?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Annona muricata L. (soursop fruit) is a seasonal plant with bio-geographical distribution in tropical and subtropical climates. This study evaluated the proximate, phenolic, and antioxidant properties of different parts of soursop fruit. The influence of oven and solar drying on phenolic and antioxidant activities of extracts (pulp, seed, and peel) were assessed. Proximate analysis on different parts of the soursop fruit showed the pulp to have high carbohydrate (32.9% ± 0.3) and moisture (19.1% ± 1.0). The seeds however contained higher crude fat (37.5% ± 1.2) and protein (25.0% ± 0.1) contents. Total phenolic content of the seeds (8.7 mgGAE g−1 ± 0.1) was higher than that of the pulp and the peel (8.6 mgGAE g−1 ± 0.1). The solar-dried fruit parts demonstrated superior antioxidant activity to the oven-dried parts. Extracts from the solar-dried soursop fruit samples, especially the seed, can serve as useful therapeutic ingredient for diet supplementation and could also be exploited for its high oil and protein contents.
1. Introduction
Soursop is cultivated in most parts of the world owing to its potential health benefits. It is known scientifically as Annona muricata L. and belongs to the family Annonaceae (Coria-Téllez et al., Citation2018; Moghadamtousi et al., Citation2015). Soursop is described by other common names such as guayabano (Philippines), guanabana (Spanish), corossol epineux (French), sirsak (Indonesia), and graviola (Brazil). In Ghana, the soursop fruit is popularly known as “aluguntugui” in Ga, “aborofontungu” (Twi) and in Ewe as “evo”. The fruit is dark green, prickly, and ovoid with juicy, acidic, whitish, and aromatic pulp (Coria-Téllez et al., Citation2018; Moghadamtousi et al., Citation2015). Soursop has increasingly gained global importance due to its medicinal, nutritional, flavor and color attributes (Adeola & Aworh, Citation2010). Annona muricata L. has been attributed with numerous health benefits, which are linked to its antioxidant potentials (Agu et al., Citation2017). The fruit is reported to be rich in flavonoids and phenols (Agu et al., Citation2017).
Soursop has a high moisture content, which makes the fruit deteriorate a few days after harvest. Post-harvest losses of the fruit have been estimated to range from 40% to 60% due to improper processing, handling, packaging, and transportation (Gustavsson et al., Citation2011). Soursop waste generated during fruit processing into juice, puree, and jellies is about 33% of the whole fruit (Mesquita et al., Citation2021). The fruit is highly susceptible to spoilage and softens very rapidly during ripening (Okigbo & Obire, Citation2009). Due to its perishable nature, physical and biochemical changes lower the organoleptic acceptability and commercial value of the fruit (Neta et al., Citation2019; Okigbo & Obire, Citation2009; Vwioko et al., Citation2013). Post-harvest treatments such as drying can be used to increase the shelf stability and concurrently maintain the nutritional quality of the soursop fruit (Moreno-Hernández et al., Citation2014). Fruits have been subjected to varied drying techniques to reduce moisture, slow degradative reactions, substantially reduce weight, and minimize costs associated with packaging, storage, and transportation (Borsini et al., Citation2021). The drying technique and conditions used can nonetheless negatively influence organoleptic, nutritional, and bioactive properties of the fruits. Hot air drying is the most commonly used drying technique (Borsini et al., Citation2021). Hot air temperatures between 30 to 60°C have generally demonstrated and maintained high levels of bioactive compounds, whereas extreme temperature treatments (80 to 100°C) induced structural changes and resulted in loss of bioactive compounds (Tsurunaga et al., Citation2022). Comparatively, although solar drying has a slower drying rate, the energy source is environmentally friendly and economically viable for developing countries in the tropics and subtropics (Hossain & Bala, Citation2007). The aim of the present work, therefore, was to investigate the proximate and antioxidant properties of different parts of the soursop fruit subjected to varied drying conditions in order to evaluate their potential as useful functional ingredients in food formulations.
