ABSTRACT
Camellia oleifera(C. oleifera)is one of the four main woody oils of the world. Research on improving the productivity of C. oleifera has focused on the growth process, with much less research on the mechanism of lipid accumulation post-ripening. The present study analyzed the metabolism of C. oleifera fruit under light and temperature stress and identified the primary and secondary metabolites of C. oleifera fruit using UPLC-MS. A total of 878 metabolites were detected, including 152 primary metabolites and 726 secondary metabolites, with a total of 32 core differential metabolites analyzed using a Venn diagram. After KEGG database matching, the differential metabolites were mainly enriched in the aminoacyl-tRNA synthesis pathway, the arginine and proline metabolism pathway and other pathways. Light and temperature stresses may have induced the expression of abscisic acid, arginase, and synthesized upstream substances to increase the accumulation of oils, squalene and sterols.
Introduction
The synthesis of lipids in plant fruits during their growth is very complex and involves the catalysis of a range of enzymes, including key enzymes that affect the partitioning of carbon sources involved in fatty acid synthesis and triglyceride assembly, and related transcription factors (Cui et al., Citation2017; Mcglew et al., Citation2015; Shockey et al., Citation2016; Li et al., Citation2010; Xu et al., Citation2016). Lipids can participate in and regulate several physiological activities such as lipid metabolism, which is an important way for plants to respond to stresses induced by temperature, salt or drought. Therefore, the quantitative and qualitative analyses of all the metabolites in plant fruits using metabolomics techniques is particularly important for determining the mechanisms by which plants respond to stress factors.
Camellia oleifera is a woody oil crop unique to China and one of the four main woody oil tree species of the world. The annual production of C. oleifera seeds in China increased from 2.164 to 2.80 million tonnes between 2016 and 2020, forming about 94% of the total woody oilseed crop production in China. C. oleifera seed oil, also known as Oriental olive oil, is made by pressing or extracting the seeds (Wang, Citation2021). It can have contents of oleic and linoleic acids greater than 90%, with a fatty acid composition comparable to that of olive oil. The cultivation of C. oleifera does not compete with that of grain for land, and is therefore an important supplement to global sources of edible oil. The post-ripening treatment is crucial for preparing C. oleifera seed oil because it improves the levels of oil and fat-soluble components, thus increasing the output value of the C. oleifera industry.
Most related studies have explored the effects of abiotic stress on lipid metabolism during plant growth, for example, Katam et al. (Citation2020) reported that the levels of saturated fatty acids are significantly elevated in soybeans under drought stress because the phospholipid molecules, mainly phosphatidylcholine, phosphatidylethanolamine and phosphatidylglycerol, are degraded after drought stress in plants (Wang et al., Citation2020). Teixeira et al. (Citation2009) reported that the content of unsaturated fatty acids in Portulaca oleracea increased under low temperature stress. In contrast to low temperature stress, high temperature stress increases the saturation of membrane lipids, (Schroda et al., Citation2015) causing a significant decrease in unsaturated fatty acids in Triticum aestivum L., for example (Djanaguiraman et al., Citation2020). The post-ripening process for the fresh fruits of C. oleifera involves several different abiotic stresses but the molecular regulatory network of the lipid response to stress has not yet been studied.
Therefore, this study aimed to reveal the metabolic response mechanism of C. oleifera fresh fruit under light and high temperature stress through the use of liquid chromatography-mass spectrometry to reveal differences in the metabolites. These different metabolites may be responsible for changes in the oil and nutrient content of C. oleifera seeds. The metabolites from C. oleifera fruit treated either by storage in the dark or under white light will be analyzed by liquid chromatography (UHPLC-ESIMS, ultrahigh performance liquid chromatography – electrospray ionization tandem mass spectrometry) to determine the accumulation of lipid-related metabolites. This study will provide basic data for improving the stress response of C. oleifera fresh fruit postharvest, and for its processing technology.
