 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Heating by microwave irradiation could be an alternative for cooking quinoa and improving its content of phenolic compounds, which have health-promoting properties. The goal of this study was to improve the bioaccessibility of phenolics content and antioxidant activity of quinoa using an optimized method of microwave irradiation. The best microwave irradiation conditions obtained by maximizing the responses were 100% irradiation power and 14 min of irradiation time. The predicted and experimentally validated values for total phenols, flavonoids, and antioxidant activity were as follows: 686.46 vs 749.67 ± 36.62 µg GAE/g; 743.94 vs 803.37 µg QE/g and 4.20 vs 4.61 µM TE/g, respectively. At the gastric level, heating by microwave irradiation promoted the bioaccessibility (>100%) of total phenols, flavonoids, and antioxidant activity, as well as gallic and coumaric acids, quercetin, and kaempferol greater. The heating treatment by microwave in quinoa could be useful to obtain new products with bioactivity potential at gastric and intestinal levels.
1. Introduction
In recent years, quinoa has become very popular in human nutrition, particularly due to its high nutritional value and content of bioactive compounds (Repo-Carrasco-Valencia et al., Citation2010; Vidaurre-Ruiz et al., Citation2017). Clinical and experimental studies have shown that the consumption of pseudocereal grains is inversely associated with the development of several diseases, and the protective effects of the antioxidant activity are one of the most studied mechanisms (Ragaee et al., Citation2014). Several research studies have shown that the main phenolic compounds found in quinoa seeds are phenolic acids such as ferulic, caffeic, vanillic, and gallic acids and flavonoids such as routine, quercetin, kaempferol, myricetin among others (Hidalgo et al., Citation2018; Pellegrini et al., Citation2018; Tang et al., Citation2015; Vega Gálvez et al., Citation2018). The main interest of the study of bioactive compounds present in quinoa is due to its high biological potential and its protective effect attributed to antioxidant, anti-inflammatory, and gastroprotective activity (Hernández-Ledesma, Citation2019; Khursheed et al., Citation2020; Mariod & Salama, Citation2020; Pásko et al., Citation2019). In agreement with Gu et al. (Citation2021), these compounds can be free (65%) or bound (35%) depending on the quinoa cultivar studied. Thermal treatment in cereals and pseudocereals is considered an obligatory step prior to their consumption because it improves the sensory properties of the product as well as the nutritional and bioactive characteristics (Ragaee et al. Citation2014). Nickel et al. (Citation2016), Sharma et al. (Citation2022), and Zhang et al. (Citation2022) tested several thermal treatments in quinoa seeds such as moist heating – boiling and autoclaving; and dry heating – roasting and microwave processing. Huge variability was observed in these studies, particularly regarding the content of total phenols and antioxidant activity, which could be attributed to the quinoa cultivar used, as well as the type of extraction performed for the solubilization of phenolic compounds. Among thermal treatments applied to quinoa, boiling, roasting, and microwave irradiation stand out, as better promoters of a formation and/or release of phenolic compounds. Boiling involves immersing food in hot water, which can result in the leaching of water-soluble bioactive compounds. These compounds, such as vitamins and phenolic compounds, can be sensitive to heat and easily dissolve in cooking water, leading to their loss. In addition, the boiling often requires a relatively long cooking time, which can contribute to the degradation of heat-sensitive bioactive compounds. Prolonged exposure to high temperatures can result in the breakdown of certain compounds, diminishing their nutritional and health benefits (Dini et al., Citation2010; Vidaurre-Ruiz et al., Citation2017).
On the other hand, the use of roasting as a heat treatment can cause the thermal degradation of certain heat-sensitive bioactive compounds. This can result in a loss of nutritional value and diminished levels of bioactive compounds in the final product. It is also important to consider the formation of potentially harmful compounds attributed to the Maillard reaction (Sharanagat et al., Citation2019).
The microwave method involves a short cooking time compared to boiling or roasting. This can help preserve heat-sensitive bioactive compounds by minimizing their exposure to high temperatures and reducing their potential for degradation. Microwaving often requires minimal or no additional water, which helps retain water-soluble bioactive compounds. Since the food is cooked using the moisture already present (Guzik et al., Citation2022; Huang et al., Citation2022).
The interest in improving the content of phenolic compounds in quinoa seeds lies in promoting a better release of these compounds under conditions of gastrointestinal digestion. This requires evaluation of the potential absorption of the released component, which can mainly occur at the level of gastric and intestinal digestion. Despite advances in research on this topic, little information is available on the recovery of polyphenols present in quinoa after the gastrointestinal digestion process (in vitro). Pellegrini et al. (Citation2017) evaluated the effect of simulated gastrointestinal conditions on the total phenols content, flavonoids, phenolic profile, and antioxidant activity of six cultivars of quinoa seeds (colored or not colored). They reported that the total phenol and flavonoids content showed variability depending on the quinoa cultivar studied and the gastrointestinal phase (oral, gastric, and intestinal) compared with undigested samples.
