?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Common beans are a source of phenolic compounds. However, they are sensitive to different factors. Microencapsulation may protect those bioactive compounds (BC). Hence, it is important to study the effects of microencapsulation and increase BC stability. A mix of methanolic extract of bean coat flour was prepared with N-lok as wall material. Inlet air temperature and total solids of wall material were evaluated. Air inlet temperature (115°C) and 28.54% total solids were the optimal conditions for the drying process, obtaining the highest concentration of BC. The particle size of microcapsules was ≈20 μm, and microencapsulation efficiency reached 96.29%. The stability conditions at two temperatures (30°C and 7°C) and two water activities (0.329 and 0.765) showed that encapsulation maintained BC stability in time as compared to control. The bean coat extract can be used as a functional microencapsulate with high BC content in the food and pharmaceutical sectors.

1. Introduction
Common beans are the most-consumed legume worldwide. It is native to Mexico, and its largest cultivation areas are currently found in Latin America (Gomes-Basso et al., Citation2018). Common bean seeds are an important source of bioactive compounds, such as peptides and polyphenols, among which are phenolic acids (caffeic, p-coumaric, synaptic, gallic, and ferulic) and flavonoids ((+)-catechin, quercetin, kaempferol, and rutin) (Do Evangelho et al., Citation2017; Petropoulos et al., Citation2019; Yang et al., Citation2018). The anthocyanin profiles in black bean coats included petunidin-2 G, petunidin-G, malvidin-G, and delphinidin-G (Aguilera et al., Citation2016). Phenolic compounds from plants have multiple positive effects on human health due to their antioxidant, anti-inflammatory, and antitumoral properties, among others (Liu, Citation2013). These effects largely depend on the bioavailability of the compounds in the organism which, in turn, varies according to the structure and shape with which they are introduced to the organism, for example, through a complex food matrix or purified isolates (Grgić et al., Citation2020). The economic implications of polyphenolic compounds are thus substantial since they are used in the food-processing industry as natural additives. However, it is probably in the field of human health that the economic implication of polyphenols is the most important (Munin & Edwards-Lévy, Citation2011).
According to D’Archivio et al. (Citation2010), several factors can alter the bioavailability of polyphenolic compounds. They are external (sun exposure, degree of maturity) and related to food processing (thermal treatments, storage, homogenization, and cooking), food (compounds potentially absorbing fiber and fat), polyphenols (chemical structure), consumers (intestinal and systemic), and the interaction with other compounds (binding to proteins or polyphenols with a similar absorption mechanism). Phenolic compounds are sensitive to light, heat, and oxygen; they are also volatile and unstable (Sansone et al., Citation2011; Trucillo et al., Citation2018). Encapsulation can be defined as a process that creates a barrier with a matrix or polymer coating around the bioactive compounds (D. R. Dias et al., Citation2017). It can positively affect phenolic compounds and prevent their degradation, reduce reactivity, and ensure bioaccessibility and bioavailability. This is because this process ensures a full coating of the active component, a delivery in a specific area of the digestive tract, and a controlled release (Grgić et al., Citation2020; Macías-Cortés et al., Citation2020; Sansone et al., Citation2011). Spray drying consists in the atomization of an emulsion or solution; the atomized droplets find hot air, leading to the formation of microparticles through thermodynamic phenomena. It is the most commonly used method to encapsulate bioactive compounds given its low cost (D. R. Dias et al., Citation2017; Macías-Cortés et al., Citation2020). There are few studies on the microencapsulation and technology process of bean compounds studies made by Tovar Benitez et al. (Citation2016) and Cian et al. (Citation2019) focus on the study of peptide encapsulation. Therefore, according to the benefits for health and composition of bioactive compounds, this work proposed to apply technology on beans for the conservation and use of bioactive compounds in the food sector. The aim of this study was to evaluate the effect of spray drying when microencapsulating bioactive compounds (total phenols, total flavonoids, and anthocyanins and radical scavenging capacity) of methanolic extract of bean (Phaselus vulgaris, L.) coat flour, and the stability of the microencapsulated compounds was evaluated under controlled storage conditions.
2. Materials and methods
2.1. Biological material
Common bean Negro San Luis was acquired from a local producer. The seeds were cleaned, and the coat of the cotyledons was separated using a dehulling machine. The coat was ground to obtain a fine flour and hermetically stored from light at 10°C until use. The N-lok – Code: 31111101 (blend of modified starch food grade and corn syrup solids) starch used was obtained from Ingredion, Mexico City.
