 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Maca is rich in bioactive compounds and has considerable potential for commercial application as a functional food material. In this study, maca root polysaccharides (MRPs) were prepared via hot water extraction, enzyme-assisted extraction, ultrasound-assisted extraction (UAE), and deep eutectic solvent (DES)-based UAE, and their physicochemical properties and biological activities were compared. The MRP obtained using DES-based UAE (MRP-DU) exhibited the highest yield (30.85%), oil-holding capacity (5.21 g/g), emulsifying capacity (50.58%), and emulsion stability (48.14%). Additionally, MRP-DU exhibited the highest antidiabetic and absorption-retarding effects on glucose and bile acid. These results suggest that MRP obtained using DES-based UAE have high application potential as functional food materials and could also serve as antidiabetic and cholesterol-lowering agents.
Introduction
Maca (Lepidium meyenii), known as Peruvian ginseng, is a cruciferous plant native to the high Andes mountain ranges of Peru and Bolivia, where it grows at altitudes above 4000 m. It contains various bioactive ingredients, such as macaenes, macamides, glucosinolates, and polysaccharides (Wang & Zhu, Citation2019), with a wide range of biological activities, including antioxidant, antifatigue, antitumor, and immunomodulatory effects as well as hepatoprotective and regulatory functions (Li, Xu, et al., Citation2018). Therefore, maca root polysaccharides (MRPs) are considered useful functional food materials.
Polysaccharides, sugar complexes with numerous bound monosaccharides, are representative materials derived from nature and are widely present in animals, plants, and algal microorganisms. Among these, plant polysaccharides have attracted considerable attention as pharmaceuticals and functional food materials because of their low toxicity and varying therapeutic properties (Schepetkin & Quinn, Citation2006). Sufficient intake of non-starch polysaccharides derived from plants has been positively associated with the prevention of many nutritional disorders like gut related problems, type 2 diabetes, cardiovascular diseases, and obesity (Ahmed et al., Citation2011; An et al., Citation2022).
Polysaccharides possess excellent physical properties and are used in the food industry for various purposes. For example, polysaccharides are used as thickeners because they increase water retention and gel formation. They are used as dispersion stabilizers for foods, compositional agents, emulsifiers, and stabilizers for suspended substances, as they exhibit physical properties such as foaming and emulsifying. The addition of polysaccharide such as starch, cellulose, and hemicellulose to meat products increases their water retention capacity, improves mouthfeel, increases the thickness, and increase water holding capacity (Kaur & Sharma, Citation2019).
Various methods are used to extract polysaccharides from plants. As a traditional polysaccharide extraction method, the hot water extraction (HWE) can promote solvent penetration, polysaccharide dissolution, and polysaccharide diffusion through thermal effects (Wu et al., Citation2020). Enzyme-assisted extraction (EAE) promotes the release of intracellular components by decomposing the cell walls of the plants and has advantages such as high extraction efficiency and improved biological activity of the extract (Nadar et al., Citation2018). Ultrasound-assisted extraction (UAE) is a method wherein the plant cell wall is destroyed by the cavitation phenomenon of ultrasonic waves; thus, the diffusion of the target component through the cell membrane is accelerated. Consequently, the volume of solvent required and energy consumption are lower than those required in conventional extraction techniques (Nuerxiati et al., Citation2019).
To extract polysaccharides from plants, both the extraction technique and the selection of the extraction solvent are very important. Most organic solvents that have been widely used as extraction solvents in the past are flammable, volatile, explosive, and toxic and cause environmental pollution and greenhouse effects. Recently, interest in extraction technologies that use deep eutectic solvents (DESs), which have low toxicity, has increased because they are eco-friendly and simple to manufacture (Kantar et al., Citation2019). Screening for highly efficient technologies for the extraction of MRPs may help in further unleashing the bioactive potential of maca roots.
Therefore, the aim of this study was to evaluate the effects of different extraction methods on the yield, physicochemical properties, and biological activities of MRPs. First, the MRPs were extracted using HWE, EAE, UAE, and DES-based UAE methods. Subsequently, we evaluated the yield, chemical composition, monosaccharide composition, and physical properties of the four MRPs. Finally, the antidiabetic activities of the four MRP samples and retardation of glucose and bile acid absorption by these were compared to determine the optimal technique for extracting polysaccharides from maca roots.
Materials and methods
Materials
The maca (L. meyenii) used in this experiment was purchased from a farm in Gyeongsan (Republic of Korea). Maca leaves were removed and washed, and only the root part was freeze-dried. The dried roots were ground using a food mixer (FM-681C, Hanil, Incheon, Republic of Korea), sieved with a 45-mesh screen (Chung Gye Indus, MFG Co., Seoul, Republic of Korea), and stored at −45°C for further analysis.
Extraction of polysaccharides from maca roots
Hot water extraction
HWE was performed by referring to the research results optimized for maca polysaccharide (Li, Xin, et al., Citation2018). Sample powder (2.0 g) was mixed with 80 mL distilled water (DW) and shaken in a shaking water bath (BS-11, JeioTech, Seoul, Republic of Korea) at 100 rpm and 90°C for 3 h. Then, the supernatant obtained via centrifugation at 4000 ×g for 20 min was precipitated with three volumes of 95% (v/v) ethanol and stored at 4°C for 12 h. Precipitates were obtained via centrifugation at 4000 ×g for 15 min, washed with 77% (v/v) ethanol, and freeze-dried. The precipitates prepared via hot water extraction are denoted as MRP-H.
Enzyme-assisted extraction
In a preliminary experiment based on a previous study (Oh & Yoon, Citation2018), the type of enzyme (Table S1) and hydrolysis time (Table S2) with the highest yield were determined, and EAE was performed under the selected conditions. The sample (2 g) was added to 80 mL acetate buffer (0.1 M, pH 4.5), and 1% (v/w) of Shearzyme Plus (Novozymes A/S, Bagsvaerd, Denmark) was added to the mixture.
Thereafter, the mixture was stirred at 50°C and 100 rpm for 12 h in a shaking water bath (BS-11, JeioTech), and the reaction solution was heated at 100°C for 5 min to inactivate the enzyme. The precipitates were treated according to the post-extraction steps of the HWE method; the polysaccharides obtained using EAE are denoted as MRP-E.