In a similar study, Balakrishnan and Schneider (Citation2020) evaluated effect of simulated gastrointestinal digestion of quinoa (seeds, sprouts, and flakes) on release flavonoids, they found an increase of quercetin and kaempferol on the gastric phase compared with no digested quinoa. Gu et al. (Citation2021) studied the effects of four cooking methods including germination, baking, normal pressure steaming, and high-pressure steaming treatments, on antioxidant capacity and bioactive compounds bioaccessibility of different colored quinoa. They found that germination treatment was a process that showed better results under simulated gastrointestinal digestion conditions. Referring to these studies shown above could be of great interest to generate information on the content of bioactive compounds after simulated gastrointestinal digestion of quinoa thermally treated with an optimized method of microwave irradiation.
Therefore, the aim of our study was to show the bioactive potential of quinoa that has been thermally treated by an optimized microwave process and its behavior under in vitro gastrointestinal digestion conditions.
2. Materials and methods
2.1. Sample collection
Quinoa (Chenopodium quinoa Willd) was obtained commercially from a local market in the city of Hermosillo, Sonora, Mexico. According to the specifications shown on the product label, it was whole organic quinoa, white and free of saponins.
2.2. Optimization process
To perform the optimization study, a central composite design was carried out, which consisted of 13 treatments evaluating the following factors: Time (T) and Power (P) with maximum and minimum levels and a central point (five repetitions). The response variables considered for the optimization study were total phenol content (TPC), total flavonoid content (TFC), and antioxidant activity (ABTS). Microwave heating was applied to each of the treatments according to the previously established conditions, as shown in . Raw quinoa (RQ) without heating treatment was used as control.
Table 1. Effects of the time and power factors of microwave cooking of quinoa on TPC, TFC, and TEAC.a,b
2.3. Microwave treatment
For the application of the microwave treatment, 10 g of quinoa were used, which were placed in a microwave container, and purified water was added (the volume of water added was variable depending on the conditions established for each treatment). Then the sample was placed in a conventional microwave oven (LG, model MS2047GR, with maximum power of 1650 watts equivalent to 100% of power) and microwave irradiation was subsequently applied. To stop cooking, the container was subjected to rapid cooling in an ice bath. Then, the sample cooked was placed in aluminum trays and dried at a temperature of 45–55°C, dried sample was grounded into powder, and labeled as MQ (microwaved quinoa) and stored at −20°C.
2.4. Extraction of free phenolics
Methanolic extraction was carried out on the MQ and RQ samples in accordance with the procedure described by Salazar Lopez et al. (Citation2016). In a 15-mL conical tube, 1 g of sample was weighed and mixed with 15 mL of 80% methanol. The mixture was sonicated at room temperature for 60 min. Next, the sample was centrifuged at 150 × g/15 min, and the supernatant was separated. The residue was subjected to the same procedure, and the supernatants were collected and filtered on Whatman Num 1 paper. The supernatants of the sample were evaporated in a rotavapor (BUCHI Labortechnik AG R-100, Flawil, Switzerland) until dry and resuspended in 10 mL of methanol at 50% (final concentration of 0.1 g/mL). This extract was stored in amber tubes at −20°C until further analysis.
2.5. Determination of total phenolic content (TPC) and total flavonoids content (TFC)
For TPC, the procedure reported by Ruiz-Hernández et al. (Citation2022) was followed. Thirty microliters of extracts were placed in microplate wells and subsequently mixed with 120 µL of 7% sodium carbonate solution. Then, 150 µL of Folin-Ciocalteu reagent (diluted 1:9) was added. The mixture was allowed to incubate at room temperature for 60 min before absorbance readings were taken using a microplate reader (FluoStar Omega, BMG Labtech Inc., Ortenberg, Germany) at a wavelength of 765 nm. The results of TPC were expressed as µg of gallic acid equivalents (GAE) per gram.
For TFC quantification, the aluminum chloride method was used following the procedure described by Valenzuela-González et al. (Citation2022). The assay consisted of placing 30 µL of sample from each of the extracts in microplate wells, adding 9 µL NaNO2 and then 120 µL H2O, incubating for 5 min, then adding 9 µL AlCl3, and then incubating again for 5 min before 60 µL 1 M NaOH + 72 µL H2O were added. After mixing, the absorbance readings at 415 nm were immediately taken using a microplate reader (FluoStar Omega, BMG Labtech Inc., Ortenberg, Germany). The results of TFC were expressed as µg of quercetin equivalents (QE) per gram.
2.6. Antioxidant capacity to sequester 2,2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS • +) (ABTS)
The ABTS assay was performed based on a previous study by Ruiz-Hernández et al. (Citation2022). A stable ABTS stock solution was prepared by mixing 5 mL of an aqueous ABTS solution (7 mM) with 0.088 mL of potassium persulfate (148 mM) and incubating in the dark at room temperature for 12–16 h. The ABTS•+ working solution was prepared before use by diluting the stock solution in ethanol (1:88, v/v), and its absorbance was adjusted to 0.7 ± 0.02 at 734 nm. A total of 280 μL of the working solution with 20 μL of extract was placed in a microplate and allowed to react at rest for 5 min. The results of antioxidant activity were expressed as TEAC (Trolox equivalents antioxidant capacity) (µM TE/g) according to a Trolox calibration curve.
