ABSTRACT
Incorporation of the baobab fruit pulp in innovative formulations remains limited, despite its abundance of various nutrients and bioactive components. The objective of this study was to enhance the utilization of the baobab fruit pulp through the development of baobab supplemented cultured milk products. The physicochemical, microbial characteristics and consumer acceptability of treatments were studied. In the treatments, an increase in baobab pulp concentration led to an increase in some proximate components. A significant increase in the calcium, zinc, and vitamin C content was also observed with increased baobab concentration at different culture concentrations. Formulations were free from pathogenic bacteria and hence safe for human consumption. It was also established that Leuconostoc mesenteroides were responsible for the fermentation of the cultured formulations. Incorporating baobab fruit pulp in milk has proven to have a positive effect on the overall nutritional value of the milk, enhancing its suitability in combating micronutrient deficiencies.
Introduction
Baobab fruit pulp has been reported to supplement food products such as cereals, snack bars, and other baked products due to its excellent nutritional properties (A. Aluko, J. Kinyuru, L. M. Chove, et al., Citation2016). Various macronutrients and micronutrients are abundantly present in the baobab fruit pulp. For instance, the pulp is a rich source of dietary fiber, iron, zinc (Muthai et al., Citation2017), vitamin C, magnesium, calcium, and potassium (Stadlmayr et al., Citation2020). Previous studies indicate that the baobab fruit pulp contains more significant quantities of all the essential nutrients required for optimal nutrition than widely consumed fruits such as mangoes, bananas, guavas, and berries (Muthai et al., Citation2017). For example, according to FAO (Citation2020), the amount of vitamin C present in the baobab fruit pulp is three times more than that occurring in oranges. Therefore, baobab supplementation in various formulations provides an opportunity to address the various micronutrient deficiencies in diets. Additionally, the baobab fruit pulp is considered a nutraceutical as it contains various bioactive natural components with health promoting properties (Gahane and Kogje, Citation2013). Outstanding amounts of flavonoids, vitamin C, phenolic compounds such as gallic acid, procyanidin B2, and epicatechins are reportedly present in the fruit pulp (Habte & Krawinkel, Citation2017; Tembo et al., Citation2017). These bioactive components are capable of inhibiting oxidation activities thereby reducing the risk of inflammation-related illnesses, cardiovascular dysfunction, cancer, diabetes, and many more (Ndiaye et al., Citation2021). In 2008, the Commission of the European Union approved the utilization of the baobab fruit as a novel food ingredient (Stadlmayr et al., Citation2020; Vassiliou, Citation2008), after which in 2009 the pulp was accorded a Generally Recognized as Safe status allowing its approval by the FDA as a food ingredient (Ismail et al., Citation2019). Since then, this commodity has gained popularity in European, Asian, and U.S.A markets where it is being promoted as a superfood and has constantly been used in the formulation of various products (Chadare et al., Citation2009; Vassiliou, Citation2008). Further, a transition has been witnessed from traditional utilization to a higher retail segment (Darr et al., Citation2020) with more than three hundred products containing baobab as an ingredient being reported in the European market (Gebauer et al., Citation2014; Meinhold & Darr, Citation2019). This has provided a vital incentive in the realization of the baobab fruit pulp’s economic value, (Gebauer et al., Citation2014) prompting efforts towards domestication of the tree in countries such as Cairo, Mauritius, Malaya, Java, New Caledonia, Hawaii, Philippines, West Indies, the Antilles, and Guyana (Wickens, Citation2008). Similarly in the Savannah regions of Sub-Saharan Africa, the baobab fruit pulp has been appreciated as a source of food through its incorporation into various traditional delicacies (Buchmann et al., Citation2010). However, consumption and value addition activities remain limited in East African countries and thus fail to tap into the full potential and commercialization of baobab products (Gebauer et al., Citation2016; Kaimba et al., Citation2020). In Kenya particularly, the baobab fruit pulp remains underutilized despite its potential in combating various nutritional deficiencies (Wairimu et al., Citation2022). Spontaneously fermented milk products are staples and are part of the cultural heritage among diverse Kenyan communities (Nduko et al., Citation2016). Locally in Kenya, maziwa lala is an example of a spontaneously fermented milk product widely consumed by rural communities. Communities living along baobab growing regions often use the baobab fruit pulp to hasten the spontaneous fermentation of this product due to its abundance of organic acids (Donkor et al., Citation2014; Sabina et al., Citation2020). However, spontaneously fermented dairy products have limited shelf life, are inconsistent in terms of quality and organoleptic properties (Nduko et al., Citation2016) and have demonstrated potential health hazards. Whereas modern interventions such as the use of starter cultures consisting of a definite microbial composition result in a high-quality safe food product with a prolonged shelf life (Marco et al., Citation2017; Teshome, Citation2015). Fermentation of milk at ambient temperatures is initiated by mesophilic lactic cultures of the Leuconostoc and Lactococcus genera, capable of inhibiting both spoilage and pathogenic bacteria (Shah & Champagne, Citation2015; Şanlier et al., Citation2019). In addition, these microbiotas produce key flavor compounds yielding dairy products with a characteristic flavor (Wairimu et al., Citation2022). Further, these microorganisms confer certain health benefits to consumers such as boosted immunity, anti-microbial, and antidiabetic activities as a result of some bioactive components yielded during the fermentation process (Şanlier et al., Citation2019). This study therefore aimed at developing a cultured bovine milk product supplemented with baobab fruit pulp and determining the physicochemical, microbial, and sensory characteristics of the developed product. Owing to the nutritional and functional properties associated with baobab fruit pulp, cultured milk supplemented with baobab fruit pulp can be termed as a functional food (Lee & Foo, Citation2014). Therefore, the product under study will be useful in combating micronutrient deficiencies prevalent among the rural poor living along baobab growing regions. Additionally, the functional product has the potential to foster economic development among rural communities living along baobab regions and inevitably increase utilization of the baobab fruit pulp while promoting adequate health and nutrition.