2.2. Microencapsulation of bean coat extract
The bean coat flour (106.8 g db) was weighed in a flask and added to 1000 mL 80% methanol; the mix was left in refrigeration and away from light for 24 h. Afterward, the extract was evaporated in a Buchi Rotavapor to eliminate waste solvent. The evaporated extract (200 mL) was placed in a beaker protected from light and mixed with commercial N-Lok starch (Ingredion, Mexico), considering the total solids of the final solution (20–30 g wall material w/v) and the inlet dryer temperature (100°C–140°C) based on the experimental design (). The mix (extract-wall material) was homogenized in a homogenizer (IKA T25, Ultra-Turrax, Germany) at 10 000 rpm for 1 min. The microencapsulation process was carried out in a spray dryer (Buchi B-191, Labortechnik, Flawil, Switzerland) using a sample speed (inlet) of 3.5 mL/min.
Table 1. Central composite design for two factors: inlet air temperature (100°C–140°C) and total solids in the mix (20–30%).
2.3. Microencapsulation efficiency (ME%)
The microencapsulation efficiency (ME%) was calculated based on the phenolic compound content. To do so, Equations 1 and 2 were used (Robert et al., Citation2010).
To quantify the total bioactive compounds in the microcapsules, the structure of the microcapsule was entirely destroyed. An Eppendorf tube was used to weigh 200 mg microcapsules, and 2.0 mL of water was added. The mix was stirred vigorously for 10 min and centrifuged at 7000 × g (MiniSpin plus, Eppendorf, Germany) for 10 min. The supernatant was recovered, and two consecutive washes were done to obtain a final volume of 6.0 mL. Finally, the volume of the mix was measured in a 50-mL flask containing water for phenol quantification. To assess the superficial bioactive compounds in the microcapsule, 200 mg microcapsules was weighed with 2.0 mL of 50% methanol in an aqueous solution. The dispersions were agitated in a vortex for 10 s and centrifuged at 7000 × g for 10 min. The supernatant was placed in a 50-mL flask to measure the volume and quantify phenols.
2.4. Quantification of total phenolic compounds
Quantification of total phenolic compounds was made according to the methodology described by Pękal and Pyrzynska (Citation2014). The total and superficial extracts obtained (250 µl) were placed in an Eppendorf tube with 625 µL Folin-Ciocalteu reagent and 500 µL 7.5% Na2CO3. The tubes were stirred and left to stand for 2 h. Absorbance was measured at 760 nm (Genesys 10S UV – vis, Thermo Fisher Scientific, U.S.A). A gallic acid standard curve (0–100 ppm) was used to determine the concentration of total phenolic compounds, and the result was expressed as mg gallic acid equivalents per g microcapsule (mg GAE/g).
2.5. Total flavonoids in microencapsulates
The total flavonoid content was assessed following the methodology described by Pękal and Pyrzynska (Citation2014) with modifications. The extract (1000 µL) was placed in different Eppendorf tubes in the presence of 125 μL 5% sodium nitrite (NaNO2) with 125 μL AlCl3 in an alkaline medium (200 μL 1 M NaOH). Absorbance was measured at 510 nm, and a catechin curve from 0 to 50 ppm was used. The results were reported in mg of catechin equivalents/g of powder (mg EC/g).
2.6. Total monomeric anthocyanins in microencapsulates
The total monomeric anthocyanins were determined in the encapsulates according to the methodology by Del Carpio-Jimenez et al. (Citation2009) with modifications. A pH 1 buffer solution (1 mL) was placed in Eppendorf tubes; 1 mL pH 4.5 buffer solution was placed in another tube. The extract (500 µL) obtained from breaking the microcapsule was added, and the absorbance was measured at 520 nm (wavelength of maximum absorbance for anthocyanins) and 700 nm in the spectrophotometer, using the corresponding buffer as target. The final absorbance was calculated using EquationEquation (3)(3)
(3) :
The value of the absorbance was substituted in EquationEquation (4)(4)
(4) to obtain the concentration of anthocyanins:
where and MW correspond to the molar absorptivity and molecular weight of anthocyanin predominant in the sample, while DF is the dilution factor (total volume/extract volume). The cyanidin-3-glucoside, its molar absorptivity and weight (
26 900 and MW 449.2 g/mol) were used. The result obtained was expressed as mg of cyanidin-3-glucoside equivalent per g of microcapsule.