Ultrasound-assisted extraction
UAE was performed using water or DES. DES based-UAE was conducted based on preliminary experiment and previous studies (Wang et al., Citation2022; Zang & Wang, Citation2017). The DES was prepared using choline chloride and urea in a molar ratio of 1:2 and then mixed with water at a ratio of 7:3 (DES:water). The powdered sample (2 g) was mixed with 80 mL DW or DES and extracted at 300 W and 20 kHz for 20 min using an ultrasonic probe device (KFS-600 N, Korprotech, Seoul, Republic of Korea). To prevent an excessive increase in the extract temperature during extraction, a beaker containing the extract was placed in an ice bath and monitored using a temperature-sensing device to maintain a temperature of 40 ± 2°C. After extraction, the precipitates were treated according to the post-extraction steps of the HWE method to obtain the MRPs. The polysaccharides extracted using water and DES are denoted as MRP-U and MRP-DU, respectively.
Yield determination
The extraction yield of each MRP was calculated by comparing the weight of the original dried ground maca roots to that of the freeze-dried polysaccharides using EquationEquation (1)(1)
(1) .
Total sugar, uronic acid, and protein contents
The total sugar content of MRPs was determined using the phenol – sulfuric acid method (Nielsen, Citation2010) and was calculated from a glucose standard curve. The uronic acid content was measured using the method described by Cesaretti et al. (Citation2003), with galacturonic acid as the standard. Protein content was determined as described by Lowry et al. (Citation1951), with bovine serum albumin as the standard.
Molecular weight determination
The molecular weight of MRPs was determined by gel permeation chromatography (GPC) system (Breeze System, Waters, Milford, MA, U.S.A.), using a Waters Ultrahydrogel 120 column (7.8 mm × 300 mm, 6 μm, Waters, U.S.A.). The data were calibrated with PEG standards (peak average molecular weights of 106, 430, 960, 1400, 4290, 6690, 12600, and 20,600 Da); a refractive index detector (RID) was used. The elution was conducted using 0.2 N NaNO3 at a flow rate of 0.8 mL/min.
Monosaccharide composition
MRP sample (0.2 g) was hydrolyzed with 3 mL of 72% H2SO4 at 45°C for 1 h and then diluted with 84 mL DW. The hydrolysates were re-hydrolyzed at 121°C and neutralized to pH 7 using a 2 N NaOH solution. The resulting supernatant was filtered (0.2 μm filter) and analyzed using a high-performance anion-exchange chromatography system (HPAEC, Dionex ICS-5000, Thermo Scientific, Sunnyvale, CA, U.S.A.) equipped with a CarboPac PA-1 column (250 × 4 mm, Dionex, Thermo Scientific). The monosaccharides were eluted from the column using 18 mM NaOH at a flow rate of 1.0 mL/min. Arabinose, galactose, glucose, and rhamnose, which were used as standards, were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). Monosaccharide content was calculated from the calibration curve obtained using each standard.
Water solubility and water- and oil-holding capacities
The water solubility (WS) of MRPs was measured as described by Abuduwaili et al. (Citation2019), with some modifications. MRP sample (100 mg) was mixed with 10 mL DW, heated at 90°C for 30 min, and then cooled to room temperature. The supernatant obtained by centrifugation (16000 ×g for 15 min) was freeze-dried and weighed. WS was calculated using EquationEquation (2)(2)
(2) .
The water-holding capacity (WHC) was determined as described by Hwang et al. (Citation2020), with slight modifications. First, MRP sample (0.5 g) was hydrated in 10 mL DW at room temperature for 1 h. After centrifugation at 3000 ×g for 15 min, the residue was weighed and lyophilized. Thereafter, the lyophilized residue was weighed. The WHC was expressed as the volume of water retained per gram of dry sample (g/g dry matter).
The oil-holding capacity (OHC) was measured as described by Robertson et al. (Citation2000), with some modifications. First, MRP sample (0.5 g) and soybean oil (100 mL) were mixed in centrifuge tubes and incubated at room temperature for 1 h. Next, the mixture was centrifuged at 2000 ×g for 20 min; the supernatant was carefully discarded, and the centrifuge tube was placed upside-down on a piece of filter paper and left for 1 h to remove grease. The weight of the tube was measured, and the OHC was expressed as the amount of oil retained per gram of dry sample (g/g dry matter).
Emulsifying properties
The emulsifying capacity (EC) was determined following the method described by Jindal et al. (Citation2013), with some modifications. An MRP suspension (3%, 20 mL) was mixed with 5 mL of soybean oil using a homogenizer (AM-1, Nissei, Tokyo, Japan) at 1,000 rpm for 5 min and then centrifuged at 4000 ×g for 10 min. The volume of the emulsified layer and the total volume of the mixture were determined, and the EC was calculated as follows (3):
To measure the emulsion stability (ES), emulsions were prepared according to the method used for EC measurement. The emulsion was then heated in a hot water bath at 80°C for 30 min, cooled to 25°C, and centrifuged at 4000 ×g for 5 min. The height of the emulsion layer was measured, and the ES was calculated using EquationEquation (4)(4)
(4) :
Foaming properties
The foaming capacity (FC) was determined using the method reported by Rezaei et al. (Citation2016), with modifications. First, MRP sample (1 g) was added to 49 mL DW and bubbled at 3000 rpm for 1 min using a homogenizer (AM-1, Nihonseiki Kaisha LDD, Nissei, Japan). The corresponding increase in volume (mL) was measured, and the FC was calculated as follows:
The foam stability (FS) was measured by comparing the foam volume after allowing the foam to stand with the initial foam volume of the sample. The foam was created using the same method as that used for determining FC, and the initial volume of the bubbles was measured. Then, the foam was allowed to stand (for 10, 30, 60, 90, or 120 min) at room temperature, and the volume was measured again. The FS was calculated using EquationEquation (6)(6)
(6) as follows:
Antidiabetic activity
The inhibitory activity against porcine pancreatic α-amylase (type VI-B; Sigma – Aldrich Co., St. Louis, MO, U.S.A.) was measured using the method described by Lee and Yoon (Citation2022), with modifications. An α-amylase enzyme solution (100 mL of 5 U/mL in 0.02 M sodium phosphate buffer, pH 6.9) was added to 100 µL of MRP sample prepared by concentration and left at room temperature for 5 min. Then, 100 µL of 1% starch was added, mixed, and allowed to react at room temperature for 5 min, after which 200 µL of 48 mM 3,5-dinitrosalicylic acid reagent (DNS, 30% sodium potassium tartarate in 0.5 M NaOH) was added and mixed. The mixture was heated in boiling water for 10 min and cooled for 10 min, after which DW (1.5 mL) was added and mixed. The absorbance was measured at 540 nm using a U-2900 spectrophotometer (Hitachi, Tokyo, Japan).