Materials and methods
Sample collection and preparation
Baobab fruits were collected from Kibwezi location in Makueni County, Kenya. The baobab fruit pulp was separated from the shells, fibers, and seeds according to the method of Kinuthia (Citation2018). The baobab fruit pulp was stored in airtight zip-loc bags awaiting further processing. On the other hand, raw milk was collected from local farmers in Kiambu County and subjected to various platform quality tests to determine suitability for processing. Parameters recorded included: butter fat content (3.5%), density (1.028 g/cm3), pH = 6.6, and violet color score in the resazurin test indicating good quality milk.
Production of cultured milk supplemented with baobab
Cultured milk supplemented with baobab was produced according to the method of Aluko (Citation2017), with slight modifications. The temperature of the milk was slightly raised to 30°C after which baobab pulp and milk were blended into five different formulations (10:990), (15:985), (20:980), (25:975), (30:970). Baobab fruit pulp quantities were determined based on their contribution to the recommended daily intake (RDI) of zinc, calcium, and iron in children and pregnant women. One batch was subjected to spontaneous fermentation, while two batches were fermented using a mesophilic culture concentration of 0.01 g and 0.005 g. The mixture was pasteurized at 85°C, rapid cooling and mesophilic culture inoculation was done at 25°C. Incubation was carried out at ambient temperatures for 16–18 hours, after which the curd was broken and the product cooled to 4°C to stop further fermentation until further analysis.
Physicochemical characteristics of the baobab-based cultured milk
Total titratable acidity
The total titratable acidity of the formulation was done according to the AOAC method (Citation2012).
Textural properties
The textural properties of the formulated samples were analyzed by the back-extrusion method using TA. XT plus texture analyzer (Stable Microsystems, Godalming, UK) (Joon et al., Citation2017).
Proximate composition
All proximate components were analyzed according to the AOAC (Citation2005) methods. Carbohydrate and energy content were calculated by reducing the other compositions as shown below:
Carbohydrates = (100% ₋ [% protein + % fiber + % moisture + % fat + % ash]).
Energy = [(4 kcal/g (protein)+ 9 kcal/g (fat) + 4 kcal/g (carbohydrates)]
Micronutrient composition of the baobab based cultured milk
Analyses of calcium, iron, and zinc were determined by Atomic Absorption Spectrophotometry as per the AOAC (Citation2010) method. Vitamin C was determined according to the AOAC method 967.21 (Citation2006).
Microbiological analysis
Bacterial counts
Total lactic acid bacteria counts were determined as per the ISO 152,141,998 1–7, total coliforms ISO 4832: 2006, Staphylococcus aureus ISO 6888–1:1999, yeasts and molds counts ISO 21,527–2. The analysis for each test microbe was carried out in triplicate under aseptic conditions.
Isolation and identification of LAB
Isolation of lactic acid bacteria from the test samples was carried out according to the ISO 15,214: 1998 method. Pure lactic acid bacteria isolated from streak plates were further tested for cell morphology, motility, gram reaction, catalase production, and acid production from glucose (Abegaz, Citation2007).
Molecular detection of lactic acid bacteria isolates
DNA extraction
A loopful of the pure bacterial isolate was aseptically picked from each of the petri dishes and transferred to Eppendorf tubes containing sterile distilled water. The isolates were subjected to a hot water bath at 100°C for 30 min and centrifugation was done at 1500 rpm for 5 min at 20°C. The supernatant was drawn aseptically and served as a DNA template for further analysis (Silva et al., Citation2012).
PCR amplification
PCR amplification of the lactic acid bacteria isolate was done according to the method of Kaur et al. (Citation2017). Four RAPD primers were used in this experiment for the amplification of two microorganisms namely, Leuconostoc mesenteroides 699 (GCTAAATACGTGCCAGCAGC and GATGATCTGACGTCGTCCCC) as forward and reverse primers respectively while Lactococcus lactis 460 (TCACCTATGCAAGCTCGGAC and FTCACCTATGCAAGCTCGGAC) as forward and reverse primers respectively, at a concentration of 10 µl. A master mix kit containing 10 mM HCL, 4.0 mM KCL, 1.5 mM MgCl2, 250 µM dNTPs mix and 1 U Taq polymerase of a pH of 9.0 was used for amplification. Then, 0.8 µl of each primer and 5 µl of the DNA template was added into 8 µl premix vials and the final volume made up to 16 µl through the addition of 1.4 µl of distilled water. The PCR conditions involved pre-denaturation at 95°C for 15 s, 35 cycles of denaturation at 94°C for 30 s, annealing at 39°C for 15 s, extension at 72°C for 1 min and final extension at 72°C for 7 min for Leuconostoc mesenteroides and pre-denaturation at 94°C for 15 s, 35 cycles of denaturation at 94°C for 30 s, annealing at 45°C for 15 s, extension at 72°C for 2 min, and final extension at 72°C for 8 min for Lactococcus lactis.
Agarose gel electrophoresis
After amplification, the PCR products were fractionated on a 1.5% agarose gel using a 100 ml TBE buffer containing 10 mg/ml ethidium bromide. The products were visualized under UV light, and the gels were photographed using a UV gel documentation system (GELMAX-PC). Nuclease-free water was used as the negative control. The band pattern was analyzed and confirmed using 100 base pair and 1kb ladders (Kaur et al., Citation2017).
Consumer acceptability of cultured milk supplemented with baobab pulp
Consumer acceptability tests were carried out at The University of Nairobi sensory evaluation laboratory. The test involved a hundred randomly selected untrained panelists. Coded products were uniformly served in a counterbalanced order to avoid bias. Six attributes namely: mouthfeel, taste, aroma, appearance, consistency, and overall acceptability were assessed by each panelist. Panelists rated the product attributes based on a 5-point hedonic scale ranging from 1 (Dislike extremely) to 5 (Like extremely). Consumer acceptability data were further explored using Principal Component Analysis.
Development of yeasts and molds in cultured formulations supplemented with baobab fruit pulp during storage
A longitudinal study design involving real-time monitoring was performed on selected samples according to the method of Yimenu et al. (Citation2019). Only samples that recorded the highest consumer acceptability and nutritional value were taken for analysis. After carrying out the initial analysis immediately after processing, samples were subjected to cold storage at 4°C while packaged in glass, carton, and white pigmented PET plastic containers. Yeast and mold counts were determined at intervals of 2 days according to the ISO 215 27–2:2008 method in samples stored for 14 days.