2.7. Free-radical scavenging capacity
The ABTS radical scavenging capacity was evaluated according to Re et al. (Citation1999). The radical was obtained through the reaction of 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (7 mM) with K2S2O8 (2.45 mM) incubated at room temperature in the dark for 24 h. A Trolox curve (0–200 ppm) was used as a reference antioxidant. The standard or extract from the breaking of the microcapsule (50 µL) was placed in Eppendorf tubes with 1.1 mL ABTS (previously formed). They were left to stand for 30 min, and the absorbance was measured at 735 nm in the spectrophotometer. The radical scavenging capacity of the extracts was determined, and results were reported in Trolox equivalents per g of microcapsules (mg TE/g). A DPPH (2,2-diphenyl-1-picrylhydrazyl) solution (100 µM) was prepared and dissolved in methanol. The standard or extract obtained from the breaking of the microcapsules (50 µL) was placed in Eppendorf tubes with 1.1 mL DPPH. The samples were left to stand for 30 min, and absorbance was measured at 510 nm in the spectrophotometer. The results were expressed in mg of ascorbic acid equivalents per g of microcapsule flour (mg AAE/g).
2.8. Humidity and water activity in microcapsules
To determine humidity, 1 g microcapsules was weighed and placed on a thermoscale (OHAUS, Switzerland). Water activity (aw) was assessed weighing 1 g sample in the Aqua LAB V. 2.2 (Mod. 3TE, Decagon Devices, Inc., U.S.A.). Both tests were done in triplicate.
2.9. Scanning electron microscopy (SEM)
The microcapsules were observed in a scanning electron microscope (Jeol, JSM-6300, Japan). The powders were placed on a sample holder using carbon conductive tape and coated with gold (0.5–1 nm thick) for 3 min. The samples were observed at 15 A and 20 kV.
2.10. Experimental design and optimization process
For the microencapsulation process, a central composite rotary model was used, including two independent variables: temperature of inlet air in drier chamber (ºC) and total solids in the mix (% weight/volume) (). The data were analyzed using the response surface methodology (RSM) in Design-Expert v7.1.5. The experimental data were adjusted to a second-order polynomial model, as shown in EquationEquation (5)(5)
(5) :
where Y is the response variable, X1 is the inlet temperature in the drier chamber, and X2 are the total solids in the mix. The significance of the models was tested using an analysis of variance (ANOVA, test F). The optimal conditions of the drying process were determined through the maximum slope method and the desirability function of the same software.
2.11. Stability under controlled conditions
The stability of bioactive compounds was evaluated under controlled conditions according to the method proposed by Rascón et al. (Citation2011) with some modifications. The microencapsulated powders were evaluated at two water activities (aw) for each temperature: 0.329 (MgCl2) and 0.765 (NaCl) equilibrated at 30°C and 4°C. The powder containing microencapsulated cells (1 g dw) was placed in aluminum containers and stored in desiccators containing saturated solutions, as previously mentioned, for 40 days. The bean coat flour (original food matrix) was used as a control under the same conditions. The half-life of the bioactive compounds (t ½) was calculated according to Modesto Junior et al. (Citation2023).
3. Results and discussion
3.1. Microencapsulation efficiency (ME%)
The ME (89.7–97.5%) was determined in the powder obtained based on total phenol concentration. shows the estimated coefficients of the adjusted model and significance level for the response variable of ME%. The independent variables were observed to have a significant effect (p < .05) in linear terms and interactions between them. The response surface of this variable () shows that an ME% higher than 95% was obtained at 105°C, that is, within the lower temperature range of the design. The ANOVA of ME% showed a significant regression model with a value of R2 ≥ 0.70, a coefficient of variation of 1.13, and a model value of p < .0001. Similar results were reported by Vargas-Campos et al. (Citation2018) who used commercial N-Lok starch to microencapsulate phytochemicals as pitaya (Stenocereus pruinosus) betalains. The feed flow rate of the sample, the temperature in the drying chamber, the inlet/outlet temperatures, and the concentration of the wall material are among the major factors to consider in the optimization of spray drying.
Figure 1. Response surface of dependent variables: (a) microencapsulation efficiency (%), (b) total phenolic compounds (mg GAE/g), (c) total flavonoids (mg CE/g), and (d) total monomeric anthocyanins (mg C3GE/g), based on the temperature of drying air and total solids of N-Lok as wall material.
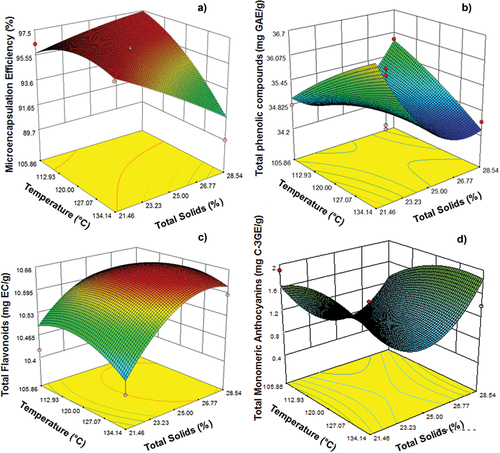
Table 2. Estimated coefficients of the adjusted model and significance level of response variables: microencapsulation efficiency, total phenols, flavonoids, monomeric anthocyanins, radical scavenging capacity (ABTS and DPPH), water activity, and humidity in microencapsulates with bean coat extract.