2.7. Phenolic compounds determination by Ultra high-performance liquid chromatography (UHPLC-DAD)
Phenolic compounds were quantified following the methodology described by Lee et al. (Citation2012) with light modifications. Briefly, the components were separated using an Agilent Binary LC system (1260 Infinity, Santa Clara, CA, U.S.A.) coupled to diode array detector (1290 Infinity, Santa Clara, CA, U.S.A.). Three phenolic acids and four flavonoids were separated with a Zorbax Eclipse Plus C18 (2.1 × 50 mm 1.8 Micron) at 30°C. The mobile phase consisted of a linear gradient of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) as follows: 97–93% A, 0–3min; 93–90% A, 3–5min; 90–88% A, 5–8min; 88–85% A, 8–10 min; 85% A, 10–15 min; 85–45% A, 15–18 min; 45–10% A, 18–20 min; 10–97% A, 20–23 min. The flow rate was 0.4 mL/min, and the injection volume was 10 μL. The phenolic compounds were identified at different wavelengths as follows: gallic acid (280 nm); coumaric and ferulic acids (320 nm); routine, myricetin, quercetin, and kaempferol (360 nm) by comparing the retention time and UV spectra peaks of samples with those of pure reference standards. The results were expressed as µg phenolic acid per gram of dry weight.
2.8. In vitro gastrointestinal digestion assay
The in vitro gastrointestinal digestion of samples was performed following the method described by Salazar-López et al. (Citation2018) subjecting the samples to oral, gastric, and intestinal digestion phases. It is important to highlight that all tests followed ethical guidelines (Council for International Organizations of Medical Sciences, Citation2016). For the oral phase, the participation of two apparently fasting healthy volunteers (normal weight and without the presence of chronic non-communicable diseases) belonging to the Functional Food Laboratory staff was required. This process was simulated by chewed one gram of MQ or RQ samples for 15 s. Subsequently, the subject expelled the chewed sample, and it was placed in 50 mL conical tubes, and rinsed their mouth twice with 5 mL of water for 60 s, and the liquid was collected and kept at 37°C for 3 min. The phenolics released to the supernatant at this stage were considered as the phenolics present in the oral fraction. Subsequently, the samples were subjected to an enzymatic hydrolysis process with 5 mL of 0.2 M HCl-KCl buffer solution and adjusted pH to 1.5 with HCl (6 M). Then, 667 μL of pepsin solution (300 mg/mL) were added, and the tubes were incubated for 1 h in a water bath with constant shaking at 37°C (Precision Scientific Mod. 66800 Winchester, VA, U.S.A.). The phenolics released to the supernatant at this stage were considered as phenolics present in the gastric fraction.
For the intestinal digestion nine mL of phosphate solution (0.1 M, pH 7.5) was added to each of the tubes from gastric digestion, and the pH was adjusted to 7.5. Then, 1 mL of pancreatin solution (17 mg/mL) and bile salts (80 mg) were added, and the mixture was incubated for 6 h in a shaking water bath at 37°C. Each phase of the digestion had a reagent blank, and all tubes from each one digestion phases were taken to centrifugation for 10 min at 700 × g/4°C; The recovered supernatants were frozen at −80°C and subsequently lyophilized. Lyophilized digests and blanks were re-dissolved in 50% methanol, filtered (Econofltr Nyln 0.25 mm 0.45 μm, Santa Clara, CA, United States), and stored at −15°C in amber vials until the corresponding analyses.
The bioaccessibility expressed in terms of percentage of the phenolic compounds present in MQ or RQ was calculated according to the following equation:
TEAC values were also analyzed by this procedure and expressed as an antioxidant activity recovery percentage.
2.9. Statistical analysis
To evaluate the conditions of microwave irradiation for optimizing the TPC, TFC and TEAC of quinoa, a two-factor and three-level, face centered central composite design that included five central points was used. The factors evaluated were the radiation time (X1: 14, 16, and 18 min) and power (X2: 60, 80 and 100%). Thirteen experiments were performed according to the experimental design, and a repetition of the experiment was considered (). To analyze the experimental data, regression analysis using response surface methodology was used. The experimental data were fitted to a second-order polynomial model, and regression coefficients were obtained; the model is shown in EquationEquation 1(1)
(1) :
where Y is the predicted response variable (TPC, TFC, and TEAC), β0 (intercept), β1 and β2 (linear terms) are regression coefficients, β11 and β22 (quadratic terms), β12 (interaction term), and X1 and X2 are the independent variables. All experimental runs were carried out in triplicate. The statistical significance of the terms in the regression equations was examined by variance analysis (ANOVA) for each response variable. The goodness of fit of the obtained models was evaluated by calculating the coefficients of multiple determination (R2). The data were analyzed using Minitab statistical software (Minitab LLC, State College, PA, U.S.A.), and statistical software (STATISTICA 10, TIBCO, Palo Alto, CA, U.S.A.) was used to generate the response surface. A validation test was carried out using the optimal conditions obtained by the prediction models.
3. Results
3.1. Optimization study
In , the average values obtained from the evaluated treatments for all response variables are shown. The ANOVA results show that the selected quadratic models adequately represented the data obtained for all responses (). Multiple regression coefficients were determined using the least squares technique to predict the quadratic polynomial models for the TPC, TFC, and TEAC of the microwave-irradiated quinoa. The results suggest that the quadratic term of power and interaction term had a negligible effect on the TPC (p > .05). All terms except the interaction term of the second-order polynomial equation had a strong significant influence on the TFC and TEAC. The values observed for the adjusted determination coefficient R2 = 0.30, 0.61 and 0.40 for TPC, TFC, and antioxidant activity, respectively, demonstrate the adequacy of the quadratic model for explaining the variation in the content of response variables because of the microwave radiation conditions. The regression analysis applied to the TPC, TFC, and TEAC results shows that the lack of fit was significant at p < .001, and the models could explain 34%, 64%, and 44%, respectively. The relationship between independent and dependent variables is illustrated in a three-dimensional representation on the response surface generated by the prediction models. shows the quadratic effect of time (T) and power (P) irradiation on the TPC (), TFC (), and TEAC (). Low values were observed during the first minutes of microwave irradiation until reaching maximum values at increased irradiation times.