Data analysis
Data from the various experiments were subjected to two-way ANOVA using STATA version 12. Mean separation was done using Bonferroni’s method at p ≤ 0.05 and data were reported in means and standard error at a 95% confidence interval.
Results
Physicochemical characteristics of cultured milk supplemented with baobab fruit pulp
Total titratable acidity
In milk samples fermented using a culture concentration of 0.01 g and 0.005 g, the total titratable acidity significantly increased with an increase in baobab pulp concentration. The highest values 1.28 ± 0.004 and 1.25 ± 0.004 g/100 ml lactic acid were recorded in samples that had 3.0% baobab pulp as shown in . In milk samples subjected to spontaneous fermentation for 48 h, values ranged from 0.81 ± 0.004 to 1.05 ± 0.004 g/100 ml lactic acid and no significant increase in total titratable acidity with further increase in the rate of baobab pulp addition was observed.
Table 1. Total Titratable Acidity (g/100 ml lactic acid) of cultured milk supplemented with baobab fruit pulp at different concentrations.
Textural properties
The firmness of fermented milk samples was significantly affected by baobab pulp and culture concentrations (p < .0001). In samples fermented using a culture concentration of 0.01 g and 0.005 g, a significant increase in firmness from 29.31 ± 0.49 to 43.18 ± 0.49 and 35.03 ± 0.49 was observed upon supplementation with 1.0% baobab pulp. This was then followed by a significant decrease with further increase in baobab pulp with the least values 20.23 ± 0.49 and 20.19 ± 0.49 being observed in samples containing 3.0% baobab pulp as shown in . Interestingly in milk samples subjected to spontaneous fermentation, no significant differences were observed among formulations.
Table 2. Firmness of cultured milk supplemented with baobab fruit pulp at different concentrations.
On the other hand, baobab pulp and culture concentrations significantly affected the consistency of the samples as shown in . Compared to the control (714.92 ± 7.7), the addition of 1% baobab pulp in 0.01 g and 0.005 g cultured formulations resulted in a significant increase in consistency of 861.02 ± 7.7 and 872 ± 7.7, respectively. This was followed by a significant decline in consistency with further baobab pulp addition with the least value (415.39 ± 7.7) being recorded in milk fermented with 0.01 g culture containing 3.0% baobab pulp. For milk samples subjected to spontaneous fermentation constant consistency with values ranging from 400.75 ± 7.7 and 427.40 ± 7.7 was recorded.
Table 3. Consistency of cultured milk supplemented with baobab fruit pulp at different concentrations.
In addition, the cohesiveness was significantly affected by culture concentrations (p < .0001) as indicated in . In milk samples fermented using culture concentrations of 0.01 g and 0.005 g and 1.0% baobab pulp addition, there was a significant increase (27.50 ± 0.49 and 28.35 ± 0.49), respectively in cohesiveness compared to the control (22.37 ± 0.49 g). Further, baobab pulp addition resulted in a significant increase in cohesiveness with the highest value of 15.43 ± 0.49 being observed in milk samples containing 3.0% baobab pulp and 0.01 g culture. In milk samples subjected to spontaneous fermentation, the highest cohesiveness was observed upon the addition of 2.0% and 3.0% baobab pulp.
Table 4. Cohesiveness of cultured milk supplemented with baobab fruit pulp at different concentrations.
Further, no significant difference was observed in the work of cohesion in milk supplemented with 1.0% and 3.0% baobab pulp at the different culture concentrations (p < 0.0001). However, significant differences existed in the cultured samples containing 1.5%, 2.0%, and 2.5% baobab pulp. In milk samples subjected to spontaneous fermentation, a varying work of cohesion was observed with further baobab pulp addition as shown in
Table 5. Work of cohesion of cultured milk supplemented with baobab fruit pulp at different concentrations.
Proximate composition of formulated samples
Moisture content in all formulations ranged between 84.28% and 87.91% and no significant differences existed. In cultured formulations and spontaneously fermented samples respectively, the highest protein content (4.8 ± 0.07 g/100 g and 4.44 ± 0.07 g/100 g) was recorded in milk samples containing 3.0% baobab pulp. Higher values of crude fat were observed in all samples containing 3.0% baobab pulp with values ranging from 1.59 ± 0.02 g/100 g to 2.69 ± 0.02 g/100 g. Higher crude fiber values were observed in all formulations containing 3.0% baobab pulp ranging from 1.64 ± 0.02 g/100 g and 2.22 ± 0.02 g/100 g. The ash content in various formulations ranged from 0.72 ± 0.006 g/100 g and 0.91 ± 0.006 g/100 g. The highest energy levels were yielded at 2.0%, 2.5%, and 3.0% baobab pulp concentrations as indicated in .
Table 6. Proximate composition of cultured milk supplemented with baobab fruit pulp at different concentrations.
Micronutrient content of formulated samples
Culture concentrations had a significant influence on the micronutrient content of the fermented milk supplemented with baobab fruit pulp (p < .05). Higher levels of micronutrients were recorded in baobab supplemented milk samples containing 0.005 g culture. For vitamin C, the highest levels (10.09 ± 0.10 mg/100 g) were reported in milk fermented using a culture concentration of 0.005 g supplemented with 3.0% baobab while the least value (2.47 ± 0.10 mg/100 g) was reported in the control. Spontaneously fermented milk supplemented with 3.0% baobab fruit pulp yielded the highest calcium content (36.25 ± 0.03 mg/100 g), while milk fermented using a culture concentration of 0.005 g supplemented with 3.0% baobab pulp, yielded the highest iron content (1.51 ± 0.02 mg/100 g). On the other hand, milk fermented using a culture concentration of 0.005 g supplemented with 3.0% baobab fruit pulp recorded the highest zinc content (1.19 ± 0.01 mg/100 g) as shown in .