3.2. Total phenolic compounds in microencapsulates
The concentration of solids in the mix, in linear and quadratic terms, showed a significant effect (p < .05) on the total phenolic compounds in the microcapsules (). The interaction between the independent variables showed a significant effect (p < .05). However, the temperature in the evaluated range (100°C–140°C), in linear and quadratic terms, showed no effect (p > .05) on these compounds. The ANOVA of the total phenolic compounds showed a significant regression model with a value of R2 ≥ 0.75, a coefficient of variation of 1.31, and a model value of p < .0001. The surface plot () shows that the highest concentration of total phenolic compounds (36.7 mg GAE/g powder) was found at a lower concentration of total solids in the mix. Then, a higher solid content was obtained with lower total phenol values. This is likely due to the dilution of these compounds with the wall material. Similar results were obtained by Nguyen et al. (Citation2022) when they evaluated the effect of the spray drying conditions on the encapsulated contents of phenolic compounds from a Hibiscus sabdariffa L. extract using maltodextrin as wall material. Mohammed et al. (Citation2017) state that the wall material concentration affects the retention of bioactive compounds due to its viscosity properties in the feeding solution. Based on the results obtained, the spray drying conditions used in this study could be applied in the encapsulation of coat flour/bean flour extracts to obtain high concentrations of phenolic compounds in the microencapsulates. Rahmati et al. (Citation2020) optimized the encapsulation process of a Moldavian balm extract to conserve phytochemicals as phenols. They reported that the best drying conditions were 140°C and 18% wall material to obtain a total phenol content of 6.25 GAE/g dry powder.
3.3. Total flavonoids in microencapsulates
shows a significant effect (p < .05) obtained from total solids in their linear and quadratic terms in total flavonoids. The ANOVA of the total phenolic compounds showed a significant regression model with a value of R2 ≥ 0.77, a coefficient of variation of 0.41, and a model value of p < .0001. shows that the largest amount of flavonoids was found at higher percentages of solids (26–28.5%). The maximum concentration of total flavonoids in the encapsulates was 10.66 mg CE/g encapsulate powder. These results are high compared to the studies carried out by Papoutsis et al. (Citation2018), who microencapsulated using spray drying citrus extracts using combinations of maltodextrin with soybean protein and ι-carrageenan. They reported values of 0.34 ± 0.01 mg CE/g db.
3.4. Total monomeric anthocyanins in microencapsulates
As shown in , the temperature and solids affected quadratic terms (p < .05). The ANOVA of the monomeric anthocyanins showed a significant regression model with a value of R2 ≥ 0.66, a coefficient of variation of 35.45, and a value of p < .0001 for the model. According to , a higher percentage of solids led to higher values of these compounds. In this work, values of 0.4–2 mg C3GE/g dry powder were obtained. Spray drying is the most common technique to encapsulate anthocyanins, and around 80–90% of the encapsulates are spray dried (D. R. Dias et al., Citation2017; Mahdavi et al., Citation2014). Nguyen et al. (Citation2022) microencapsulated anthocyanins of Roselle extract (Hibiscus sabdariffa L.), and they evaluated the effect of the spray drying process on the physicochemical and antioxidant properties of dry powders. Three inlet air temperatures (150°C, 160°C, and 170°C) and the relationship between anthocyanins and wall material (maltodextrin) were analyzed. They found that a higher wall material concentration led to a higher protection of anthocyanins. Kalušević et al. (Citation2017) microencapsulated black soybean (legume) coat extract, rich in anthocyanins, by spray drying (140°C inlet temperature). They used gum Arabic and powdered skim milk as wall materials and reported values of 1780, 1591, and 1923 µ CGE/g dry microparticles, respectively. These results are similar to those reported in this work.
