
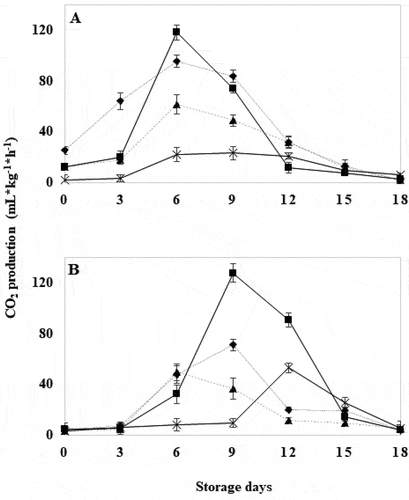
WT control tomatoes showed an increase in CO2 production the first 6 days in which the maximum value was reached; decreasing to very low values on day 18 (). The CO2 production of WT tomatoes treated for 10 seconds reached its highest production on day 6, showing an acceleration of the respiratory rate. WT tomatoes treated for 20 and 30 seconds showed significantly lower respiration rates (P ≤ .05) than the control (), indicating an altered respiratory metabolism that coincided with delayed color change [Supplemental content 2]. The variation of respiratory rate by HWT has been previously reported by Wei et al. (Citation2018). The control GM tomatoes produced the characteristic climacteric respiratory curve (), reaching the maximum value on day 9, '3 days after the WT control (), and with a lower value, which explains the difference in the ripening process. GM tomatoes with HWT for 10 seconds showed the highest respiration peak of all treated samples (). This effect can be attributed to changes in intracellular conditions associated with the exposure time of the fruit in the treatment, that caused disorders in transpiration and respiration mechanisms, water loss and evidenced symptoms of deterioration (Mama et al., Citation2016). GM tomatoes treated for 20 seconds caused a maximum peak on day 6, but with a lower concentration than the control GM. The GM tomatoes with HWT for 30 seconds evidenced a significant delay in the implementation of the maximum peak and a significantly lower value than that of the control GM tomatoes (P ≤ .05). This may be attributed to the effect of HWT by inhibition of mitochondrial enzymes (Paull & Chen, Citation2000), decreased CO2 production, that caused a delay in the appearance of the climacteric peak in the fruit, and thus a pause in its ripening (Cheng et al., Citation1988; Jing et al., Citation2009).
Figure 4. CO2 production in wild tomatoes (a) and genetically modified tomatoes (b) with hot water treatment at 40°C for 18 days. Control (![]()
3.5. Ethylene production rate
WT tomatoes showed an adverse effect by HWT exposure, modifying the typical peak of ethylene production associated with ripening, compared to the rest of the treatments (). The climacteric peak usually manifests during the pink stage in tomato (L. Wang et al., Citation2019). In this study, it appeared from day 6 in control WT fruits, treated for 10 and 20 seconds, with a coloration characteristic of the pink stage [Supplemental content 2], while in WT tomatoes with HWT for 30 seconds it appeared on day 9 (), observing a delay in ripening because the maximum ethylene concentration is closely related to tomato ripening (Workman & Pratt, Citation1957).