Table 7. Micronutrient content of cultured milk supplemented with baobab fruit pulp at different concentrations.
Microbial analysis of formulated products
Total coliforms
Total coliforms in cultured samples supplemented with baobab fruit pulp were not detected. The highest counts (3.19 ± 0.002 CFU-G) were detected in spontaneously fermented milk containing 1.0% baobab fruit pulp as shown in .
Table 8. Total coliforms (log10 CFU in cultured milk supplemented with baobab fruit pulp at different concentrations.
Staphylococcus aureus, yeasts and molds were not detected in any of the test samples.
Isolation and identification of lactic acid bacteria
Lactic acid bacteria counts ranged from 6.22 ± 0.01CFU/G and 8.40 ± 0.01CFU/G as indicated in
Table 9. Lactic Acid Bacteria (log10 CFU) in cultured milk supplemented with baobab fruit pulp at different concentrations.
Isolation and molecular identification of lactic acid bacteria
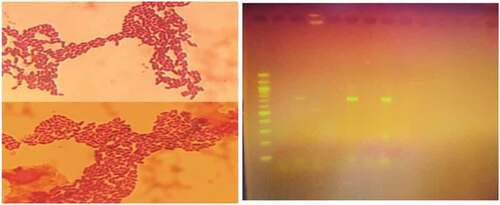
Typical lactic acid bacteria colonies upon microscopic examination exhibited cocci cell morphology as shown in , non-motility, and gram-positive reaction. Biochemical tests indicated negative catalase reaction and glucose utilization. Based on these characteristics, the isolates were considered as lactic acid bacteria. Further PCR amplification confirmed Leuconostoc mesenteroides to be present in the formulated samples with the length slightly above 699 base pair.
Consumer acceptability of cultured milk supplemented with baobab fruit pulp
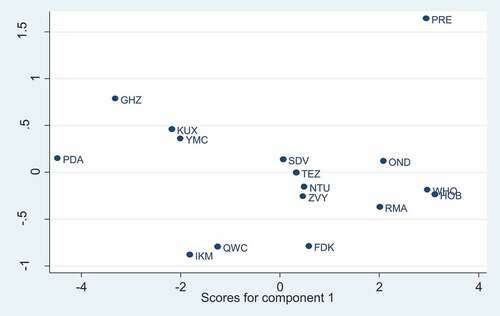
Two principal components were generated and accounted for 96% of the variation. Mouthfeel, taste, aroma, consistency, and overall acceptability had a strong loading on component 1 while appearance had a strong loading in component 2 as shown in .
Table 10. Principal components (eigenvectors).
The highest rating regarding mouthfeel, taste, aroma, consistency and overall acceptability were recorded in cultured milk supplemented with 1.5% baobab pulp and 2.0% baobab pulp fermented using culture concentrations of 0.005 g and 0.01 g, respectively. However, the control scored higher in all attributes as indicated in .
Figure 2. Scores for cultured formulations supplemented with baobab fruit pulp.
Development of yeasts and molds in cultured formulations supplemented with baobab fruit pulp during storage
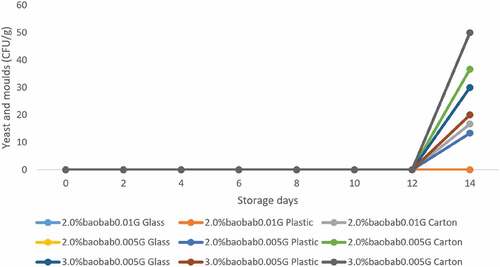
Yeast and molds were not observed during the entire storage period in samples packaged using glass and plastic, containing 2.0% baobab pulp and 0.01 g culture. On the 14th day of storage, samples containing 3.0% baobab and 0.005 g culture packaged in carton tubs, recorded the highest-level yeast and molds. Samples containing 2.0% baobab and 0.01 g culture packaged in plastic recorded the lowest yeast and mold counts as shown in .
Discussion
Physico-chemical characteristics of baobab-based formulations
The increase in total titratable acidity with an increase in baobab concentration may be attributed to organic acids such as malic, succinic, tartaric, citric, and ascorbic acids (Mpofu et al., Citation2014) present in the pulp. Values yielded in the formulated samples were higher than 0.7% recommendations by the Kenya Bureau of Standards (KS 941, Citation2018). Additional fermentable sugars due to baobab pulp addition allowed more biochemical activity of the starter cultures consequently increasing the total titratable acidity (Dabora, Citation2016). In this study, texture profiling was characterized using force and deformation as the major parameters. In addition, these properties are largely affected by the structure, spatial distribution, and bond strength of the casein micelles (A. Aluko, J. Kinyuru, L. M. Chove, et al., Citation2016). Increasing the baobab concentration in formulated samples may have resulted in an interruption of the gel structure due to the rearrangement of the protein-protein matrix in the products (Izadi et al., Citation2015).