Figure 1. Response surfaces for the TPC (total phenol content) (a), TFC (total flavonoid content) (b), and antioxidant activity determined by TEAC assay (c) in quinoa as a function of the microwave irradiation time and power.
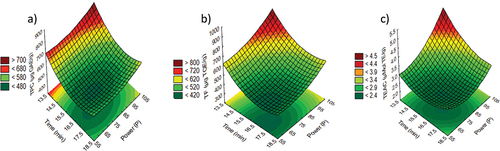
Table 2. Coefficients of the regression equations for TPC, TFC, and TEAC.a
Unlike the TFC and TEAC, the quadratic term of power was not significant for the TPC, which increased linearly with increasing time. The values predicted by the polynomial equations were optimized for each of the dependent variables, and it was found that the same conditions of time (14 min) and power (100%) had a higher influence on the TPC (686.46 µg GAE/g), TFC (743.94 µg QE/g) and TEAC (4.20 μM TE/g), to exception TFC, these values were higher than that RQ. These results were validated experimentally (TPC: 749.67 ± 36.62 µg GAE/g, TFC: 803.37 ± 27.0 µg QE/g, TEAC: 4.61 ± 0.16 μM TE/g), and the percentages of variation between the predicted and experimental values were not greater than 10%. The experimental values were considered for subsequent discussions.
shows the TPC, TFC, and antioxidant activity values for RQ and MQ samples, as well as the individual phenolics. The use of microwaves to cook quinoa to exception TFC promoted a greater release of TPC, antioxidant activity and all individual phenolic compounds evaluated than that reported for RQ (p < .05).
Table 3. Total phenol content (TPC), total flavonoid content (TFC), antioxidant activity (TEAC), and individual phenolic compounds of methanolic extracts of microwave-optimized quinoa (MQ) and raw quinoa (RQ).a
3.2. In vitro gastrointestinal digestion and bioaccessibility
It is interesting to show that the application of a treatment to food can improve the phenolic profile and eventually antioxidant activity. This could mean that the applied treatment makes the bioactive compound more accessible. However, the term changes to “bioaccessible” when the food is subjected to a gastrointestinal digestion process.
Therefore, bioaccessibility can be defined as the ability of a nutrient or bioactive compound to be released from the food matrix by the action of gastrointestinal digestion and be available for absorption (Shahidi & Peng, Citation2018). shows the values of TPC, TFC, and TEAC (lines) and their respective bioaccessibility (bars) for RQ and MQ samples after each of the gastrointestinal digestion phases. Oral digests of both RQ and MQ showed lower TPC, TFC, and TEAC values than those of gastric and intestinal digestions. From oral to gastric digestion, an increase was observed in TPC, TFC, and TEAC values for RQ and MQ samples, this increase was notably higher in MQ samples (TPC: 2973.35 ± 58.4 µg GAE/g, TFC: 796.93 ± 28.8 µg QE/g and TEAC: 1.51 ± 0.10 µM TE/g). For the intestinal phase, RQ showed the highest values of TPC (1979.26 ± 71 µg GAE/g), TFC (516.49 ± 12.6 µg QE/g), and TEAC (4.44 ± 0.08 µM TE/g) ().
Figure 2. Total phenolic content (TPC) (a,b), Total flavonoid content (TFC) (c,d) and antioxidant activity (TEAC) (e,f) (lines), after simulated gastrointestinal digestion of RQ (raw quinoa) and MQ (microwaved quinoa). Bars represent bioaccessibility of TPC, TFC and TEAC calculated from undigested extract concentrations considered as 100%.
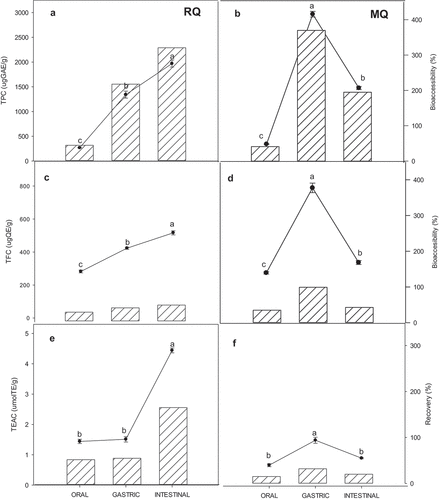
In the same figure, it is possible to observe the bioaccessibility values (%) for each variable, considering the values reported before digestion (methanolic extracts) as 100%. In the RQ digests, the bioaccessibility of TPC, TFC, and TEAC was higher in the intestinal phase than in the oral and gastric digestions, with values that exceeded 100% (TPC: 318%, TEAC: 170%). In the MQ digests, higher bioaccessibility values were found in the gastric phase.
Regarding individual phenolic acids and flavonoid contents and their bioaccessibiliy, these were examined during the in vitro digestion process for RQ and MQ, and the results are shown in .