Materials and methods
Plant materials
C.oleifera fresh fruits (100 kg), of a similar morphological maturity and size, consisting of the peel and seeds were selected and placed in a constant temperature incubator at 35°C under dark conditions and at 35°C under white light irradiation for the post-ripening treatment for 0, 9 and 16 h. The group with no treatment (0 h) was defined as the control group. The groups exposed to light for 9 and 16 h were labeled as LT-9 and LT-16, respectively, and the group kept in the dark for 9 and 16 h were labeled as NLT-9 and NLT-16, respectively. The kernel of Camellia oleifera fruit is used in the metabolome. The fresh fruits of Camellia oleifera used in our experiment were collected from Guangxi Forestry Research Institute. These fresh fruits of Camellia oleifera are planted by ourselves and are specially used for scientific research experiments.
Extraction of metabolites
The freeze-dried samples were crushed with a mixer mill for 60 s at 45 Hz then a 50 mg aliquot of each sample was weighed precisely and transferred to an Eppendorf tube. After adding 500 μL of the extraction solution (methanol/water = 3:1, precooled to −40°C, containing the internal standard). After 30 s of vortexing, the samples were homogenized at 40 Hz for 4 min then sonicated for 5 min in an ice-water bath. They were then homogenized again at 40 Hz for 4 min and sonicated for 10 min in an ice-water bath. The samples were extracted overnight at 4°C using a shaker. After centrifugation at 12,000 rpm (RCF = 13800 (×g), R = 8.6 cm) for 15 min at 4°C, the supernatant was carefully filtered through a 0.22 μm microporous membrane. The resulting supernatants were then diluted 5 times with methanol/water mixture (v:v = 3:1, containing the internal standard) and vortexed for 30 s. After transfer to 2 mL glass vials, 40 μL was taken from each sample and pooled for use as the QC samples. The samples were stored at −80°C until the UHPLC- MS analysis.
UHPLC-MS analysis
The UHPLC separation was carried out using an EXIONLC System (Sciex Technologies, Framingham, MA, USA). The mobile phase A was 0.1% formic acid in water, and the mobile phase B was acetonitrile. The column temperature was set at 40°C. The auto-sampler temperature was set at 4°C and the injection volume was 2 μL.
A QTrap 6500+ (Sciex Technologies) was used for the assay development. Typical ion source parameters were: Ion spray voltage, +5500/-4500 V; Curtain gas, 35 psi; Temperature: 400°C; Ion source gas, 1:60 psi; Ion source gas 2, 60 psi; and Declustering Potential, ±100 V.
We confirm that all methods involving plants were performed in accordance with the relevant guidelines and regulations.
Data preprocessing and annotation
SCIEX Analyst Work Station Software (Version 1.6.3) was used for multiple reaction monitoring data acquisition and processing. MS raw data (.wiff) files were converted to the TXT format using MSconventer. An in-house R program and database were used for peak detection and annotation.
Results
Composition of C. oleifera fruit
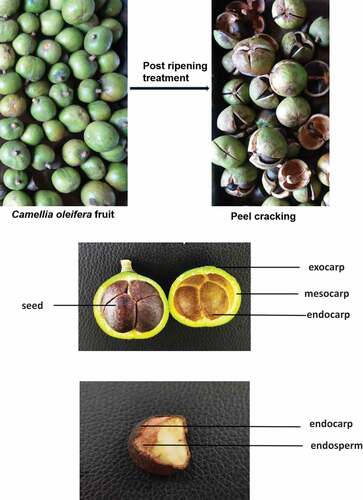
The experimental variety selected in this study was common C. oleifera, which is mainly composed of the pericarp, seed coat and kernel. The fresh pericarp accounts for about 65% and the seed about 35% of the fresh fruit weight ().