α-Glucosidase inhibitory activity was measured according to the method described by Lee and Yoon (Citation2022). First, 50 µL aliquots each of the MRP solution, 0.2 U/mL α-glucosidase enzyme solution, and 200 mM potassium phosphate buffer (pH 6.8) were mixed and incubated at 37°C for 15 min. Then, 100 µL of 3 mM p-nitrophenyl-α-D-glucopyranoside (Sigma – Aldrich Co., St. Louis, MO, U.S.A.) was added to the mixture and allowed to react at 37°C for 10 min. To stop the reaction, 50 µL of 0.1 M NaOH was added, and the absorbance was measured at 405 nm. Acarbose (Sigma – Aldrich Co.), used as an α-amylase inhibitor for treating diabetes, was used as the positive control.
Retarding effect of MRPs on glucose absorption
The retarding effects of MRPs on glucose absorption in the gastrointestinal tract were measured by determining the glucose permeation rate (GPR) in the dialysate, as described by Adiotomre et al. (Citation1990). To measure the GPR, MRP sample (0.2 g) and glucose (36 mg) were dissolved in 0.1% sodium azide and added to dialysis bags (D7884: MW cut-off ≤1200; Sigma – Aldrich Co.). Each dialysis bag was immersed in 100 mL phosphate buffer, containing 0.1% sodium azide, and dialyzed at 37°C for 12 h. The dialysate (1 mL) was collected at regular intervals, and the glucose content of the dialysate was analyzed using the DNS method. The dialysate obtained by the same process but without a sample was used as the control, and the dialysate obtained using carboxymethyl cellulose (CMC; Sigma – Aldrich Co.) were used as positive control.
Retarding effect of MRPs on bile acid absorption
The delaying effect of MRPs on bile acid absorption was evaluated based on the principle that free bile acid binds to polysaccharides and delays dialysis membrane permeation (Adiotomre et al., Citation1990). To measure the bile acid permeation rate (BPR), 1 L phosphate buffer (50 mM, pH 7.0), containing 0.1% sodium azide, and 6 mL of 15 mM taurocholic acid-containing sample (0.2 g) were added to the dialysis bag. Each dialysis bag was immersed in 100 mL phosphate buffer containing 0.1% sodium azide and dialyzed at 37°C for 12 h. The dialysate (1 mL) was collected at regular intervals to allow the measurement of bile acid, and taurocholic acid content was determined as described by Boyd et al. (Citation1966). The dialysate obtained using the same process but without a sample was used as the control, and the dialysate obtained by adding CMC (Sigma – Aldrich Co.) was used as the positive control.
Statistical analysis
Results except for average molecular weight are expressed as the mean and standard deviation of values obtained from triplicate experiments. Statistical analyses were performed using the SPSS software (Ver. 21; SPSS Inc., Chicago, IL, U.S.A.). The statistical significance was set to p < .05. Significant differences between the mean values of the tests were verified using Duncan’s multiple-range test.
Results and discussion
Extraction yield, basic components and average molecular weight
The extraction yield, basic components and average molecular weight of the MRPs obtained using the four extraction methods are shown in . MRP-DU had the highest yield (30.85 ± 0.37%) (p < .05), followed by MRP-U (15.16 ± 0.29%), MRP-H (13.08 ± 0.09%), and MRP-E (6.22±0.21%). MRP-DU produced approximately twice the yield as did MRP-U. This suggests that the extraction yield can be increased by applying DES as the extraction solvent for UAE. Total sugar content of MRPs ranged 79.86 ± 1.31–89.39 ± 0.38%, and MRP-H had the highest. Both MRP-U and MRP-DU had high protein contents (7.71 ± 0.21% and 7.63 ± 0.11%, respectively), while MRP-E (1.71 ± 0.06%) had the lowest protein content (p < .05). Nuerxiati et al. (Citation2019) reported that the polysaccharides extracted with UAE (3.16%) from Orchis chusua D. Don had higher protein contents than those extracted with HWE (2.64%), which is consistent with the results of this study. These findings reflect the improved protein solubility due to the exposure of the hydrophilic portions of amino acids to ultrasound (Singh et al., Citation2018). Chen and Huang (Citation2018) reported that proteins decrease the homogeneity and refinement of polysaccharides. Wang et al. (Citation2019) reported that proteins exhibit surface tension by interacting with polysaccharides, which affects the physical properties such as emulsion formation ability. Uronic acid content was the highest in MRP-DU (7.05 ± 0.08%), followed by MRP-E (6.78 ± 0.02%), MRP-U (5.77 ± 0.04%), and MRP-H (4.44 ± 0.12%). Uronic acid refers to acidic sugars and compounds that have gained carboxyl groups through the oxidation of an alcohol group at the end of a monosaccharide molecule. Uronic acids include galacturonic and glucuronic acids, and the polysaccharide fractions containing them exhibit various physiological activities (Kumar & Kumar, Citation2017). The average molecular weight (Mw) of MRPs was highest in MRP-DU (18.17 KDa), followed by MPR-U (14.60 KDa), MRP-H (13.75 KDa), and MRP-E (11.76 KDa). MW is an important factor related to the functional properties of polysaccharides, and it has been reported that emulsification and foamability increase as the MW increases (Shen et al., Citation2019).
Table 1. Extraction yield, basic components and average molecular weight (Mw) of maca root polysaccharides extracted using various extraction methods.
Monosaccharide composition
The constituent monosaccharide content of the MRPs obtained using the four different extraction methods is listed in . All MRPs were mostly composed of glucose; therefore, the MRPs were confirmed to be neutral polysaccharides. Glucose content was highest in MRP-H (88.66 ± 0.17 mg/100 mg), followed by MRP-E (85.461 ± 0.09 mg/100 mg), MRP-DU (81.70±0.03 mg/100 mg), and MRP-U (80.47±0.11 mg/100 mg), and MRP-U (80.465 ± 0.110 mg/100 mg). Galactose content was the next highest. However, the average content was 0.76 ± 0.01 mg/100 mg, and small quantities of arabinose and rhamnose were also present. Zhang et al. (Citation2016) reported that the maca polysaccharide treated using HWE, separation, and purification was a neutral polysaccharide containing large quantities of glucose (53.66%), along with arabinose (26.21%), mannose (11.81%), and galactose (8.32%); these results are similar to those of the present study. Maca polysaccharides are generally heteropolysaccharides composed of glucose, arabinose, and galactose; however, there are differences in the seeds, cultivation area, time, and cultivation conditions (Tang et al., Citation2017).
Table 2. Monosaccharide composition (mg/100 mg) of maca root polysaccharides extracted using various methods.