Table 4. Phenolic acids and flavonoids (µg/g) and bioaccessibility (%) in microwaved quinoa after in vitro gastrointestinal digestion.a
The digestion of MQ in the oral phase promoted the increase in the content of coumaric acid, ferulic acid, routine, and myricetin, while in digests from the gastric-phase routine and myricetin did not show changes with respect to RQ digests, while for the flavonoid quercetin, it was observed that gastric digestion conditions did not favor a greater release in MQ. In MQ intestinal digests, no increases in phenol content were found, except for coumaric acid and kaempferol. Considering as 100% the content of these compounds in the MQ samples prior to the digestion process, it is possible to determine the percentage of bioaccessibility (% B) of the same, and despite the fact that most of the phenolic compounds did not exceed the values presented for RQ, it is relevant to mention that in the gastric phase the bioaccessibility of gallic acid, coumaric acid, quercetin, and kaempferol reached values greater than 100%. While in the intestinal phase only ferulic acid reached values greater than 100%.
4. Discussion
Several studies have shown that quinoa cultivars used for microwave irradiation treatment can show ample variability in phenolic content. Zhang et al. (Citation2022) evaluated the effect of microwave irradiation in black quinoa. They reported results notably higher for TPC (2 640.00 ± 30.0 µg GAE/g), TFC (3 880.0 ± 270.0 µg RE/g) and TEAC (76.77 ± 6.55 µM TE/g), that obtained in our study (749.67 ± 36.6 µg GAE/g; 803.37 ± 27.0 QE/g; and 4.61 ± 0.16 µM TE/g), in which white quinoa was used.
However, the quinoa cultivar could not be a main factor of variation in phenolic content since that a similar study carried out by Sharma et al. (Citation2022) in white quinoa microwaved for 5 min after moisture conditioning reported similar higher values for total phenols and flavonoids content (2 230.0 ± 60.0 µg GAE/g; 13400.0 ± 0.0 µg QE/g respectively) than those reported in our study. For the above, the microwave quinoa processing conditions as well as phenolic extraction conditions could be also factors promoting variability in the results of total phenols. In our study, organic white quinoa free of saponins was used, and the microwave process was applied with high humidity to achieve more uniform cooking of the product. It is worth mentioning that we used an extraction system based on aqueous methanol, a system regularly used for the extraction of free phenols that differs from other studies where acidified methanol or ethanol have been used.
As shown in , the TPC and TEAC values evaluated in quinoa treated by an optimized method of microwaved increased by approximately 20% y 77%, respectively, compared to quinoa without thermal treatment. Similarly, the individual phenols evaluated in this study increased significantly in relation to raw quinoa, in terms of percentage the values in descending order were as follows: gallic acid (66%), ferulic acid (63%), coumaric acid (39%), routine (28%), myricetin (10%), and quercetin (2%), while kaempferol was not detected in RQ. Various studies have shown that different cooking methods cause differences in the physicochemical and functional properties of food components, depending on processing parameters, conditions, and food sources. The varied composition of polyphenols provided by different types of cooking methods can also modify the biological activities of foods (Dini et al., Citation2010; Nickel et al., Citation2016; Sharma et al., Citation2022; Zhang et al., Citation2022). On the other hand, it is important to point out that since there should not be an appreciable biosynthesis of phenolic compounds during cooking, an increase in phenolic content is indicative of an increase in recoverable compounds (Burgos et al., Citation2013).
Microwave is a method that differs from conventional heat treatments, it is based on the application of microwaves to cook food by exposing it to electromagnetic radiation in the microwave frequency range and induces polar molecules in food to rotate and produce thermal energy in a process known as dielectric heating (Huang et al., Citation2022; Zhang et al., Citation2022).
The way in which this process promotes loss and/or gain of phenolic compounds is explained by the fact that plants contain moisture (micro or macroscopic) that serves as a target for microwave heating. Plant tissues, cell walls, and their by-products within the plant matrix interact with the emitted radiation waves. This interaction results in the heating of moisture trapped within the plant matrix which causes evaporation of moisture from the plant matrix. This process causes considerable pressure to be exerted on plant cell walls at the cellular and subcellular level, resulting in swelling of the plant cells during the process. This swelling ultimately causes structural changes in the plant matrix, thus promoting greater solute mass transfer due to cell rupture. This, in turn, facilitates the leaching of phytochemicals from the plant cell matrix to the outside (Ameer et al., Citation2017).
Although the effects on the composition of phenols in foods treated by microwaves can be controversial, various studies have consistently explained how losses in these components could occur. Some explanations could be that (a) water-soluble polyphenols leach into the cooking water, (b) the decomposition of the polyphenols (c) the polyphenols that participate in the Maillard reaction could contribute to the decrease in TPC values after cooking (Tian et al., Citation2016).
On the other hand, there have been studies related to the increase in phenolic compounds after a thermal action including microwave process, such as (a) the release of polyphenols from the plant cell matrix and intracellular complexes, forming the corresponding free phenolic compounds and improved the extract ability of the phenolic compounds from the cellular matrix and release of dietary fiber-bound polyphenols (Ruiz-Rodríguez et al., Citation2008).
