ABSTRACT
The extraction of bioactive compounds from natural food sources through non-conventional novel methods is gaining popularity due to their numerous advantages over conventional methods. In this review article, various extraction methods such as conventional and non-conventional novel methods were discussed with emphasis on green extraction methods. The use of hybrid extraction techniques has an effective and novel method of extraction. The bioactive compounds extracted by such novel techniques have great potential in functional food market, as they are considered as safer. Additionally, with an increasing interest of consumers toward food product, these novel greener technologies would be safer since it has less chemical interference. The outcome of this review will give an idea for appropriate extraction process with better efficiency and eco-friendly, which could be beneficial for extraction industry. Further research is required to validate these green extraction techniques in terms of their safety, consumer acceptability, challenges, and legal feasibility.
1. Introduction
In recent years, it’s been seen as a surge for the use of various bioactive compounds such as pigments, minerals, polysaccharides, organic acids, dietary fibres, sugars, lipids, and phytochemicals (polyphenols, carotenoids) for various purposes, which has led to the adoption of suitable extraction technique in order to confer the desired health benefits (Granato et al., Citation2017; Valdes & Garrigos, Citation2016). These bioactive compounds have diverse applications in foods such as antioxidants, coloring agents, and preservatives (Franco et al., Citation2018). Bioactive compound extraction was performed by conventional methods (e.g. Soxhlet extraction, hydro distillation, water distillation, and steam distillation), combined water and steam distillation, maceration, cohobation, enfleurage and heating reflux extraction has been in wide use in food industry. The application of heat, mixing as well as extracting power of solvents is the driving force for conventional extraction methods (Tongnuanchan & Benjakul, Citation2014). However, the problem with these methods is that they are laborious, time-consuming, and expensive, and have low extraction selectivity, cause thermal degradation of thermolabile compounds, and pose environmental disposal problem (Koubaa et al., Citation2015; Qi et al., Citation2015; Roohinejad et al., Citation2014). Thus, there arises the need to explore novel techniques for bioactive compounds extraction. The potential non-conventional novel extraction techniques nowadays in use are ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), high-pressure (HP), pressurized liquid extraction (PLE), negative pressure cavitations-assisted extraction (NPCE), subcritical water extraction (SWE), supercritical fluid extraction (SFE), enzyme-assisted extraction (EAE), pulsed electric field-assisted extraction (PEF), and accelerated solvent extraction (ASE) (Azmir et al., Citation2013). The non-conventional extraction techniques are non-thermal and “green” in concept, as they comply with Environmental Protection Agency (Putnik et al., Citation2018). These green techniques also utilize relatively lower amounts of solvents, energy lesser extraction time and provide higher yields, and are environment-friendly (Giacomettia et al., Citation2018). The concept of green extraction techniques puts an impetus on the creation of sustainable extraction techniques and intends to follow the strategy of the United Nations for 2030. This concept is called as e3 principle and includes six principles: (a) well-defined sourcing; (b) limiting the organic solvent usage; (c) minimizing consumption of energy; (d) by-product generation with high potential of value addition; (e) lessening the duration of extraction; and (f) natural ingredient recovery (Chemat et al., Citation2017; Mnayer et al., Citation2017). The current review article presents an updated overview of green extraction techniques, their principles, applications, and future prospective, as a technological imperative for the extraction of bioactive compounds.
2. Extraction of bioactive compounds by conventional extraction methods
The effective extraction techniques for bioactive compounds have been a study of interest. However, the conventional methods using solvent still exist, namely, boiling, Soxhlet extraction, heat reflux, soaking etc. due to their ease in operation. Among these, organic solvent extraction is widely used despite being time-consuming due to resistance of plant tissues to solvent penetrations (Tiwari, Citation2015). Some of the conventional extraction techniques are discussed below:
2.1. Soxhlet extraction
The extraction of valuable bioactives from plant sources has been carried out by Soxhlet extraction since decades for fat extraction and is still limited for this purpose. The method is currently used as a comparative method for novel extraction methods. The technique involves putting dried sample inside thimble and then put in distillation flask containing desired organic solvent. The solution is aspirated off by means of siphon into distillation flask once it reaches the overflow point. The extracted fat/solute is retained in the distillation flask as it is carried into the solvent by the solution. The solvent is pushed back to the solute and process is continued until complete extraction.
2.2. Maceration
Maceration has been the prevalent and cheap technique for the extraction of bioactive compounds and essential oils. The maceration process involves several steps namely grinding, addition of suitable solvent, and straining off the liquid. The pressed out liquid and strained mass are mixed and separated by means of filtration.
2.3. Hydrodistillation
Hydrodistillation as conventional extraction method has also been used for bioactive and essential oil extraction. The method does not require drying of material and organic solvents. The process can be performed by three ways: water and steam distillation, water distillation, and direct steam distillation (Vankar, Citation2004). The hydrodistillation involves packing of sample in compartment and then boiling in sufficient amount of water. Conversely, direct steam can be injected into the plant material. The use of hot water and steam is the major extractants for bioactive compound separation from plant tissues. Cooling can be performed indirectly by the use of water which results in condensing water and oil vapor mixture. The condensed mixture is passed to a separator, which separates the oil and bioactive compounds from water (Masango, Citation2005).
3. Novel extraction techniques
The extraction is a crucial process for isolation, identification, and separation of bioactive compounds prior to their analysis. In this context, novel extraction techniques with better yields, safety, and eco-friendly nature with wider applicability and optimization have been developed (Giacomettia et al., Citation2018). These innovative non-thermal extraction techniques decrease mass transfer limitations and enable sustainable production of green chemical engineering (Chemat et al., Citation2017a). However, there are drawbacks associated with these innovative methods, which include high initial capital investment and lack of control of all process variables that minimize their applicability at large-scale level (Chemat et al., Citation2011). The promising green extraction methods includes MAE, UAE, HP assisted extraction (PLE), SWE, SFE, electrically assisted extraction (PEF, and high voltage electric charges) and EAE are discussed in detail in below sections. (Barba et al., Citation2015; Xie et al., Citation2015; Xynos et al., Citation2012).
3.1. Ultrasound-assisted extraction of bioactive compounds
Ultrasound-assisted extraction (UAE) at the frequency range of about 35 kHZ is most popular novel extraction technique for natural materials. The process is carried out at around 70°C for 2 hrs which is relatively lesser than used in conventional extraction methods. The selectivity of solvent ascribes to the extraction efficiency of UAE (Banožić et al., Citation2019) and minimizes the thermal degradation of bioactive compounds through cavitation mechanism (Ameer et al., Citation2017; Vardanega et al., Citation2014). In recent years, there has been number of studies on the use of ultrasound-assisted extraction as an alternative to conventional extraction techniques due to higher extraction efficiency, less energy consumption, and use of safe and non-toxic solvents (Chemat et al., Citation2017; Shirsath et al., Citation2012; Tiwari, Citation2015). The target bioactive compounds are released due to induction of cavitation (production, growth and collapse of bubbles), pressure thermal and mechanical effects by ultrasonic waves (Rosello-Soto et al., Citation2015; Zhu et al., Citation2017). The selection of suitable solvent in ultrasound-assisted extraction is crucial process as it affects the cavitation mechanism and acoustic transfer of energy (Lupacchini et al., Citation2017). The promising solvents used in UAE are ionic liquids, glycerol, water, oligomers of ethylene glycol, and various other biomass-based solvents (Veillet et al., Citation2010). The diffusion of solvent within the sample is accelerated through propagation of ultrasonic waves by inducing radiation pressure, which creates friction between sample and medium molecules. This results in efficient extraction of compounds from plant tissues at the cost of their deformation. The UAE methods, however, result in low extraction efficiency due to least contact between sample and ultrasonic field (Chen et al., Citation2017). Although cavitation effect can be induced in liquid and liquid-containing solid materials but ultrasound results in effective extraction of bioactive compounds from solid materials. UAE has limited applicability in industries due to confinement of cavitation mechanism to lab scale extractors only which is now being resolved by flow batch reactors with reduced extraction time (Cravotto et al., Citation2018). The operating parameters, namely, temperature, time, power, frequency, solvent type, solvent/solid ratio etc., can be monitored during the extraction of bioactive compounds by UAE (Banožić et al., Citation2019). The UAE is proven as efficient technique for the extraction of phenolic-rich compounds and its numerous applications in extraction of food components are listed in . UAE has been used to extract isoflavone derivatives namely, genistin, glycitin, daidzin, and malonyl genistin from soybean, phenolic compounds from strawberries, namely, rutin, quercetin, kaempferol, naringin, naringenin, and ellagic acid, chlorogenic acid from leaves and bark, quercetin and rutin from Euonymus alatus, alkaloids from Catharanthusroseus, anthocyanins from grape peel rosmarinic acid from Rosmarinus officinalis and essential oil from cloves (Azmir et al., Citation2013; Rostagno et al., Citation2007; Tekin et al., Citation2015). Ultrasound-assisted green extraction technique is widely accepted as sustainable processing technique for extraction of wide range of natural derivatives (Tiwari, Citation2015).
Table 1. Novel techniques for extraction of bioactive compounds and essential oils from foods.