3.5. Free-radical scavenging capacity in microcapsules
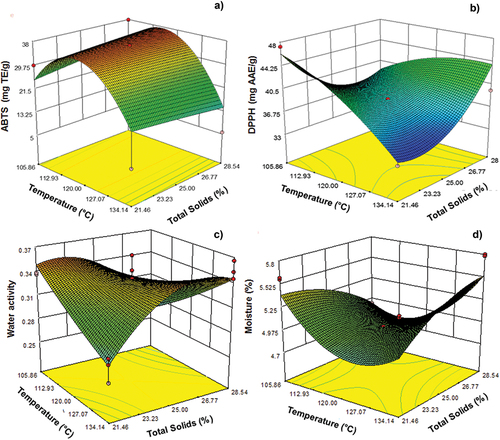
Temperature, in its linear and quadratic terms, significantly affected (p < .05) the radical scavenging capacity of ABTS (). The ANOVA of the free-radical scavenging by ABTS showed a significant regression model with a value of R2 ≥ 0.72, a coefficient of variation of 27.58%, and a value of p < .0001 for the model. shows that, at temperatures above 127°C, there was a decrease in the powder radical scavenging capacity. Temperature, in its linear and quadratic terms, and the interaction between independent variables showed a significant effect (p < .05) on free-radical scavenging by DPPH, as shown in . The ANOVA of free-radical scavenging by DPPH showed a significant regression model with a value of R2 ≥ 0.81, a coefficient of variation of 5.54%, and a value of p < .0001 for the model. shows that free-radical scavenging was reduced as temperature increased, as in ABTS. The free-radical scavenging capacity decreased as the temperature rose (134°C). Similar results were obtained by Bakowska-Barczak and Kolodziejczyk (Citation2011), Rigon and Noreña (Citation2016), and Laokuldilok and Kanha (Citation2017).
3.6. Water activity and humidity in microcapsules
Water activity significantly affected (p < .05) the interaction between independent variables (). We obtained R2 ≥ 0.89, a coefficient of variation of 3.82, and a value of p < .0001 for the model. shows the water activity was reduced as the temperature increased with a low amount of total solids. This behavior can be explained by the fact that a higher drying temperature leads to a greater heat transfer to the particles. This increases the driving force of humidity evaporation, leading to greater water elimination from the food matrix (Martins et al., Citation2019). Values under 0.3 aw promote powder stability and extend useful life by lowering the presence of available free water for chemical reactions and the growth of pathogenic microorganisms (Atalar & Dervisoglu, Citation2015). shows that the humidity values were 4–5.6%. The lowest humidity values were obtained at higher temperatures (120°C–130°C). The increase in total solids also increased the percentage of humidity. shows that the interaction between independent variables has a significant effect (p < .05) as the temperature in its quadratic terms. We obtained an R2 ≥ 0.69, a coefficient of variation of 4.60, and a value of p < .0001 for the model. According to the water activity and humidity values obtained in this research work, stability could be increased during the storage of the microencapsulated bioactive compounds.
3.7. Drying process optimization
The responses selected for optimization were the highest bioactive compound content in microcapsules; total phenols, flavonoids, and monomeric anthocyanins; the highest free-radical scavenging activity with both methods; and the lowest contents of water activity and humidity. The conditions obtained for spray drying were 115°C with 28.5% of solids. The conditions obtained in the software were experimentally validated ().
Table 3. Experimental verification of predicted response variables in microcapsules.
3.8. Bioactive compound stability under controlled conditions
The stability of the encapsulated compounds (total phenols, flavonoids, and anthocyanins) and bean coat flour was analyzed under controlled temperature and water activity conditions (). In general, anthocyanins presented the greatest degradation during storage under all conditions. For water activities of 0.765 (), the degradation of the compounds was higher compared to that of 0.329. Still, the microencapsulated compounds presented the highest concentration in end storage with respect to control (p < .05). presents the differences (∆) in bioactive compound concentration (initial – final) in microencapsulates and bean coat flour under different storage conditions, as well as the half-life of the bioactive compounds (t ½). Finally, the best conditions for the stability of phenolic compounds were 0.329 and 7 and 30°C. These results are useful to determine the food products in which microencapsulates can be incorporated, under storage conditions that guarantee the stability of the compounds. According to M. I. Dias et al. (Citation2015), some matrices for microencapsulates obtained by spray-dried are bread, ice cream, pasta, soup, yogurt, and natural food dyes (Mojica et al., Citation2017).
Figure 3. Stability of encapsulated compounds (total phenols, flavonoids, and anthocyanins) and bean coat flour analyzed under controlled conditions of temperature and water activities for 40 days: (a, d) total phenolic compounds (mg GAE/g); (b, e) total flavonoids (mg CE/g); and (c, f) total monomeric anthocyanins (mg C3GE/g).
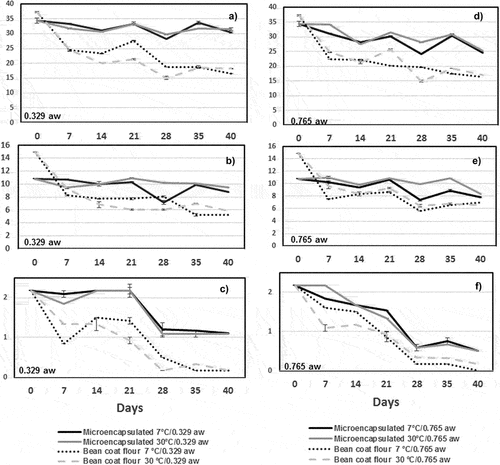
Table 4. Differences (∆) in concentration of bioactive compounds (initial – final) in microencapsulates and bean coat flour under different storage conditions and half-life of the bioactive compounds (t ½).