The findings found in our study in relation to the effect of applying heat treatment by microwave irradiation under optimized conditions on the content of phenolic compounds and antioxidant activity in white quinoa are relevant. However, great interest has been aroused in evaluating the effect of these phenolic compounds in heat-treated quinoa once they have been subjected to gastrointestinal digestion processes. In vitro methods to simulate the gastrointestinal digestion of food matrices have become a good tool as an alternative to the use of in vivo methods. Static-type tests such as those used in our study give the possibility of evaluating the behavior of phenolic compounds in food matrices under gastrointestinal digestion conditions, as well as offering information about the release capacity of phenolic compounds and potentially being absorbable (Minekus et al., Citation2014; Rasera et al., Citation2023).
It is well known that gastrointestinal digestion conditions (pH, enzymatic activity), the possible interactions that occur between the components during digestion, and the structure of the food matrix, among other factors, can have a relevant influence on the reduction and/or increase of phenolic components (Velderrain-Rodríguez et al., Citation2014).
In our study, notably, the recovery of phenolic compounds at the intestinal level derived from the digestion of RQ and MQ could potentially be absorbed and thus exert their systemic action on target organs. But no less relevant could be finding a greater recovery of total phenols at the gastric level, particularly due to the gastroprotective effect these compounds could exert. Several studies report gastroprotective activity attributed to phenolic compounds present in various food matrices including quinoa subjected to various types of processes (fermented, germinated, heating under pressure) (Mariod & Salama, Citation2020; Ruiz-Hurtado et al., Citation2021; Sun et al., Citation2021). As well as individual phenolics such as quercetin and kaempferol have been studied by their gastroprotective effects (Chiu et al., Citation2021; de Lira et al., Citation2009). According to the findings found in our study, white quinoa subjected to microwave heating could offer a promising new therapy in the treatment of the acute gastric injury.
5. Conclusions
As outlined in this paper, the recoveries of phenolic compounds found in quinoa seeds (raw and microwaved) after an in vitro digestion process varied widely. The intestinal digestion conditions were more favorable to release phenolics found in RQ, and although raw quinoa seeds may not be sensorial acceptable, it is suggested that they could possibly be used as a food additive.
On the other hand, gastric digestion conditions were favorable to release phenolics found in MQ, particularly gallic acid, coumaric acid, quercetin, and kaempferol. Future studies could be focused on the beneficial effects of the compounds present in microwaved quinoa and their products exploring the influence in vivo as gastroprotective agents.
Author contributions
M.V.G; R.M.R.S conceived and designed the experiment, M.V.G. performed the experiment, M.V.G., R.M.R.S, and J.L.C.L analyzed the data and wrote the paper. All authors read and improved the manuscript.
Acknowledgment
Valenzuela Gonzalez M. received a scholarship from CONAHCyT (National Research, Technology and Humanities Council), Mexico.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Council for International Organizations of Medical Sciences (CIOMS). (2016). International ethical guidelines for health-related research involving humans (4th ed.). CIOMS
- Ameer, K., Shahbaz, H. M., & Kwon, J. H. (2017). Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Comprehensive Reviews in Food Science and Food Safety, 16(2), 295–315. https://doi.org/10.1111/1541-4337.12253
- Balakrishnan, G., & Schneider, R. G. (2020). Quinoa flavonoids and their bioaccessibility during in vitro gastrointestinal digestion. Journal of Cereal Science, 95(May), 103070. https://doi.org/10.1016/j.jcs.2020.103070
- Burgos, G., Amoros, W., Muñoa, L., Sosa, P., Cayhualla, E., Sanchez, C., Díaz, C., & Bonierbale, M. (2013). Total phenolic, total anthocyanin and phenolic acid concentrations and antioxidant activity of purple-fleshed potatoes as affected by boiling. Journal of Food Composition and Analysis, 30(1), 6–12. https://doi.org/10.1016/j.jfca.2012.12.001
- Chiu, H. F., Venkatakrishnan, K., Golovinskaia, O., & Wang, C. K. (2021). Gastroprotective effects of polyphenols against various gastro-intestinal disorders: A mini-review with special focus on clinical evidence. Molecules, 26(7), 2090. https://doi.org/10.3390/molecules26072090
- de Lira Mota, K. S., Dias, G. E. N., Pinto, M. E. F., Luiz-Ferreira, Â., Monteiro Souza-Brito, A. R., Hiruma-Lima, C. A., Barbosa-Filho, J. M., & Batista, L. M. (2009). Flavonoids with gastroprotective activity. Molecules, 14(3), 979–1012. https://doi.org/10.3390/molecules14030979