3.2. Microwave-assisted extraction of bioactive compounds
The microwave-assisted extraction (MAE) utilizing microwave energy at a frequency of 2.45 GHz is considered as a non-conventional method of extraction for natural products (Pimentel-Moral et al., Citation2018). This type of extraction is low energy, solvent, and reduced time extraction involving a blend of solvent extraction and extraction using microwaves (Banozic et al., Citation2020). The process involves the conversion of electromagnetic energy to heat through the mechanism of dipole rotation and ionic conduction. The mechanism of extraction in microwave-assisted extraction involves three steps (Ani et al., Citation2011): firstly, separation of solutes under accelerated temperature and pressure from active sites of sample occurs; secondly solvent diffuses across the sample matrix; and thirdly the solutes are released from sample within solvent. The advantages of MAE over conventional methods include faster heating, lesser thermal degradation, and enhanced yield (Cravottoa et al., Citation2008). MAE is a green extraction technology that minimizes the use of organic solvents and is an efficient extraction technique for organometallic and organic compounds (Vinatoru et al., Citation2017). Microwave-assisted extraction has proven to be a potential effective method for the extraction () of caffeine and polyphenols from green tea (Pan et al., Citation2003), bioactive compounds from edible flowers Hibiscus sabdariffa (Pimentel-Moral et al., Citation2018), essential oil from lilac buds, (Kapadiya et al., Citation2018), ginsenosides from ginseng root (Shu et al., Citation2003), silybinin, flavolignin from Silybum marianum, phenolic compounds from bran and flour of maize and sorghum (Chiremba et al., Citation2012), flavonoids and phenols from Chinese quince (Teng et al., Citation2009), tannins and cinnamaldehyde from various plant extracts (Jahanshaei et al., Citation2012). Microwave-assisted green extraction technology has been reported as sustainable food processing technique (Anne et al., Citation2013).
3.3. High-pressure-assisted extraction of bioactive compounds
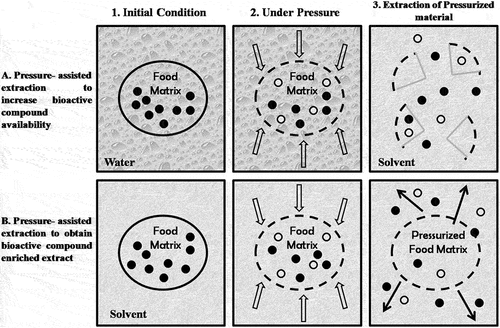
HP processing applied at the pressure range of 100 MPa to 1000 MPa has emerged as novel green technology in food processing (Barba et al., Citation2015) with no reported adverse effects on human health (Barba et al., Citation2016; Pereira & Vicente, Citation2010). The application of HP as an aid to extraction process for the separation of bioactive compounds has proven to be promising method as it minimizes the temperature surge and thereby, maintains the functionality of natural bioactives (Alexandre et al., Citation2017; Barba et al., Citation2015). The process of extraction through HP can be either negative pressure, HP (above 100 MPa) or medium- to HP at moderate pressure of 10 MPa, which includes negative pressure extraction (NPE), pressurized hot water extraction (PHWE), and pressurized liquid extraction (PLE). The extraction occurs in three steps (): come up step, pressure upholding step, and pressure relief step (Huang et al., Citation2013). The length of the phase is an absolute indicator of yield of extraction and in maintenance step, both inside and outside cell pressure is balanced which allows rapid solvent permeation thereby, dissolving the active compound in a shorter duration. However, the pressure relief stage lasts few seconds due to rapid decrease in pressure within cells concomitant with the changes in the non-covalent bonds. Due to HP, the process of extraction takes place at faster rate and higher yields than conventional extraction methods (Ferrentino et al., Citation2016; Prasad et al., Citation2011). The higher yields could be ascribed not only to inactivation of degrading enzymes (Chakraborty et al., Citation2014) but to decreased pH value also which enhances the bioactivity (Ahmed & Ramaswamy, Citation2006; Corrales et al., Citation2009). The magnitude of applied pressure and structural and physiological composition of natural source containing bio active compound is determinant factor for their dissolution rate (Serment-Moreno et al., Citation2017).
Figure 1. Schematic representation of the steps commonly applied in studies reporting effect of high-pressure processing (HPP) on food compound extractability (Jung, Citation2016).
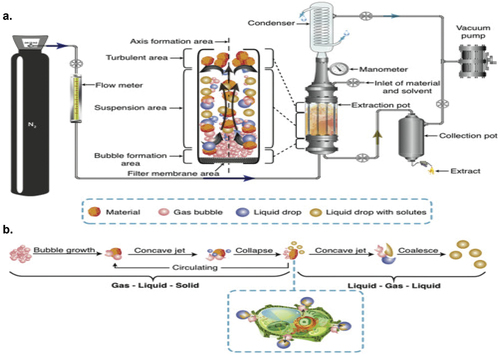
3.4. Negative pressure cavitation-assisted extraction of bioactive compounds
Negative pressure extraction is among the novel green extraction technologies in which cavitation is produced by negative pressure, which results in mass transfer, turbulence, and collision between solid matrix and solvent (Ramos et al., Citation2019), (). This extraction technique is economical with higher extraction yields (Luo et al., Citation2014; Wang et al., Citation2015; Zhao et al., Citation2011) in which negative pressure is induced into nitrogen solvent system and sample, creating collision between them. In addition to collision turbulence, cavitations and gas-liquid-solid systems result in rupture and expansion of cells, thereby enhanced mass transfer (. There also occurs complete contact between dispersed sample and the solvent in case of negative pressure extraction (Liu et al., Citation2009). This negative pressure cavitation-based extraction is coupled with ultrasound-assisted extraction (NPC-UAE) in which sample is allowed to get deposited at the base of the ultrasonic device in which solvent system is dispersed to encounter gigantic ultrasonic field (Zhang et al., Citation2011). Many studies have reported the success of this technique both alone as well as in combination with other extraction methods, such as enzymatic extraction, homogenization, mechanical pulverization, microwave extraction, ionic liquid solvents, and deep eutectic solvents in extracting bioactive compounds from foods, namely, isoflavonoids, flavonoids, saponins, polyphenols, stilbenes, and alkaloids. The NPC extraction technique because of its easy procedure, economical, and high efficiency has explored its potential industrial applications than other extraction techniques (Ramos et al., Citation2019). The applications of negative pressure cavitation (NPC) extraction include () the extraction of phytochemicals (alkaloids, polyphenols, polysaccharides, flavonoids) from roots, leaves, and seeds (Liu et al., Citation2009; Zhang et al., Citation2010). Negative pressure cavitation-based ultrasound-assisted extraction has been used to extract flavonoids (rutin, nicotiflorin, narcissin, quercetin, kaempferol, and isorhamnetin) from Flos sophorae immature (Wang et al., Citation2019).
3.5. Pressurized liquid and hot water extraction of bioactive compounds
The combined effect of increased pressure for enhanced extraction represents the method known as Pressurized Liquid Extraction (PLE), or Pressurized Solvent Extraction (PSE), or Accelerated Solvent Extraction (ASE) (Zhu et al., Citation2017). The unique feature of this extraction is the requirement of less solvent while maintaining the solvent-liquid state even above the normal boiling point (Azmir et al., Citation2013). The extraction yield of bioactive compounds from solid matrices can further be increased by choosing suitable solvent, optimum solid-liquid ratio, temperature, pressure and length of extraction cycle. The range of pressure (3.5–20 MPa) and temperature from normal room temperature to 200°C is used in PLE (Mustafa & Turner, Citation2011). If in case of pressurized extraction, the solvent used is water, it is called as pressurized hot water extraction (PHWE). During extraction, the low dielectric constant of water (solvent) associated with high temperatures results in decrease of viscosity, thereby decreasing the surface tension and ultimately increased diffusion of bio actives (Herrero et al., Citation2013). Pressurized hot water extraction is suitable for extraction of polar, non-polar, and moderately polar compounds (Bursać et al. Citation2018). Accelerated solvent extraction method coupled with UHPLC-DAD has been declared to be an efficient method for identifying the phytochemical profile of various foods (Ahmad et al., Citation2019). Many applications of PLE for extracting natural products have been cited in the literature (Kaufmann & Christen, Citation2002). Some of the applications are listed in as isoflavones from soybeans (Rostagno et al., Citation2004), thymoquinone (THQ) from black seeds (Ahmad et al., Citation2019), terpenoids and sterols from tobacco (Shen & Shao, Citation2005), flavonoids from spinach (Howard & Pandjaitan, Citation2008), phenolic compounds from parsley (Petroselinum crispum), lycorine and galanthamine (Amaryllidaceae alkaloids) from Narcissus jonquilla (Mroczek & Mazurek, Citation2009), phenolic compounds (gallocatechin (GCT), catechin, epicatechin gallate, caffeic acid, chlorogenic acid, and myricetin) from Anatolia propolis, polyphenols from olive leaves (Xynos et al., Citation2014), bioactive compounds from rosemary leaves (Rodriguez-Meizoso et al., Citation2012), antioxidants (carnosic and rosmarinic acids) (Herrero et al., Citation2010), phenolic compounds from olive leaves of Croatia, and bioactives from marine sponges (Putnik & Kovačevć, Citation2017).