Figure 5. Ethylene production rate in wild tomatoes (a) and genetically modified tomatoes (b) with hot water treatment at 40°C for 18 days. Control (![]()
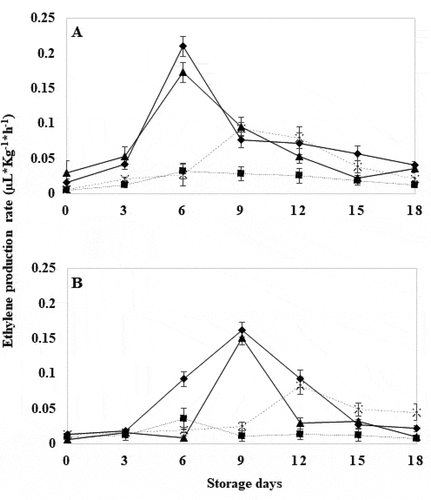
GM tomatoes exhibited lower ethylene production compared to unmodified fruit; furthermore, their climacteric peak emerged on day 9 for the GM control and treated for 20 seconds, for the HWT for 30 seconds it occurred on day 12 (). This reduction in ethylene production is a characteristic physiological response in fruit subjected to heat stress, in which the synthesis of mRNA is altered, which encodes biochemical and physiological changes that regulate fruit ripening. This is further coupled with silencing of the TomLoxB gene, which has been able to reduce ACC oxidase activity, and thus ethylene production rate (Biggs et al., Citation1988; Bouzayen et al., Citation2010; Klein & Lurie, Citation1991; Paull & Chen, Citation2000; Velázquez-López et al., Citation2020). Ethylene production in WT and GM tomatoes with treatment for 10 seconds was the lowest compared to the rest of the treatments, no significant differences were found between them (P ≤ .05). This may be attributed to the fact that at 35°C and above there is an accumulation of ACC synthase in tomato, that induced a reduction in ethylene production, and become vulnerable under these conditions (Atta-Aly, Citation1992; Biggs et al., Citation1988). On the other hand, ACC may have other derivatives such as MACC (1-malonylamino-1-aminocyclopropane-1-carboxylic acid) during ethylene biosynthesis, which can be reconverted to ACC through the action of MACC hydrolase, thus regulating the ACC pool for ethylene production (Van De Poel et al., Citation2014) that could have been affected by the heat stress by the treatment.
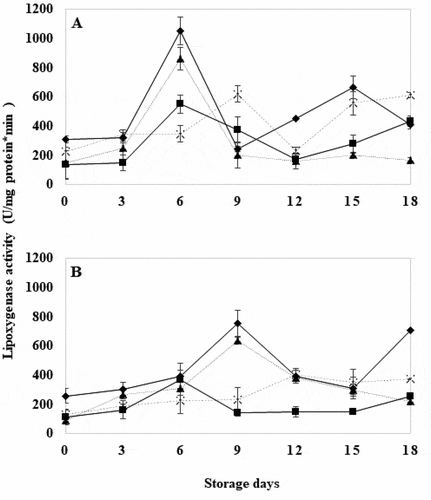
3.6. Lipoxygenase (LOX) activity
Lipoxygenase (LOX) activity in control WT tomatoes was significantly higher (P ≤ .05) compared to GM fruits at day 6 (). This increase occurred abruptly during the break stage and decreased as ripening progressed. These results were in agreement with data reported by other authors (Ealing, Citation1994; Sheng et al., Citation2000; Xue et al., Citation2009). On the other hand, WT tomatoes with HWT for 30 seconds showed significantly higher LOX activity (P ≤ .05) compared to GM tomatoes with the same treatment at day 9 (). At the same time, both treatments recorded the lowest activity among treatments. This may be attributed to the effect of TomLoxB gene silencing, which reduces mRNA accumulation, but does not totally inhibit LOX synthesis (León-García et al., Citation2017; Velázquez-López et al., Citation2020). Paull and Chen (Citation2000) mentioned that HWT at 40°C effects a reversible interruption in protein synthesis, producing new polypeptides after the induction of the heat shock, thus expressing the enzyme again. This heat shock attenuates the enzyme, which acted with less activity on treated tomatoes, and thus reduce fruit ripening during storage (Ferrie et al., Citation1994; Griffiths et al., Citation1999). On the other hand, Yilmaz (Citation2001) reported that the optimum temperature of activity for LOX is 25°C, to be successfully measured is at 35°C; however, at 40°C, LOX activity was only 38%, the temperature applied in this study. Therefore, it is inferred that LOX activity was partially inhibited during the time of exposure to HWT, but once the tomato fruits were placed at 25°C, they recovered their activity together with normal fruit ripening.