Description of metabolite detection
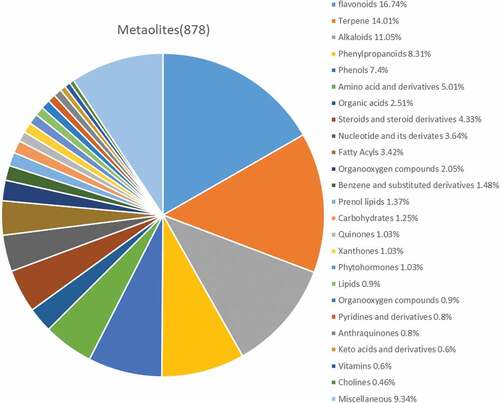
To better understand the pattern of metabolite changes in the C. oleifera fruits under different post-ripening treatments, the primary and secondary metabolites in the samples were identified by the broad-targeted metabolomics technology of UPLC-MS. A total of 878 metabolites were detected (), including 147 flavonoids, 123 terpenoids, 97 alkaloids, 73 phenylpropanoids, 65 phenols, 44 amino acids and their derivatives, 38 steroids and their derivatives, 32 nucleotides and their derivatives, 30 fatty acyls, 22 organic acids and their derivatives, 18 organic oxygen compounds, 13 aromatic compounds, 12 pregnenolone lipids and 11 sugars, 9 other quinones, 9 ×anthones, 9 plant hormones, 8 lipids, 8 organic nitrogen compounds, 7 pyridines and their derivatives, 7 anthraquinones, 5 keto acids and their derivatives, 5 vitamins, 4 choline and 82 other categories of substances.
Multivariate statistical analysis
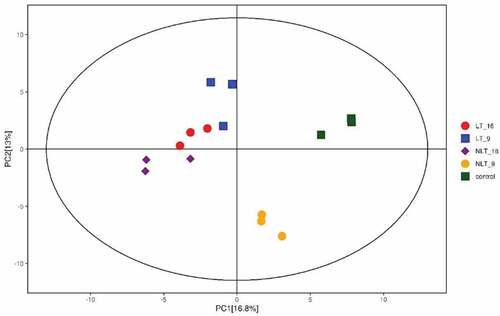
PCA analysis reflects the characteristics of metabolomics multidimensional data through several principal components. Therefore, we can observe the differences between different groups through the PCA diagram. In the present study, the PCA results showed obvious separation between groups treated with or without light and between different treatment times. This showed that the metabolites in the sample changed significantly after the post-ripening treatment of C. oleifera fruit (). The first principal component (PC1) explained 16.8% of the characteristics of the original data set. It showed that the different post-ripening times were separated(except for the light treatment group), while the groups with or without light treatment were separated on the second principal component, which explained 13% of the characteristics of the original data set.
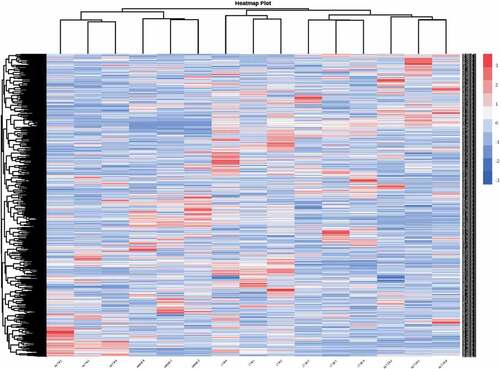
The differences between the accumulation patterns of metabolites from C. oleifera fruits for the five different post-ripening treatments could be analyzed by the cluster heat map (). The results from the heatmap cluster analysis showed 9 clusters in total, with the different biological repeats also being clustered together, indicating a good homogeneity and high reliability of data. In general, both the cluster analysis and the principal component analysis showed that the metabolites of C. oleifera fresh fruits were significantly different after the different post-ripening treatments.