3.6. Supercritical and subcritical fluid extraction of bioactive compounds
Supercritical and subcritical extraction techniques are novel methods for obtaining bioactive compounds efficiently with unique properties of solvents, namely, diffusivity, density, viscosity, and dielectric constant (Rosello-Soto et al., Citation2016). Subcritical and supercritical extraction techniques, work alone or in combination with other extraction methods for enhanced yield and quality of the bioactive compounds. (Essien et al., Citation2020). These extraction methods have gained significant interest in recent years for the extraction of bioactive compounds from natural sources (Formato et al., Citation2013).
Subcritical water extraction (SWE) as the green extraction technique utilizes the unique properties of water at subcritical point (374°C, 220 bar) and is also known as hot liquid solvent or pressurised hot water extraction (Srinivas & King, Citation2010). The subcritical water extraction involves the mechanism of transfer of solutes from the sample to the extraction medium by the process of diffusion, partitioning equilibrium, and convection (Zakaria & Kamal, Citation2015).
Supercritical fluid extraction (SFE) as one of extraction method in wide use ensures efficient and fast extraction at moderate temperatures and without use of harmful solvents and clean-up steps. The ideal solvent used is carbon dioxide (CO2) at its fairly low critical temperature (32°C), pressure (7.4 MPa) and due to its non-toxic, non-explosive, and readily available nature (Pourmortazavi & Hajimirsadeghi, Citation2007). The change in different extraction parameters (temperature, flow rate, extraction time, and pressure) helps in the collection of specific fractions, as it is convenient to collect extracts and solvent separately (Koubaa et al., Citation2017). A super critical fluid is formed when the pressure and temperature are increased above critical points specific for that particular liquid or gas. In the supercritical area, the separation surface between liquid and gas disappears, and a homogeneous super critical fluid emerges (McHugh & Krukonis, Citation2013). Supercritical fluids (SFs) pose a density change in comparison with liquids, and even a slight increment in pressure leads to huge increase in density of fluid which enhances the extracting power of the super critical fluid. The volatile nature of super critical fluid enables the recovery of solvent from active extracts (Pourmortazavi & Hajimirsadeghi, Citation2007). The process of super critical fluid extraction can employ either offline or online methods. In offline method, the sample is placed in a basket within an extraction vessel which is continuously being filled from the bottom by super critical fluid. The super critical fluid containing extracted components within the extraction vessel flow to the separator through depressurization valve thereby collecting the extracts from the gaseous fluid into a collector (Fornari et al., Citation2012). In online method, the super critical fluid extraction instrument is directly connected with analytical devices like gas chromatography (GC) or liquid chromatography (LC) columns. The online mode causes a large amount of extract to flow through extraction vessel, which minimizes the loss of sample, exposure to air and light along with decrease in the analysis time (Yousefi et al., Citation2019). The mechanism of super critical fluid extraction involves heating and pressurisation whereby mass transfer occurs by diffusion, convection, and displacement by expansion of solution. (Bruno et al., Citation2019). Numerous applications of both super critical and subcritical processes in food analysis for extraction of natural extracts have been reported in , namely, antimicrobial and antioxidant compounds from natural sources (Rahal et al., Citation2015), essential oils from plant sources (Roohinejad et al., Citation2017), essential oil from Algerian rosemary leaves (Zermane et al., Citation2010), essential oils from sage, rosemary, marjoram, basil, thyme, oregano, and marigold belonging to family Lamiaceae, decaffeination of coffee preparation, purine alkaloids (caffeine, theobromine, and theophylline) from Ilex paraguaryensis (herbal matetea), naringin (flavonoid) from citrus paradise than, polyphenols, procyanidins catechin and epicatechin from grape, alkaloids from Catharanthus roseus leaves, beta-carotene and linoleic acids from Eucheuma cottonii and Gracilaria sp. (Machmudah et al., Citation2017), polyphenol from pepper-rosmarin leaves (Garmus et al., Citation2015), anthocyanins from the pericarp and cob of purple corn (Essien et al., Citation2020), extraction of bioactive compounds from flowers, bioactives from Lonicera japonica Thunberg (Hsu et al., Citation2016), eugenol from clove (Frohlich et al., Citation2018), quercetin and gallic acid from Macela flowers, chlorogenic acid from seeds of sunflower, bioactives from sunflower (Helianthus annuus L.) (Daraee et al., Citation2018), flavonoids from cole pollen, encapsulation (Cheng et al., Citation2017), development of bioactive film packaging (Zhao & Saldaña, Citation2019) and production of anti-biofilms (Zizovic et al., Citation2018).
3.7. Electrically assisted extractions of bioactive compounds
The electrically assisted extraction of bioactive compounds involves two non-thermal technologies, namely, pulsed electric field (PEF) and high-voltage electric discharge plasma (HVED). Pulsed electric field involves the short (μs) and high pulses (kV/cm) with the help of electrodes at a range of 15–35 kV/cm by generating 50–700 kJ/kg of energy (Evrendilek, Citation2017; Yan et al., Citation2017). It consists of a treatment chamber comprising of two electrodes in between which the sample is placed. The pulsed electric field extraction can be either batch or continuous mode according to the design of treatment chamber (Buckow et al., Citation2013). The efficiency of pulsed electric field-assisted extraction depends on specific energy input, temperature, field strength, pulse number, and characteristics of the sample (Soliva-Fortuny et al., Citation2009). The use of pulsed electric field has gained interest in recent years as an efficient method of bioactive extraction due to low energy consumption and capability to form metabolically new compounds along with release of intracellular compounds by enhancing cell membrane permeability (Parniakov et al., Citation2016). Pulsed electric field has also been used to enhance the yield, bio availability and solvent diffusion assisted extraction in various foods (Barba et al., Citation2015). Various advantages of pulsed electric field assisted extraction, namely, enhanced mass transfer, less extraction time, high yield, and low temperature has explored its industrial applicability (Puertolas et al., Citation2016; Yan et al., Citation2017). However, the industrial application were limited at the initial stages of adoption of this technique due to less commercial availability of treatment chambers and PEF generators (Raso et al., Citation2016; Yan et al., Citation2017). Pulsed electric field is used as a pre-treatment for various extraction methods due to fewer requirements of extraction parameters. The pulsed electric field has been used to extract olive oil (Abenoza et al., Citation2013), essential oil from rose (Tintchev et al., Citation2012), vegetable oil (Abenoza et al., Citation2013), eugenol, ellagic acid and quercetin from Emblica officinalis, total flavonoids, and antioxidants from fresh spearmint leaves (Zhou et al., Citation2017), betanin from beetroots, phytosterols from maize, isoflavonoids from soybeans, anthocyanins monoglucosides from grape, anthocyanin and polyphenols from grape skin, polyphenols and anthocyanins from Merlot skin (Puertolas et al., Citation2016), polyphenols, chlorophyll and carotenoids from Stevia rebaudiana Bertoni, extraction of sunflower oil, juice extraction from plant tissues and bioactive compounds from edible flowers (Gabrić et al., Citation2017).
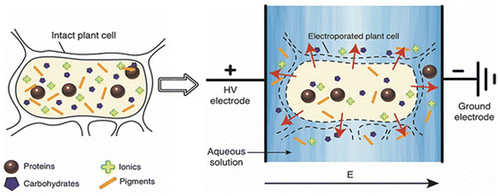
Another electrically assisted novel extraction technology involves high-voltage electric discharge plasma (HVED), which represents the pulsed mode plasma systems and is also known as “corona discharge” due to crown like discharge surrounding the cathode wire through pulsed DC power supply (Attri & Grover, Citation2015). In HVED, hot and localized plasma are generated along with release of – OH radicals and emission of UV light through photonic dissociation of water. This result in pyrolytic effect and generation of shockwaves due to electrohydraulic cavitation (Xie et al., Citation2015). This extraction process used at an electric field intensity of 20-80kV/cm causes electrical breakdown, which leads to physical and chemical changes resulting in release of intracellular components (Barba et al., Citation2015). The HVED has been used as an efficient technique for extraction of specific bioactive compounds with enhanced yield and mass transfer (Parniakov et al., Citation2016). The high-voltage pulsed electric field has been used to extract bioactive compounds from natural sources (). In one study, the HVED has been reported to be an efficient technique for extraction of polyphenols and proteins from olive pit than UAE or PEF. The HVED has been used to extract pectin from sugar beet pulp (Almohammed et al., Citation2018), polyphenols, and proteins while extracting essential oils from herbs and oilseeds and extraction of essential oils for the development of novel functional products (Giacomettia et al., Citation2018).
Figure 3. Selective extraction of bioactive and useful components from plant cells assisted by electroporation using pulsed electric field (Barba et al., Citation2016).