Water solubility and water- and oil-holding capacities
The WS, WHC, and OHC values of the MRP are shown in . The WS of MRP-E was the highest (87.37 ± 0.23%) (p < .05), followed by MRP-H (82.87 ± 0.45%), MRP-DU (76.33 ± 0.35%), and MRP-U (75.57 ± 0.21%). The WS was significantly different for the MRPs obtained from different extraction methods under various temperatures. The high WS of MRP-E is considered to be due to enzymatic degradation of insoluble polysaccharides to convert them to soluble polysaccharides, increasing their solubility (Luo et al., Citation2017). In addition, the high WS of MRP-H is considered to be due to the accelerated decomposition of high molecular weight polysaccharides into low molecular weight oligosaccharides and monosaccharides by heat treatment (Lu et al., Citation2018). The low WS of the MRP obtained using UAE may be caused by the lower degree of polysaccharide degradation that that in other extraction methods because it is extracted at a low temperature. The WS is an important factor in the preparation and application of polysaccharides. The higher the WS, the greater the applicability of the polysaccharide to water-soluble foods such as beverages, increasing its scope as a raw material with the potential for enhancing functionality (Li et al., Citation2019).
Table 3. Water solubility and water-and oil-holding capacities of maca root polysaccharides extracted using various methods.
The WHC of MRPs exhibited a trend similar to that of WS. For example, MRP-E had the highest WHC at 17.23 ± 0.50 g/g (p < .05), followed by MRP-H (15.67 ± 0.74 g/g), MRP-DU (13.93 ± 0.12 g/g), and MRP-U (13.71 ± 0.32 g/g). Water-soluble polysaccharides have higher WHC than insoluble polysaccharides; therefore, high water-soluble polysaccharide content can improve water retention (Karra et al., Citation2019). Chen et al. (Citation2015) reported that the WHC of two polysaccharide fractions purified from maca were 8.39 ± 0.05 g/g and 16.29 ± 0.69 g/g, respectively, which were lower than or similar to the results of this study. The WHC of the polysaccharide fraction obtained from potato peel using HWE was 4.10 ± 0.54 g/g (Jeddou et al., Citation2016), which was very low compared to the results of this study.
As such, the high WHC of MRP is considered to be because the polysaccharides of the previous studies (Chen et al., Citation2015; Jeddou et al., Citation2016) contain both soluble and insoluble dietary fiber, whereas MRPs are composed of soluble polysaccharide. In particular, MRP-E had the highest WHC, which was attributed to an increase in the content of low-molecular-weight water-soluble polysaccharides due to enzymatic hydrolysis. The WHC also contributes to moisturizing the food, improving the texture and viscosity, and preserving the flavor (Trigui et al., Citation2018). Therefore, MRPs can be used in various food products because of their high WHC.
The OHC of MRP-DU was 5.21 ± 0.10 g/g, which was the highest (p < .05), followed by that of MRP-U (4.00 ± 0.16 g/g). The OHC of MRP-E and MRP-H was low (3.14 ± 0.08 and 2.73 ± 0.82 g/g, respectively). These results were similar to those of Chen et al. (Citation2015), who reported that the OHC of dietary fiber fractions isolated and purified from maca was 3.91 ± 0.25 g/g and 5.79 ± 0.24 g/g, respectively. Abuduwaili et al. (Citation2019) reported that the OHC of Fritillaria pallidiflora Schrenk polysaccharide obtained using various extraction methods was 5.46–9.53 g/g, confirming that there was a difference in OHC depending on the source and type of polysaccharides. Li et al. (Citation2019) reported that the OHC decreased as the protein content decreased in the polysaccharide fraction obtained from okara, indicating that the OHC is related to protein content. Therefore, the OHC of the MRP extracted using UAE was high because of the increased protein content. However, our results revealed only a loose correlation with protein content, which may be due to the effects of the structure and surface area of the polysaccharide on the OHC in addition to the protein content.
Emulsifying properties
EC refers to the force that forms an emulsion by reducing the surface tension between hydrophilic and hydrophobic materials. Two immiscible components can be mixed by homogenization to form an emulsion; however, they can be easily separated by external forces such as temperature and pressure. Therefore, emulsifiers are added to increase the emulsifying power when preparing foods in the form of emulsions. The most commonly used emulsifiers in the food industry include phospholipids, proteins, and polysaccharides (Pereira et al., Citation2019). MRP-DU had the highest EC, at 50.58 ± 1.21% (p < .05), followed by MRP-U (29.29 ± 1.39%), whereas MRP-E and MRP-H had low EC values of 8.67 ± 0.61% and 5.97 ± 0.79%, respectively (). The EC of polysaccharides is affected by the protein content; in the presence of proteins, the EC is enhanced owing to the polysaccharide-protein interactions such as polysaccharide-protein electrostatic complexes and Maillard conjugates (Kontogiorgos, Citation2019). EC is also affected by uronic acids in polysaccharides, which are important components of plant cell walls and exhibits surface activity by interacting with proteins (Schmidt et al., Citation2015). Therefore, the high EC of the MRPs extracted using UAE was attributed to their high protein content. Particularly, MRP-DU had a high EC, which was most likely caused by the strong interactions between amino group and carboxyl group owing to the high uronic acid content of MRP-DU.
Table 4. Emulsifying properties of maca root polysaccharides extracted using various methods.
ES refers to the degree to which an emulsifier maintains surface activity when heat is applied to the emulsion; generally, for a polysaccharide to be used as an emulsifier, it must have stabilizing properties for the emulsion as well as emulsion-forming ability (Hou et al., Citation2019). The ES of the MRPs exhibited the same relationship with the extraction methods as did the EC, in the order of MRP-DU (48.14 ± 1.15%) > MRP-U (27.82 ± 1.09%) > MRP-E (8.23 ± 0.62%) > MRP-H (5.31 ± 0.66%) (). Wu et al. (Citation2022) reported that the ES of three polysaccharides obtained from soy hull was proportional to their molecular weight and uronic acid concentration. Therefore, the high ES of MRP-DU is attributed to its high uronic acid content and large molecular weight. As polysaccharides with suitable emulsifying properties are likely to be used as stabilizers and emulsifiers, the MRP-DU is expected to be extensively applicable in the food industry.