Proximate composition
The moisture content in all the samples ranged from 86.17% to 84.44%, slightly lower than findings from a similar study conducted by Dabora (Citation2016), who reported a moisture content of 86.75% to 87.97%. A reduction in moisture content with an increase in baobab pulp concentration was due to the absorption of moisture by the baobab fruit pulp powder (Wairimu et al., Citation2022). Increasing the baobab pulp concentration led to an increase in protein in all samples. This study agrees with the findings by Dabora (Citation2016), who reported an increase in the protein content from 3.67% to 4.59% with an increased rate of baobab fruit pulp addition in bovine milk. Higher protein levels were observed in cultured milk compared to spontaneously fermented milk since starter cultures can increase the protein availability in milk (Teshome, Citation2015) and are capable of secreting proteolytic enzymes that hydrolyze caseins to peptides and amino acids (Aluko, Citation2017; Zumunta & Umar, Citation2020). However, a study conducted by Aluko (Citation2017), and Adelekan and Saleh (Citation2020) reported a decrease in protein content with an increase in baobab fruit pulp as a result of fermentation. The fat content increased in all the samples with an increase in baobab pulp concentration due to additional fat from the pulp that was imparted to the product. This finding is similar to a study conducted by Wairimu et al. (Citation2022). Spontaneously fermented milk and 0.01 g cultured milk showed the highest levels of fat, which could be due to increased lipase activity which hydrolyses fat in fatty acids and glycerol (Adelekan & Saleh, Citation2020). The fat content in the formulated samples complies with the Kenya Bureau of Standards (KS 941, Citation2018) minimum of 10%. The increase in fiber content in all the samples with an increase in baobab pulp concentration was similar to a study conducted by Chipchura (2021). This is because baobab pulp is a rich source of dietary fiber and polysaccharides synthesis by lactic acid bacteria (Korcz et al., Citation2018; Muthai et al., Citation2017). The increase in ash content implies that baobab pulp has a high mineral content (Aluko, Citation2017), similar to findings reported by Wairimu et al. (Citation2022) in baobab pulp enriched goat milk yoghurt. Lactic acid bacteria utilize minerals for growth and metabolic activity (Chen et al., Citation2017) this could be the reason why lower ash content values were recorded in 0.01 g cultured milk compared to 0.005 g and spontaneously fermented baobab supplemented milk. Lactose in milk is hydrolyzed by lactic acid bacteria resulting in the production of lactic acid (Ihemeje et al., Citation2015) accounting for the low carbohydrate content observed in the formulated samples. This work corroborates with Aluko (Citation2017) and Zumunta and Umar (Citation2020).
Micronutrient content of the baobab-based formulations
According to Zahra’u et al. (Citation2014), the baobab pulp is an amenable source of calcium and can be utilized for calcium supplementation. Calcium is vital for bone formation and mineralization and the requirements are high during growth, pregnancy, and lactation (Adelekan & Saleh, Citation2020). The addition of baobab, therefore, resulted in increased calcium content in all the cultured spontaneously fermented milk samples. Iron is vital as it is responsible for various metabolic processes in the body such as DNA synthesis, oxygen, and electron transport (Abbaspour et al., Citation2014). Iron is the most abundant microelement in the baobab fruit pulp (Muthai et al., Citation2017) and an increase in baobab pulp concentration resulted in increased iron levels in the products under study. On the other hand, zinc is an essential trace element that promotes enzymatic activity, DNA synthesis, immune system function, tissue growth, and maintenance as well as the development of male reproductive organs (Deshpande et al., Citation2013; Kwamboka, Citation2020). Zinc in the formulated samples ranged from 0.74 to 1.19 mg/100 g quite similar to results in a study reported by Aluko (Citation2017). The baobab pulp has an outstanding content of vitamin C (238 mg/100 g), an antioxidant with biochemical and molecular roles in the body (Habte & Krawinkel, Citation2017). Even though the vitamin C increases with an increase in baobab concentration, the vitamin C content was lower as it is heat labile and quantities may have been lost during the pasteurization process (Tembo et al., Citation2017).
Microbiological characteristics of the baobab-based formulations
Spontaneous fermentation of milk is conducted by natural microflora in the environment. Coliforms participate in this kind of fermentation and tend to multiply rapidly since the numbers of lactic acid bacteria are very low (Mwangi et al., Citation2016). Total coliforms were not detected in milk fermented with mesophilic cultures supplemented with baobab. This could be because lactic acid bacteria used in fermentation secrete antimicrobial metabolites which create an unfavorable environment for coliforms, yeasts and molds that could be pathogenic or cause spoilage (Teshome, Citation2015). The formulated products were free from Staphylococcus aureus indicating that the samples were processed under hygienic conditions. All formulated products met the minimum CFU/g of 108 according to the Kenya Bureau of Standards (KS 941, Citation2018). Lactic acid bacteria were also evident in spontaneously fermented milk samples containing baobab fruit pulp since they form part of the inherently mixed microflora in milk (Mwangi et al., Citation2016). Lactic acid bacteria CFU/g increased with an increase in baobab pulp concentration since the pulp possesses up to about 25% of soluble fiber capable of stimulating the proliferation and metabolic activities of the lactic acid bacteria (Abdalla et al., Citation2010). Leuconostoc mesenteroides evident in formulated samples have health benefits and play an important role in the enhancing textural and aromatic properties of buttermilk, cheese, butter, and kefir particularly (Kaur et al., Citation2017). Leuconostoc mesenteroides has superior functional properties compared to other strains as well as high probiotic potential according to De Paula et al. (Citation2015).
Consumer acceptability of cultured milk supplemented with baobab
Sensory characteristics often have a strong influence on the consumer perception of food product quality and often determine the success of products in the market segment (Sharif et al., Citation2017). In the formulated samples, mouthfeel scores were greatly affected by increased baobab concentration suggesting that higher values recorded in milk containing 2.0% baobab fermented with 0.01 g baobab could have been due to the high total solids and pectin content that impart a gelling characteristic to milk (Ndabikunze et al., Citation2011). Compared to controlled fermentation, spontaneous fermentation results in inconsistencies in terms of texture, flavor, and aroma (Moslehishad et al., Citation2013) therefore recording low scores in the formulations. Generally, the control scored better in all the sensory attributes compared to supplemented samples. This may have been due to increased acidity, color alteration, and texture changes due to baobab addition. This further indicates that baobab and culture levels used during fermentation influence consumer preference.
Effect of packaging and storage time on the development of yeast and molds in cultured milk supplemented with baobab pulp
Yeast and molds are considered to be frequent natural contaminants in fermented dairy products and are responsible for their spoilage (Nwagu & Amadi, Citation2010). Increased acidity with increased storage time as well as reduction of potential oxygen during fermentation facilitates their proliferation (Sengupta et al., Citation2013). In all the test samples, yeasts and molds were observed on the 14th day of storage, corroborating with a study conducted by Aluko (Citation2017) who also reported yeasts and molds development on the 14th day of storage in probiotic yoghurt fortified with baobab fruit pulp. Carton packaging material was not an ideal candidate for the keeping quality of the test samples due to a high number of yeasts and molds counts as they are permeable to gases and when there is a lack of oxygen and carbon IV oxide balance proliferation of yeasts and molds precedes (Singh et al., Citation2011).