Differential metabolite analysis
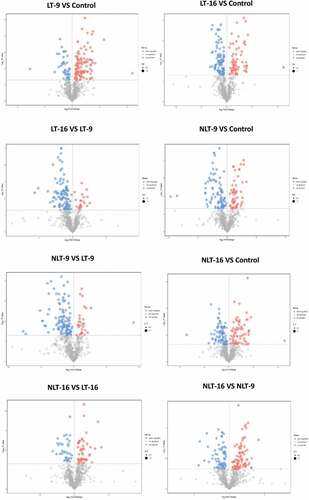
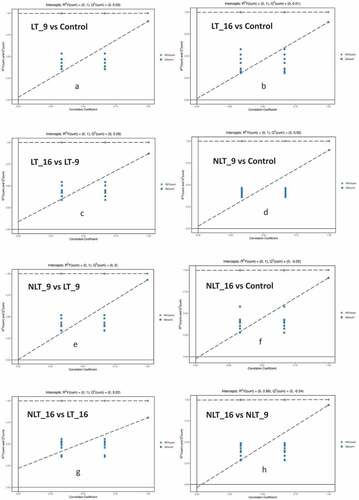
Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) is a multivariate statistical analysis technique with supervised pattern recognition, which can effectively eliminate influences irrelevant to the study and thus enable the screening of differential metabolites (Xiao et al., Citation2021). OPLS-DA was used to analyze different stress treatment groups in pairs then the score map was produced. In this model, R2X and R2Y, respectively, represent the interpretation rate of the built model to the X and Y matrices and Q2 represents the prediction ability of the model. The value of Q2 for all comparison groups was higher than 0.8, indicating that the built model was appropriate. The OPLS-DA score plot showed a significant separation between the different comparison groups. The results of the permutation test indicated that the model was extremely robust and that there was no overfitting (). In , the X-axis represents the replacement retention of the permutation test, the Y-axis represents the value of R2Y or Q2. The green dot represents the R2Y value obtained by the permutation test, the blue square dot represents the Q2 value obtained by the permutation test, and the two dotted lines represent the regression lines of R2 Y and Q2 respectively. The Q2 values of the permutation test stochastic model are all smaller than those of the original model. Meanwhile, the Q2 of the stochastic model gradually decreases as the replacement retention gradually decreases and the proportion of replaced Y variables increases. It shows that the original model has good robustness, and there is no over fitting phenomenon.
Figure 5. Permutation test of the OPLS-DA model for comparing the treatment groups of Camellia oleifera fresh fruits.(a,b,c,d,e,f,g,h: Permutation test of OPLS-DA model for group LT_9 vs Control, LT_16 vs Control, LT_16 vs LT_9, NLT_9 vs Control, NLT_9 vs LT_9, NLT_16 vs Control, NLT_16 vs LT_16, NLT_16 vs NLT_9.).
In the present study, the p value of the Student t-test was less than 0.05, and the variable projection importance (VIP) of the first principal component of the OPLS-DA model was greater than 1 for screening the differential metabolites (). The results showed a total of 145 differential metabolites (102 metabolites up-regulated expression and 43 metabolites down-regulated expression) in the comparison of LT-9 with the control group (). Other control groups were used in a similar fashion.
Table 1. Differential metabolites expression in the post-ripening treatment groups of C. oleifera fresh fruits.
Core differential metabolites
The Venn diagram () shows the numbers of differential metabolites between LT-9 vs control, LT-16 vs control, NLT-9 vs control and NLT-16 vs control. In these paired comparisons, 32 overlapping differential metabolites were considered to be the key metabolites affecting the post-ripening treatment of C. oleifera fresh fruits ().For the different comparison groups more overlapping differential metabolites appeared, indicating the similarity of the changes between the different groups under light and temperature stress, and the changed metabolites included some functional substances, including flavonoid, such as isoquercitrin and epicatechin. The synthesis of plant flavonoids is influenced by external environmental factors such as temperature, light, abscisic acid(ABA) and gibberellin(GA), among which light is a very important factor (Loreti et al., Citation2008).