Foaming properties
Polymeric substances in food, such as proteins and polysaccharides, are essential factors that determine the foaming properties. To determine the foaming properties of these components, their foaming ability and foam stability are measured (Jeddou et al., Citation2016). The relationships between the FC and FS of the MRPs and the extraction methods are shown in . MRP-U had the highest FC (19.60 ± 0.35%; p < .05), followed by MRP-DU (9.73 ± 0.46%), MRP-E (8.87 ± 0.50%), and MRP-H (6.47 ± 0.42%). Akhtar et al. (Citation2019) reported that the FC of polysaccharide fractions obtained from chickpeas was approximately 6% and 15% at concentrations of 2% and 4%, respectively, which is similar to the results of this study. Trigui et al. (Citation2018) reported that the FC of water-soluble polysaccharides obtained from black cumin seeds was 58.98 ± 1.95% at a concentration of 3%, which was higher than the results of this study. The difference in FC depending on the source is influenced by various factors such as polysaccharide concentration, molecular weight and structure, and protein content (Akhtar et al., Citation2019).
Table 5. Foaming properties of maca root polysaccharides extracted using various methods.
FS refers to the ability to stabilize the surface film of a gas-liquid to maintain a foam for a certain duration. MRP-DU had the highest FS, regardless of the standing time, and maintained 50% foam even after 120 min. MRP-H had the second-highest FS and retained 28.79% foam after 120 min. MRP-U maintained 73.80% of its foam when left for 10 min but had a very low FS of 3.73% after 120 min. Tan and Gan (Citation2016) reported that the FS of a 1% polysaccharide obtained from Momordica charantia was 87.9% after being left for 1 h and was much higher than that obtained in this study. The FS is strongly related to the molecular weight of the polysaccharide, and the higher the molecular weight, the better the FS (Shen et al., Citation2019). The reason for the large difference in FS between the previous study (Tan & Gan, Citation2016) and MRPs is considered to be due to the difference in molecular weight of the polysaccharide.
Antidiabetic activity
α-Amylase is a digestive enzyme that first acts on ingested carbohydrates and hydrolyzes α-D-(1,4)-glucan in starch. α-Glucosidase is an enzyme present in the brush border membrane of the epithelial cells of the small intestine that hydrolyzes polysaccharides and disaccharides into monosaccharides to increase blood glucose levels. Therefore, as the inhibition of these enzymes can lower blood sugar levels, antidiabetic activity can be evaluated by measuring the degree of inhibition of α-amylase and α-glucosidase activities (Lee & Yoon, Citation2022).
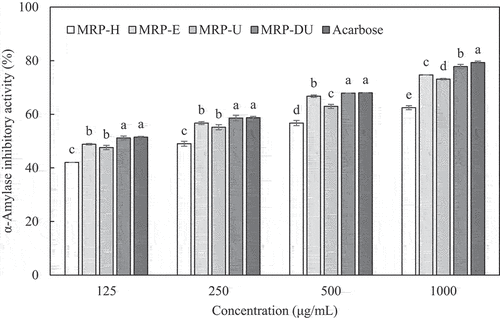
The α-amylase inhibitory activity of the MRP obtained using the extraction method increased with increasing concentration, as shown in . At all concentrations, MRP-DU exhibited the highest inhibitory activity among the extracts, and there was no significant difference in the inhibitory activity of the positive control (acarbose 125–500 µg/mL). At 1000 µg/mL, acarbose demonstrated the highest activity (79.31 ± 0.52%), and the MRP-DU was 77.81 ± 0.79%, corresponding to 98.1% of acarbose. Next, MRP-E (74.68 ± 0.00%), MRP-U (73.11 ± 0.28%), and MRP-H (62.46 ± 0.75%) demonstrated decreasing α-amylase inhibition in the order listed. These results indicate that all MRPs, except MRP-H, exhibited >90% inhibitory activity against acarbose.
Figure 1. α-Amylase inhibitory activity of maca root polysaccharides extracted using various methods. Results are expressed as the mean ± SD of values from triplicate experiments. Values with different letters are significantly different (p < .05). MRP-H, MRP-E, MRP-U, and MRP-DU are maca root polysaccharides extracted using HWE-, EAE-, UAE-, and DES-based UAE, respectively.
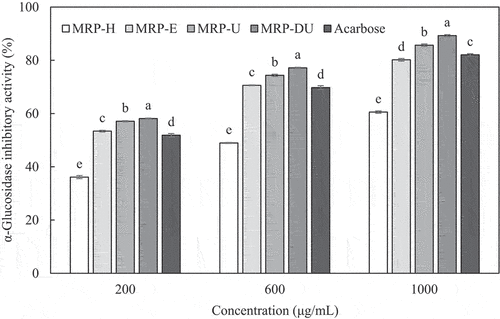
The α-glucosidase inhibitory activity of the MRPs obtained using various extraction methods is shown in . The α-glucosidase inhibitory activity increased with increasing concentration, similar to the α-amylase inhibitory activity. At 200 µg/mL concentration, MRP-DU demonstrated the highest inhibitory activity, at 58.13 ± 0.19% (p < .05), followed by MRP-U (57.11 ± 0.22%), MRP-E (53.41 ± 0.29%), acarbose (51.88 ± 0.61%), and MRP-H (36.14 ± 0.57%). All the MRPs, except MRP-H, demonstrated higher inhibitory activities than acarbose. Additionally, the MRPs obtained using UAE showed higher α-glucosidase inhibitory activities at all concentrations than acarbose. These findings indicate that the MRPs, especially MRP-DU, obtained using UAE inhibit the activity of α-glucosidase and prevent postprandial blood glucose levels from increasing. Thus, they act as therapeutic agents against diabetes when ingested.
Figure 2. α-Glucosidase inhibitory activity of maca root polysaccharides extracted using various methods.Bars represent the mean ± SD of values from triplicate experiments. MRP-H, MRP-E, MRP-U, and MRP-DU were maca root polysaccharides extracted using HWE-, EAE-, UAE-, and DES-based UAE, respectively.