Conclusion
Incorporation of the baobab pulp as a food ingredient in various formulations increases the consumption and commercial value of the highly underutilized baobab fruit. When incorporated into cultured milk an increase in the nutritional value was witnessed. Therefore, cultured milk supplemented with baobab pulp serves as a vehicle in the delivery of all essential nutrients to support vulnerable populations. In addition, the product possesses the qualities of a functional-based drink due to the presence of probiotic bacteria (Leuconostoc mesenteroides) besides the functional properties of baobab pulp. Therefore, this product can promote the health and wellness of vulnerable populations living along baobab growing regions. Baobab-based cultured milk, when adopted for commercialization, will have a greater market potential as consumer needs are changing to convenient and healthier versions.
Ethical clearance
No human or animal subjects were used in this study.
Acknowledgment
We acknowledge the laboratory technicians for their technical guidance in this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The original research generated data used to support the findings of this study are included in this article.
Additional information
Funding
References
- Abbaspour, N., Hurrell, R., & Kelishadi, R. (2014). Review on iron and its importance for human health. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences, 19(2), 164. http://etd.repository.ugm.ac.id/penelitian/unduh/310778
- Abdalla, A. A., Mohammed, M. A., & Mudawi, H. A. (2010). Production and quality assessment of instant baobab (Adansonia digitata L.). Advance Journal of Food Science and Technology, 2(2), 125–133. https://www.airitilibrary.com/Publication/alDetailedMesh?docid=20424876-201003-201009060053-201009060053-125-133
- Abegaz, K. (2007). Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. African Journal of Biotechnology, 6(12). https://www.ajol.info/index.php/ajb/article/view/57589
- Adelekan, A. O., & Saleh, A. A. (2020). Chemical composition and microbiological quality of baobab (Adansonia digitata L.) fruit fortified yoghurt. Nigerian Journal of Microbiology, 34(1), 4998–5006. http://www.nsmjournal.org.ng/2020-34-1/17.pdf
- Aluko, A. (2017). Probiotic viability and storage stability of yoghurt enriched with baobab pulp (Adansonia digitata L.) [ Doctoral dissertation]. http://ir.jkuat.ac.ke/handle/123456789/2825
- Aluko, A., Kinyuru, J., Chove, L. M., Kahenya, P., & Owino, W. (2016). Nutritional quality and functional properties of baobab (Adansonia digitata L.) pulp from Tanzania. Journal of Food Research, 5(5), 23. https://doi.org/10.5539/jfr.v5n5p23
- AOAC. (2005). The official methods of analysis of association of official analytical chemist.
- AOAC. (2006) Official method 967.21. Ascorbic acid in vitamins preparations and juices, 2, 6-dichloroindophenol titrimetric method. Chapter. 45, pp. 1–16. Official methods of analysis of AOAC International, 17.
- AOAC. (2010). The official methods of analysis. Association of official analytical chemist (19th ed.).
- AOAC. (2012). Official Methods of Analysis. Association of Official Analytical Chemist (20th ed.).
- Buchmann, C., Prehsler, S., Hartl, A., & Vogl, C. R. (2010). The importance of baobab (Adansonia digitata L.) in rural West African subsistence—suggestion of a cautionary approach to international market export of baobab fruits. Journal of Ecology of Food and Nutrition, 49(3), 145–172. https://doi.org/10.1080/03670241003766014
- Chadare, F. J., Linnemann, A. R., Hounhouigan, J. D., Nout, M. J. R., & Van Boekel, M. A. J. S. (2009). Baobab food products: A review on their composition and nutritional value. Critical. Reviews of Food Science and Nutrition, 49(3), 254–274. https://doi.org/10.1080/10408390701856330
- Chen, C., Zhao, S., Hao, G., Yu, H., Tian, H., & Zhao, G. (2017). Role of lactic acid bacteria on the yogurt flavour: A review. International Journal of Food Properties, 20(sup1), S316–330. https://doi.org/10.1080/10942912.2017.1295988
- Dabora, S. A. M. A. (2016). Assessment of the effect of addition of Baobab (Adansonia digitata L.) fruit pulp on properties of camel milk yoghurt [ Doctoral dissertation]. Sudan University of Science and Technology. http://repository.sustech.edu/handle/123456789/14515
- Darr, D., Chopi-Msadala, C., Namakhwa, C. D., Meinhold, K., & Munthali, C. (2020). Processed baobab (Adansonia digitata L.) food products in Malawi: From poor men to premium-priced specialty food. Forests, 11(6), 698. https://doi.org/10.3390/f11060698
- De Paula, A. T., Jeronymo-Ceneviva, A. B., Silva, L. F., Todorov, S. D., Franco, B. D. G. M., & Penna, A. L. B. (2015). Leuconostoc mesenteroides SJRP55: A potential probiotic strain isolated from Brazilian water buffalo mozzarella cheese. Annals of Microbiology, 65(2), 899–910. https://doi.org/10.1007/s13213-014-0933-9
- Deshpande, J. D., Joshi, M. M., & Giri, P. A. (2013). Zinc: The trace element of major importance in human nutrition and health. International Journal of Medical Sciences and Public Health, 2(1), 1–6. https://doi.org/10.5455/ijmsph.2013.2.1-6
- Donkor, A. M., Tei, M., Suurbaar, J., Yakubu, A., & Addae, D. (2014). Stability evaluation and degradation kinetics of ascorbic acid in baobab fruit pulp formulated with the seed oil. British Biotechnology Journal, 4(5), 566–578. https://doi.org/10.9734/BBJ/2014/9837
- FAO. (2020). FAO/INFOODS food composition table for Western Africa (2019) user guide and condensed food composition table. 8 October 2022.