Figure 7. Venn diagram indicating the differential and common metabolites in the post-ripening treatment groups of Camellia oleifera fresh fruits.
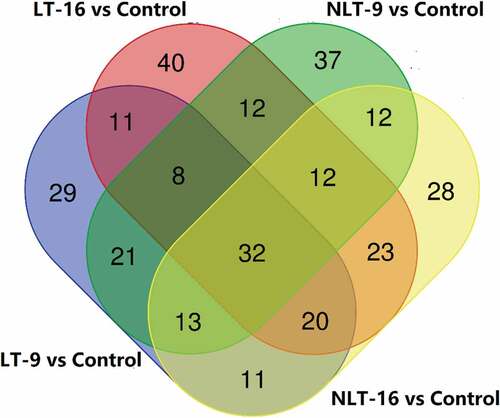
Table 2. 32 core differential metabolites in the post-ripening treatment groups of C. oleifera fresh fruits.
All the differential metabolites in the different comparison groups were matched to the KEGG database to obtain information on the pathways in which the metabolites were involved. Enrichment analysis was performed on the annotated results to identify those pathways with a high enrichment of the differential metabolites (). In these comparison groups, some metabolic pathways overlapped, such as aminoacyl-tRNA biosynthesis pathway (overlapping 7 times), arginine and proline metabolism pathway (overlapping 7 times), phenylalanine metabolism pathway (overlapping 4 times).
Table 3. Metabolic pathway enrichment information of differential metabolites in different comparison groups.
Discussion
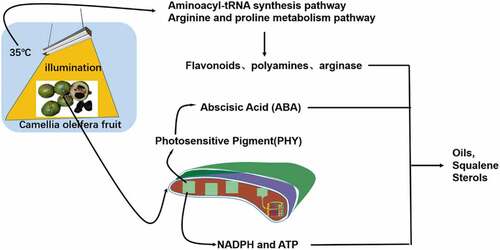
In the present study, a comparative analysis of differences in the metabolites in green C. oleifera fruits during post-ripening was made between different light and temperature treatments. The experimental objects were “ordinary” C. oleifera fruits, where the peel of the fresh fruit accounted for 65% of the weight of the whole fruit. Although these seeds are predominantly sink tissues, they contain chloroplasts with thylakoid structures and enzymes of typical photosynthetic machinery. However, rather than producing carbon for export as in source tissues, photosynthesis in seeds can participate in metabolism in at least three ways. First, light reactions can produce NADPH and ATP for energetically expensive fatty acid synthesis; second, 02 evolution may help prevent anoxia inside the seeds; (Rolletschek et al., Citation2002) and third, the dark reactions of photosynthesis could improve the biosynthetic efficiency of seeds by refixing respiratory CO2 and providing intermediates for metabolism. Ruuska et al. (Citation2004) have also shown that under normal growth conditions, even the low level of light reaching seeds plays a substantial role in activating light-regulated enzymes, increasing fatty acid synthesis, and potentially powering the refixation of CO2. A previous study reported that the oil content of C. oleifera seed oil was increased by 1% due to light factors (Wu et al., Citation2021). Light may not only activate the light-regulating enzyme in C. oleifera peel, but also provide NADPH and ATP for the new synthesis of fatty acids. As a next step in this research, enzyme activity experiments are required for verification.
In addition, flavonoids were an important class of 32 core differential metabolites in the post-ripening process of C. oleifera fresh fruits, with 10 substances, seven of which were down-regulated and three of which were up-regulated. Flavonoids inhibit fatty acid synthesis by competing with fatty acids for synthetic substrates, and down-regulation of flavonoid expression leads to an increase in seed fatty acid levels (Li et al., Citation2018). ABA, GA and other plant hormones can directly regulate the metabolism of flavonoids, and they have an irreplaceable role in the negative regulation of oil content by the flavonoid (Jeong et al., Citation2004).LIAN showed that genes involved in flavonoid synthesis also regulate the accumulation of fatty acids, which in turn affects fatty acid fractions, and that when flavonoids were absent or synthesis was reduced, oleic acid (C18:1) content was significantly reduced and linoleic acid (C18:2) and α-linolenic acid (C18:3) content was significantly increased in rapeseed (Lian et al., Citation2017).