2. Materials and methods
2.1. Materials
The chemicals and reagents used for analysis included vitamin C, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis,3-ethylbenzothiazoline-6-sulfonic acid (ABTS), acetate buffer, TPTZ, and gallic acid purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A). Folin Ciocalteu phenolic reagent was purchased from Merck (Darmstadt, Germany). Sodium carbonate (Na2CO3), sodium nitrate, aluminum chloride, sodium bicarbonate, HCl, and FeCl3 were bought from May & Baker Chemicals (Dagenham, E.). All the chemicals used were of analytical grade.
2.2. Sample source
The soursop fruits were obtained from a farm at Bame in the Ho West district of the Volta Region of Ghana. The region has a bi-modal climatic condition with two rainy seasons (major season from March to June, and minor season from July to November). Mean annual rainfall figures are between 120.1 mm and 192 mm (Darko et al., Citation2021; Ghana Statistical Service [GSS], Citation2014). Annual mean temperature ranged from 22 to 32°C, and humidity from 68% to 83%. Annual mean sunshine hours is between 319 and 371 h. The soursop fruits were harvested in January, 2021, and kept in a −20°C freezer (Protech PRCF-500, China) until use. The fruit was separated into different parts to investigate the fruit component with optimum phenolic and antioxidant compounds.
2.3. Study size and design
Proximate analysis was carried out on oven-dried soursop pulp and seed samples (n = 2). For antioxidant activity and phenolic and flavonoid contents analysis, a 3 × 2 factorial design was used, resulting in a total sample size of six (n = 6). The factors used were fruit parts (pulp, seed, and peel) and drying technique (oven and solar). Oven drying was done at 60°C for 24 h and solar drying was done between 30 and 36°C for 120 h (5 days).
2.4. Proximate analysis of fruit pulp and seeds
Proximate composition of the fruit was carried out using Association of Official Analytical Chemists methods, to determine the moisture, ash, crude fat, crude fiber, crude protein, and carbohydrate contents of the samples (AOAC, Citation2005). Protein was calculated from total nitrogen using the conversion factor 6.25. Carbohydrate was determined by difference.
2.5. Phenol extraction
The peel, seed, and pulp of the soursop fruit were milled into fine powder. Samples (15 mg) were transferred into a conical flask and 75 ml of absolute methanol was added (Darko et al., Citation2021). The samples were covered with aluminium foil to minimize evaporation and allowed to stand for 24 h under ambient temperature (~25°C), away from light. After 24 h, the mixtures were filtered using a filter paper. The filtrates were transferred into pre-weighed petri dishes and evaporated at 45°C using an oven (Binder Heating and Drying Oven, Tuttlingen, Germany) to remove the methanol. The extracts obtained after drying were used for total phenol content and antioxidant activity determinations (Obeng et al., Citation2020).
2.6. Antioxidant activity determination
2.6.1. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay
According to methods described by Dzah et al. (Citation2019) with some modification, 20 μL of extract (2mgmL−1) and 980 μL of 0.1 mM DPPH solution were mixed. The reaction mixture was allowed to stand at 4°C for 30 min and absorbance measured spectrophotometrically at 516 nm against blanks. The decrease in absorbance was proportional to antioxidant activity (AA) of extracts, calculated according to EquationEquation (1)(1)
(1) .