Figure 6. Lipoxygenase activity in wild tomatoes (a) and genetically modified tomatoes (b) with hot water treatment at 40°C for 18 days. Control (![]()
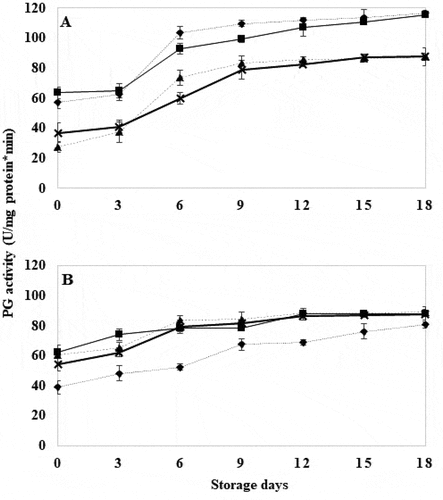
3.7. Polygalacturonase (PG) activity
WT tomatoes showed that at longer exposure time to HWT, polygalacturonase (PG) activity was inhibited (). Untreated WT tomatoes showed higher PG activity values (P ≤ .05) compared to WT tomatoes treated for 20 and 30 seconds (). These results agree with those reported by Yoshida et al. (Citation1984), where PG activity in tomatoes decreased upon heat treatment. On the other hand, GM tomatoes treated for 30 seconds showed significantly lower PG activity (P ≤ .05) compared to the control WT tomatoes (). These results could be related to the silencing of the TomLoxB gene, which reduces the activity of the LOX that in turn influences the ethylene hormone (Velázquez-López et al., Citation2020). The function of LOX is to peroxidize the fatty acids present in the tomato cell membrane; the radicals produced by this reaction tend to affect the activity of 1-aminocyclopropane-1-carboxylic acid oxidase (ACCO), a precursor enzyme of ethylene formation in the Yang cycle. There is an increase in ACCO accumulation and because climacteric fruits display an autocatalytic pathway for ethylene, ethylene production is decreased by ACCO accumulation, which in turn regulates PG expression and activity (Alexander & Grierson, Citation2002; Velázquez-López et al., Citation2020; Xue et al., Citation2009). Because of the above, control GM fruits recorded lower PG activity than GM tomatoes with HWT (). The PG activity in the GM lines with HWT did not show significant differences, and remained constant until the end of storage and was higher than the GM control fruits. The results of firmness and polygalacturonase activity showed that they were inversely proportional and it is inferred that HWT together with TomLoxB gene silencing had an effect on polygalacturonase activity, which is one of the enzymes found in the cell wall that provides membrane integrity to preserve firmness in tomatoes.
Figure 7. Polygalacturonase activity in wild tomatoes (a) and genetically modified tomatoes (b) with hot water treatment at 40°C for 18 days. Control (![]()
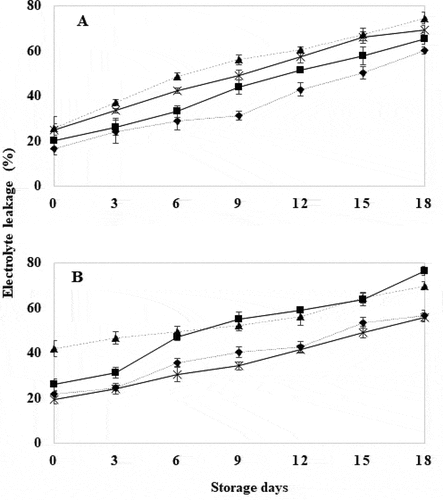
3.8. Electrolyte leakage (EL)
Electrolyte leakage in WT tomatoes treated for 10 and 30 seconds showed significantly lower values (P ≤ .05) compared to WT tomatoes treated for 20 seconds (). HWT applied to tomato attenuated electrolyte leakage by increasing the concentration of calcium ions that bind the pectin chains present in the fruit, which provides greater stability to membrane structure (Côté et al., Citation1993; East et al., Citation2011; Jing et al., Citation2009; McDonald et al., Citation1999; Saltveit, Citation2005). In GM tomatoes treated for 30 seconds electrolyte leakage was similar to control GM tomatoes and significantly lower than the rest of the treatments (P ≤ .05) (). These results can be attributed to the characteristics of GM tomatoes that according to León-García et al. (Citation2017) display greater firmness and integrity caused by TomloxB silencing. We can add to this fact the advantage provided by HWT that is able to decrease electrolyte leakage (Côté et al., Citation1993; McDonald et al., Citation1999; Salazar-Salas et al., Citation2017). However, for the rest of the treatments with higher electrolyte leakage, this effect may be related to a disruption of the cell membrane by the loss of phospholipids, galactolipids and unsaturated glycolipids present in the cell membrane (Bergevin et al., Citation1993; Saltveit, Citation2005). The increase in membrane permeability may also be related to the elevated PG activity described above.