Light also has a role in reversing seed dormancy and promoting germination, with its molecular mechanism achieving this by stimulating the photosensitive pigment (PHY) signaling pathway (Yan & Chen, Citation2020). PHY regulates the synthesis of gibberellin (GA) and the decomposition of abscisic acid (ABA), (Lettuce et al., Citation2008) which plays an important role in oil accumulation and maturation in C. oleifera seeds. The metabolomics analysis revealed that ABA was detected in the metabolites of the different LT groups but not in the metabolites of the NLT groups. The comparative analysis of differences in metabolites also found that quercetin was up-regulated after 9 h of post-ripening, but there was no significant difference after 16 h. Ma et al. (Citation2018) have reported that quercetin up-regulated the expression pattern of key genes involved in cellular signaling mechanisms such as phosphatidylinositol 4-kinase α, thus enhancing cell growth and lipid accumulation in the green microalga, Chlorella vulgaris. Kusano et al. (Citation2011) reported that the levels of two quercetin derivatives of wild-type Arabidopsis were dramatically enhanced following UV-B treatment. Therefore, the increase in fatty acids in the LT groups may be related to the metabolism of ABA and quercetin.
Arginine decarboxylase (ADC), arginase and nitric oxide synthase (NOS) are the key enzymes for L-arginine catabolism. Arginine can form polyamine (PA) and proline through the ADC or arginase ornithine decarboxylase (ODC) pathways (Cantón et al., Citation2005). NO can also be formed through the NOS pathway. The relative strength of the activities of these three enzymes determines the metabolic direction of arginine. Arginase induction is part of the defense mechanism against biological and abiotic stresses (Zhang et al., Citation2013). Of the differential metabolites of the NLT16 and LT16 treatment groups, proline was up-regulated. High temperature stress decreased the water content of C. oleifera fruit and enhanced the arginase activity induced by hot air, which promoted the metabolism of arginine to produce polyamine and proline. We can therefore speculate that the metabolic direction of arginine in fruit under light and temperature stress is the ODC pathway, which needs to be further verified by enzyme activity experiments in the later stage. The increase in polyamines and the synthesis and accumulation of secondary metabolites are two common active defense responses of plants under stress. Adding polyamines to cultured plant cells can lead to an increase in secondary metabolites such as flavonoids and terpenoids (Shinde et al., Citation2009). Squalene, a triterpenoid, is also an upstream substance in the synthesis of sterols. Squalene and sterols are important fat-soluble nutrients in oil. Of the 32 core metabolites, steroids and their derivatives were up-regulated, and the upstream terpenes were likely to be up-regulated, which needs clarification by further research. The arginine and proline metabolic pathways synthesize upstream substances of lipid-soluble components, and the aminoacyl-tRNA biosynthesis pathway reacts mainly in the metabolism of a variety of amino acids, which may be related to the metabolism of related enzymes, so these metabolic pathways are relevant to the present study.
Credit author statement
Jianwen Wu: Conceptualization, Writing-original draft.
Guiqing Li: Data curation.
Jihua Guan: Investigation.
Xingyue Tang: Formal analysis.
Mi Qiu: Validation.
Suhua Yang: Methodology.
Shunzhong Lu: Resources.