2.6.2. 2,2’-Azino-bis(3-ethylbenzthiazoline-6-sulphonic) (ABTS) acid scavenging activity
The ABTS scavenging activity was determined using methods reported by Re et al. (Citation1999) with slight modifications. ABTS stock solution (7 mM) and potassium persulfate (2.4 mM) were mixed according to 1:1 ratio to generate ABTS free radicals after 16-h incubation in the dark. A working solution of 0.1 mM ABTS solution was obtained from the ABTS radical solution. Vitamin C (50 μl) and 50 μl of extracts each were added to 150 μl of ABTS in a 96-well microtiter plate. Samples were incubated at room temperature for 30 min. The absorbance of the samples was read at 734 nm. The radical scavenging activity was calculated as percentage of the control sample as:
2.6.3. Ferric reducing antioxidant potential (FRAP) analysis
This method is based on the reduction of colorless ferric complex (Fe3+ tripyridyltriazine) to blue-colored ferrous complex (Fe2+ tripyridyltriazine) by the action of electron-donating antioxidants at low pH. The working FRAP reagent was prepared by mixing 30 ml of acetate buffer (pH 3.68) and 3 ml of 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) in 3 ml of 20 mM ferric chloride and 40 mM HCl (Obeng et al., Citation2020). All the required solutions were freshly prepared before being used. FRAP working solution (0.3 ml) was placed in a test tube and 30 μl of the sample was added. The solution was vortexed and incubated for 30 min at 37°C and absorbance was determined at 593 nm. The value for FRAP was calculated by:
2.7. Determination of total phenolic content
Total phenol content was estimated using the Folin Ciocalteu method (Darko et al., Citation2021). Folin reagent (10%) and sodium carbonate (20%) were freshly prepared. Samples (100 μl) were placed in test tubes and 500 μl of distilled water was added, subsequently followed with 100 μl of Folin Ciocalteu’s reagent. The mixture was vortexed and allowed to stand for 6 min. Sodium carbonate (20%) and additional 500 μl of distilled water was added, and the resulting solution was incubated for 90 min at 25°C. The mixture (200 μl) was placed in a 96-well microtiter plate and absorbance (750 nm) was read. Total phenol content of each sample was determined in Gallic Acid Equivalent (GAE) mgg−1 on dry weight basis.
2.8. Statistical analysis
Statgraphics (Graphics Software System, STCC, Inc. U.S.A) was used to analyse data. Comparisons between the different samples were done using analysis of variance (ANOVA) and differences between means were determined with LSD. Correlation tests were also carried out to establish the relationship between the antioxidant properties of the different dried soursop fruit parts. A probability value of p ≤ .05 was considered to be statistically significant for the tests carried out. The statistical software XLSTAT was used to perform Principal Component Analysis (PCA) among the antioxidant properties. All measurements were calculated from values obtained from duplicate assays.
3. Results and discussion
3.1. Proximate composition of soursop fruit pulp and seed
Results of the proximate composition of oven-dried soursop fruit pulp and seeds are shown in . The proximate analysis of the soursop pulp revealed a decreasing trend, which was carbohydrate > fat > moisture > protein > crude fiber > ash whilst soursop seed demonstrated an increasing trend (ash < moisture < carbohydrate < crude fiber < protein < fat). Although fat content was appreciably high in both seed (37.5% ± 1.2) and pulp (24.07% ± 0.1) samples, the pulp had a higher carbohydrate content (32.9% ± 0.33), whereas soursop seeds demonstrated higher protein content (25.0% ± 0.0). The crude fat content for the pulp (24.1% ± 0.1) exceeded values (9.8%) obtained by Akomolafe and Ajayi (Citation2015). Also, the high carbohydrate content of the pulp contributes to its sweet taste and energy value. The protein content of the seeds in this study was higher than the value (17.6%) obtained by Olabinjo (Citation2019) and the reported protein contents of watermelon (21.5%), orange (20.2%), grape (21.4%) and white roselle (22.7%) seeds (Cho et al., Citation2004). Postharvest quality of fruits is significantly influenced by the moisture content. The moisture content of the soursop seed (4.7%) was relatively lower than the pulp (19.1%), and this is indicative of their higher microbial and shelf life stability. The moisture contents of the oven-dried soursop fruit pulp in this study were lower than values reported for freshly harvested Nigerian soursop fruit (80.3%; Ekpete et al., Citation2013), Annona squamosa (78.5%; Abdualrahman et al., Citation2019). Dried fruits with adequate nutritional quality have been produced by employing appropriate drying techniques, temperature, and time (Abe-Inge et al., Citation2018). Ash levels of the pulp and seed samples were within the same range (3.2 to 3.5%), and this is indicative of the mineral content of the soursop fruit that can act as inorganic co-factors (Iheanacho & Udebuani, Citation2009). For crude fiber composition, the seeds recorded a higher value of 15.4% than that of the pulp (6.5%). Soursop seeds are therefore rich in dietary fiber and can be exploited for utilization in dietary formulations to positively influence health.