3.8. Enzyme-assisted extraction of bioactive compounds
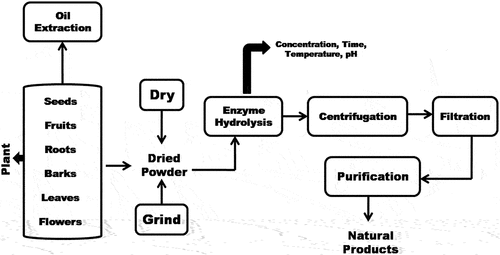
Enzymatic-assisted extraction is one among the novel eco-friendly technique for increased yield and extraction of cell bound compounds, oil and bioactive compounds as it utilizes water as solvent rather than organic chemicals (Puri et al., Citation2012). In normal extraction processes, the sample matrix is not in close contact with solvent system, while in case of enzyme-assisted extraction, the cytoplasm bound bioactive compounds are made accessible to solvent. Various enzymes for hydrolyzing cell wall and polysaccharides in plant materials are being used in enzymatic extraction such as α-amylase, pectinase, and cellulose. The enzyme-assisted extraction can be performed either by enzyme-assisted aqueous extraction (EAAE) or by enzyme-assisted cold pressing (EACP) (Latif & Anwar, Citation2009). The enzyme-assisted aqueous extraction involves the formation of colloidal polysaccharide-protein complex while in enzyme-assisted cold pressing, the enzyme used hydrolyses the cell wall without the formation of colloidal polysaccharide-protein (Concha et al., Citation2004). In enzymatic assisted-extractions, the concentration and composition of enzyme, sample to solvent ratio, hydrolysis time, particle size, and moisture content of sample and are crucial factors for extraction (Niranjan & Hanmoungjai, Citation2004). The EAAE has been mainly explored for the extraction of oils from different oil seeds (), (Concha et al., Citation2004). EACP has also been demonstrated as an efficient method for extraction of bioactive compounds from oilseeds with higher free fatty acids and phosphorus content (Latif & Anwar, Citation2009). The enzyme-assisted-extraction has been used in extraction of bioactive compounds () namely, phenolic compounds, anthocyanins, non-anthocyanin flavonoids, and anti-oxidants from pomace of grapes, phenolic compounds from Ribes nigrum pomace (Maier et al., Citation2008), phenolic compounds from citrus peels (Li et al., Citation2013), phenolic antioxidants from solid wastes of raspberry, and phenolic compounds from grape waste (Laroze et al., Citation2010).
Figure 4. The process of enzyme-assisted extraction method from the natural products (Selvamuthukumaran & Shi, Citation2017).
3.9. Other methods for extraction of bioactive compounds
There are many other extraction methods for bioactive compound extraction from natural sources which include ohmic accelerated steam distillation (OASD), Ohmic-assisted hydrodistillation (OAHD), ionic liquids (ILs), and thermo mechanical transient control pressure drop (DIC). Ohmic accelerated steam distillation (OASD) unlike traditional steam distillation makes use of volumetric ohmic heating to enhance the extraction rate.
The method has been successfully employed for extraction of lavender essential oil (Gavahian & Chu, Citation2018). Another method that involves the combination of distillation and ohmic heating is known as Ohmic-assisted hydrodistillation (OAHD). The method is energy economical with less extraction time and has been used for essential oil extraction from Zataria multiflora Boiss (Chemat et al., Citation2012). Ionic liquids (ILs) are now a days being used in green extraction techniques and are replacing organic solvents (Ramos et al., Citation2019). ILs as green solvents in extraction processes are gaining popularity due to their non-volatility, low toxicity, high ionic conductivity, non-flammability, and hydrophobicity, different polarity, and selectivity (Liu et al., Citation2019; Zlabur et al., Citation2018). Ionic liquids (ILs) in combination with novel extraction techniques such as ultrasound-assisted extraction, microwave-assisted extraction, and subcritical water extraction are increasing tremendously (Ullah et al., Citation2019). The ionic liquids in combination with novel extraction techniques have been used to extract the bioactive compounds from food by-products and natural sources as well as in the processing of poultry feathers, crustacean shells, peanut hulls, potato peels, and citrus (Arshadi et al., Citation2016). Further, ionic liquids are used as mobile or stationary phase in different analytical techniques (Nan & Anderson, Citation2018; Zhang, Citation2018) and green solvents in liquid-phase microextraction and liquid/liquid extraction (Mei et al., Citation2019). Further applications of ILs include additives, reactants, microwave absorbers or templates for preparation of polymers, biomass-based composites, carbon-derived composites, and inorganic nanomaterials (Pathak et al., Citation2016; Wang et al., Citation2019). ILs has been used for the extraction of phenolic compounds, anthocyanins, and proanthocyanidins from grape skin (Curko et al., Citation2017), lingo cellulosic materials (Gillet et al., Citation2017), hemicellulose, cellulose, lignin, furfural, 5-hydroxymethylfurfural (5-HMF) and reducing sugars (Wang et al., Citation2019), fermentable sugars from eucalyptus biomass residues and wheat straw (Bernardo et al., Citation2019), phenolic compounds (Lopes et al., Citation2018), alkaloids, terpenoids, flavonoids, saponins, and essential oils (Passos et al., Citation2014; Xiao et al., Citation2018).
Another method is thermomechanical transient control pressure drop (DIC) in which plant structure is expanded that increases the rate of diffusion (Zhang et al., Citation2020). The DIC method has been used to extract anthocyanin and anthocyanins in rose calyces (Tiwari, Citation2015). The crucial factors for this type of extraction include pressure, temperature, time duration, solvent, ratio of solvent to matrix and matrix properties (Shukla et al., Citation2019).
4. Challenges and perspectives
Although the novel extraction technologies are spreading like a raging fire, in response to increasing customer demand but at the same time they pose certain challenges. The commercialization of most of these novel methods takes time before all quality issues related to these techniques are resolved. In addition to this, it becomes mandatory in near future to couple various novel extraction techniques for effective, sustainable, and safe production of bioactive compounds. Further studies are needed to explore other green techniques for the extraction of bioactive compounds. The challenges proposed by the environmental protection and competent global world intensely suggest the innovations in existing methods that are efficient than traditional ones rather than simply their continuity (Chemat, Vian, Citation2019). Green extraction techniques respond to challenges by extending shelf life and maintaining nutritional value of food products, mean while using less energy and unit operations there by contributing to sustainable food processing by utilizing naturally ingredients (bioactives and essential oils) (Chemat, Vorobiev, Citation2019).
5. Conclusion
The ever-rising demand for bioactive compounds from natural sources has resulted in various novel extraction techniques. This surge in demand due to well-established and well-known benefits of bioactive compounds may lead to exploration of further novel extraction techniques in future. However, it is imperative to understand every facet of these non-conventional extraction processes as these extraction methods differ in their mechanism as well as extraction behavior. The development as well as application of hybrid methods involving combination of different extraction methods needs to be investigated keeping in view the properties of material. The bioactive compounds extracted by novel techniques have great potential in functional food market as they extracted under mild processing conditions for better retention of functionality. Further research is required to evaluate these green extraction techniques in terms of their safety, scalability, consumer acceptability, challenges, legal aspects, and potential feasibility.
Acknowledgement
The authors acknowledge the TEQUIP-III for financial assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abenoza, M., Benito, M., Saldaña, G., Álvarez, I., Raso, J., & Sánchez-Gimeno, A. C. (2013). Effects of pulsed electric field on yield extraction and quality of olive oil. Food and Bioprocess Technology, 6(6), 1367–1373. https://doi.org/10.1007/s11947-012-0817-6
- Ahmad, R., Ahmad, N., & Shehzad, A. (2019). Solvent and temperature effects of Accelerated solvent extraction (ASE) coupled with ultra-high pressure liquid chromatography (UHPLC-DAD) technique for determination of Thymoquinone in commercial food samples of black seeds (Nigella sativa). Food Chemistry, 309, 125740. https://doi.org/10.1016/j.foodchem.2019.125740
- Ahmed, J., & Ramaswamy, H. S. (2006). High pressure processing of fruits and vegetables. Stewart Postharvest Review, 2(1), 1–8. https://doi.org/10.2212/spr.2006.1.8
- Alexandre, E. M. C., Araújo, P., Duarte, M. F., de Freitas, V., Pintado, M., & Saraiva, J. A. (2017). Experimental design, modeling, and optimization of high-pressure-assisted extraction of bioactive compounds from pomegranate peel. Food and Bioprocess Technology, 10(5), 886–900. https://doi.org/10.1007/s11947-017-1867-6
- Almohammed, F., Kouba, M., & Khelfa, A. (2018). Pectin recovery from sugar beet pulp enhanced by high-voltage electrical discharges. Food and Bioproducts Processing, 103, 95–103. https://doi.org/10.1016/j.fbp.2017.03.005
- Ameer, K., Shahbaz, H. M., & Kwon, J.-H. (2017). Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Comprehensive Reviews in Food Science and Food Safety, 16(2), 295–315. https://doi.org/10.1111/1541-4337.12253
- Ani, T. A., Calinescu, L., & Lavric, V. (2011). Microwave extraction of active principles from medicinal plants.Chemistry and. Materials Science, 74(2), 129–142.