Figure 8. Percentage of electrolyte leakage in wild tomatoes (a) and genetically modified tomatoes (b) with hot water treatment at 40°C for 18 days. Control (![]()
4. Conclusions
Hot water treatment for 30 seconds extended postharvest life in the GM tomatoes to 18 days, while 20 seconds did it for 15 days, and 10 seconds for 12 days, compared to the WT tomato that lasted 9 days. The best treatment applied to the GM tomatoes at the break-ripe stage was hot water treatment at 40°C for 30 seconds. This hot water treatment resulted in a slow decrease in °hue angle in the first few days of storage. Fruits showed greater firmness and less weight loss over time. As well, CO2 and ethylene production rates were reduced. LOX and PG activities were partially inhibited. This treatment allowed for normal ripening, decreased metabolic activity, maintained membrane integrity and thereby increased the postharvest life of the genetically modified tomatoes.
Supplemental Material
Download Zip (163.1 KB)Acknowledgments
The authors thank CONACyT for providing graduate studies grant to the first author (271647).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- Alexander, L., & Grierson, D. (2002). Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. Journal of Experimental Botany, 53(377), 2039–2055. https://doi.org/10.1093/jxb/erf072
- Atta-Aly, M. A. (1992). Effect of high temperature on ethylene biosynthesis by tomato fruit. Postharvest Biology and Technology, 2(1), 19–24. https://doi.org/10.1016/0925-5214(92)90023-I
- Baghbani-Arani, A., Poureisa, M., Alekajbaf, H., Borz-Abad, R. K., & Khodadadi-Dashtaki, K. (2021). Investigating the status of transgenic crops in Iran in terms of cultivation, consumption, laws and rights in comparison with the world. Scientific Reports, 11(1), 1–10. https://doi.org/10.1038/s41598-021-88713-7
- Bergevin, M., L’Heureux, G. P., Thompson, J. E., & Willemot, C. (1993). Effect of chilling and subsequent storage at 20 C on electrolyte leakage and phospholipid fatty acid composition of tomato pericarp. Physiologia Plantarum, 87(4), 522–527. https://doi.org/10.1111/j.1399-3054.1993.tb02502.x
- Biggs, M. S., Woodson, W. R., & Handa, A. K. (1988). Biochemical basis of high‐temperature inhibition of ethylene biosynthesis in ripening tomato fruits. Physiologia Plantarum, 72(3), 572–578. https://doi.org/10.1111/j.1399-3054.1988.tb09167.x
- Bouzayen, M., Latché, A., Nath, P., & Pech, J. C. (2010). Mechanism of fruit ripening Eng Chong, Pua, and Michael R., Davey. In Plant developmental biology-biotechnological perspectives (pp. 319–339). Springer.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
- Cheng, T. S., Floros, J. D., Shewfelt, R. L., & Chang, C. J. (1988). The effect of high-temperature stress on ripening of tomatoes (Lycopersicon esculentum). Journal of Plant Physiology, 132(4), 459–464. https://doi.org/10.1016/S0176-1617(88)80063-4
- Côté, F., Thompson, J. E., & Willemot, C. (1993). Limitation to the use of electrolyte leakage for the measurement of chilling injury in tomato fruit. Postharvest Biology and Technology, 3(2), 103–110. https://doi.org/10.1016/0925-5214(93)90002-K
- De La Cruz, J., Vela, G., Dorantes, L., & García, H. S. (2010). Efecto del etileno sobre el ACC y ACC oxidasa en la maduración de papaya ‘Maradol’. Revista Fitotecnia Mexicana, 33(2), 133–140. https://doi.org/10.35196/rfm.2010.2.133
- Ealing, P. M. (1994). Lipoxygenase activity in ripening tomato fruit pericarp tissue. Phytochemistry, 36(3), 547–552. https://doi.org/10.1016/S0031-9422(00)89772-1
- East, A. R., Hewett, E. W., Heyes, J. A., & Biswas, P. (2011). Increase in electrolyte leakage as a function of chilling stress and ripening of tomato. In IV international conference postharvest unlimited 2011 945 International Society for Horticultural Science (ISHS) (pp. 283–290).