Table 1. Proximate composition of oven-dried soursop fruit pulp and seed.
3.2. Antioxidant activity under varied drying conditions
Three different oxidant system assays were used to investigate the effect of fruit part and drying treatment on the antioxidant potential of soursop fruits because the food matrix contained a mixture of different antioxidants with varied reaction mechanisms (Almeida et al., Citation2011). shows the result of DPPH scavenging activity on oven-dried and solar-dried soursop fruit parts (peel, pulp, and seeds). The DPPH scavenging activities of the different fruit samples were significantly different (p < .05). Irrespective of the drying condition used, the soursop fruit pulp had the least DPPH scavenging activity (37.8–67.8%), whereas the seeds demonstrated the highest activity (90.5–94.8%). The DPPH scavenging activity generally ranged from 37.8% to 94.8% for oven dried samples and 67.8% to 94.4% for solar-dried samples. The DPPH radical activity of the oven dried samples demonstrated a decreasing trend (pulp < peel < seed). The solar-dried samples also showed a similar trend except that values reported for the radical activity of the peel (94.4%) was slightly higher than the seed (90.5%), although not significantly different (p > .05). Subsequently, it was observed that except for soursop seeds, the solar-dried peel and pulp had a higher DPPH scavenging activity than their respective oven-dried samples. Thus, drying technique and temperature employed significantly influenced the DPPH scavenging activity of the samples analyzed. Drying at higher temperatures (60–120°C) has been shown to drastically produce lower antioxidant values due to degradation of bioactive compounds (Borsini et al., Citation2021). However, increased degradation of antioxidants as a function of increased temperature had been reported in watermelon (Salin et al., Citation2022). The DPPH values obtained for the solar-dried samples were generally comparable with methanolic extracts from the aerial part of different root vegetables (Mohammed et al., Citation2022) but higher than the DPPH of both ripe (17.7%) and unripe (29.24%) Chrysophyllum albidum fruit (Darko et al., Citation2021). The results obtained demonstrated that soursop seeds and solar-dried fruit parts are good sources of antioxidants. Solar drying, a relatively cheaper preservative method, proved more advantageous in conserving antioxidant compounds compared to oven drying at 60°C.
Figure 1. The antioxidant activities (DPPH and ABTS) of oven- and solar-dried soursop seeds, pulp, and peel. Different letters on bars with the same pattern indicate significant differences (p < .05; n = 6).
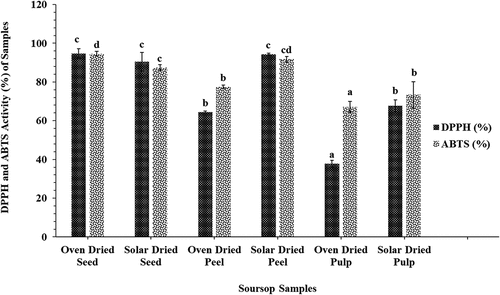
shows the free radicals scavenging activity of oven-dried and solar-dried soursop pulp, peel, and seeds on ABTS. The ABTS scavenging activity of all samples studied were significantly different (p < .05) and ranged between 67.2% and 94.6% for oven-dried samples and 73.4% to 91.8% for solar-dried samples. Generally, the solar-dried peel (91.8%) and solar-dried pulp (73.4%) samples exhibited high ABTS scavenging activities when compared with corresponding oven-dried samples (77.5%, 67.2%); however, the seeds demonstrated the opposite trend. ABTS activity of the oven dried samples increased in the order of fruits parts (pulp < peel < seed). The solar-dried samples showed a decreasing trend from pulp to peel, and the ABTS activity of the solar-dried peel (91.8%) was significantly higher than the corresponding solar-dried seed (87.4%).