- Anne, Y. L., Tixier, S. F., Vian, M. A., & Chemat, F. (2013). Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. Trends in Analytical Chemistry, 47, 1–11. https://doi.org/10.1016/j.trac.2013.02.007
- Arshadi, M., Attard, T. M., Lukasik, R. M., Brncic, M., Lopes, A. M. D., Finell, M., Geladi, P., Gerschenson, L. N., Gogus, F., Herrero, M., Hunt, A. J., Ibanez, E., Kamm, B., Mateos-Aparicio, I., Matías, A., Mavroudis, N. E., Montoneri, E., Morais, A. R. C., Nilsson, C. … Yuste-Cordoba, F. J. (2016). Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chemistry, 18(23), 6160–6204. https://doi.org/10.1039/C6GC01389A
- Attri, R., & Grover, S. (2015). Analyzing the scheduling system stage of production system life cycle. Management Science Letters, 5(5), 431–442. https://doi.org/10.5267/j.msl.2015.3.011
- Azmir, J., Zaidul, I. S. M., Rahman, M. M., Sharif, K. M., Mohamed, A., Sahena, F., Jahurul, M. H. A., Ghafoor, K., Norulaini, N. A. N., & Omar, A. K. M. (2013). Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering, 117(4), 426–436. https://doi.org/10.1016/j.jfoodeng.2013.01.014
- Banozic, M., Babic, J., & Jokic, S. (2020). Recent advances in extraction of bioactive compounds from tobacco industrial waste-a review. Industrial Crops & Products, 144, 112009. https://doi.org/10.1016/j.indcrop.2019.112009
- Banožić, M., Banjari, I., Jakovljević, M., Šubarić, D., Tomas, S., Babić, J., & Jokić, S. (2019). Optimization of ultrasound-assisted extraction of some bioactive compounds from tobacco waste. Molecules, 24(8), 1611. https://doi.org/10.3390/molecules24081611
- Barba, F. J., Brianceau, S., Turk, M., Boussetta, N., & Vorobiev, E. (2015). Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food and Bioprocess Technology, 8(5), 1139–1148. https://doi.org/10.1007/s11947-015-1482-3
- Barba, F. J., Zhu, Z., Koubaa, M., de Souza Sant’Ana, A., & Orlien, V. (2016). Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends in Food Science & Technology, 49, 96–109. https://doi.org/10.1016/j.tifs.2016.01.006
- Bernardo, J. R., Gírio, F. M., & Łukasik, R. M. (2019). The effect of the chemical character of ionic liquids on biomass pre-treatment and posterior enzymatic hydrolysis. Molecules, 24(4), 808–825. https://doi.org/10.3390/molecules24040808
- Bruno, S. F., Ekorong, F. J. A. A., Karkal, S. S., Cathrine, M. S. B., & Kudre, T. G. (2019). Green and innovative techniques for recovery of valuable compounds from seafood by-products and discards: A review. Trends in Food Science & Technology, 85, 10–22. https://doi.org/10.1016/j.tifs.2018.12.004
- Buckow, R., Ng, S., & Toepfl, S. (2013). Pulsed electric field processing of orange juice: A review on microbial, enzymatic, nutritional, and sensory quality and stability. Comprehensive Reviews in Food Science and Food Safety, 12(5), 455–467. https://doi.org/10.1111/1541-4337.12026
- Chakraborty, S., Kaushik, N., Rao, P. S., & Mishra, H. N. (2014). High-pressure inactivation of enzymes: A review on its recent applications on fruit purees and juices. Comprehensive Reviews in Food Science and Food Safety, 13(4), 578–596. https://doi.org/10.1111/1541-4337.12071
- Chemat, F., Rombaut, N., Meullemiestre, A., Turk, M., Perino, S., Fabiano-Tixier, A. S., & Abert-Vian, M. (2017a). Review of green food processing techniques. Preservation, transformation, and extraction. Innovative Food Science & Emerging Technologies, 41, 357–377. https://doi.org/10.1016/j.ifset.2017.04.016
- Chemat, F., Rombaut, N. A., Sicaire, A.-G., Meullemiestre, A., Fabiano-Tixier, A.-S., & Abert-Vian, M. (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry, 34, 540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
- Chemat, F., Vian, M. A., & Cravotto, G. (2012). Green extraction of natural products: Concept and principles. International Journal of Molecular Sciences, 13(7), 8615–8627. https://doi.org/10.3390/ijms13078615
- Chemat, F., Vian, M. A., Tixier, A. S. F., Strube, J., Uhlenbrock, L., Gunjevic, V., & Cravotto, G. (2019). Green extraction of natural products. Origins, current status, and future challenges. TrAc Trends in Analytical Chemistry, 118, 248–263. https://doi.org/10.1016/j.trac.2019.05.037
- Chemat, F., & Vorobiev, E. (2019). Green food processing techniques preservation, transformation and extraction. Elsevier.
- Chemat, F., Zill-E, H., & Khan, M. K. (2011). Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonics Sonochemistry, 18(4), 813–835. https://doi.org/10.1016/j.ultsonch.2010.11.023
- Cheng, Y.-S., Lu, P.-M., Huang, C.-Y., & Wu, J.-J. (2017). Encapsulation of lycopene with lecithin and α-tocopherol by supercritical antisolvent process for stability enhancement. The Journal of Supercritical Fluids, 130, 246–252. https://doi.org/10.1016/j.supflu.2016.12.021
- Chen, F. L., Zhang, Q., Liu, J. L., Gu, H. Y., & Yang, L. (2017). An efficient approach for the extraction of orientin and vitexin from Trollius chinensis flowers using ultrasonic circulating technique. Ultrasonics Sonochemistry, 37, 267–278. https://doi.org/10.1016/j.ultsonch.2017.01.012
- Chiremba, C., Taylor, J. R. N., Rooney, L. W., & Beta, T. (2012). Phenolic acid content of sorghum and maize cultivars varying in hardness. Food Chemistry, 134(1), 81–88. https://doi.org/10.1016/j.foodchem.2012.02.067
- Concha, J., Soto, C., Chamy, R., & Zuniga, M. E. (2004). Enzymatic pretreatment on rose-hip oil extraction: Hydrolysis and pressing conditions. Journal of the American Oil Chemists’ Society, 81(6), 549–552. https://doi.org/10.1007/s11746-006-0939-y
- Corrales, M., Garcia, A. F., Butz, P., & Tauscher, B. (2009). Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. Journal of Food Engineering, 90(4), 415–421. https://doi.org/10.1016/j.jfoodeng.2008.07.003
- Cravottoa, G., Boffa, L., & Mantegna, S. (2008). Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrasonics Sonochemistry, 15(5), 898–902. https://doi.org/10.1016/j.ultsonch.2007.10.009
- Cravotto, G., Mariatti, F., Gunjevic, V., Secondo, M., Villa, M., Parolin, J., & Cavaglià, G. (2018). Pilot scale cavitational reactors and other enabling technologies to design the industrial recovery of polyphenols from agro-food by-products, a technical and economical overview. Foods, 7(9), 130. https://doi.org/10.3390/foods7090130
- Curko, N., Tomasevi, M., Bubalo, M. C., Gracin, L., Redovnikovi, I. R., & Gani, K. K. (2017). Extraction of proanthocyanidins and anthocyanins from grape skin by using ionic liquids. Food Technology and Biotechnology, 55(3), 429–437. https://doi.org/10.17113/ftb.55.03.17.5200
- Daraee, A., Ghoreishi, S. M., & Hedayati, A. (2018). Supercritical CO2 extraction of chlorogenic acid from sunflower (Helianthus annuus) seed kernels: Modeling and optimization by response surface methodology. The Journal of Supercritical Fluids, 144, 19–27. https://doi.org/10.1016/j.supflu.2018.10.001
- Essien, S. O., Young, B., & Baroutian, S. (2020). Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends in Food Science & Technology, 97, 156–169. https://doi.org/10.1016/j.tifs.2020.01.014
- Evrendilek, G. A. (2017). Impacts of pulsed electric field and heat treatment on quality and sensory properties and microbial inactivation of pomegranate juice. Food Science and Technology International, 23(8), 668–680. https://doi.org/10.1177/1082013217715369
- Ferrentino, G., Asaduzzaman, M., & Scampicchio, M. M. (2016). Current technologies and new insights for the recovery of high valuable compounds from fruits by-products. Critical Reviews in Food Science and Nutrition, 58(3), 386–404. https://doi.org/10.1080/10408398.2016.1180589
- Formato, A., Gallo, M., Ianniello, D., Montesano, D., & Naviglio, D. (2013). Supercritical fluid extraction of α- and β-acids from hops compared to cyclically pressurized solid–liquid extraction. The Journal of Supercritical Fluids, 84, 113–120. https://doi.org/10.1016/j.supflu.2013.09.021
- Fornari, T., Vicente, G., Vazquez, E., García-Risco, M. R., & Reglero, G. (2012). Isolation of essential oil from different plants and herbs by supercritical fluid extraction. Journal of Chromatography A, 1250, 34–48. https://doi.org/10.1016/j.chroma.2012.04.051
- Franco, D., Rodríguez-Amado, I., Agregán, R., Munekata, P. E. S., Vázquez, J. A., Barba, F. J., & Lorenzo, J. M. (2018). Optimization of antioxidants extraction from peanut skin to prevent oxidative processes during soybean oil storage. LWT - Food Science and Technology, 88, 1–8. https://doi.org/10.1016/j.lwt.2017.09.027
- Frohlich, P. C., Santos, K., & Palu, F. (2018). Evaluation of the effects of temperature and pressure on the extraction of eugenol from clove (Syzygium aromaticum) leaves using supercritical CO2. The Journal of Supercritical Fluids, 143, 313–320. https://doi.org/10.1016/j.supflu.2018.09.009
- Gabrić, D., Barba, F., Roohinejad, S., Gharibzahedi, S. M. T., Radojčin, M., Putnik, P., & Bursać Kovačević, D. (2017). Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. Journal of Food Process Engineering, 41(1), e12638. https://doi.org/10.1111/jfpe.12638
- Garmus, T. T., Paviani, L. C., Queiroga, C. L., & Cabral, F. A. (2015). Extraction of phenolic compounds from pepper-rosmarin (Lippia sidoides Cham.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents. The Journal of Supercritical Fluids, 99, 68–75. https://doi.org/10.1016/j.supflu.2015.01.016
- Gavahian, M., & Chu, Y. (2018). Ohmic accelerated steam distillation of essential oil from lavender in comparison with conventional steam distillation. Innovative Food Science & Emerging Technologies, 50, 34–41. https://doi.org/10.1016/j.ifset.2018.10.006
- Giacomettia, J., Kovacevicb, D. B., Putnikb, P., Gabrićb, D., Bilušićc, T., Krešićd, G., Stulićb, V., Barbae, F. J., Chematf, F., Barbosa-Cánovasg, G., & Jambrak, A. R. (2018). Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Research International, 113, 245–262. https://doi.org/10.1016/j.foodres.2018.06.036
- Gillet, S., Aguedo, M., Petitjean, L., Morais, A. R. C., Lopes, A. M. D., Lukasik, R. M., & Anastas, P. T. (2017). Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chemistry, 19(18), 4200–4233. https://doi.org/10.1039/C7GC01479A
- Gopalasatheeskumar, K. (2018). Significant role of soxhlet extraction process in phytochemical research. Mintage Journal of Pharmaceutical & Medical Sciences, 7(1), 43–47.