- Fallik, E. (2004). Prestorage hot water treatments (immersion, rinsing and brushing). Postharvest Biology and Technology, 32(2), 125–134. https://doi.org/10.1016/j.postharvbio.2003.10.005
- Ferrie, B. J., Beaudoin, N., Burkhart, W., Bowsher, C. G., & Rothstein, S. J. (1994). The cloning of two tomato lipoxygenase genes and their differential expression during fruit ripening. Plant Physiology, 106(1), 109–118. https://doi.org/10.1104/pp.106.1.109
- Griffiths, A., Barry, C., Alpuche-Solis, A. G., & Grierson, D. (1999). Ethylene and developmental signals regulate expression of lipoxygenase genes during tomato fruit ripening. Journal of Experimental Botany, 50(335), 793–798. https://doi.org/10.1093/jxb/50.335.793
- Guevara, P. A. & Ramos, R. M. E. (2014). Post harvest effects of the hot water treatment of tomatoes ‘nabateo’ (Solanum lycopersicum L.) cv. conservation in their green maturity state. Anales Científicos, 75(2), 403–412. https://doi.org/10.21704/ac.v75i2.981
- Jing, Y., Fu, M. R., Zhao, Y. Y., & Mao, L. C. (2009). Reduction of chilling injury and ultrastructural damage in cherry tomato fruits after hot water treatment. Agricultural Sciences in China, 8(3), 304–310. https://doi.org/10.1016/S1671–2927(08)60213-8
- Kalantari, S., Hatami, M., & Delshad, M. (2015). Diverse postharvest responses of tomato fruits at different maturity stages to hot water treatment. International Journal of Horticultural Science and Technology, 2(1), 67–74 https://dx.doi.org/10.22059/IJHST.2015.54265 URL HTTPS://IJHST.UT.AC.IR/ARTICLE_54265_7334.HTML.
- Klein, J. D., & Lurie, S. (1991). Postharvest heat treatment and fruit quality. Postharvest News and Information, 2(1), 15–19 https://volcaniarchive.agri.gov.il/skn/c6/%d7%90%d7%a1%d7%99%d7%a3_%d7%9e%d7%90%d7%92%d7%a8_%d7%94%d7%9e%d7%97%d7%a7%d7%a8_%d7%94%d7%97%d7%a7%d7%9c/e38900/postharvest_heat_treatment_and_fruit_quality?nsid=2#cq=ASabpostharvestSa4heatSa9treatmentSa3andSa5fruitSa7quality%7C&search=f5ed1093b4f29afa0ec9d5eab3502cc3&entity_number=1.
- Klümper, W., & Qaim, M. (2014). A meta-analysis of the impacts of genetically modified crops. PloS One, 9(11), e111629. https://doi.org/10.1371/journal.pone.0111629
- Kumar, K., Gambhir, G., Dass, A., Tripathi, A. K., Singh, A., Jha, A. K., Yadava, P., Choudhary, M., & Rakshit, S. (2020). Genetically modified crops: Current status and future prospects. Planta, 251(4), 1–27. https://doi.org/10.1007/s00425-020-03372-8
- León-García, E., Vela-Gutiérrez, G., Del Ángel-Coronel, O. A., Torres-Palacios, C., De La Cruz-Medina, J., Gómez-Lim, M. A., & García, H. S. (2017). Increased postharvest life of TomLox B silenced mutants of tomato (Solanum lycopersicum) Var. TA234. Plant Foods for Human Nutrition, 72(4), 380–387. https://doi.org/10.1007/s11130-017-0629-y
- Li, P., Yin, F., Song, L., & Zheng, X. (2016). Alleviation of chilling injury in tomato fruit by exogenous application of oxalic acid. Food Chemistry, 202 (14) , 125–132. https://doi.org/10.1016/j.foodchem.2016.01.142
- Lobato, M., Hewitt, S., Capell Capell, T., Christou, P., Dhingra, A., & Girón-Calva, P. S. (2021). Transgenic and genome-edited fruits: Background, constraints, benefits, and commercial opportunities. Horticultural Research, 8(1), 166–178. https://doi.org/10.1038/s41438-021-00601-3
- Lurie, S., Klein, J. D., Fallik, E., & Varjas, L. (1998). Heat treatment to reduce fungal rots, insect pests and to extend storage. In International Postharvest science conference Postharvest 96 464 (International Society for Horticultural Science (ISHS)) (pp. 309–314).