Xing Fan: Project administration, Software, Supervision, Writing-review & editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Additional information
Funding
References
- Cantón, F. R., Suárez, M. F., & Cánovas, F. M. (2005). Molecular aspects of nitrogen mobilization and recycling in trees. Photosynthesis Research, 83(2), 265–278. https://doi.org/10.1007/s11120-004-9366-9
- Cui, Y., Liu, Z., Zhao, Y., Wang, Y., Huang, Y., Li, L., Wu, H., Xu, S., & Hua, J. (2017). Overexpression of heteromeric GhAccase subunits enhanced oil accumulation in upland cotton. Plant Molecular Biology Reporter, 35(2), 287–297. https://doi.org/10.1007/s11105-016-1022-y
- Djanaguiraman, M., Narayanan, S., Erdayani, E., & Prasad, P. V. V. (2020). Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC plant biology, 20(1), 1–12. https://doi.org/10.1186/s12870-020-02479-0
- Jeong, S., Goto-Yamamoto, N., Kobayashi, S., & Esaka, M. (2004). Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Science, 167(2), 247. https://doi.org/10.1016/j.plantsci.2004.03.021
- Katam, R., Shokri, S., Murthy, N., Singh, S. K., Suravajhala, P., Khan, M. N., Bahmani, M., Sakata, K., & Reddy, K. R. (2020). Proteomics, physiological, and biochemical analysis of cross tolerance mechanisms in response to heat and water stresses in soybean[j/OL]. Plos One, 15(6), 1–29. https://doi.org/10.1371/journal.pone.0233905
- Kusano, M., Tohge, T., Fukushima, A., Kobayashi, M., Hayashi, N., Otsuki, H., Kondou, Y., Goto, H., Kawashima, M., Matsuda, F., Niida, R., Matsui, M., Saito, K., & Fernie, A. R. (2011). Metabolomics reveals comprehensive reprogramming involving two independent metabolic responses of Arabidopsis to UV-B light. The Plant Journal, 67(2), 354–369. https://doi.org/10.1111/j.1365-313X.2011.04599.x
- Lettuce, P., Oa, S., Sawada, Y., Mitsuhashi, W., Tatematsu, K., Kushiro, T., Koshiba, T., Kamiya, Y., Inoue, Y., Nambara, E., & Toyomasu, T. (2008). Phytochrome- and Gibberellin-mediated regulation of abscisic acid metabolism during germination of. Plant Physiology, 146(3), 1386–1396. https://doi.org/10.1104/pp.107.115162
- Li, C., Zhang, B., Chen, B., Ji, L., & Yu, H. (2018). Site-specific phosphorylation of TRANSPARENT TESTA GLABRA1mediates carbon partitioning in Arabidopsis seeds. Nature Communications, 9(1), 571. https://doi.org/10.1038/s41467-018-03013-5
- Li, X. R., Wang, L., & Ruan, Y. L. (2010). Developmental and molecular physiological evidence for the role of phosphoenolpyruvate carboxylase in rapid cotton fibre elongation. Journal of Experimental Botany, 61(1), 287–295. https://doi.org/10.1093/jxb/erp299
- Lian, J., Lu, X., Yin, N., Ma, L., Lu, J., Liu, X., Li, J., Lu, J., Lei, B., Wang, R., & Chai, Y. (2017). Silencing of BnTT1 family genes affects seed flavonoid biosynthesis and alters seed fatty acid composition in Brassica napus. Plant Science, 254, 32–47. https://doi.org/10.1016/j.plantsci.2016.10.012
- Loreti, E., Povero, G., Novi, G., Solfanelli, C., Alpi, A., & Perata, P. (2008). Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. The New Phytologist, 179(4), 1004. https://doi.org/10.1111/j.1469-8137.2008.02511.x
- Ma, Y., Balamurugan, S., Yuan, W., Yang, F., Tang, C., Hu, H., Zhang, H., Shu, X., Li, M., Huang, S., Li, H., & Wu, L. (2018). Quercetin potentiates the concurrent hyper-accumulation of cellular biomass and lipids in Chlorella vulgaris. Bioresource Technology, 269, 434–442. https://doi.org/10.1016/j.biortech.2018.07.151