- Granato, D., Nunes, D. S., & Barba, F. J. (2017). An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends in Food Science & Technology, 62, 13–22. https://doi.org/10.1016/j.tifs.2016.12.010
- Herrero, M., Castro-Puyana, M., Mendiola, J. A., & Ibañez, E. (2013). Compressed fluids for the extraction of bioactive compounds. TrAc Trends in Analytical Chemistry, 43, 67–83. https://doi.org/10.1016/j.trac.2012.12.008
- Herrero, M., Plaza, M., Cifuentes, A., & Ibáñez, E. (2010). Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in-vitro assays. Journal of Chromatography A, 1217(16), 2512–2520. https://doi.org/10.1016/j.chroma.2009.11.032
- Howard, L., & Pandjaitan, N. (2008). Pressurized liquid extraction of flavonoids from Spinach. Journal of Food Science, 73(3), 151–157. https://doi.org/10.1111/j.1750-3841.2007.00658.x
- Hsu, H., Hsiao, P., Kuo, T., Chiang, S., Chend, S., Chioue, S., Linga, X., Liang, M., Cheng, W., & Jer-Yiing Houng, J. (2016). Antioxidant and anti-inflammatory activities of Lonicera japonica Thunb. var. sempervillosa Hayata flower bud extracts prepared by water, ethanol and supercritical fluid extraction techniques. Industrial Crops and Products, 89, 543–549. https://doi.org/10.1016/j.indcrop.2016.05.010
- Huang, H. W., Hsu, C. P., Yang, B. B., & Wang, C. Y. (2013). Advances in the extraction of natural ingredients by high pressure extraction technology. Trends in Food Science & Technology, 33(1), 54–62. https://doi.org/10.1016/j.tifs.2013.07.001
- Jahanshaei, S., Tabarsa, T., & Asghari, J. (2012). Eco-friendly tannin-phenol formaldehyde resin for producing wood composites. Pigment and Resin Technology, 41(5), 296–301. https://doi.org/10.1108/03699421211264857
- Jung, S. (2016). Applications and opportunities for pressure-assisted extraction. In V. M. Balasubramaniam, Gustavo V. Barbosa-Cánovas, & Huub L. M. Lelieveld (Eds.), High pressure processing of food (pp. 173–191). Springer.
- Kapadiya, S. M., Parikh, J. K., & Desai, M. A. (2018). A greener approach towards isolating clove oil from buds of Syzygium aromaticum using microwave radiation. Industrial Crops and Products, 112, 626–632. https://doi.org/10.1016/j.indcrop.2017.12.060
- Kaufmann, B., & Christen, P. (2002). Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochemical Analysis, 13(2), 105–113. https://doi.org/10.1002/pca.631
- Koubaa, M., Mhemdi, H., Barba, F. J., Angelotti, A., Bouaziz, F., Chaabouni, S. E., & Vorobiev, E. (2017). Seed oil extraction from red prickly pear using hexane and supercritical CO2: Assessment of phenolic compound composition, antioxidant and antibacterial activities. Journal of the Science of Food and Agriculture, 97(2), 613–620. https://doi.org/10.1002/jsfa.7774
- Koubaa, M., Roselló-Soto, E., Šic Žlabur, J., Režek Jambrak, A., Brnčić, M., Grimi, N., & Barba, F. J. (2015). Current and new insights in the sustainable and green recovery of nutritionally valuable compounds from Stevia rebaudiana Bertoni. Journal of Agricultural and Food Chemistry, 63(31), 6835–6846. https://doi.org/10.1021/acs.jafc.5b01994
- Kovačević D, B., Barba, F. J., Granato, D., Galanakis, C. M., Herceg, Z., Dragović-Uzelac, V., & Putnik, P. (2018). Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chemistry, 254 150–157. https://doi.org/10.1016/j.foodchem.2018.01.192
- Laroze, L., Soto, C., & Zuniga, M. (2010). Phenolic antioxidants extraction from raspberry wastes assisted by-enzymes. Electronic Journal of Biotechnology, 13(6), 11–12. https://doi.org/10.2225/vol13-issue6-fulltext-12
- Latif, S., & Anwar, F. (2009). Physicochemical studies of hemp (Cannabis sativa) seed oil using enzyme‐assisted cold‐pressing. European Journal of Lipid Science and Technology, 111(10), 1042–1048. https://doi.org/10.1002/ejlt.200900008
- Li, B. B., Smith, B., & Hossain, M. (2013). Extraction of phenolics from citrus peels. I. Solvent extraction method. Separation and Purification Technology, 48(2), 182–188. https://doi.org/10.1016/j.seppur.2005.07.005
- Liu, W., Fu, Y. J., Zu, Y. G., Kong, Y., Zhang, L., Zu, B. S., & Efferth, T. (2009). Negative-pressure cavitation extraction for the determination of flavonoids in pigeon pea leaves by liquid chromatography–tandem mass spectrometry. Journal of Chromatography A, 1216(18), 3841–3850. https://doi.org/10.1016/j.chroma.2009.02.073
- Liu, X., Jing, X., & Li, G. (2019). A process to acquire essential oil by distillation concatenated liquid-liquid extraction and flavonoids by solid-liquid extraction simultaneously from Helichrysum arenarium (L.) Moench inflorescences under ionic liquid-microwave mediated. Separation and Purification Technology, 209, 164–174. https://doi.org/10.1016/j.seppur.2018.07.028
- Lopes, A. M. D., Lins, R. M. G., Rebelo, R. A., & Lukasik, R. M. (2018). Biorefinery approach for lignocellulosic biomass valorisation with and acidic ionic liquid. Green Chemistry, 20(17), 4043–4057. https://doi.org/10.1039/C8GC01763H
- Luo, M., Yang, L. Q., Yao, X. H., Mu, F. S., Zhang, D. Y., Song, Z. Y., Qiao, Q., Fu, Y. J., & Zu, Y. G. (2014). Optimization of enzyme-assisted negative pressure cavitation extraction of five main indole alkaloids from Catharanthus roseus leaves and its pilot-scale application. Separation and Purification Technology, 125, 66–73. https://doi.org/10.1016/j.seppur.2013.12.034
- Lupacchini, M., Mascitti, A., Giachi, G., Tonucci, L., D’Alessandro, N., Martinez, J., & Colacino, E. (2017). Sonochemistry in non-conventional, green solvents or solvent free reactions. Tetrahedron, 73(6), 609–653. https://doi.org/10.1016/j.tet.2016.12.014
- Machmudah, S., Widiyastuti, S. W., Wahyudiono, H. K., & Goto, M. (2017). Sub and supercritical fluids extraction of phytochemical compounds from Eucheuma cottonii and Gracilaria sp. Chemical Engineering Transactions, 56, 1291–1296. https://doi.org/10.3303/CET1756216
- Maier, T., Göppert, A., Kammerer, D. R., Schieber, A., & Carle, R. (2008). Optimization of a process for enzyme-assisted pigment extraction from grape (Vitis vinifera L.) pomace. European Food Research and Technology, 227, 267–275. https://doi.org/10.1007/s00217-007-0720-y
- Masango, P. (2005). Cleaner production of essential oils by steam distillation. Journal of Cleaner Production, 13(8), 833–839. https://doi.org/10.1016/j.jclepro.2004.02.039
- McHugh, M., & Krukonis, V. (2013). Supercritical fluid extraction: Principles and practice (2nd ed.). Elsevier.