- Lurie, S., & Klein, J. D. (1992). Ripening characteristics of tomatoes stored at 12 C and 2 C following a prestorage heat treatment. Scientia Horticulturae, 51(1–2), 55–64. https://doi.org/10.1016/0304-4238(92)90103-J
- Mahajan, P. V., Caleb, O. J., Singh, Z., Watkins, C. B., & Geyer, M. (2014). Postharvest treatments of fresh produce. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 372(2017), 20130309. https://doi.org/10.1098/rsta.2013.0309
- Mama, S., Yemer, J., & Woelore, W. (2016). Effect of hot water treatment on shelf life of tomato (Lycopersicon esculentum Mill). Journal of Nature of Science Research, 6 (17) , 69–77 https://iiste.org/Journals/index.php/JNSR/article/view/33130.
- McDonald, R. E., McCollum, T. G., & Baldwin, E. A. (1999). Temperature of water heat treatments influences tomato fruit quality following low-temperature storage. Postharvest Biology and Technology, 16(2), 147–155. https://doi.org/10.1016/S0925-5214(99)00008-3
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. https://doi.org/10.1021/ac60147a030
- Pathare, P. B., Opara, U. L., & Al-Said, F. A. J. (2013). Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology, 6(1), 36–60. https://doi.org/10.1007/s11947-012-0867-9
- Paull, R. E., & Chen, N. J. (2000). Heat treatment and fruit ripening. Postharvest Biology and Technology, 21(1), 21–37. https://doi.org/10.1016/S0925-5214(00)00162-9
- Pinheiro, J., Alegria, C., Abreu, M., Sol, M., Gonçalves, E. M., & Silva, C. L. (2015). Postharvest quality of refrigerated tomato fruit (Solanum lycopersicum, cv. Zinac) at two maturity stages following heat treatment. Journal of Food Processing and Preservation, 39(6), 697–709. https://doi.org/10.1111/jfpp.12279
- Polenta, G., Lucangeli, C., Budde, C., Gonzalez, C. B., & Murray, R. (2006). Heat and anaerobic treatments affected physiological and biochemical parameters in tomato fruits. LWT-Food Science and Technology, 39(1), 27–34. https://doi.org/10.1016/j.lwt.2004.11.003
- Rodríguez, R., Picó, Y., & Mañes, J. J. (1999). Ingeniería genética e industria agroalimentaria: Ventajas e inconvenientes genetic engineering and food industry: Advantages and inconveniences exeñería xenética e industria agroalimentaria: Ventaxas einconvintes. CYTA-Journal of Food, 2(3), 143–151 https://doi.org/10.1080/11358129909487596 URL https://www.tandfonline.com/doi/abs/10.1080/11358129909487596.
- Salazar-Salas, N. Y., Valenzuela-Ponce, L., Vega-Garcia, M. O., Pineda-Hidalgo, K. V., Vega-Alvarez, M., Chavez-Ontiveros, J., Delgado-Vargas, F., & Lopez-Valenzuela, J. A. (2017). Protein changes associated with chilling tolerance in tomato fruit with hot water pre-treatment. Postharvest Biology and Technology, 134 (12) , 22–30. https://doi.org/10.1016/j.postharvbio.2017.08.002
- Saltveit, M. E. (2005). Influence of heat shocks on the kinetics of chilling-induced ion leakage from tomato pericarp discs. Postharvest Biology and Technology, 36(1), 87–92. https://doi.org/10.1016/j.postharvbio.2004.10.007
- Sheng, J., Luo, Y., & Wainwright, H. (2000). Studies on lipoxygenase and the formation of ethylene in tomato. The Journal of Horticultural Science and Biotechnology, 75(1), 69–71. https://doi.org/10.1080/14620316.2000.11511202
- Sheng, J., Ye, J., Shen, L., & Luo, Y. (2002, August). Effect of lipoxygenase and jasmonic acid on ethylene biosynthesis during tomato fruit ripening. In XXVI international horticultural congress: Asian plants with unique horticultural potential: Genetic resources, cultural 620 (Taylor & Francis Online) (pp. 119–125).