Retarding effect of MRPs on glucose absorption
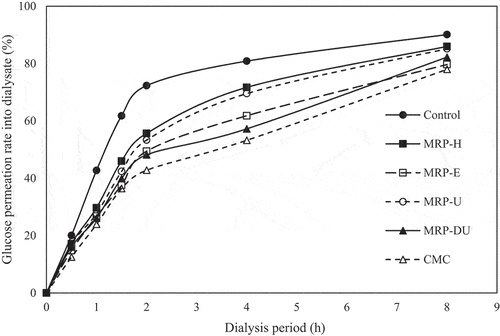
The retarding effect on glucose absorption can be tested in vitro using the entrapping effect of dietary fiber and has a very high correlation with the in vivo hypoglycemic test (Baek et al., Citation2010). The relationship between the GPR and the dialysis time of the MRP obtained using each extraction method is shown in . During up to 2 h of dialysis, all samples exhibited rapid GPR; particularly, the GPR of the control increased rapidly and was highest at 72.30%. The GPRs of CMC, MRP-H, MRP-E, MRP-U, and MRP-DU were 42.81%, 55.66%, 49.44%, 53.34%, and 48.18% after 2 h of dialysis, respectively, all of which were lower than the GPR of the control. Subsequently, as the dialysis time increased for all groups, the GPR continued to increase slowly. After 8 h of dialysis, all MRPs had lower GPRs than the control (90.01%); MRP-E had the lowest GPR (79.67%), and MRP-DU had the second lowest GPR (85.23%). Polysaccharides form a viscous gel in aqueous solutions, reducing the access of glucose to the epithelial cells of the small intestine, thereby delaying absorption (Ahmed et al., Citation2011). Daou and Zhang (Citation2014) reported that the polysaccharides with high water retention capacity and solubility delay glucose absorption. Rodríguez-Gutiérrez et al. (Citation2014) reported that the uronic acid content of polysaccharides and the internal structure of the polysaccharide particles affect glucose absorption. The GPR of MRP-E was high because of its high WHC, WS, and uronic acid content. However, MRP-DU, with relatively low water retention and solubility, might have exhibited higher values than MRP-E because of its different uronic acid content, surface area, and polysaccharide structure.
Figure 3. Passive transport of glucose in the presence of maca root polysaccharides extracted using various methods. Results are expressed as the mean ± SD of values from triplicate experiments. %, ratio of glucose in dialysate out of total glucose added.
Retarding effect of MRP on bile acid absorption
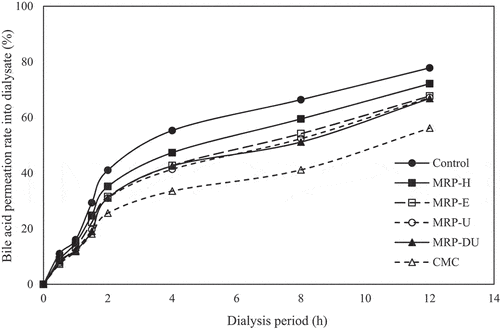
The BPR according to the dialysis time of the MRPs obtained using each extraction method is shown in . After 30 min of dialysis, the BPR of the control group was highest, at 11.00%, and the CMC and MRPs ranged from 7.28 to 8.90%. After 2 h of dialysis, the BPR of the control group had increased significantly to 41.03%, and the BPR of the CMC and MRP were 25.60–35.18%, which was very low compared to that of the control. This difference continued to occur for 12 h of dialysis; the BPR of the control was 77.81%, and the BPR of the CMC, MRP-DU, MRP-U, MRP-E, and MRP-H were 56.16, 66.18, 67, 14, and 67.72, and 72.12% respectively. The BPR exhibited a gentle increase relative to the GPR with increasing dialysis time, possibly due to the slow dialysis due to the higher molecular weight of bile than that of glucose (Oh & Lee, Citation1996). Polysaccharides adsorb free bile acids, inhibit the reabsorption of bile acids, and induce cholesterol consumption for bile acid synthesis in the body. Consequently, blood cholesterol levels are reduced, which reduces the risk of cardiovascular diseases, such as arteriosclerosis and heart disease (Baek et al., Citation2010). Ma et al. (Citation2015) reported that a large number of hydrophobic groups present in polysaccharides form hydrophobic bonds through interactions with bile acid, thereby increasing the adsorption capacity, and that the small size of dietary fiber particles increases the adsorption capacity by widening the surface area. Therefore, MRPs, especially MRP-DU and MRP-U, are expected to have a positive effect on lipid metabolism, as they are highly effective in delaying bile acid absorption.
Figure 4. Passive transport of bile acid in the presence of maca root polysaccharides extracted using various methods. Results are expressed as the mean ± SD of values from triplicate experiments. %, ratio of bile acid in dialysate out of total bile acid added. MRP-H, MRP-E, MRP-U, and MRP-DU: maca root polysaccharides extracted using HWE-, EAE-, UAE-, and DES-based UAE, respectively.
Conclusion
Here, we compared the physicochemical activity, antidiabetic activity, and retarding effects of polysaccharides extracted from maca roots using four extraction methods on the dialysis membrane transport of glucose and bile acid. The data from this study demonstrated the feasibility of producing polysaccharides with significant physical and biological activities. Particularly, MRPs extracted using UAE, had not only a high yield but also suitable emulsifying properties, foaming properties, antidiabetic activity, and retarding effects on glucose and bile acid. Additionally, MRP-DU inhibited the α-amylase activity at a level similar to that of acarbose. Therefore, MRP is a novel material that has antidiabetic and hypoglycemic effects and may be used in the food industry to prepare functional foods and nutraceutical products.
Supplemental Material
Download MS Word (14.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2023.2252879.