- Mei, M., Huang, X., & Chen, L. (2019). Recent development and applications of polyionic liquids in microextraction techniques. TrAc Trends in Analytical Chemistry, 112, 123–134. https://doi.org/10.1016/j.trac.2019.01.003
- Mnayer, D., Fabiano-Tixier, A.-S., Petitcolas, E., Ruiz, K., Hamieh, T., & Chemat, F. (2017). Extraction of green absolute from thyme using ultrasound and sunflower oil. Resource-Efficient Technologies, 3(1), 12–21. https://doi.org/10.1016/j.reffit.2017.01.007
- Mroczek, T., & Mazurek, J. (2009). Pressurized liquid extraction and anticholinesterase activity-based thin-layer chromatography with bioautography of Amaryllidaceae alkaloids. Analytica Chimica Acta, 633(2), 188–196. https://doi.org/10.1016/j.aca.2008.11.053
- Mustafa, A., & Turner, C. (2011). Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Analytica Chimica Acta, 703(1), 8–18. https://doi.org/10.1016/j.aca.2011.07.018
- Nan, H., & Anderson, J. L. (2018). Ionic liquid stationary phases for multidimensional gas chromatography. TrAc Trends in Analytical Chemistry, 105, 367–379. https://doi.org/10.1016/j.trac.2018.03.020
- Niranjan, K., & Hanmoungjai, P. (2004). Enzyme-aided aquous extraction. In N. T. Dunford and H. B. Dunford (Eds.), Nutritionally enhanced edible oil processing (p. 314 pages). AOCS Publishing.
- Pan, X., Niu, G., & Liu, H. (2003). Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chemical Engineering and Processing-Process Intensification, 42(2), 129–133. https://doi.org/10.1016/S0255-2701(02)00037-5
- Parniakov, O., Barba, F. J., Grimi, N., Lebovka, N., & Vorobiev, E. (2016). Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chemistry, 192, 842–848. https://doi.org/10.1016/j.foodchem.2015.07.096
- Passos, H., Freire, M. G., & Coutinho, J. A. P. (2014). Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chemistry, 16(12), 4786–4815. https://doi.org/10.1039/C4GC00236A
- Pathak, A. K., Ameta, C., Ameta, R., & Punjabi, P. B. (2016). Microwave-assisted organic synthesis in ionic liquids. Journal of Heterocyclic Chemistry, 53(6), 1697–1705. https://doi.org/10.1002/jhet.2515
- Pereira, R. N., & Vicente, A. A. (2010). Environmental impact of novel thermal and non-thermal technologies in food processing. Food Research International, 43(7), 1936–1943. https://doi.org/10.1016/j.foodres.2009.09.013
- Pimentel-Moral, S., Borrás-Linares, I., Lozano-Sánchez, J., Arráez-Román, D., Martínez-Férez, A., & Segura-Carretero, A. (2018). Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. Journal of Pharmaceutical and Biomedical Analysis, 156, 313–322. https://doi.org/10.1016/j.jpba.2018.04.050
- Pourmortazavi, S. M., & Hajimirsadeghi, S. S. (2007). Supercritical fluid extraction in plant essential and volatile oil analysis. Journal of Chromatography A, 1163(1–2), 2–24. https://doi.org/10.1016/j.chroma.2007.06.021
- Prasad, M. K. N., Ismail, A., & Ming, J. Y. (2011). High pressure- assisted extraction: Method, technique, and application. In N. Lebovka; E. Vorobiev, and E. Chemat (Eds.), Enhancing extraction processes in the food industry (pp. 303–322). CRC Press.
- Puertolas, E., Koubaa, M., & Barba, F. J. (2016). An overview of the impact of electrotechnologies for the recovery of oil and high-value compounds from vegetable oil industry: Energy and economic cost implications. Food Research International, 80, 19–26. https://doi.org/10.1016/j.foodres.2015.12.009
- Puri, M., Sharma, D., & Barrow, C. J. (2012). Enzyme-assisted extraction of bioactives from plants. Trends in Biotechnology, 30(1), 37–44. https://doi.org/10.1016/j.tibtech.2011.06.014
- Putnik, P., & Kovačevć, D. B. (2017). Fresh-cut apples spoilage and predictive microbial growth under modified atmosphere packaging. In R. Rai and J. B. Aswathanarayan (Eds.), Food safety and protection (p. 728). CRC Press.
- Putnik, P., Lorenzo, J. M., Barba, F. J., Roohinejad, S., Jambrak, A. R., Granato, D., Montesano, D., & Kova_cevíc, D. B. (2018). Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods, 7(7), 106–122. https://doi.org/10.3390/foods7070106
- Qi, X. L., Peng, X., Huang, Y. Y., Li, L., Wei, Z.-F., Zu, Y. G., & Fu, Y. J. (2015). Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Industrial Crops and Products, 70, 142–148. https://doi.org/10.1016/j.indcrop.2015.03.026
- Rahal, N. B., Barba, F. J., Barth, D., & Chevalot, I. (2015). Supercritical CO2 extraction of oil, fatty acids and flavonolignans from milk thistle seeds: Evaluation of their antioxidant and cytotoxic activities in Caco-2 cells. Food and Chemical Toxicology, 83, 275–282. https://doi.org/10.1016/j.fct.2015.07.006
- Ramos, M., Jimenez, A., & Garrigos, M. C. (2019). IL-based advanced techniques for the extraction of value-added compounds from natural sources and food by-products. TrAc Trends in Analytical Chemistry, 119, 115616. https://doi.org/10.1016/j.trac.2019.07.027
- Raso, J., Frey, W., Ferrari, G., Pataro, G., Knorr, D., Teissie, J., & Miklavčič, D. (2016). Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innovative Food Science & Emerging Technologies, 37, 312–321. https://doi.org/10.1016/j.ifset.2016.08.003
- Rodriguez-Meizoso, I., Castro-Puyana, M., Börjesson, P., Mendiola, J. A., Turner, C., & Ibanez, E. (2012). Life cycle assessment of green pilot-scale extraction processes to obtain potent antioxidants from rosemary leaves. The Journal of Supercritical Fluids, 72, 205–212. https://doi.org/10.1016/j.supflu.2012.09.005
- Roohinejad, S., Koubaa, M., Barba, F. J., Leong, S. Y., Khelfa, A., Greiner, R., & Chemat, F. (2017).In: S. M. Hashemi, A. M. Khaneghah, & A. de Souza Sant'Ana (Eds.), Extraction methods of essential oils from herbs and spices. In Essential oils in food processing: Chemistry, safety and applications (Ist ed., pp. 21–55). John Wiley & Sons Ltd.
- Roohinejad, S., Oey, I., Everett, D. W., & Niven, B. E. (2014). Evaluating the effectiveness of β-carotene extraction from pulsed electric field-treated carrot pomace using oil-in-water microemulsion. Food and Bioprocess Technology, 7(11), 3336–3348. https://doi.org/10.1007/s11947-014-1334-6
- Rosello-Soto, E., Koubaa, M., Moubarik, A., Lopes, R. P., Saraiva, J. A., Boussetta, N., & Barba, F. J. (2015). Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends in Food Science & Technology, 45(2), 296–310. https://doi.org/10.1016/j.tifs.2015.07.003
- Rosello-Soto, E., Parniakov, O., Deng, Q., Patras, A., Koubaa, M., Grimi, N., & Barba, F. J. (2016). Application of non-conventional extraction methods: Toward a sustainable and green production of valuable compounds from mushrooms. Food Engineering Reviews, 8(2), 214–234. https://doi.org/10.1007/s12393-015-9131-1
- Rostagno, M., Palma, M., & Barrosa, C. G. (2004). Pressurized liquid extraction of isoflavones from soybeans. Analytica chimica acta, 522(2), 169–177. https://doi.org/10.1016/j.aca.2004.05.078
- Rostagno, M. A., Palma, M., & Barroso, C. G. (2007). Ultrasound-assisted extraction of isoflavones from soy beverages blended with fruit juices. Analytica chimica acta, 597, 265–272. https://doi.org/10.1016/j.aca.2007.07.006
- Selvamuthukumaran, M., & Shi, J. (2017). Recent advances in extraction of antioxidants from plant by-products processing industries. Food Quality and Safety, 1(1), 61–81. https://doi.org/10.1093/fqs/fyx004
- Serment-Moreno, V., Jacobo-Velázquez, D. A., Torres, J. A., & Welti-Chanes, J. (2017). Microstructural and physiological changes in plant cell induced by pressure: Their role on the availability and pressure-temperature stability of phytochemicals. Food Engineering Reviews, 9(4), 314–334. https://doi.org/10.1007/s12393-017-9158-6
- Shen, J., & Shao, X. (2005). A comparison of accelerated solvent extraction, Soxhlet extraction, and ultrasonic-assisted extraction for analysis of terpenoids and sterols in tobacco. Analytical and Bioanalytical Chemistry, 383(6), 1003–1008. https://doi.org/10.1007/s00216-005-0078-6
- Shirsath, S. R., Sonawane, S. H., & Gogate, P. R. (2012). Intensification of extraction of natural products using ultrasonic irradiations - a review of current status. Chemical Engineering and Processing: Process Intensification, 53, 10–23. https://doi.org/10.1016/j.cep.2012.01.003
- Shukla, S. K., Khokarale, S. G., Bul, T. Q., & Mikkola, J.-P. T. (2019). Ionic liquids: Potential materials for carbon dioxide capture and utilization. Frontiers in Materials, 6(42), 1–8. https://doi.org/10.3389/fmats.2019.00042
- Shu, Y., Ko, M. Y., & Chang, Y. S. (2003). Microwave-assisted extraction of ginsenosides from ginseng root. Microchemical Journal, 74(2), 131–139. https://doi.org/10.1016/S0026-265X(02)00180-7
- Soliva-Fortuny, R., Balasa, A., Knorr, D., & Martín-Belloso, O. (2009). Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends in Food Science & Technology, 20(11–12), 544–556. https://doi.org/10.1016/j.tifs.2009.07.003
- Srinivas, K., & King, J. W. (2010). Supercritical carbon dioxide and subcritical water: Complementary agents in the processing of functional foods. In J. Smith and E. Charter (Eds.), Functional food products development (pp. 39–78). Blackwell Publishing Ltd.