- Gahane, R. N., & Kogje, K. K. (2013). Effect of pre-treatments for enhancing the germination of Adansonia digitata L. and Cochlospermum religiosum L. Indian Forester, 139(7), 648–651. http://www.indianforester.co.in
- Gebauer, J., Adam, Y. O., Sanchez, A. C., Darr, D., Eltahir, M. E., Fadl, K. E., & Kehlenbeck, K. (2016). Africa’s wooden elephant: The baobab tree (Adansonia digitata L.) in Sudan and Kenya: A review. Genetic Resources and Crop Evolution, 63(3), 377–399. https://doi.org/10.1007/s10722-015-0360-1
- Gebauer, J., Assem, A., Busch, E., Hardtmann, S., Möckel, D., Krebs, F., & Kehlenbeck, K. (2014). Der Baobab (Adansonia digitata L.): Wildobst aus Afrika für Deutschland und Europa. Erwerbs-Obstbau, 56(1), 9–24. https://doi.org/10.1007/s10341-013-0197-8
- Habte, T. Y., & Krawinkel, M. B. (2017). Metaphysical analysis of the nutritional and therapeutic value of baobab (Adansonia Digitata L.). Journal of Nutritional Health Sciences, 5(1), 101. https://doi.org/10.15744/2393-9060.5.101
- Ihemeje, A., Nwachukwu, C. N., & Ekwe, C. C. (2015). Production and quality evaluation of flavoured yoghurts using carrot, pineapple, and spiced yoghurts using ginger and pepper fruit. African Journal of Food Science, 9(3), 163–169. https://doi.org/10.5897/AJFS2014.1244
- Ismail, B. B., Pu, Y., Guo, M., Ma, X., & Liu, D. (2019). LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata L.) fruit pulp. Food Chemistry, 277, 279–288. https://doi.org/10.1016/j.foodchem.2018.10.056
- ISO. (1998). Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of mesophilic lactic acid bacteria — Colony count technique at 30 degrees C. ISO, 152141998, 1–7.
- ISO. (2006). Microbiology of food and animal feeding stuffs–horizontal method for the enumeration of coliforms—colony-count technique. ISO, 4832.
- ISO. (2008). Microbiology of food and animal feeding stuffs: Horizontal method for the enumeration of yeasts and moulds. ISO. 21527-2.
- ISO 6888-1:1999. Microbiology of food and animal feeding stuffs - Horizontal method for the enumeration of coagulase-positive staphylococci– Part 1: Technique using Baird-Parker agar medium.
- Izadi, Z., Nasirpour, A., Garoosi, G. A., & Tamjidi, F. (2015). Rheological and physical properties of yogurt enriched with phytosterol during storage. Journal of Food Science and Technology, 52(8), 5341–5346. https://doi.org/10.1007/s13197-014-1593-2
- Joon, R., Mishra, S. K., Brar, G. S., Singh, P. K., & Panwar, H. (2017). Instrumental texture and syneresis analysis of yoghurt prepared from goat and cow milk. The Pharma Innovation, 6(7, Part G), 971. https://www.thepharmajournal.com/archives/2017/vol6issue7/PartG/6-7-73-280.pdf
- Kaimba, G. K., Muendo, K. M., & Mithofer, D. (2020). Marketing of baobab pulp in Kenya: Collectors’ choice of rural versus urban markets. African Journal of Agricultural and Resource Economics, 15(311–2020–1789), 194–212. https://doi.org/10.53936/afjare.2020.15(3).13
- Kaur, J., Lee, S., Park, Y. S., & Sharma, A. (2017). RAPD analysis of Leuconostoc mesenteroides strains associated with vegetables and food products from Korea. LWT, 77, 383–388. https://doi.org/10.1016/j.lwt.2016.11.078
- Kinuthia, U. M. (2018). Nutritional characterisation of Baobab (Adansonia digitata l.) fruits based on Africa geographical regions (Doctoral dissertation, Egerton University).
- Korcz, E., Kerényi, Z., & Varga, L. (2018). Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food & Function, 9(6), 3057–3068. https://doi.org/10.1039/C8FO00118A
- KS 941. (2018). Kenya Standard — Fermented (cultured) milk — Specification (Second Ed.).
- Kwamboka, M. D. (2020). Potential role of baobab in household food security in Kilifi and Kitui Counties of Kenya [ Doctoral dissertation]. JKUAT-Agriculture. http://ir.jkuat.ac.ke/handle/123456789/5299.
- Lee, S. C., & Foo, M. H. (2014). Functional foods and its biomarkers. introduction to functional food science: Textbook (2nd ed.). Functional Food Center.
- Marco, M. L., Heeney, D., Binda, S., Cifelli, C. J., Cotter, P. D., Foligné, B., & Hutkins, R. (2017). Health benefits of fermented foods: Microbiota and beyond. Current Opinion in Biotechnology, 44, 94–102. https://doi.org/10.1016/j.copbio.2016.11.010
- Meinhold, K., & Darr, D. (2019). The processing of non-timber forest products through small and medium enterprises—a review of enabling and constraining factors. Forests, 10(11), 1026. https://doi.org/10.3390/f10111026.