References
- Abuduwaili, A., Rozi, P., Mutailifu, P., Gao, Y., Nuerxiati, R., Aisa, H. A., & Yili, A. (2019). Effects of different extraction techniques on physicochemical properties and biological activities of polysaccharides from Fritillaria pallidiflora schrenk. Process Biochemistry, 83, 189–197. https://doi.org/10.1016/j.procbio.2019.05.020
- Adiotomre, J., Eastwood, M. A., Edwards, C., & Brydon, W. G. (1990). Dietary fiber: In vitro methods that anticipate nutrition and metabolic activity in humans. The American Journal of Clinical Nutrition, 52(1), 128–134. https://doi.org/10.1093/ajcn/52.1.128
- Ahmed, F., Sairam, S., & Urooj, A. (2011). In vitro hypoglycemic effects of selected dietary fiber sources. Journal of Food Science and Technology, 48(3), 285–289. https://doi.org/10.1007/s13197-010-0153-7
- Akhtar, H. M. S., Abdin, M., Hamed, Y. S., Wang, W., Chen, G., Chen, D., Chen, C., Li, W., Mukhtar, S., & Zeng, X. (2019). Physicochemical, functional, structural, thermal characterization and α-amylase inhibition of polysaccharides from chickpea (Cicer arietinum L.) hulls. LWT – Food Science and Technology, 113, 108265. https://doi.org/10.1016/j.lwt.2019.108265
- An, Y., Lu, W., Li, W., Pan, L., Lu, M., Cesarino, I., Li, Z., & Zeng, W. (2022). Dietary fiber in plant cell walls—The healthy carbohydrates. Food Quality and Safety, 6, 1–17. https://doi.org/10.1093/fqsafe/fyab037
- Baek, J. H., Cha, T. Y., Heo, J. C., Lee, S. H., & Lee, S. Y. (2010). In vitro and in vivo physiological characteristics of dietary fiber from by-product of aloe vera gel processing. Food Engineering Progress, 14(2), 173–182. https://db.koreascholar.com/Article/Detail/285638
- Boyd, G. S., Eastwood, M. A., & Maclean, N. (1966). Bile acids in the rat: Studies in experimental occlusion of the bile duct. Journal of Lipid Research, 7(1), 83–94. https://doi.org/10.1016/S0022-2275(20)39589-4
- Cesaretti, M., Luppi, E., Maccari, F., & Volpi, N. (2003). A 96-well assay for uronic acid carbazole reaction. Carbohydrate Polymers, 54(1), 59–61. https://doi.org/10.1016/S0144-8617(03)00144-9
- Chen, F., & Huang, G. (2018). Extraction and antioxidant activities of cushaw polysaccharide. International Journal of Biological Macromolecules, 120(Pt B), 1646–1649. https://doi.org/10.1016/j.ijbiomac.2018.09.200
- Chen, J., Zhao, Q., Wang, L., Zha, S., Zhang, L., & Zhao, B. (2015). Physicochemical and functional properties of dietary fiber from maca (Lepidium meyenii Walp.) liquor residue. Carbohydrate Polymers, 132, 509–512. https://doi.org/10.1016/j.carbpol.2015.06.079
- Daou, C., & Zhang, H. (2014). Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. Journal of Food Science and Technology, 51(12), 3878–3885. https://doi.org/10.1007/s13197-013-0925-y
- Hou, Y., Gong, T., Zhang, J., Yang, X., & Guo, Y. (2019). Structural characterization and emulsifying properties of thinned-young apples polysaccharides. Biochemical and Biophysical Research Communications, 516(4), 1175–1182. https://doi.org/10.1016/j.bbrc.2019.07.019
- Hwang, Y. J., Kim, J. M., & Yoon, K. Y. (2020). Characteristics of water-soluble polysaccharide extracts produced from perilla seed meal via enzymatic hydrolysis. CyTA-Journal of Food, 18(1), 653–661. https://doi.org/10.1080/19476337.2020.1814420
- Jeddou, K. B., Chaari, F., Maktouf, S., Ellouz, O. N., Helbert, C. B., & Ghorbel, R. E. (2016). Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chemistry, 205, 97–105. https://doi.org/10.1016/j.foodchem.2016.02.108
- Jindal, M., Kumar, V., Rana, V., & Tiwary, A. K. (2013). Exploring potential new gum source Aegle marmelos for food and pharmaceuticals: Physical, chemical and functional performance. Industrial Crops and Products, 45, 312–318. https://doi.org/10.1016/j.indcrop.2012.12.037
- Kantar, S. E., Rajha, H. N., Boussetta, N., Vorobiev, E., Maroun, R. G., & Louka, N. (2019). Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chemistry, 295, 165–171. https://doi.org/10.1016/j.foodchem.2019.05.111
- Karra, S., Sebii, H., Borchani, C., Danthine, S., Blecker, C., Attia, H., Besbes, S., & Bouaziz, M. A. (2019). Physico-chemical and functional properties of dried male date palm flowers. Food Bioscience, 31, 100441. https://doi.org/10.1016/j.fbio.2019.100441
- Kaur, R., & Sharma, M. (2019). Cereal polysaccharides as sources of functional ingredient for reformulation of meat products: A review. Journal of Functional Foods, 62, 103527. https://doi.org/10.1016/j.jff.2019.103527
- Kontogiorgos, V. (2019). Polysaccharides at fluid interfaces of food systems. Advances in Colloid and Interface Science, 270, 28–37. https://doi.org/10.1016/j.cis.2019.05.008
- Kumar, P., & Kumar, V. (2017). Estimation of uronic acids using diverse approaches and monosaccharide composition of alkali soluble polysaccharide from Vitex negundo linn. Carbohydrate Polymers, 165(1), 205–212. https://doi.org/10.1016/j.carbpol.2017.02.034
- Lee, J. J., & Yoon, K. Y. (2022). Ultrasound-assisted extractions for improving the recovery of phenolics and charantin from bitter melon and for increasing the antioxidant, antidiabetic and anti-obesity activities of its extracts. Polish Journal of Food and Nutrition Sciences, 72(3), 141–150. https://doi.org/10.31883/pjfns/149434
- Li, Y., Xin, Y., Xu, F., Zheng, M., Xi, X., Cui, X., Cao, H., Guo, H., & Han, C. (2018). Maca polysaccharides: Extraction optimization, structural features and anti-fatigue activities. International Journal of Biological Macromolecules, 115, 618–624. https://doi.org/10.1016/j.ijbiomac.2018.04.063
- Li, Y., Xu, F., Zheng, M., Xi, X., Cui, X., & Han, C. (2018). Maca polysaccharides: A review of compositions, isolation, therapeutics and prospects. International Journal of Biological Macromolecules, 111, 894–902. https://doi.org/10.1016/j.ijbiomac.2018.01.059
- Li, B., Yang, W., Nie, Y., Kang, F., Goff, H. D., & Cui, S. W. (2019). Effect of steam explosion on dietary fiber, polysaccharide, protein and physicochemical properties of okara. Food Hydrocolloids, 94, 48–56. https://doi.org/10.1016/j.foodhyd.2019.02.042
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193(1), 265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
- Lu, X., Li, N., Qiao, X., Qiu, Z., & Liu, P. (2018). Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT - Food Science and Technology, 95, 223–229. https://doi.org/10.1016/j.lwt.2018.04.059