- Dini, I., Tenore, G. C., & Dini, A. (2010). LWT - Food science and technology antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT - Food Science and Technology, 43(3), 447–451. https://doi.org/10.1016/j.lwt.2009.09.010
- Gu, R., Chang, X., Bai, G., Li, X., Di, Y., Liu, X., Sun, L., & Wang, Y. (2021). Effects of household cooking methods on changes of tissue structure, phenolic antioxidant capacity and active component bioaccessibility of quinoa. Food Chemistry, 350(October 2020), 129138. https://doi.org/10.1016/j.foodchem.2021.129138
- Guzik, P., Szymkowiak, A., Kulawik, P., Zając, M., & Migdał, W. (2022). The confrontation of consumer beliefs about the impact of microwave-processing on food and human health with existing research. Trends in Food Science & Technology, 119(May 2021), 110–121. https://doi.org/10.1016/j.tifs.2021.11.011
- Hernández-Ledesma, B. (2019). Quinoa (Chenopodium quinoa Willd.) as a source of nutrients and bioactive compounds: A review. Bioactive Compounds in Health and Disease, 2(3), 27–47. https://doi.org/10.31989/bchd.v2i3.556
- Hidalgo, A., Ferraretto, A., De Noni, I., Bottani, M., Cattaneo, S., Galli, S., & Brandolini, A. (2018). Bioactive compounds and antioxidant properties of pseudocereals-enriched water biscuits and their in vitro digestates. Food Chemistry, 240, 799–807. https://doi.org/10.1016/j.foodchem.2017.08.014
- Huang, K., Shi, J., Li, M., Sun, R., Guan, W., Cao, H., Guan, X., & Zhang, Y. (2022). Intervention of microwave irradiation on structure and quality characteristics of quinoa protein aggregates. Food Hydrocolloids, 130(February), 107677. https://doi.org/10.1016/j.foodhyd.2022.107677
- Khursheed, R., Singh, S. K., Wadhwa, S., Gulati, M., & Awasthi, A. (2020). Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems. Drug Discovery Today, 25(1), 209–222. https://doi.org/10.1016/j.drudis.2019.11.001
- Lee, K. M., Kalyani, D., Tiwari, M. K., Kim, T. S., Dhiman, S. S., Lee, J. K., & Kim, I. W. (2012). Enhanced enzymatic hydrolysis of rice straw by removal of phenolic compounds using a novel laccase from yeast yarrowia lipolytica. Bioresource Technology, 123, 636–645. https://doi.org/10.1016/j.biortech.2012.07.066
- Mariod, A. A., & Salama, S. M. (2020). The efficacy of processing strategies on the gastroprotective potentiality of Chenopodium quinoa seeds. Scientific World Journal, 2020, 1–16. https://doi.org/10.1155/2020/6326452
- Minekus, M., Alminger, M., Alvito, P., Ballance, S., Bohn, T., Bourlieu, C., Carrière, F., Boutrou, R., Corredig, M., Dupont, D., Dufour, C., Egger, L., Golding, M., Karakaya, S., Kirkhus, B., Le Feunteun, S., Lesmes, U., MacIerzanka, A., MacKie, A., … Brodkorb, A. (2014). A standardised static in vitro digestion method suitable for food – an international consensus. Food and Function, 5(6), 1113–1124. https://doi.org/10.1039/c3fo60702j
- Nickel, J., Spanier, L. P., Botelho, F. T., Gularte, M. A., & Helbig, E. (2016). Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd grains. Food Chemistry, 209, 139–143. https://doi.org/10.1016/j.foodchem.2016.04.031
- Paśko, P., Tyszka-Czochara, M., Namieśnik, J., Jastrzębski, Z., Leontowicz, H., Drzewiecki, J., Martinez-Ayala, A. L., Nemirovski, A., Barasch, D., & Gorinstein, S. (2019). Cytotoxic, antioxidant and binding properties of polyphenols from the selected gluten-free pseudocereals and their by-products: In vitro model. Journal of Cereal Science, 87, 325–333. https://doi.org/10.1016/j.jcs.2019.04.009
- Pellegrini, M., Lucas-Gonzales, R., Ricci, A., Fontecha, J., Fernandez-Lopez, J., Perez-Alvarez, J. A., & Viuda-Martos, M. (2018). Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Industrial Crops and Products, 111, 38–46. https://doi.org/10.1016/j.indcrop.2017.10.006
- Pellegrini, M., Lucas-Gonzalez, R., Fernández-López, J., Ricci, A., Pérez-Álvarez, J. A., Sterzo, C. L., & Viuda-Martos, M. (2017). Bioaccessibility of polyphenolic compounds of six quinoa seeds during in vitro gastrointestinal digestion. Journal of Functional Foods, 38, 77–88. https://doi.org/10.1016/j.jff.2017.08.042
- Ragaee, S., Seetharaman, K., & Abdel-Aal, E. S. M. (2014). The impact of milling and thermal processing on phenolic compounds in Cereal grains. Critical Reviews in Food Science and Nutrition, 54(7), 837–849. https://doi.org/10.1080/10408398.2011.610906
- Rasera, G. B., de Camargo, A. C., & de Castro, R. J. S. (2023). Bioaccessibility of phenolic compounds using the standardized INFOGEST protocol: A narrative review. Comprehensive Reviews in Food Science and Food Safety, 22(1), 260–286. https://doi.org/10.1111/1541-4337.13065
- Repo-Carrasco-Valencia, R., Hellström, J. K., Pihlava, J. M., & Mattila, P. H. (2010). Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chemistry, 120(1), 128–133. https://doi.org/10.1016/j.foodchem.2009.09.087