3.9. Scanning electron microscopy (SEM)
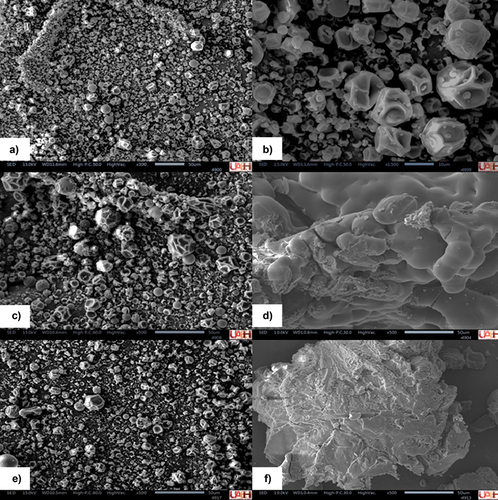
Microcapsules obtained through spray drying at 115°C with 28.54% of solids in wall material showed surface cavities (irregular and shrunken) as a result of the fast evaporation in the dryer () at time zero of storage. Microcapsules measured ≈ 10–30 µm, according to the scale in the micrograph. Micrographs obtained at the end of storage (40 days) were used to observe capsule integrity. It is important to mention that capsule morphology changed to an agglomerated polymeric matrix at high water activities and the two temperatures were evaluated (). This occurred because of water absorption from the storage microenvironment. In contrast, the morphology of the microcapsules remained intact, as at the beginning of storage, when water activities were low at both temperatures (). However, stability was preserved according to the results obtained from the bioactive compounds in all storage conditions evaluated.
Figure 4. Micrograph of microcapsules with extract of bean coat flour and N-Lok starch obtained by spray drying using inlet air temperature of 115°C and 28.5% solids in wall material: (a) After spray drying (zero time) 300×; (b) (zero time) 500×; (c) After 40 days at 7°C and 0.329 aw; (d) After 40 days at 7°C and 0.765 aw; (e) After 40 days at 30°C and 0.329 aw, and (f) After 40 days at 30°C and 0.765 aw.
4. Conclusions
Common bean coat flour is a raw material that can be used to extract bioactive compounds, which can be encapsulated using spray drying. In this work, the optimal temperature and total solids in wall material (N-Lok) were obtained for a maximum encapsulation efficiency and high bioactive compound concentrations (phenols, flavonoids, and anthocyanins). In addition, microencapsulates with radical scavenging capacity were obtained. If the drying temperature in the optimization process is increased, the bioactive compounds are reduced. Bioactive compounds and functional activity are protected as the concentration of solids increases. The optimal spray-drying conditions are as follows: 115°C with 28.5% of solids. The microcapsules present an adequate particle size (≈10–30 µm) for incorporation into a food system. The process of spray drying yields functional powders in the food technology sector.
Acknowledgments
The authors would like to acknowledge the contribution of Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aguilera, Y., Mojica, L., Rebollo-Hernanz, M., Berhow, M., De Mejía, E. G., & Martín-Cabrejas, M. A. (2016). Black bean coats: New source of anthocyanins stabilized by β-cyclodextrin copigmentation in a sport beverage. Food Chemistry, 212, 561–570. https://doi.org/10.1016/j.foodchem.2016.06.022
- Atalar, I., & Dervisoglu, M. (2015). Optimization of spray drying process parameters for kefir powder using response surface methodology. LWT - Food Science and Technology, 60(2), 751–757. https://doi.org/10.1016/j.lwt.2014.10.023
- Bakowska-Barczak, A. M., & Kolodziejczyk, P. P. (2011). Black currant polyphenols: Their storage stability and microencapsulation. Industrial Crops and Products, 34(2), 1301–1309. https://doi.org/10.1016/j.indcrop.2010.10.002
- Cian, R. E., Campos‐Soldini, A., Chel‐Guerrero, L., Drago, S. R., & Betancur‐Ancona, D. (2019). Bioactive Phaseolus lunatus peptides release from maltodextrin/gum Arabic microcapsules obtained by spray drying after simulated gastrointestinal digestion. International Journal of Food Science & Technology, 54(6), 2002–2009. https://doi.org/10.1111/ijfs.14031
- D’Archivio, M., Filesi, C., Varì, R., Scazzocchio, B., & Masella, R. (2010). Bioavailability of the polyphenols: Status and controversies. International Journal of Molecular Sciences, 11(4), 1321–1342. https://doi.org/10.3390/ijms11041321
- Del Carpio-Jimenez, C., Serrano, C., & Giusti, M. (2009). Caracterización de las antocianinas de los frutos de Berberis boliviana Lechler. Revista de la Sociedad Química del Perú, 75(1), 76–86.