3.3. Ferric reducing ability of plasma (FRAP)
shows the ferric reducing ability of oven-dried and solar-dried soursop pulp, peel, and seeds. Values were compared with ascorbic acid. FRAP activity of the samples ranged from 0.147 mg mL−1 (oven-dried pulp) to 1.667 mg mL−1 (oven-dried seed). Irrespective of drying method used, the seed samples had the highest ferric reducing ability (0.667 to 1.667 mg mL−1), compared to both the peel and pulp, with the oven-dried pulp recording the least ferric reducing ability (0.147 mg mL−1). However, for the seeds, the oven dried had high ferric reducing ability of 1.667 mg mL−1 as compared to the respective solar-dried sample (0.667 mg mL−1). The FRAP activity of the seed samples were higher than values reported for C. albidum fruits studied at different maturity stages (0.25 to 0.51 mg mL−1). Again generally as observed for DPPH and ABTS activities, the solar-dried samples recorded high ferric reducing ability than the oven-dried samples. For instance, the solar-dried pulp and peel samples, respectively, recorded FRAP values of 0.343 and 0.387 mg mL−1, whereas oven-dried samples had FRAP values of 0.266 mg mL−1 for the pulp and 0.147 mg mL−1 for the peel.
Figure 2. FRAP and phenolic activity of oven- and solar-dried soursop seeds, pulp, and peel. Different letters on bars indicate significant differences (p < .05; n = 6).
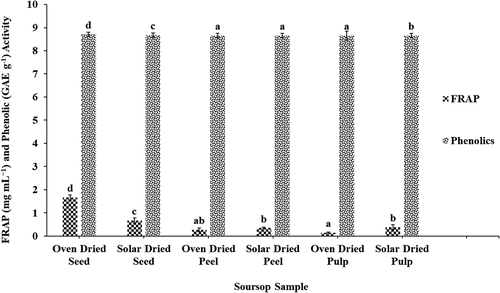
Results of the total phenolic composition of oven-dried and solar-dried soursop seed, peel, and pulp are presented in . From the figure, soursop seeds had high phenolic activities in terms of both oven- and solar-dried samples, as compared to both peel and pulp, with the peel having the least phenolic activity. The oven-dried seeds had the highest activity (p ≤ .05) of 8.701 mg GAE g−1 compared to the solar-dried samples (8.673 mg GAE g−1). With regard to the fruit peel, the phenolic activity of the solar-dried sample (8.655 mg GAE g−1) did not differ significantly (p ≥ .05) from the oven-dried sample, which recorded a value of 8.653 mg GAE g−1. Oven drying at 60 and 70°C have been reported to reduce phenolic compounds in mango and artichoke by-products (Borsini et al., Citation2021; Fratianni et al., Citation2020). Phenolic compounds are heat sensitive and their chemical structural properties are significantly altered under extreme temperature conditions, contributing to reduced TPC retention (Llavata et al., Citation2022; Salin et al., Citation2022). The degradation effect of high temperatures on TPC were also observed in edible Agastache flowers (Marchioni et al., Citation2021). The reported TPC of the Agastache (32.26–32.58 mg GAE g−1) (Marchioni et al., Citation2021) and that of Hibiscus powder (31.5–41.9 mg GAE g−1) (Nguyen et al., Citation2021) were higher than values obtained for the soursop fruit parts. However, values reported in Sargassum fusiforme, a healthy edible seaweed, were lower (7.85 mg GAE g−1). The results are in agreement with findings of Hazarikaa et al. (Citation2022) that reported significant loss of active ingredients when herbs and spices are dried at a temperature of 60°C.