- Moslehishad, M., Mirdamadi, S., Ehsani, M. R., Ezzatpanah, H., & Moosavi‐movahedi, A. A. (2013). The proteolytic activity of selected lactic acid bacteria in fermenting cow’s and camel’s milk and the resultant sensory characteristics of the products. International Journal of Dairy Technology, 66(2), 279–285. https://doi.org/10.1111/1471-0307.12017
- Mpofu, A., Linnemann, A. R., Nout, M. J., Zwietering, M. H., & Smid, E. J. (2014). Mutandabota, a food product from Zimbabwe: Processing, composition, and socioeconomic aspects. Ecology of Food and Nutrition, 53(1), 24–41. https://doi.org/10.1080/03670244.2013.767802
- Muthai, K. U., Karori, M. S., Muchugi, A., Indieka, A. S., Dembele, C., Mng’omba, S., & Jamnadass, R. (2017). Nutritional variation in baobab (Adansonia digitata L.) fruit pulp and seeds based on Africa geographical regions. Food Science and Nutrition, 5(6), 1116–1129. https://doi.org/10.1002/fsn3.502
- Mwangi, L. W., Matofari, J. W., Muliro, P. S., & Bebe, B. O. (2016). Hygienic assessment of spontaneously fermented raw camel milk (suusa) along the informal value chain in Kenya. International Journal of Food Contamination, 3(1), 1–9. https://doi.org/10.1186/s40550-016-0040-8
- Ndabikunze, B. K., Masambu, B. N., Tiisekwa, B. P. M., & Issa-Zacharia, A. (2011). The production of jam from indigenous fruits using baobab (Adansonia digitata L.) powder as a substitute for commercial pectin. African Journal of Food Science, 5(3), 168–175. https://academicjournals.org/journal/AJFS/article-full-text-pdf/51E20113089
- Ndiaye, E. M., Yousra, Y. E. I., Alioune, S., Ayessou, N. C., Harhar, H., Cisse, M., & Tabyaoui, M. (2021). Secondary metabolites and antioxidant activity of different parts of the baobab fruit (Adansonia digitata L.). Food and Nutrition Sciences, 12(7), 732–741. https://doi.org/10.4236/fns.2021.127055
- Nduko, J. M., Matofari, J. W., Nandi, Z. O., & Sichangi, M. B. (2016). Spontaneously fermented Kenyan milk products: A review of the 800 current state and future perspectives. African Journal of Food Science, 11(1), 1–11. https://doi.org/10.5897/AJFS2016.1516
- Nwagu, T. N., & Amadi, E. C. (2010). Bacteria population of some commercially prepared yoghurt sold in Enugu State, Eastern Nigeria. African Journal of Microbiology Research, 4(10), 984–988. https://academicjournals.org/journal/AJMR/article-full-text-pdf/B5454F313298
- Sabina, I., Nihmot, A., & Ifechukwu, J. (2020). African baobab: Its role in enhancing nutrition, health, and the environment. Trees, Forests and People, 3, 100043. https://doi.org/10.1016/j.tfp.2020.100043
- Şanlier, N., Gökcen, B. B., & Sezgin, A. C. (2019). Health benefits of fermented foods. Critical Reviews in Food Science and Nutrition, 59(3), 506–527. https://doi.org/10.1080/10408398.2017.1383355
- Sengupta, S., Bhowal, J., Bhattacharyya, D. K., & Bengal, W. (2013). Development of new kinds of soy yogurt containing functional lipids as superior quality food. Annals of Biological Research, 4(4), 144–151. http://scholarsresearchlibrary.com/ABR-vol4-iss4/ABR-2013-4-4-144151.pdf
- Shah, N. P., & Champagne, C. P. (2015). Cultured milk and yogurt. Dairy Processing and Quality Assurance, 235–265. https://doi.org/10.1002/9781118810279.ch10
- Sharif, M. K., Butt, M. S., Sharif, H. R., & Nasir, M. (2017). Sensory evaluation and consumer acceptability. Handbook of food science and technology, 361–386. https://www.researchgate.net/profile/HafizSharif/publication/320466080Sensory_Evaluation_and_Consumer_Acceptability/links/59e705b94585151e54658b81/Sensory-Evaluation-and-Consumer-Acceptability.pdf
- Silva, G. A. D., Bernardi, T. L., Schaker, P. D. C., Menegotto, M., & Valente, P. (2012). Rapid yeast DNA extraction by boiling and freeze-thawing without using chemical reagents and DNA purification. Brazilian Archives of Biology and Technology, 55(2), 319–327. https://doi.org/10.1590/S1516-89132012000200020
- Singh, P., Wani, A. A., Saengerlaub, S., & Langowski, H. C. (2011). Understanding critical factors for the quality and shelf-life of MAP fresh meat: A review. Critical Reviews in Food Science and Nutrition, 51(2), 146–177. https://doi.org/10.1080/10408390903531384
- Stadlmayr, B., Wanangwe, J., Waruhiu, C. G., Jamnadass, R., & Kehlenbeck, K. (2020). Nutritional composition of baobab (Adansonia digitata L.) fruit pulp sampled at different geographical locations in Kenya. Journal of Food Composition and Analysis, 94, 103617. https://doi.org/10.1016/j.jfca.2020.103617
- Tembo, D. T., Holmes, M. J., & Marshall, L. J. (2017). Effect of thermal treatment and storage on bioactive compounds, organic acids and antioxidant activity of baobab fruit (Adansonia digitata L.) pulp from Malawi. Journal of Food Composition and Analysis, 58, 40–51. https://doi.org/10.1016/j.jfca.2017.01.002
- Teshome, G. (2015). Review on lactic acid bacteria function in milk fermentation and preservation. African Journal of Food Science, 9(4), 170–175. https://doi.org/10.5897/AJFS2015.1276
- Vassiliou, A. (2008). Commission decision. European Union C (2008) 30462008/575/EC. 38–39
- Wairimu, N., Owaga, E. E., & Koskei, K. (2022). Development and evaluation of goat milk yoghurt enriched with baobab fruit pulp. European Journal of Agriculture and Food Sciences, 4(2), 100–105 . https://doi.org/10.24018/ejfood.2022.4.2.485
- Wickens, G. E. (2008). The baobabs: Pachycauls of Africa, Madagascar and Australia. Springer Science & Business Media. https://doi.org/10.1007/978-1-4020-6431-9
- Yimenu, S. M., Koo, J., Kim, B. S., Kim, J. H., & Kim, J. Y. (2019). Freshness-based real-time shelf-life estimation of packaged chicken meat under dynamic storage conditions. Poultry Science, 98(12), 6921–6930. https://doi.org/10.3382/ps/pez461
- Zahra’u, B., Mohammed, A. S., Ghazali, H. M., & Karim, R. (2014). Baobab tree (Adansonia digitata L.) parts: Nutrition, applications in food and uses in ethno-medicine–a review. Annals of Nutritional Disorders & Therapy, 1(3), 1011. https://www.doc-developpement-durable.org/file/Culture/ArbresFruitiers/FICHES_ARBRES/baobab/Nutrition,%20Applications%20in%20Food%20and%20Uses%20in%20Ethno.pdf
- Zumunta, J. D., & Umar, A. F. (2020). Biochemical changes during the fermentation of baobab (Adansonia digitata L.) fruit pulp yoghurt. Nigerian Journal of Microbiology, 34(1), 5018–5024. https://nsmjournal.org.ng/2020-34-1/19.pdf