- Dias, D. R., Botrel, D. A., Fernandes, R. V., & Borges, S. V. (2017). Encapsulation as a tool for bioprocessing of functional foods. Current Opinion in Food Science, 13, 31–37. https://doi.org/10.1016/j.cofs.2017.02.001
- Dias, M. I., Ferreira, I. C., & Barreiro, M. F. (2015). Microencapsulation of bioactives for food applications. Food & Function, 6(4), 1035–1052. https://doi.org/10.1039/c4fo01175a
- Do Evangelho, J. A., Vanier, L. N., Pinto, V. Z., De Berrios, J. J., Dias, A. R., & Zavareze, E. D. (2017). Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chemistry, 214, 460–467. https://doi.org/10.1016/j.foodchem.2016.07.046
- Gomes-Basso, L. F., Zielinski, A. F., Wojeicchowski, J. P., Nogueira, A., & Demiate, I. M. (2018). Beans (Phaseolus vulgaris L.): Whole seeds with complex chemical composition. Current Opinion in Food Science, 19, 63–71. https://doi.org/10.1016/j.cofs.2018.01.010
- Grgić, J., Šelo, G., Planinić, M., Tišma, M., & Bucić-Kojić, A. (2020). Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants, 9(10), 923. https://doi.org/10.3390/antiox9100923
- Kalušević, A., Lević, S., Čalija, B., Pantić, M., Belović, M., Pavlović, V., Nedović, V., Milić, J., Žilić, S., & Nedović, V. (2017). Microencapsulation of anthocyanin-rich black soybean coat extract by spray drying using maltodextrin, gum Arabic and skimmed milk powder. Journal of Microencapsulation, 34(5), 475–487. https://doi.org/10.1080/02652048.2017.1354939
- Laokuldilok, T., & Kanha, N. (2017). Microencapsulation of black glutinous rice anthocyanins using maltodextrins produced from broken rice fraction as wall material by spray drying and freeze drying. Journal of Food Processing and Preservation, 41(1), e12877. https://doi.org/10.1111/jfpp.12877
- Liu, R. H. (2013). Dietary bioactive compounds and their health implications. Journal of Food Science, 78(s1), A18–A25. https://doi.org/10.1111/1750-3841.12101
- Macías-Cortés, E., Gallegos-Infante, J. A., Rocha-Guzmán, N. E., Moreno-Jiménez, M. R., Medina-Torres, L., & González-Laredo, R. F. (2020). Microencapsulation of phenolic compounds: Technologies and novel polymers. Revista Mexicana de Ingeniería Química, 19(2), 491–521. https://doi.org/10.24275/rmiq/Alim642
- Mahdavi, S. A., Jafari, S. M., Ghorbani, M., & Assadpoor, E. (2014). Spray-drying microencapsulation of anthocyanins by natural biopolymers: A review. Drying Technology, 32(5), 509–518. https://doi.org/10.1080/07373937.2013.839562
- Martins, E., Cnossen, D. C., Silva, C. R., Cezarino, J. C., Nero, L. A., Perrone, I. T., & Carvalho, A. F. (2019). Determination of ideal water activity and powder temperature after spray drying to reduce Lactococcus lactis cell viability loss. Journal of Dairy Science, 102(7), 6013–6022. https://doi.org/10.3168/jds.2019-16297
- Modesto Junior, E. N., Martins, M. G., Pereira, G. A., Chisté, R. C., & Pena, R. D. S. (2023). Stability kinetics of anthocyanins of grumixama berries (Eugenia brasiliensis Lam.) during thermal and light treatments. Foods, 12(3), 565. https://doi.org/10.3390/foods12030565
- Mohammed, N. K., Tan, C. P., Manap, Y. A., Alhelli, A. M., & Hussin, A. S. (2017). Process conditions of spray drying microencapsulation of nigella sativa oil. Powder Technology, 315, 1–14. https://doi.org/10.1016/j.powtec.2017.03.045
- Mojica, L., Berhow, M., & de Mejia, E. G. (2017). Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chemistry, 229, 628–639. https://doi.org/10.1016/j.foodchem.2017.02.124
- Munin, A., & Edwards-Lévy, F. (2011). Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics, 3(4), 793–829. https://doi.org/10.3390/pharmaceutics3040793