3.4. Correlation between TPC, DPPH, ABTS, and FRAP antioxidant activities
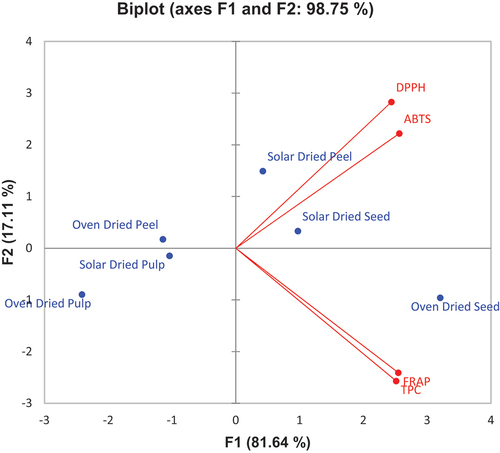
Pearson’s correlation between total phenolic content (TPC), free radical scavenging (DPPH), ABTS, and ferric reducing antioxidant power (FRAP) is presented in . There was a significant positive relationship (r = 0.943) between ABTS and DPPH. There was also a positive significant relationship (r = 0.829) between FRAP and DPPH. Additionally, results from the table revealed a significant positive relationship (r = 0.986) between TPC and FRAP. The relationship between FRAP and ABTS as well as TPC and ABTS was positive but insignificantly related.
Table 2. Correlation between TPC, DPPH, ABTS acid scavenging activity, and FRAP.
3.5. Principal components analysis (PCA) among the antioxidant properties
PCA of the antioxidant properties discriminated against the oven- and solar-dried peel, pulp, and seed samples in this study (). Results from the PCA revealed that solar-dried seed and solar-dried peel samples contributed to the free radical scavenging (DPPH and ABTS) activities, the oven-dried seed also contributed to ferric reducing antioxidant power and TPC of the fruit parts.
4. Conclusion
The different parts of the soursop fruit (skin, pulp, and seeds) under different drying regimens (solar and oven drying) demonstrated high nutritive value and good antioxidant ability. The potential antioxidant activities of the solar-dried samples were generally higher compared to the oven-dried treatment. The soursop seeds also demonstrated superior antioxidant properties comparative to other fruit parts. The different fruit parts can therefore be introduced into human diet, to combat oxidative stress and improve health.
Acknowledgement
The authors wish to express their appreciation to Mr. Danyo Emmanuel Kormla.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdualrahman, M. A. T., Ma, H., Zhou, C., Yagoub, A. E. A., Ali, A. O., Tahir, H. E., & Wali, A. (2019). Post-harvest physico-chemical properties of the pulp and seed oil from Annona squamosa L. (Gishta) fruit in darfur region, Sudan. Arabian Journal Chemistry, 12(8), 4514–4521. https://doi.org/10.1016/j.arabjc.2016.07.008
- Abe-Inge, V., Agbenorhevi, J. K., Kpodo, F. M., & Adzinyo, O. A. (2018). Effects of different drying techniques on quality characteristics of African palmyra palm (Borassus aethiopum) fruit flour. Food Research, 2(4), 331–339. https://doi.org/10.26656/fr.2017.2(4).050
- Adeola, A. A., & Aworh, O. C. (2010). Development and sensory evaluation of an improved beverage from Nigeria’s tamarind (Tamarindus indica l.) fruit. African Journal of Food Agriculture Nutrition & Development, 10(9), 4079–4092. https://doi.org/10.4314/ajfand.v10i9.62888
- Agu, K. C., Okolie, N. P., Eze, G. I., Anionye, J. C., & Falodun, A. (2017). Phytochemical analysis, toxicity profile, and hemomodulatory properties of Annona muricata (Soursop). Egyptian Journal of Haematology, 42(1), 36–44. https://doi.org/10.4103/1110-1067.206431
- Akomolafe, S. F., & Ajayi, O. B. (2015). A comparative study on antioxidant properties, proximate and mineral compositions of the peel and pulp of ripe Annona muricata (L.) fruit. International Food Research Journal, 22(6), 2381–2388.
- Almeida, M. M. B., Sousa, P. H. M., Arriaga, A. M. C., Prado, G. M., Magalhaes, C. E. C., & Maia, G. A. (2011). Bioactives compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Research International, 44(7), 2155–2159. https://doi.org/10.1016/j.foodres.2011.03.051
- AOAC. (2005). Official methods of analysis of the association of official analytical chemist (18th ed.). Horwitz William Publication.