- Luo, X., Wang, Q., Zheng, B., Lin, L., Chen, B., Zheng, Y., & Xiao, J. (2017). Hydration properties and binding capacities of dietary fibers from bamboo shoot shell and its hypolipidemic effects in mice. Food and Chemical Toxicology, 109(P2), 1003–1009. https://doi.org/10.1016/j.fct.2017.02.029
- Ma, M., Mu, T., Sun, H., Zhang, M., Chen, J., & Yan, Z. (2015). Optimization of extraction efficiency by shear emulsifying assisted enzymatic hydrolysis and functional properties of dietary fiber from deoiled cumin (Cuminum cyminum L.). Food Chemistry, 179, 270–277. https://doi.org/10.1016/j.foodchem.2015.01.136
- Nadar, S. S., Rao, P., & Rathod, V. K. (2018). Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Research International, 108, 309–330. https://doi.org/10.1016/j.foodres.2018.03.006
- Nielsen, S. S. (2010). Phenol-sulfuric acid method for total carbohydrates. In S. S. Nielsen (Ed.), Food analysis laboratory manual. Food science texts series (pp. 47–53). Springer US. https://doi.org/10.1007/978-1-4419-1463-7_6
- Nuerxiati, R., Abuduwaili, A., Mutailifu, P., Wubulikasimu, A., Rustamova, N., Jingxue, C., Aisa, H. A., & Yili, A. (2019). Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep). International Journal of Biological Macromolecules, 141(1), 431–443. https://doi.org/10.1016/j.ijbiomac.2019.08.112
- Oh, H. J., & Lee, S. R. (1996). Physiological function in vitro of β-glucan isolated from barley. Korean Journal of Food Science & Technology, 28(4), 689–695. https://koreascience.kr/article/JAKO199603042041374.pdf
- Oh, M. H., & Yoon, K. Y. (2018). Comparison of the biological activity of crude polysaccharide fractions obtained from Cedrela sinensis using different extraction methods. Polish Journal of Food and Nutrition Sciences, 68(4), 327–334. https://doi.org/10.1515/pjfns-2018-0007
- Pereira, G. A., Silva, E. K., Araujo, N. M. P., Arruda, H. S., Meireles, M. A. A., & Pastore, G. M. (2019). Mutamba seed mucilage as a novel emulsifier: Stabilization mechanisms, kinetic stability and volatile compounds retention. Food Hydrocolloids, 97, 105190. https://doi.org/10.1016/j.foodhyd.2019.105190
- Rezaei, A., Nasirpour, A., & Tavanai, H. (2016). Fractionation and some physicochemical properties of almond gum (Amygdalus communis L.) exudates. Food Hydrocolloids, 60, 461–469. https://doi.org/10.1016/j.foodhyd.2016.04.027
- Robertson, J. A., Monredon, F. D., Dysseler, P., Guillon, F., Amadò, R., & Thibault, J. F. (2000). Hydration properties of dietary fibre and resistant starch: A European collaborative study. LWT-Food Science and Technology, 33(2), 72–79. https://doi.org/10.1006/fstl.1999.0595
- Rodríguez-Gutiérrez, G., Rubio-Senent, F., Lama-Muñoz, A., García, A., & Fernández-Bolaños, J. (2014). Properties of lignin, cellulose, and hemicelluloses isolated from olive cake and olive stones: Binding of water, oil, bile acids, and glucose. Journal of Agricultural and Food Chemistry, 62(36), 8973–8981. https://doi.org/10.1021/jf502062b
- Schepetkin, I. A., & Quinn, M. T. (2006). Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. International Immunopharmacology, 6(3), 317–333. https://doi.org/10.1016/j.intimp.2005.10.005
- Schmidt, U. S., Schmidt, K., Kurz, T., Endreß, H. U., & Schuchmann, H. P. (2015). Pectins of different origin and their performance in forming and stabilizing oil-in-water-emulsions. Food Hydrocolloids, 46, 59–66. https://doi.org/10.1016/j.foodhyd.2014.12.012
- Shen, S. G., Lin, Y. H., Zhao, D. X., Wu, Y. K., Yan, R. R., Zhao, H. B., Tan, Z. L., Jia, S. R., & Han, P. P. (2019). Comparisons of functional properties of polysaccharides from Nostoc flagelliforme under three culture conditions. Polymers, 11(2), 263–274. https://doi.org/10.3390/polym11020263
- Singh, A., Benjakul, S., & Kijroongrojana, K. (2018). Effect of ultrasonication on physicochemical and foaming properties of squid ovary powder. Food Hydrocolloids, 77, 286–296. https://doi.org/10.1016/j.foodhyd.2017.10.005
- Tan, H. F., & Gan, C. Y. (2016). Polysaccharide with antioxidant, α-amylase inhibitory and ACE inhibitory activities from Momordica charantia. International Journal of Biological Macromolecules, 85, 487–496. https://doi.org/10.1016/j.ijbiomac.2016.01.023
- Tang, W., Jin, L., Xie, L., Huang, J., Wang, N., Chu, B., Dai, X., Liu, Y., Wang, R., & Zhang, Y. (2017). Structural characterization and antifatigue effect in vivo of maca (Lepidium meyenii Walp) polysaccharide. Journal of Food Science, 82(3), 757–764. https://doi.org/10.1111/1750-3841.13619
- Trigui, I., Yaich, H., Sila, A., Rouhou, S. C., Bougatef, A., Blecker, C., Attia, H., & Ayadi, M. A. (2018). Physicochemical properties of water-soluble polysaccharides from black cumin seeds. International Journal of Biological Macromolecules, 117, 937–946. https://doi.org/10.1016/j.ijbiomac.2018.05.202
- Wang, Y., Xu, F., Cheng, J., Wu, X., Xu, J., Li, C., Li, W., Xie, N., Wang, Y., & He, L. (2022). Natural deep eutectic solvent-assisted extraction, structural characterization, and immunomodulatory activity of polysaccharides from Paecilomyces hepialid. Molecules, 27(22), 8020. https://doi.org/10.3390/molecules27228020
- Wang, S., Yang, J., Shao, G., Qu, D., Zhao, H., Yang, L., Zhu, L., He, Y., Liu, H., & Zhu, D. (2019). Soy protein isolated-soy hull polysaccharides stabilized O/W emulsion: Effect of polysaccharides concentration on the storage stability and interfacial rheological properties. Food Hydrocolloids, 101, 105490. https://doi.org/10.1016/j.foodhyd.2019.105490
- Wang, S., & Zhu, F. (2019). Chemical composition and health effects of maca (Lepidium meyenii). Food Chemistry, 288, 422–443. https://doi.org/10.1016/j.foodchem.2019.02.071
- Wu, X. H., Luo, M. S., Zhao, L., Wang, S. N., Zhu, D. S., Yang, L. N., & Liu, H. (2022). Emulsification characteristics of soy hull polysaccharides obtained by membrane separation. International Food Research Journal, 29(5), 1215–1225. https://doi.org/10.47836/ifrj.29.5.22
- Wu, H., Shang, H., Guo, Y., Zhang, H., & Wu, H. (2020). Comparison of different extraction methods of polysaccharides from cup plant (Silphium perfoliatum L.). Process Biochemistry, 90, 241–248. https://doi.org/10.1016/j.procbio.2019.11.003
- Zang, L., & Wang, M. (2017). Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita thunb. International Journal of Biological Macromolecules, 95, 675–681. https://doi.org/10.1016/j.ijbiomac.2016.11.096
- Zhang, M., Wang, G., Lai, F., & Wu, H. (2016). Structural characterization and immunomodulatory activity of a novel polysaccharide from Lepidium meyenii. Journal of Agricultural and Food Chemistry, 64(9), 1921–1931. https://doi.org/10.1021/acs.jafc.5b05610