- Mcglew, K., Shaw, V., Zhang, M., Kim, R. J., Yang, W., Shorrosh, B., Suh, M. C., & Ohlrogge, J. (2015). An annotated database of Arabidopsis mutants of acyl lipid metabolism. Plant cell reports, 34(4), 519–532. https://doi.org/10.1007/s00299-014-1710-8
- Rolletschek, H., Borisjuk, L., Koschorreck, M., Wobus U, & Weber H (2002). Legume embryos develop in a hypoxic environment. Journal of Experimental Botany, 53(371), 1099–1107. https://doi.org/10.1093/jexbot/53.371.1099
- Ruuska, S. A., Schwender, J., & Ohlrogge, J. B. (2004). The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiology, 136(1), 2700–2709. https://doi.org/10.1104/pp.104.047977
- Schroda, M., Hemme, D., & Mühlhaus, T. (2015). The Chlamydomonas heat stress response. The Plant Journal, 82(3), 466–480. https://doi.org/10.1111/tpj.12816
- Shinde, A. N., Malpathak, N., & Fulzele, D. P. (2009). Optimized production of isoflavones in cell cultures of Psoralea corylifolia L. Using elicitation and precursor feeding. Biotechnology and Bioprocess Engineering, 14(5), 612–618. https://doi.org/10.1007/s12257-008-0316-9
- Shockey, J., Regmi, A., Cotton, K., Adhikari, N., Browse, J., & Bates, P. D. (2016). Identification of arabidopsis GPAT9 (At5g60620) as an essential gene involved in triacylglycerol biosynthesis1[open]. Plant Physiology, 170(1), 163–179. https://doi.org/10.1104/pp.15.01563
- Teixeira, M. C., Coelho, N., Olsson, M. E., Brodelius, P. E., Carvalho, I. S., & Brodelius, M. (2009). Molecular cloning and expression analysis of three omega-6 desaturase genes from purslane (Portulaca oleracea L.). Biotechnology Letters, 31(7), 1089–1101. https://doi.org/10.1007/s10529-009-9956-x
- Wang, R. (2021). China’s grain and oil production and marketing in 2020. China Oils and Fats, 46(08), 1–5.
- Wang, Y., Zhang, X., Huang, G., Feng, F., Liu, X., Guo, R., Gu, F., Zhong, X., & Mei, X. (2020). Dynamic changes in membrane lipid composition of leaves of winter wheat seedlings in response to PEG-induced water stress. BMC Plant Biology, 20(1), 84. https://doi.org/10.1186/s12870-020-2257-1
- Wu, J., Fan, X., Huang, X., Li, G., Guan, J., Tang, X., Qiu, M., Yang, S., & LU, S. (2021). Effect of different drying treatments on the quality of camellia oleifera seed oil. South African Journal of Chemical Engineering, 35(October 2020), 8–13. https://doi.org/10.1016/j.sajce.2020.10.003
- Xiao, J., Gu, C., He, S., Zhu, D., Huang, Y., & Zhou, Q. (2021). Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Research International, 141(prepublish), 110–128. https://doi.org/10.1016/j.foodres.2021.110128
- Xu, Z., Li, J., Guo, X., Jin, S., & Zhang, X. (2016). Metabolic engineering of cottonseed oil biosynthesis pathway via RNA interference. Scientific Reports, 6(1), 1–14. https://doi.org/10.1038/srep33342
- Yan, A., & Chen, Z. (2020). The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. The Botanical Review, 86(1), 39–75. https://doi.org/10.1007/s12229-020-09220-4
- Zhang, X., Shen, L., F, L. I., Meng, D., & Sheng, J. (2013). Arginase induction by heat treatment contributes to amelioration of chilling injury and activation of antioxidant enzymes in tomato fruit. Postharvest Biology and Technology, 79, 1–8. https://doi.org/10.1016/j.postharvbio.2012.12.019