- Borsini, A. A., Llavata, B., Uma˜na, M., & C´arcel, J. A. (2021). Artichoke by products as a source of antioxidant and fiber: How it can be affected by drying temperature. Foods, 10(2), 1–13. https://doi.org/10.3390/foods10020459
- Cho, E., Seddon, J. M., Rosner, B., Willet, W. C., & Hankinson, S. E. (2004). Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. American Medical Association, 122(6), 83–92. https://doi.org/10.1001/archopht.122.6.883
- Coria-Téllez, A., Montalvo-Gonzalez, E., Yahia, E., & Obledo-Vázquez, E. (2018). Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arabian Journal Chemistry, 11(5), 662–691. https://doi.org/10.1016/j.arabjc.2016.01.004
- Darko, D. A., Kpodo, F. M., Duah, J., Essuman, E. K., Kortei, N. K., Tettey, C. O., & Nuro-Ameyaw, P. (2021). Antioxidant and physicochemical properties of Chrysophyllum albidum fruit at different ripening stages. African Journal of Food Agriculture Nutrition & Development, 21(9), 18694–18710. https://doi.org/10.18697/ajfand.104.19055
- Dzah, C. S., Duan, Y., Zhang, H., Golly, M. K., & Ma, H. (2019). Enhanced screening of key ultrasonication parameters: Total phenol content and antioxidant activity assessment of tartary buckwheat (Fagopyrum tataricum) water extract. Separation Science and Technology, 55(17), 3242–3325. https://doi.org/10.1080/01496395.2019.1675704
- Ekpete, O. A., Edori, O. S., & Fubara, E. P. (2013). Proximate and mineral composition of some Nigerian fruits. British Journal of Applied Science & Technology, 3(4), 1447–1454. https://doi.org/10.9734/BJAST/2014/4431
- Fratianni, A., Adiletta, G., DiMatteo, M., Panfili, G., Niro, S., Gentile, C., Farina, V., Cinquanta, L., & Corona, O. (2020). Evolution of carotenoid content, antioxidant activity and volatile compounds in dried mango fruits (Mangifera indica L.). Foods, 9(10), 1424. https://doi.org/10.3390/foods9101424
- Ghana Statistical Service. (2014). Population and housing census, district analytical report. Ho west district.
- Gustavsson, J., Cederberg, C., Sonesson, U., Otterdijk, R., & Meybeck, A. (2011). Global food losses and food waste: Extent causes and prevention. Rome: Food and Agriculture Organization (FAO) of the United Nations, 1–29.
- Hazarikaa, U., Kov´acs, Z., Bodor, Z., & Gosztola, B. (2022). Phytochemicals and organoleptic properties of French tarragon (Artemisia dracunculus L.) influenced by different preservation methods. LWT-Food Science and Technology, 169, 114006. https://doi.org/10.1016/j.lwt.2022.114006
- Hossain, M. A., & Bala, B. K. (2007). Drying of hot chilli using solar tunnel drier. Solar Energy, 89(1), 85–92. https://doi.org/10.1016/j.solener.2006.06.008
- Iheanacho, M. E., & Udebuani, A. C. (2009). Nutritional composition of some leafy vegetables consumed in Imo State, Nigeria. Journal of Applied Sciences and Environmental Management, 13(3), 35–38. https://doi.org/10.4314/jasem.v13i3.55349
- Llavata, B., Picinelli, A., Simal, S., & Carcela, J. A. (2022). Cider apple pomace as a source of nutrients: Evaluation of the polyphenolic profile, antioxidant and fiber properties after drying process at different temperatures. Food Chemistry, 15, 100403. https://doi.org/10.1016/j.fochx.2022.100403
- Marchioni, I., Dimita, R., Gioè, G., Pistelli, L., Ruffoni, B., Pistelli, L., & Najar, B. (2021). The effects of post-harvest treatments on the quality of Agastache aurantiaca edible flowers. Horticulture, 7(4), 83. https://doi.org/10.3390/horticulturae70400083