- Nguyen, Q. D., Dang, T. T., Nguyen, T. V., Nguyen, T. D., & Nguyen, N. N. (2022). Microencapsulation of Roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of drying conditions on some physicochemical properties and antioxidant activities of spray-dried powder. Food Science Nutrition, 10(1), 191–203. https://doi.org/10.1002/fsn3.2659
- Papoutsis, K., Golding, J. B., Vuong, Q., Pristijono, P., Stathopoulos, C. E., Scarlett, C. J., & Bowyer, M. (2018). Encapsulation of citrus by-product extracts by spray-drying and freeze-drying using combinations of maltodextrin with soybean protein and ι-carrageenan. Foods, 7(7), 115. https://doi.org/10.3390/foods7070115
- Pękal, A., & Pyrzynska, K. (2014). Evaluation of aluminium complexation reaction for flavonoid content assay. Revista de la Sociedad Química del Perú, 7(9), 1776–1782. https://doi.org/10.1007/s12161-014-9814-x
- Petropoulos, S. A., Taofiq, O., Fernandes, Â., Tzortzakis, N., Ciric, A., Sokovic, M., Barros, L., & Ferreira, I. C. (2019). Bioactive properties of greenhouse-cultivated green beans (Phaseolus vulgaris L.) under biostimulants and water-stress effect. Journal of the Science of Food and Agriculture, 99(13), 6049–6059. https://doi.org/10.1002/jsfa.9881
- Rahmati, E., Sharifian, F., & Fattahi, M. (2020). Process optimization of spray-dried Moldavian balm (Dracocephalum moldavica L.) extract powder. Food Science & Nutrition, 8(12), 6580–6591. https://doi.org/10.1002/fsn3.1949
- Rascón, M. P., Beristain, C. I., García, H. S., & Salgado, M. A. (2011). Carotenoid retention and storage stability of spray-dried encapsulated paprika oleoresin using gum Arabic and soy protein isolate as wall materials. LWT - Food Science and Technology, 44(2), 549–557. https://doi.org/10.1016/j.lwt.2010.08.021
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237. https://doi.org/10.1016/S0891-58499800315-3
- Rigon, R. T., & Noreña, C. P. (2016). Microencapsulation by spray-drying of bioactive compounds extracted from blackberry (Rubus fruticosus). Journal of Food Science and Technology, 53(3), 1515–1524. https://doi.org/10.1007/s13197-015-211
- Robert, P., Gorena, T., Romero, N., Sepulveda, E., Chavez, J., & Saenz, C. (2010). Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. International Journal of Food Science & Technology, 45(7), 1386–1394. https://doi.org/10.1111/j.1365-2621.2010.02270.x
- Sansone, F., Picerno, P., Mencherini, T., Villecco, F., D’ursi, A. M., Aquino, R. P., & Lauro, M. R. (2011). Flavonoid microparticles by spray-drying: Influence of enhancers of the dissolution rate on properties and stability. Journal of Food Engineering, 103(2), 188–196. https://doi.org/10.1016/j.jfoodeng.2010.10.015
- Tovar Benitez, T., Jimenez-Martinez, C., Perea-Flores, M. J., Tellez-Medina, D. I., & Davila-Ortiz, G. (2016). Microencapsulation of bayo bean (Phaseolus vulgaris) protein hydrolysate with inhibitory activity on angiotensin-converting enzyme through freeze-drying. Revista Mexicana de Ingeniería Química, 15(3), 797–807. https://doi.org/10.24275/rmiq/Alim1038
- Trucillo, P., Campardelli, R., Aliakbarian, B., Perego, P., & Reverchon, E. (2018). Supercritical assisted process for the encapsulation of olive pomace extract into liposomes. The Journal of Supercritical Fluids, 135, 152–159. https://doi.org/10.1016/j.supflu.2018.01.018
- Vargas-Campos, L., Valle-Guadarrama, S., Martínez-Bustos, F., Salinas-Moreno, Y., Lobato-Calleros, C., & Calvo-López, A. D. (2018). Encapsulation and pigmenting potential of betalains of pitaya (Stenocereus pruinosus) fruit. Journal of Food Science and Technology, 55(7), 2436–2445. https://doi.org/10.1007/s13197-018-3161-7
- Yang, Q. Q., Gan, R. Y., Ge, Y. Y., Zhang, D., & Corke, H. (2018). Polyphenols in common beans (Phaseolus vulgaris L.): Chemistry, analysis, and factors affecting composition. Comprehensive Reviews in Food Science and Food Safety, 17(6), 1518–2153. https://doi.org/10.1111/1541-4337.12391