- Tucker, G. A., Robertson, N. G., & Grierson, D. (1980). Changes in polygalacturonase isoenzymes during the ‘ripening’of normal and mutant tomato fruit. European Journal of Biochemistry, 112(1), 119–124. https://doi.org/10.1111/j.1432-1033.1980.tb04993.x
- Van De Poel, B., Bulens, I., Hertog, M. L., Nicolai, B. M., & Geeraerd, A. H. (2014). A transcriptomics‐based kinetic model for ethylene biosynthesis in tomato (Solanum lycopersicum) fruit: Development, validation and exploration of novel regulatory mechanisms. New Phytologist, 202(3), 952–963. https://doi.org/10.1111/nph.12685
- Velázquez-López, A. A., La Cruz-Medina, D., García, H. S., Vela-Gutiérrez, G., Torres-Palacios, C., & León-García, E. (2020). Lipoxygenase and its relationship with ethylene during ripening of genetically modified tomato (Solanum lycopersicum). Food Technology and Biotechnology, 58(2), 223–229. https://doi.org/10.17113/ftb.58.02.20.6207
- Wang, L., Baldwin, E., Luo, W., Zhao, W., Brecht, J., & Bai, J. (2019). Key tomato volatile compounds during postharvest ripening in response to chilling and pre-chilling heat treatments. Postharvest Biology and Technology, 154 (8) , 11–20. https://doi.org/10.1016/j.postharvbio.2019.04.013
- Wang, S., Tang, J., & Cavalieri, R. P. (2001). Modeling fruit internal heating rates for hot air and hot water treatments. Postharvest Biology and Technology, 22(3), 257–270. https://doi.org/10.1016/S0925-5214(01)00085-0
- Wei, Y., Zhou, D., Wang, Z., Tu, S., Shao, X., Peng, J., & Tu, K. (2018). Hot air treatment reduces postharvest decay and delays softening of cherry tomato by regulating gene expression and activities of cell wall‐degrading enzymes. Journal of the Science of Food and Agriculture, 98(6), 2105–2112. https://doi.org/10.1002/jsfa.8692
- Workman, M., & Pratt, H. K. (1957). Studies on the physiology of tomato fruits. II. Ethylene production at 20° C as related to respiration, ripening, and date of harvest. Plant Physiology, 32(4), 330. https://doi.org/10.1104/pp.32.4.330
- Xue, Z., Kou, X., Luo, Y., Zhu, B., & Xu, W. (2009). Effect of ethylene on polygalacturonase, lipoxygenase and expansin in ripening of tomato fruits. Transactions of Tianjin University, 15(3), 173–177. https://doi.org/10.1007/s12209-009-0031-4
- Yilmaz, E. (2001). Kinetic studies with crude tomato lipoxygenase. Turkish Journal of Agriculture and Forestry, 25(5), 291–296 https://dergipark.org.tr/en/pub/tbtkagriculture/issue/11649/138696.
- Yoshida, O., Nakagawa, H., Ogura, N., & Sato, T. (1984). Effect of heat treatment on the development of polygalacturonase activity in tomato fruit during ripening. Plant and Cell Physiology, 25(3), 505–509 https://doi.org/10.1093/oxfordjournals.pcp.a076739.
- Zhang, Y., Ji, H., Yu, J., & Zhang, Z. (2018). Effect of cold and heat shock treatment on the color development of mature green tomatoes and the roles of their antioxidant enzymes. Food and Bioprocess Technology, 11(3), 705–709. https://doi.org/10.1007/s11947-017-2053-6