- Mesquita, P. C., Rodrigues, L. G. G., Mazzutti, S., da Silva, M., Vitali, L., & Lanza, M. (2021). Intensified green-based extraction process as a circular economy approach to recover bioactive compounds from soursop seeds (Annona muricata L.). Food Chemisty, X, 12, 100164. https://doi.org/10.1016/j.fochx.2021.100164
- Moghadamtousi, S. Z., Fadaeinasab, M., Nikzad, S., Mohan, G., Ali, H. M., & Kadir, H. A. (2015). Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. International Journal of Molecular Sciences, 16(7), 15625–15658. https://doi.org/10.3390/ijms160715625
- Mohammed, E. A., Abdalla, I. G., Alfawaz, M. A., Mohammed, M. A., Al Maiman, S. A., Osman, M. A., Yagoub, A. E. A., & Hassan, A. B. (2022). Effects of extraction solvents on total phenolic content, total flavonoid content, and antioxidant activity in the aerial part of root vegetables. Agriculture, 12(11), 1820. https://doi.org/10.3390/agriculture12111820
- Moreno-Hernández, C. L., Sáyago-Ayerdi, S. G., García-Galindo, H. S., Mata-Montes De Oca, M., & Montalvo-González, E. (2014). Effect of the application of 1-methylcyclopropene and wax emulsions on proximate analysis and some antioxidants of soursop (Annona muricata L.). Scientific World Journal, 896853, 1–7. https://doi.org/10.1155/2014/896853
- Neta, M. T. S. L., de Jesus, M. S., da Silva, J. L. A., Araujo, H. C. S., Sandes, R. D. D., Shanmugam, S., & Narain, N. (2019). Effects of spray drying on bioactive and volatile compounds in soursop (Annona muricata) fruit pulp. Food Research International, 124, 70–77. https://doi.org/10.1016/j.foodres.2018.09.039
- Nguyen, Q. D., Dang, T. T., Nguyen, T. V. L., Nguyen, T. T. D., & Nguyen, N. N. (2021). Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of drying conditions on some physicochemical properties and antioxidant activities of spray-dried powder. Food Science and Nutrition, 10(1), 191–203. https://doi.org/10.1002/fsn3.2659
- Obeng, E., Kpodo, F. M., Tettey, C. O., Essuman, E. K., & Adzinyo, O. A. (2020). Antioxidant, total phenols and proximate constituents of four tropical leafy vegetables. Scientific African, 7, e00227. https://doi.org/10.1016/j.sciaf.2019.e00227
- Okigbo, N. R., & Obire, O. (2009). Mycoflora and production of wine from fruits of soursop (Annona Muricata L.). International Journal of Wine Research, 1, 1–9. https://doi.org/10.2147/IJWR.S4667
- Olabinjo, O. O. (2019). Evaluation of nutritional and phytochemical properties of dried soursop seeds. Canadian Journal of Agriculture and Crops, 5(1), 25–34. https://doi.org/10.20448/803.5.1.25.34
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26(9), 1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3
- Salin, N. S., M Saad, W. M. M., Razak, H. R. A., & Salim, F. (2022). Effect of storage temperatures on physico-chemicals, phytochemicals and antioxidant properties of watermelon juice (Citrullus lanatus). Metabolites, 12(1), 75. https://doi.org/10.3390/metabo12010075
- Tsurunaga, Y., Kanou, M., Ikeura, H., Makino, M., & Oowatari, I. T. (2022). Effect of different tea manufacturing methods on the antioxidant activity, functional components, and aroma compounds of Ocimum gratissimum. LWT - Food Science and Technology, 169, 114058. https://doi.org/10.1016/j.lwt.2022.114058
- Vwioko, D. E., Osemwegie, O. O., & Akawe, J. N. (2013). The effect of garlic and ginger phytogenics on the shelf life and microbial contents of homemade soursop (Annona muricata) fruit juice. Biokemistri, 25(2), 31–38.