- Tekin, K., Akalın, M. K., & Şeker, M. G. (2015). Ultrasound bath-assisted extraction of essential oils from clove using central composite design. Industrial Crops and Products, 77, 954–960. https://doi.org/10.1016/j.indcrop.2015.09.071
- Teng, H., Ghafoor, K., & Yong-Hee, C. (2009). Optimization of Microwave-assisted extraction of active components from Chinese Quince using response surface methodology. Journal of the Korean Society for Applied Biological Chemistry, 52(6), 694–701. https://doi.org/10.3839/jksabc.2009.115
- Tintchev, F., Dobreva, A., Schulz, H., & Toepfl, S. (2012). Effect of pulsed electric fields on yield and chemical composition of rose oil (Rosa damascena Mill.). Journal of Essential Oil-Bearing Plants, 15(6), 876–884. https://doi.org/10.1080/0972060X.2012.10662589
- Tiwari, B. K. (2015). Ultrasound: A clean, green extraction technology. Trends in Analytical Chemistry, 71, 100–109. https://doi.org/10.1016/j.trac.2015.04.013
- Tongnuanchan, P., & Benjakul, S. (2014). Essential oils: Extraction, bioactivities, and their uses for food preservation. Journal of Food Science, 79(7), 1231–1249. https://doi.org/10.1111/1750-3841.12492
- Ullah, H., Wilfred, C. D., & Shaharun, M. S. (2019). Ionic liquid-based extraction and separation trends of bioactive compounds from plant biomass. Separation Science and Technology, 54(4), 559–579. https://doi.org/10.1080/01496395.2018.1505913
- Valdes, A., & Garrigos, M. C. (2016). Microencapsulation of natural antioxidant compounds obtained from biomass wastes: A review. Materials Science Forum, 875, 112–126. https://doi.org/10.4028/www.scientific.net/MSF.875.112
- Vankar, P. S. (2004). Essential oils and fragrances from natural sources. Resonance, 9(4), 30–41. https://doi.org/10.1007/BF02834854
- Vardanega, R., Santos, D., & De Almeida, M. A. (2014). Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharmacognosy Reviews, 8(16), 88–95. https://doi.org/10.4103/0973-7847.134231
- Veillet, S., Tomao, V., & Chemat, F. (2010). Ultrasound assisted maceration: An original procedure for direct aromatisation of olive oil with basil. Food Chemistry, 123(3), 905–911. https://doi.org/10.1016/j.foodchem.2010.05.005
- Vinatoru, M., Mason, T. J., & Calinescu, L. (2017). Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of functional compounds from plant materials. Trends in Analytical Chemistry, 97, 159–178. https://doi.org/10.1016/j.trac.2017.09.002
- Wang, Y., Hou, Q., Ju, M., & Li, W. (2019). New developments in material preparation using a combination of ionic liquids and microwave irradiation. Nanomaterials, 9(4), 647–673. https://doi.org/10.3390/nano9040647
- Wang, X. Q., Wei, W., Zhao, C. J., Li, C. Y., Luo, M., Wang, W., Zu, Y. G., Efferth, T., & Fu, Y. J. (2015). Negative-pressure cavitation coupled with aqueous two-phase extraction and enrichment of flavonoids and stilbenes from the pigeon pea leaves and the evaluation of antioxidant activities. Separation and Purification Technology, 156, 116–123. https://doi.org/10.1016/j.seppur.2015.09.028
- Xiao, J., Chen, G., & Li, N. (2018). Ionic liquid solutions as a green tool for the extraction and isolation of natural products. Molecules, 23(7), 1765–1788. https://doi.org/10.3390/molecules23071765
- Xie, P., Huang, L., Zhang, C., You, F., & Zhang, Y.-L. (2015). Reduced pressure extraction of oleuropein from olive leaves (Olea europaea L.) with ultrasound assistance. Food and Bioproducts Processing, 93, 29–38. https://doi.org/10.1016/j.fbp.2013.10.004
- Xynos, N., Papaefstathiou, G., Gikas, E., Argyropoulou, A., Aligiannis, N., & Skaltsounis, A. L. (2014). Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology. Separation and Purification Technology, 122, 323–330. https://doi.org/10.1016/j.seppur.2013.10.040
- Xynos, N., Papaefstathiou, G., Psychis, M., Argyropoulou, A., Aligiannis, N., & Skaltsounis, A.-L. (2012). Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. The Journal of Supercritical Fluids, 67, 89–93. https://doi.org/10.1016/j.supflu.2012.03.014
- Yan, L.-G., He, L., & Xi, J. (2017). High intensity pulsed electric field as an innovative technique for extraction of bioactive compounds - a review. Critical Reviews in Food Science and Nutrition, 57(13), 2877–2888. https://doi.org/10.1080/10408398.2015.1077193
- Yousefi, M., Rahimi-Nasrabadi, M., Pourmortazavi, S. M., Wysokowski, M., Jesionowski, T., Ehrlich, H., & Mirsadeghi, S. (2019). Supercritical fluid extraction of essential oils. Trends in Analytical Chemistry, 118, 182–193. https://doi.org/10.1016/j.trac.2019.05.038
- Zakaria, S. M., & Kamal, S. M. M. (2015). Subcritical water extraction of bioactive compounds from plants and algae: Applications in pharmaceutical and food ingredients. Food Engineering Reviews, 8(1), 23–34. https://doi.org/10.1007/s12393-015-9119-x
- Zermane, A., Meniai, A. H., & Barth, D. (2010). Supercritical CO2 extraction of essential oil from Algerian Rosemary (Rosmarinus officinalis l.). Chemical Engineering Technology, 33(3), 489–498. https://doi.org/10.1002/ceat.200900381
- Zhang, Q. (2018). Ionic liquids in capillary electrophoresis for enantioseparation. TrAc Trends in Analytical Chemistry, 100, 145–154. https://doi.org/10.1016/j.trac.2018.01.001
- Zhang, J., Wena, C., Zhanga, H., Duana, Y., & Maa, H. (2020). Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends in Food Science & Technology, 95, 183–195. https://doi.org/10.1016/j.tifs.2019.11.018
- Zhang, D.-Y., Zhang, S., Zu, Y.-G., Fu, Y.-J., Kong, Y., Gao, Y., Zhao, J.-T., & Efferth, T. (2010). Negative pressure cavitation extraction and antioxidant activity of genistein and genistin from the roots of pigeon pea [Cajanus cajan (L.) Millsp.]. Separation and Purification Technology, 74(2), 261–270. https://doi.org/10.1016/j.seppur.2010.06.015
- Zhang, D.-Y., Zu, Y. G., Fu, Y. J., Luo, M., Gu, C. B., Wang, W., & Yao, X. H. (2011). Negative pressure cavitation extraction and antioxidant activity of biochanin a and genistein from the leaves of Dalbergia odorifera T. Chen. Separation and Purification Technology, 83, 91–99. https://doi.org/10.1016/j.seppur.2011.09.017
- Zhao, B. S., Fu, Y. J., Wang, W., Zu, Y. G., Gu, C. B., Luo, M., & Efferth, T. (2011). Enhanced extraction of isoflavonoids from Radix Astragali by incubation pretreatment combined with negative pressure cavitation and its antioxidant activity. Innovative Food Science and Emerging Technologies, 12(4), 577–585. https://doi.org/10.1016/j.ifset.2011.05.003
- Zhao, Y., & Saldaña, M. D. A. (2019). Use of potato by-products and gallic acid for development of bioactive film packaging by subcritical water technology. The Journal of Supercritical Fluids, 143, 97–106. https://doi.org/10.1016/j.supflu.2018.07.025
- Zhou, Y. J., Xue, C. M., Zhang, S. S., Yao, G. M., Zhang, L., & Wang, S. J. (2017). Effects of high intensity pulsed electric fields on yield and chemical composition of rose essential oil. International Journal of Agricultural and Biological Engineering, 10(3), 295–301.
- Zhu, Z., Zhang, R., Zhan, S., He, J., Barba, F., Cravotto, G., & Li, S. (2017). Recovery of oil with unsaturated fatty acids and polyphenols from Chaenomelessinensis (Thouin) Koehne: Process optimization of pilot-scale subcritical fluid assisted extraction. Molecules, 22(10), 1788. https://doi.org/10.3390/molecules22101788
- Zizovic, I., Senerovic, L., Moric, I., Adamovic, T., Jovanovic, M., Krusic, M. K., Misic, D., Stojanovic, D., & Milovanovica, S. (2018). Utilization of supercritical carbon dioxide in fabrication of cellulose acetate films with anti-biofilm effects against Pseudomonas aeruginosa and Staphylococcus aureus. The Journal of Supercritical Fluids, 140, 11–20. https://doi.org/10.1016/j.supflu.2018.05.025
- Zlabur, J. S., Voca, S., Brncic, M., & Brncic, S. R. (2018). New trends in food technology for green recovery of bioactive compounds from plant materials. In: A. M. Grumezescu & A. M. Holban (Eds.), Role of materials science in food bioengineering. Handbook of food bioengineering (pp. 1–36). Academic Press.