- Ruiz Hernández, A. A., Rouzaud Sández, O., Frías, J., Ayala Zavala, F., Astiazarán García, H., & Robles Sánchez, M. (2022). Optimization of the duration and intensity of UV-A radiation to obtain the highest free phenol content and antioxidant activity in sprouted sorghum (sorghum bicolor L Moench). Plant Foods for Human Nutrition, 77(2), 317–318. https://doi.org/10.1007/s11130-021-00938-z
- Ruiz-Hurtado, P. A., Garduño-Siciliano, L., Dominguez-Verano, P., Martinez-Galero, E., Canales-Martinez, M. M., & Rodriguez-Monroy, M. A. (2021). Evaluation of the gastroprotective effects of Chihuahua propolis on indomethacin-induced gastric ulcers in mouse. Biomedicine & Pharmacotherapy, 137, 111345. https://doi.org/10.1016/j.biopha.2021.111345
- Ruiz-Rodríguez, A., Marín, F. R., Ocaña, A., & Soler-Rivas, C. (2008). Effect of domestic processing on bioactive compounds. Phytochemistry Reviews, 7(2), 345–384. https://doi.org/10.1007/s11101-007-9073-1
- Salazar-López, N. J., González-Aguilar, G. A., Rouzaud-Sández, O., & Robles-Sánchez, M. (2018). Bioaccessibility of hydroxycinnamic acids and antioxidant capacity from sorghum bran thermally processed during simulated in vitro gastrointestinal digestion. Journal of Food Science and Technology, 55(6), 2021–2030. https://doi.org/10.1007/s13197-018-3116-z
- Salazar Lopez, N. J., Loarca-Piña, G., Campos-Vega, R., Gaytán Martínez, M., Morales Sánchez, E., Esquerra-Brauer, J. M., Gonzalez-Aguilar, G. A., & Robles Sánchez, M. (2016). The extrusion process as an alternative for improving the biological potential of sorghum bran: Phenolic compounds and Antiradical and anti-inflammatory capacity. Evidence-Based Complementary and Alternative Medicine, 2016(September), 1–8. https://doi.org/10.1155/2016/8387975
- Shahidi, F., & Peng, H. (2018). Bioaccessibility and bioavailability of phenolic compounds. Journal of Food Bioactives, 4, 11–68. https://doi.org/10.31665/JFB.2018.4162
- Sharanagat, V. S., Suhag, R., Anand, P., Deswal, G., Kumar, R., Chaudhary, A., Singh, L., Singh Kushwah, O., Mani, S., Kumar, Y., & Nema, P. K. (2019). Physico-functional, thermo-pasting and antioxidant properties of microwave roasted sorghum [Sorghum bicolor (L.) Moench]. Journal of Cereal Science, 85(December 2018), 111–119. https://doi.org/10.1016/j.jcs.2018.11.013
- Sharma, S., Kataria, A., & Singh, B. (2022). Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (Chenopodium quinoa). Lwt, 160(February), 113256. https://doi.org/10.1016/j.lwt.2022.113256
- Sun, Y., Ma, N., Yi, J., Zhou, L., & Cai, S. (2021). Gastroprotective effect and mechanisms of Chinese sumac fruits (Rhus chinensis Mill.) on ethanol-induced gastric ulcers in mice. Food & Function, 12(24), 12565–12579. https://doi.org/10.1039/D1FO02864B
- Tang, Y., Li, X., Zhang, B., Chen, P. X., Liu, R., & Tsao, R. (2015). Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chemistry, 166, 380–388. https://doi.org/10.1016/j.foodchem.2014.06.018
- Tian, J., Chen, J., Ye, X., & Chen, S. (2016). Health benefits of the potato affected by domestic cooking: A review. Food Chemistry, 202, 165–175. https://doi.org/10.1016/j.foodchem.2016.01.120
- Valenzuela-González, M., Rouzaud-Sández, O., Ledesma-Osuna, A. I., Astiazarán-García, H., Salazar-López, N. J., Vidal-Quintanar, R. L., & Robles-Sánchez, M. (2022). Bioaccessibility of phenolic compounds, antioxidant activity, and consumer acceptability of heat-treated quinoa cookies. Food Science & Technology, 2061, 1–8. https://doi.org/10.1590/fst.43421
- Vega Gálvez, A., Zura, L., Lutz, M., Jagus, R. J., Agüero, M. V., Pastén, A., Scala, K. D., & Uribe, E. (2018). Assessment of dietary fiber, isoflavones and phenolic compounds with antioxidant and antimicrobial properties of quinoa (Chenopodium quinoa Willd). Chilean Journal of Agricultural & Animal Sciences, 34(1), 57–67. https://doi.org/10.4067/S0719-38902018005000101
- Velderrain-Rodríguez, G. R., Palafox-Carlos, H., Wall-Medrano, A., Ayala-Zavala, J. F., Chen, C. Y. O., Robles-Sánchez, M., Astiazaran-García, H., Alvarez-Parrilla, E., & González-Aguilar, G. A. (2014). Phenolic compounds: Their journey after intake. Food and Function, 5(2), 189–197. https://doi.org/10.1039/c3fo60361j
- Vidaurre-Ruiz, J. M., Días-Rojas, G., Mendoza-Llamo, E., & Solano-Cornejo, M. Á. (2017). Variación del contenido de Betalaínas, compuestos fenólicos y capacidad antioxidante durante el procesamiento de la quinua (Chenopodium quinoa W). Revista de la Sociedad Química del Perú, 83(3), 319–330. https://doi.org/10.37761/rsqp.v83i3.116
- Zhang, Y., Yan, Y., Li, W., Huang, K., Li, S., Cao, H., & Guan, X. (2022). Microwaving released more polyphenols from black quinoa grains with hypoglycemic effects compared with traditional cooking methods. Journal of the Science of Food and Agriculture, 102(13), 5948–5956. https://doi.org/10.1002/jsfa.11947
